Abstract
OBJECTIVES
The comparative effectiveness and safety of proton pump inhibitors (PPIs) versus histamine-2 receptor blockers for stress ulcer prophylaxis in the cardiac surgical intensive care unit population is uncertain. Although the Proton Pump Inhibitors versus Histamine-2 Receptor Blockers for Ulcer Prophylaxis Therapy in the Intensive Care Unit (PEPTIC) trial reported a higher risk of mortality in the PPI arm with no difference in gastrointestinal bleeding, detailed information on surgical variables and clinically relevant surgical subgroups was not available.
METHODS
The analysis included all Canadian cardiac surgery patients enrolled in the PEPTIC trial. Data were electronically linked using unique patient identifiers to a clinical information system. Outcomes of interest included in-hospital mortality, gastrointestinal bleeding, Clostridium difficile infections, ventilator-associated conditions and length of stay.
RESULTS
We studied 823 (50.6%) randomized to PPIs and 805 (49.4%) to histamine-2-receptor blockers. In the intention-to-treat analysis, there were no differences in hospital mortality [PPI: 4.3% vs histamine-2 receptor blockers: 4.8%, adjusted odds ratio (aOR) 0.97, 95% confidence interval (CI) 0.55–1.70], gastrointestinal bleeding (3.9% vs 4.8%, aOR 1.09, 95% CI 0.66–1.81), C. difficile infections (0.9% vs 0.1%, aOR 0.18, 95% CI 0.02–1.59), ventilator-associated conditions (1.6% vs 1.7%, aOR 0.92, 95% CI 0.85–1.00) or median length of stay (9.2 vs 9.8 days, adjusted risk ratio 1.06, 85% CI 0.99–1.13). No significant treatment differences were observed among subgroups of interest or per-protocol populations.
CONCLUSIONS
In a secondary analysis of cardiac surgery patients enrolled in the PEPTIC trial in Canada, no differences in effectiveness or safety were observed between use of PPIs and histamine-2 receptor blockers for stress ulcer prophylaxis.
Clinical trial registration number
anzctr.org.au identifier: ACTRN12616000481471.
Keywords: Cardiac surgical intensive care unit, Stress ulcer prophylaxis, Gastrointestinal bleeding, Ventilator-associated conditions, Clostridium difficile infections
The reported prevalence of bleeding stress ulcers among patient admitted to intensive care units is 0.6–6.0% [1–5].
BACKGROUND
The reported prevalence of bleeding stress ulcers among patient admitted to intensive care units is 0.6–6.0% [1–5]. Up to 81% of critically ill patients receive routine stress ulcer prophylaxis (SUP) and this practice is supported by randomized controlled trials that have shown proton pump inhibitors (PPIs) reducing the risk of gastrointestinal (GI) bleeding, but not mortality [4, 6–8]. Although PPIs are most commonly prescribed for SUP, there is significant institutional variation in practice with some centres routinely using histamine-2 receptor blockers (H2RBs) [7–9]. The recent Proton Pump Inhibitors versus Histamine-2 Receptor Blockers for Ulcer Prophylaxis Therapy in the Intensive Care Unit (PEPTIC) trial randomized patients to admitted to intensive care units to PPIs or H2RBs and reported no differences in all-cause mortality despite a lower risk of upper GI bleeding with use of PPIs [10]. In a pre-specified subgroup of cardiac surgical intensive care unit (CSICU) patients, the study reported a higher risk of mortality in the PPI arm with no statistically significant difference in upper GI bleeding.
There is a lack of high-quality SUP evidence in the CSICU population, and cardiac surgical clinical practice guidelines provide little guidance on best SUP practices in this population [11]. A systemic review limited to observational studies and small randomized trials in the cardiac surgical population concluded that existing evidence was marginally in favour of PPI over H2RB use for SUP, but that PPIs were associated with a higher risk of hospital-acquired pneumonia [12]. The overall incidence of bleeding stress ulcers reported in retrospective studies of <1% may suggest a lack of need for SUP for the majority of patients undergoing cardiac surgery, but it also belies the variable risk in this heterogenous patient group [13–17]. Moreover, randomized trials of non-surgical patients on dual anti-platelet therapy have reported that PPIs are superior to either placebo or H2RBs in preventing long-term outpatient GI complications, yet little is known about their comparative effectiveness or safety in the postoperative inpatient setting [18, 19]. Accordingly, we conducted a post hoc analysis to explore the effectiveness and safety of PPIs versus H2RBs for SUP across subsets of CSICU patients including surgical types, anti-coagulation and anti-platelet use, chronic kidney disease and mechanical circulatory support therapy.
MATERIALS AND METHODS
Data source
We conducted a post hoc exploratory analysis using data from the PEPTIC trial (anzctr.org.au identifier: ACTRN12616000481471). The design and primary findings of the PEPTIC trial have been previously reported [10, 20]. Briefly, it was a registry-embedded, open-label, randomized cluster cross-over trial that compared SUP with PPIs or H2RBs in 26 982 mechanically ventilated patients ≥18 years admitted to 50 intensive care units in 5 countries. The only exclusion criterion was an admission diagnosis of upper GI bleeding. Individual units were randomly allocated to 6-month alternating blocks of either a PPI or H2RB as the default SUP agent on the standardized CSICU admission order set. Given the open-label nature of the study, clinicians were discouraged from prescribing the alternate agent unless clinically indicated. The study reported no differences in upper GI bleeding rates, mortality or in-hospital infections between the study arms.
Ethics
Ethical approvals were obtained by all study sites; some regions received a full waiver of consent and others were conducted with a waiver and opportunity for patients to opt out. The study was approved by the University of Alberta’s Human Research Ethics Boards (Pro00074103).
Study population and data linkages
The present analysis was conducted using health data of the 1628 Canadian study participants enrolled at the University of Alberta Hospital CSICU October 2017 and October 2018. The PEPTIC study case report form data were electronically linked to the patients’ electronic medical records (eCritical Alberta) [21] that contained demographic information and admission Acute Physiology, Age, Chronic Health Evaluation (APACHE) III and Sequential Organ Failure Assessment scores, laboratory information, duration of mechanical ventilation, the occurrence of ventilator-associated condition (VAC) and GI bleeding, in-hospital mortality information and length of stay (LOS). These data were also linked to 4 additional administrative databases using unique patient identifiers, as previously described [22]. First, the Alberta Provincial Project for Outcomes Assessment in Coronary Heart Disease registry contains detailed cardiac surgical information including past medical variables and postoperative GI bleeding [23]. Postoperative CSICU variables and complications are extracted using the Society of Thoracic Surgeons database definitions by trained chart abstracters. Second, a provincial Clostridiumdifficile infection (CDI) quality assurance dataset which is adjudicated using Infectious Diseases Society of America standardized criteria [24]. Third, the Alberta Provincial Population Heath datasets maintain the Discharge Abstract Database, which codes the primary admission diagnosis, up to 24 secondary diagnoses, and up to 16 diagnostic and therapeutic procedures for each hospitalization [25]. These data were used to identify all comorbidities and procedures using hospitalization data 5 years preceding the index cardiac surgery. Finally, the Pharmacy Information Network was used to identify all prescription medications filled within 30 days of hospital discharge.
Outcomes of interest
The primary outcome of interest was in-hospital mortality through 90 days. Secondary outcomes included: (i) clinically important upper GI bleeding during the CSICU stay [defined as clinically overt upper GI bleeding and ≥1 of the following: (a) spontaneous drop of systolic, mean or diastolic blood pressure ≥20 mmHg maintained for ≥1 h; (b) starting a new vasopressor by intravenous infusion or ≥20% increase in the dose of existing vasopressor infusions; and (c) ≥20 g/l decrease in haemoglobin or ≥2 packed red blood cells unit transfusion], (ii) CDI, (iii) VAC [26, 27] reported as the number of VAC events among CSIUC admission with an LOS of >4 days, (iv) CSICU and hospital LOS and (v) duration of mechanical ventilation. All analyses were performed in the intention-to-treat (ITT) and the per-protocol (PP) populations; the latter analysis was performed given the imbalance in treatment adherence between the study arms.
Subgroups of interest were defined prior to the analysis and included urgent/emergent versus elective surgery, mechanical circulatory support (defined as durable or temporary surgically implanted ventricular assist device or extracorporeal membrane oxygenation), anti-coagulation use (defined as intravenous anti-coagulants or coumadin), coagulopathy (defined as an anti-Xa or partial thromboplastin time >1.5 time the upper limit or formal or international normalized ratio >1.5 without anticoagulant use), dual-anti-platelet use (defined as aspirin plus ticagrelor or clopidogrel) or preoperative chronic kidney disease (defined using Society of Thoracic Surgery Criteria).
Statistical analysis
We reported categorical variables as frequency with percentage and compared them using the chi-squared test. We reported continuous variables as median with interquartile range and compared them using non-parametric Kruskal–Wallis test. To examine the effect difference on outcomes between the treatment arms (setting PPI as reference), we adopted generalized linear regression models. We built logistic regression models for binary responses and negative binomial regression models for duration responses. To address potential confounding issues, models included the following candidate variables in selection: age, sex, APACHE III score at the time of admission, admission type, admission source, surgery priority, pre-surgical comorbidities (hypertension, diabetes mellitus, dyslipidaemia, heart failure, myocardial infarction, atrial fibrillation, prior percutaneous coronary intervention, prior coronary artery bypass grafting, peripheral vascular disease, cerebrovascular disease, chronic obstructive pulmonary disease, chronic kidney disease (without renal replacement therapy), dementia, human immunodeficiency virus, malignancy), surgical procedure and categorical Charlson comorbidity index score. We employed standard stepwise variable selection procedure (with default enter and stay criterion 0.05) to create sparse models. We performed all of the statistical analyses using Statistical Analysis System (SAS) Enterprise Guide 7.1 (Cary, NC, USA).
RESULTS
We studied 1628 patients admitted to the CSICU with 823 (50.6%) randomized to PPI treatment and 805 (49.4%) to H2RB. Baseline demographics, medical and surgical variables and laboratory values were generally well balanced between treatment arms (Table 1). Patients randomized to PPI treatment more frequently had dyslipidaemia, and elective admissions, while patients assigned to H2RB treatment more frequently had a history of heart failure, cerebrovascular disease, higher APACHE II scores and anti-Xa levels >0.3. Adherence to assigned treatment was 94.21% in the PPI arm and 90.3% in the H2RB arm.
Table 1:
Baseline characteristics, treatment assignment and outcomes between Canadian cardiac surgical intensive care unit patients stratified by proton pump inhibitor and histamine-2 receptor blocker assignment
| Proton pump inhibitor (n = 823) | Histamine-2 receptor blocker (n = 805) | P-Value | |
|---|---|---|---|
| Age, median (IQR), years | 64 (54–72) | 63 (54–72) | 0.366 |
| Female sex, n (%) | 286 (34.8) | 285 (35.4) | 0.783 |
| Comorbidities, n (%) | |||
| Hypertension | 592 (71.9) | 545 (67.7) | 0.063 |
| Diabetes mellitus | 268 (32.6) | 238 (29.6) | 0.191 |
| Dyslipidaemia | 537 (65.2) | 486 (60.4) | 0.042 |
| Myocardial infarction | 211 (25.6) | 195 (24.2) | 0.510 |
| Percutaneous coronary intervention | 112 (13.6) | 95 (11.8) | 0.274 |
| Coronary artery bypass | 346 (42.0) | 324 (40.2) | 0.462 |
| Heart failure | 162 (19.7) | 191 (23.7) | 0.048 |
| Atrial fibrillation | 156 (19.0) | 136 (16.9) | 0.279 |
| Cerebrovascular disease | 20 (2.4) | 42 (5.2) | 0.003 |
| Peripheral vascular disease | 34 (4.1) | 28 (3.5) | 0.491 |
| Chronic obstructive coronary disease | 81 (9.8) | 109 (13.5) | 0.020 |
| Chronic kidney disease | 93 (11.3) | 103 (12.8) | 0.354 |
| Cancer | 35 (4.3) | 40 (5.0) | 0.491 |
| Number of hospitalizations in prior year, median (IQR) | 1 (1–2) | 1 (1–2) | 0.644 |
| APACHE III score, median (IQR) | 55 (45–65) | 56 (47–66) | 0.027 |
| (Valid n) | (n = 748) | (n = 773) | |
| Admission SOFA score, median (IQR) | 6 (5–8) | 7 (5–8) | 0.002 |
| Source of admission to ICU, n (%) | 0.117 | ||
| Emergency department | 5 (0.6) | 5 (0.6) | |
| Hospital ward | 22 (2.7) | 40 (5.0) | |
| From operating room | 786 (95.5) | 751 (93.3) | |
| Transfer from another hospital | 10 (1.2) | 9 (1.1) | |
| Treatment adherence, n (%)a | 763 (94.2) | 707 (90.3) | <0.001 |
| Surgical priority, n (%) | 0.016 | ||
| Elective operative | 717 (87.1) | 665 (82.6) | |
| Emergent operative | 67 (8.1) | 87 (10.8) | |
| Non-operative | 39 (4.7) | 48 (6.0) | |
| Not available | 0 (0) | 5 (0.6) | |
| Type of surgery, n (%) | 0.141 | ||
| CABG | 202 (24.5) | 193 (24.0) | |
| CABG + 1 valve | 256 (31.1) | 214 (26.6) | |
| ≥ 2 valves ± CABG | 59 (7.2) | 52 (6.5) | |
| Transplant | 31 (3.8) | 50 (6.2) | |
| Mechanical circulatory support | 29 (3.5) | 37 (4.6) | |
| Congenital | 30 (3.6) | 36 (4.5) | |
| Aortic surgery | 113 (13.7) | 108 (13.4) | |
| Othersb | 103 (12.5) | 115 (14.3) | |
| Aortic cross-clamp time, median (IQR), min | 70 (48–99) | 67 (43–95) | 0.085 |
| Preoperative left ventricular ejection fraction, median (IQR), % | 50 (40–55) | 50 (36–60) | 0.609 |
| (n = 583) | (n = 278) | ||
| CSICU laboratory values | |||
| Admission haemoglobin, median (IQR) | 91 (81–104) | 91 (81–104) | 0.735 |
| Platelet count (lowest), median (IQR) | 166 (127–219) | 161 (123–219) | 0.405 |
| Lactate on admission, median (IQR) | 1.5 (1.2–2.1) | 1.3 (0.8–1.9) | 0.246 |
| Lactate highest, median (IQR) | 2.5 (1.7–3.8) | 2.5 (1.8–4.0) | 0.105 |
| INR >1.5, n (%) | 179 (21.8) | 166 (20.6) | 0.578 |
| PTT >45, n (%) | 181 (22.0) | 208 (25.8) | 0.069 |
| Anti-Xa >0.30, n (%) | 29 (3.5) | 45 (5.6) | 0.045 |
| Discharge medication, n (%) | |||
| Beta-blocker | 517 (62.8) | 490 (60.9) | 0.418 |
| Calcium channel blocker | 99 (12.0) | 89 (11.1) | 0.539 |
| Digitalis | 4 (0.5) | 8 (1.0) | 0.231 |
| ACE or ARB | 276 (33.5) | 247 (30.7) | 0.218 |
| Clopidogrel or ticagrelor | 35 (4.3) | 27 (3.4) | 0.344 |
| Warfarin or NOAC | 460 (55.9) | 440 (54.7) | 0.616 |
Missing n = 35 (13 in PPI arm; 23 in H2RB arm) based on the first medication received post admission.
Common procedures in this category included pericardiectomy, epicardial pacemaker insertions, cardiac trauma and/or cardiac tumour resections.
ACE: angiotensin converting enzyme inhibitors; APACHE: Acute Physiology and Chronic Health Evaluation; ARB: angiotensin receptor blocker; CABG: coronary artery bypass grafting; CSICU: cardiac surgical intensive care unit; H2RB: histamine-2 receptor blocker; ICU: intensive care unit; INR: international normalized ratio; IQR: interquartile range; NOAC: novel oral anticoagulant; PPI: proton pump inhibitor; PTT: partial thromboplastin time; SOFA: Sequential Organ Failure Assessment.
Outcomes in proton pump inhibitor- and histamine-2 receptor blocker-treated patients
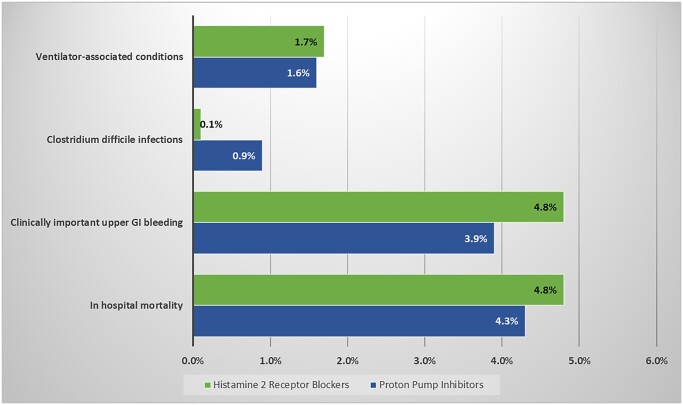
The incidence of in-hospital mortally, clinically important GI bleeding, VAC and CDI in the overall population were 4.3%, 4.5%, 1.7% and 0.5%, respectively. Outcomes in the ITT population are presented in Table 2 and Fig. 1. Among patients allocated to PPI and H2RB respectively, there were no observed differences in in-hospital mortality [4.3% vs 4.8%, adjusted odds ratio (aOR) 0.97, 95% confidence interval (CI) 0.55–1.70], clinically important GI bleeding (3.9% vs 4.8%, aOR 1.09, 95% CI 0.66–1.81), CDI (0.9% vs 0.1%, aOR 0.18, 95% CI 0.02–1.59), VAC (1.6% vs 1.7%, aOR 0.99, 95% CI 0.45–2.16), median hospital LOS (9.2 vs 9.8 days) or median CSICU LOS (9.3 vs 9.8 days). The median duration of mechanical ventilation was longer in the H2RB treated patients (7.7 vs 6.3 days, aOR 1.12, 95% CI 1.01–1.25) Results were similar in a PP analysis (Supplementary Material, Table S1).
Table 2:
Outcomes in the intention-to-treat population
| Outcomes | PPI (n = 823) | H2RB (n = 805) | H2RB versus PPI, adjusted OR/RR (95% CI)a | P-Valuea |
|---|---|---|---|---|
| Primary outcome | ||||
| In-hospital mortality, n (%) | 35 (4.3) | 39 (4.8) | 0.97 (0.55–1.7) | 0.919 |
| Secondary outcomes | ||||
| Clinically important upper GI bleeding, n (%) | 32 (3.9) | 39 (4.8) | 1.09 (0.66–1.81) | 0.732 |
| Clostridium difficile infection, n (%) | 7 (0.9) | 1 (0.1) | 0.18 (0.02–1.59) | 0.124 |
| Ventilator-associated conditions, n (%) | 13 (1.6) | 14 (1.7) | 0.99 (0.45–2.16) | 0.981 |
| ICU length of stay (days), median (IQR) | 3.0 (1.7–5.1) | 2.8 (1.1–4.9) | 0.92 (0.85–1.00) | 0.064 |
| Hospital length of stay (days), median (IQR) | 9.2 (6.7–17.4) | 9.8 (6.6–20.0) | 1.06 (0.99–1.13) | 0.075 |
| Duration of mechanical ventilation (h), median (IQR) | 6.3 (4.4–14.4) | 7.7 (4.9–19.9) | 1.12 (1.01–1.25) | 0.034 |
OR/RR (95% CI) and P-value were from modelling analysis. Binary (length of stay) outcomes were fitted using logistic (negative binomial) regression. Models included the following candidate variables in selection: age, sex, APACHE III score at the time of admission, admission type, admission source, surgery priority, pre-surgical comorbidities [hypertension, diabetes mellitus, dyslipidaemia, heart failure, myocardial infarction, atrial fibrillation, prior percutaneous coronary intervention, prior coronary artery bypass grafting, peripheral vascular disease, cerebrovascular disease, chronic obstructive pulmonary disease, chronic kidney disease (without renal replacement therapy), dementia, human immunodeficiency virus, malignancy], surgical procedure and Charlson comorbidity index category.
APACHE: Acute Physiology and Chronic Health Evaluation; CI: confidence interval; GI: gastrointestinal; H2RB: histamine-2 receptor blocker; ICU: intensive care unit; IQR: interquartile; OR: odds ratio; PPI: proton pump inhibitor; RR: risk ratio.
Figure 1:
No difference was observed in the incidence of ventilatory-associated conditions, Clostridium difficile infection, clinically important upper gastrointestinal bleeding or in-hospital mortality.
Subgroup analyses
Outcomes in pre-specified subgroups for all-cause in-hospital mortality and clinically important upper GI bleeding are presented in Table 3. No substantial heterogeneity in treatment effect between mortality and assigned treatment was observed by type of surgery, surgical priority, dual anti-platelet use or preoperative chronic renal failure. A treatment interaction was observed among patients stratified by anticoagulant use (P-interaction 0.034), though the results within the individual subgroups of PPI versus H2RB in the anticoagulated (aOR 0.55, 95% CI 0.25–1.21) and not anticoagulated cohorts (aOR 1.81, 95% CI 0.78–4.16) did not reach statistical significance. No differences in clinically important upper GI bleeding were observed in key subgroups in the PP population (Supplementary Material, Table S2).
Table 3:
Mortality and gastrointestinal bleeding outcomes in pre-specified subgroups
| CSICU subgroup | In-hospital death by 90 days |
Clinically important upper gastrointestinal bleeding |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| ITT, n | PPI (n = 823), n (%) | H2RB (n = 805), n (%) | PPI versus H2RB, OR (95% CI)a | P-Value for interactiona | PPI (n = 823), n (%) | H2RB (n = 805), n (%) | PPI versus H2RB, OR (95% CI)a | P-Value for interactiona | |
| Type of surgery | 0.250 | 0.867 | |||||||
| MCS | 66 | 3 (0.4) | 1 (0.1) | 0.23 (0.02–2.38) | 2 (0.2) | 4 (0.5) | 2.05 (0.3–13.89) | ||
| All others | 1562 | 33 (4.0) | 39 (4.8) | 1.08 (0.61–1.93) | 30 (3.7) | 35 (4.4) | 1.09 (0.65–1.85) | ||
| Surgical priority | 0.765 | 0.743 | |||||||
| Emergent/urgent | 806 | 23 (2.8) | 24 (3.0) | 1.04 (0.53–2.06) | 25 (3.0) | 30 (3.7) | 1.08 (0.61–1.93) | ||
| Elective | 822 | 13 (1.6) | 16 (2.0) | 0.82 (0.31–2.15) | 7 (0.9) | 9 (1.1) | 0.94 (0.33–2.69) | ||
| Dual anti-platelet | 1.00 | 0.928 | |||||||
| Yes | 62 | 0 (0) | 0 (0) | – | 0 (0) | 1 (0.1) | – | ||
| No | 1566 | 36 (4.4) | 40 (5.0) | 0.97 (0.56–1.69) | 32 (3.9) | 38 (4.7) | 1.07 (0.64–1.77) | ||
| Anticoagulant use | 0.034 | 0.904 | |||||||
| Yes | 293 | 22 (2.7) | 21 (2.6) | 0.55 (0.25–1.21) | 11 (1.3) | 16 (2.0) | 1.32 (0.57–3.05) | ||
| No | 1335 | 14 (1.7) | 19 (2.4) | 1.81 (0.78–4.16) | 21 (2.6) | 23 (2.9) | 1.18 (0.62–2.25) | ||
| Chronic kidney disease | 0.522 | 0.056 | |||||||
| Yes | 196 | 9 (1.1) | 5 (0.6) | 0.54 (0.14–2.15) | 3 (0.4) | 10 (1.2) | 3.33 (0.89–12.55) | ||
| No | 1432 | 27 (3.3) | 35 (4.4) | 1.07 (0.57–2,00) | 29 (3.5) | 29 (3.6) | 0.88 (0.5–1.53) | ||
OR (95% CI) and P-value for interaction were from logistic regression. Models were adjusted for age, sex, APACHE III score at the time of admission, admission type, admission source, surgery priority, pre-surgical comorbidities [hypertension, diabetes mellitus, dyslipidaemia, heart failure, myocardial infarction, atrial fibrillation, prior percutaneous coronary intervention, prior coronary artery bypass grafting, peripheral vascular disease, cerebrovascular disease, chronic obstructive pulmonary disease, chronic kidney disease (without renal replacement therapy), dementia, human immunodeficiency virus, malignancy], surgical procedure and Charlson comorbidity index category.
APACHE: Acute Physiology and Chronic Health Evaluation; CI: confidence interval; CSICU: cardiac surgical intensive care unit; H2RB; histamine-2 receptor blocker; ITT: intention-to-treat; MCS: mechanical circulatory support; OR; odds ratio; PPI, proton pump inhibitor.
DISCUSSION
In this exploratory analysis of cardiac surgery patients enrolled in the PEPTIC study, we observed no differences in all-cause in-hospital mortality or upper GI bleeding between patients admitted to the CSICU allocated to PPIs or H2RBs for routine SUP. Moreover, there were no significant differences in complications including ventilator-association conditions or CDI between treatment arms. The results were similar in the ITT and PP populations, and no differences in outcomes were observed among the small subset of patients who may have been at higher risk of GI bleeding.
The lack of an observed difference in this study differs from the subgroup analysis of the PEPTIC trial, which reported that CSICU patients treated with PPIs had a higher risk of in-hospital mortality compared with H2RBs, despite no differences in upper GI bleeding rates between treatment arms—the principal mechanism by which SUP is perceived to reduce morbidity [4, 10]. These findings that PPIs were not associated with increased risks imply that the difference in mortality found between treatment groups in the main trial was driven by unresolved confounding and regional variation. The lack of a mortality difference in the Canadian subset of cardiac surgery patients does not appear to have been driven by a chance imbalance in baseline characteristics between treatment groups. We submit this secondary analysis enabled more granular electronic health record data and linked cardiac surgical and patient medical information, which may have decreased the potential for unmeasured confounders between treatment arms. In addition, differences in patient-level risk between enrolling countries cannot be excluded, thus suggesting the need for confirmatory studies from other participating nations.
Despite SUP in both treatment arms, the incidence of upper GI bleeding observed in this analysis was more than double the incidence in the PEPTIC trial’s main analysis [10]. We hypothesize that this may due to differences in patient risk associated with the routine receipt of aspirin postoperatively and a higher prevalence of SUP risk factors such as chronic kidney disease and anti-coagulation use [14]. In addition, the PEPTIC study in Canada used a validated electronic algorithm to detect GI bleeding events, which may have been more sensitive than traditional case report forms used in other centres. Nonetheless, the point estimates for GI bleeding were numerically (but non-significantly) lower in the PPI arm of this study population which is in line with the main trial’s results. This analysis builds on the main trial’s results by reporting no bleeding differences in the cardiac surgical population, among higher-risk subgroups, or in PP population. While we did observe statistically significant heterogeneity of treatment effect based on the presence or absence baseline anticoagulation use, this subgroup was small and there was no suggestion of a substantive increased risk of mortality with PPI use. Collectively, our data suggest that either H2RBs or PPIs are reasonable first-line SUP treatments in the CSICU population. Notwithstanding, it may be still reasonable to consider PPI treatment in selected patients at higher risk of upper GI bleeding, such as those receiving system anticoagulation, mechanical circulatory support, dual antiplatelet therapy and/or recent peptic ulcer disease [14, 18, 28].
Multiple non-randomized observational studies have documented the association between more potent gastric acid suppression with PPIs and an increased risk of infectious complications including hospital- or ventilator-acquired pneumonia or CDI [29, 30]. Randomized trials conducted in intensive care patients, however, have not confirmed these findings [4, 10]. The current analysis did not detect a meaningful difference in VAC or CDI between the treatment arms suggesting potential equipoise in terms of safety between the therapeutic strategies. The median duration of mechanical ventilation was lower in the PPI arm; however, in the absence of differences in VAC, CDI, or LOS, we believe that this is more likely a spurious finding given that our secondary analyses were not adjusted for multiplicity.
Strengths and limitations
The strengths of this study include the CDI outcomes derived from a quality assurance dataset and the registry-linked granular medical and cardiac surgical information, which allowed for the adjustment of baseline differences. We acknowledge several limitations. First, although there was no interaction for the primary outcomes across study region (Australia–New Zealand, Ireland–UK and Canada) for in-hospital mortality or CDI, clinically significant upper GI bleeding rates were lower in PPI-treated patients in Australia–New Zealand and Ireland–UK, but not in Canadian sites. These findings may be due to differences in event detection and merit further electronic health record-linked analyses to explore the clinical variations underpinning these differences. Second, our findings were not adjusted for multiplicity, though we performed a PP analysis. Third, these short-term in-hospital results should not be used to inform outpatient GI prophylactic practices in patients on anti-coagulation or dual anti-platelet therapy. Fourth, no gastroscopy data were available to confirm upper GI bleeding and data on rates of ventilator-associated pneumonia meeting Centers for Disease Control definitions were not available in these datasets. Finally, the results of this pre-specified secondary analysis should be considered hypothesis generating and the subgroups were likely underpowered to detect a clinically significant effect.
CONCLUSION
In a subgroup of a randomized trial comparing PPI and H2RB SUP treatment among patients admitted to the CSICU, no differences in efficacy or safety were observed between the treatment arms. These data suggest that either PPIs or H2RBs are appropriate SUP therapeutic strategies among patients admitted to CSICUs.
SUPPLEMENTARY MATERIAL
Supplementary material is available at EJCTS online.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to gratefully acknowledge the data collection, informatics and/or study management efforts of Cathy Curr, Stanley Mathew, Avan Ricci Sargento, Danielle Southern, David Diachinsky, Mohamed Omar, Desiree Ross, Ruth Santos and Stephanie Smith, along with the Critical Care Strategic Clinical Network and eCritical Alberta Teams. We would like to acknowledge Ms. L. Soulard for copy editing the manuscript.
Funding
The PEPTIC trial was funded by the Health Research Council of New Zealand, the Canadian Institutes of Health Research, the Australian and New Zealand Intensive Care Foundation and the Health Research Board of Ireland. The PEPTIC trial was supported by the UK National Institute for Health Research.
Conflict of interest: SMB receives scientific advisory and speaking fees from Baxter and clinical adjudication fees from BioPorto. The other authors report no conflicts of interest.
Data Availability Statement
This article’s data cannot be shared publicly given the authors do not have ethics approval to share the primary trial’s raw data. The corresponding author will consider reasonable requests to share the data for peer review.
Author contributions
Sean van Diepen: Conceptualization; Formal analysis; Writing—original draft; Writing—review & editing. Tim Coulson: Writing—original draft; Writing—review & editing. Xiaoming Wang: Writing—original draft; Writing—review & editing. Dawn Opgenorth: Writing—original draft; Writing—review & editing. Danny J. Zuege: Writing—original draft; Writing—review & editing. Jo Harris: Writing—original draft; Writing—review & editing. Malik Agyemang: Writing—original draft; Writing—review & editing. Daniel J. Niven: Writing—original draft; Writing—review & editing. Rinaldo Bellomo: Writing—original draft; Writing—review & editing. Stephen E. Wright: Writing—original draft; Writing—review & editing. Paul J. Young: Writing—original draft; Writing—review & editing. Sean M. Bagshaw: Conceptualization; Writing—original draft; Writing—review & editing.
Reviewer information
European Journal of Cardio-Thoracic Surgery thanks Carlos A. Mestres, Omar A. Jarral and the other, anonymous reviewer(s) for their contribution to the peer review process of this article.
Glossary
ABBREVIATIONS
- aOR
Adjusted odds ratio
- APACHE
Acute Physiology, Age, Chronic Health Evaluation
- CDI
Clostridium difficile infections
- CSICU
Cardiac surgical intensive care unit
- GI
Gastrointestinal
- H2RB
Histamine-2 receptor blockers
- ITT
Intention-to-treat
- LOS
Length of stay
- PEPTIC
Proton Pump Inhibitors versus Histamine-2 Receptor Blockers for Ulcer Prophylaxis Therapy in the Intensive Care Unit
- PP
Per-protocol
- PPIs
Proton pump inhibitors
- SUP
Stress ulcer prophylaxis
- VAC
Ventilator-associated conditions
REFERENCES
- 1. Ben-Menachem T, Fogel R, Patel RV, Touchette M, Zarowitz BJ, Hadzijahic N. et al. Prophylaxis for stress-related gastric hemorrhage in the medical intensive care unit. A randomized, controlled, single-blind study. Ann Intern Med 1994;121:568–75. [DOI] [PubMed] [Google Scholar]
- 2. Cook DJ, Fuller HD, Guyatt GH, Marshall JC, Leasa D, Hall R. et al. Risk factors for gastrointestinal bleeding in critically ill patients. N Engl J Med 1994;330:377–81. [DOI] [PubMed] [Google Scholar]
- 3. Cook DJ, Griffith LE, Walter SD, Guyatt GH, Meade MO, Heyland DK. et al. ; Canadian Critical Care Trials Group. The attributable mortality and length of intensive care unit stay of clinically important gastrointestinal bleeding in critically ill patients. Crit Care 2001;5:368–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Krag M, Marker S, Perner A, Wetterslev J, Wise MP, Schefold JC. et al. ; SUP-ICU trial group. Pantoprazole in patients at risk for gastrointestinal bleeding in the ICU. N Engl J Med 2018;379:2199–208. [DOI] [PubMed] [Google Scholar]
- 5. Zandstra DF, Stoutenbeek CP.. The virtual absence of stress-ulceration related bleeding in ICU patients receiving prolonged mechanical ventilation without any prophylaxis. A prospective cohort study. Intensive Care Med 1994;20:335–40. [DOI] [PubMed] [Google Scholar]
- 6. Alhazzani W, Alshamsi F, Belley-Cote E, Heels-Ansdell D, Brignardello-Petersen R, Alquraini M. et al. Efficacy and safety of stress ulcer prophylaxis in critically ill patients: a network meta-analysis of randomized trials. Intensive Care Med 2018;44:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gratrix AP, Enright SM, O'Beirne HA.. A survey of stress ulcer prophylaxis in intensive care units in the UK. Anaesthesia 2007;62:421–2. [DOI] [PubMed] [Google Scholar]
- 8. Krag M, Perner A, Wetterslev J, Wise MP, Borthwick M, Bendel S. et al. ; SUP-ICU co-authors. Prevalence and outcome of gastrointestinal bleeding and use of acid suppressants in acutely ill adult intensive care patients. Intensive Care Med 2015;41:833–45. [DOI] [PubMed] [Google Scholar]
- 9. Shears M, Alhazzani W, Marshall JC, Muscedere J, Hall R, English SW. et al. Stress ulcer prophylaxis in critical illness: a Canadian survey. Can J Anaesth 2016;63:718–24. [DOI] [PubMed] [Google Scholar]
- 10. The PEPTIC Investigators. Effect of stress ulcer prophylaxis with proton pump inhibitors vs histamine-2 receptor blockers on in-hospital mortality among ICU patients receiving invasive mechanical ventilation: the PEPTIC randomized clinical trial. JAMA 2020;323:616–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Engelman DT, Ben Ali W, Williams JB, Perrault LP, Reddy VS, Arora RC. et al. Guidelines for perioperative care in cardiac surgery: enhanced recovery after surgery society recommendations. JAMA Surg 2019;154:755–66. [DOI] [PubMed] [Google Scholar]
- 12. Shin J-S, Abah U.. Is routine stress ulcer prophylaxis of benefit for patients undergoing cardiac surgery? Interact CardioVasc Thorac Surg 2012;14:622–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Aljarallah B, Wong W, Modry D, Fedorak R.. Prevalence and outcome of upper gastrointestinal bleeding post-coronary artery bypass graft. Int J Health Sci 2008;2:69–76. [PMC free article] [PubMed] [Google Scholar]
- 14. Krawiec F, Maitland A, Duan Q, Faris P, Belletrutti PJ, Kent WDT.. Duodenal ulcers are a major cause of gastrointestinal bleeding after cardiac surgery. J Thorac Cardiovasc Surg 2017;154:181–8. [DOI] [PubMed] [Google Scholar]
- 15. Filsoufi F, Rahmanian PB, Castillo JG, Scurlock C, Legnani PE, Adams DH.. Predictors and outcome of gastrointestinal complications in patients undergoing cardiac surgery. Ann Surg 2007;246:323–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Geissler HJ, Fischer UM, Grunert S, Kuhn-Regnier F, Hoelscher A, Schwinger RH. et al. Incidence and outcome of gastrointestinal complications after cardiopulmonary bypass. Interact CardioVasc Thorac Surg 2006;5:239–42. [DOI] [PubMed] [Google Scholar]
- 17. Aggarwal A, Pant R, Kumar S, Sharma P, Gallagher C, Tatooles AJ. et al. Incidence and management of gastrointestinal bleeding with continuous flow assist devices. Ann Thorac Surg 2012;93:1534–40. [DOI] [PubMed] [Google Scholar]
- 18. Bhatt DL, Cryer BL, Contant CF, Cohen M, Lanas A, Schnitzer TJ. et al. Clopidogrel with or without omeprazole in coronary artery disease. N Engl J Med 2010;363:1909–17. [DOI] [PubMed] [Google Scholar]
- 19. Almufleh A, Ramirez FD, So D, Le May M, Chong AY, Torabi N. et al. H2 receptor antagonists versus proton pump inhibitors in patients on dual antiplatelet therapy for coronary artery disease: a systematic review. Cardiology 2018;140:115–23. [DOI] [PubMed] [Google Scholar]
- 20. Young PJ, Bagshaw SM, Forbes A, Nichol A, Wright SE, Bellomo R. et al. ; Australian and New Zealand Intensive Care Society Clinical Trials Group on behalf of the PEPTIC investigators. A cluster randomised, crossover, registry-embedded clinical trial of proton pump inhibitors versus histamine-2 receptor blockers for ulcer prophylaxis therapy in the intensive care unit (PEPTIC study): study protocol. Crit Care Resusc 2018;20:182–9. [PubMed] [Google Scholar]
- 21. Brundin-Mather R, Soo A, Zuege DJ, Niven DJ, Fiest K, Doig CJ. et al. Secondary EMR data for quality improvement and research: a comparison of manual and electronic data collection from an integrated critical care electronic medical record system. J Crit Care 2018;47:295–301. [DOI] [PubMed] [Google Scholar]
- 22. van Diepen S, Lin M, Bakal JA, McAlister FA, Kaul P, Katz JN. et al. Do stable non–ST-segment elevation acute coronary syndromes require admission to coronary care units? Am Heart J 2016;175:184–92. [DOI] [PubMed] [Google Scholar]
- 23. Benoit MA, Bagshaw SM, Norris CM, Zibdawi M, Chin WD, Ross DB. et al. Postoperative complications and outcomes associated with a transition to 24/7 intensivist management of cardiac surgery patients. Crit Care Med 2017;45:993–1000. [DOI] [PubMed] [Google Scholar]
- 24. Cohen SH, Gerding DN, Johnson S, Kelly CP, Loo VG, McDonald LC. et al. ; Infectious Diseases Society of America. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA). Infect Control Hosp Epidemiol 2010;31:431–55. [DOI] [PubMed] [Google Scholar]
- 25. Woolridge S, Alemayehu W, Kaul P, Fordyce CB, Lawler PR, Lemay M. et al. National trends in coronary intensive care unit admissions, resource utilization, and outcomes. Eur Heart J: Acute Cardiovas Care 2020;9:923–30. [DOI] [PubMed] [Google Scholar]
- 26. Centers for Disease Control and Prevention National Health Safety Network. Ventilator-Associated Event (VAE). https://www.cdc.gov/nhsn/pdfs/pscmanual/10-vae_final.pdf (10 August 2021, date last accessed).
- 27. Nakahashi S, Yamada T, Ogura T, Nakajima K, Suzuki K, Imai H.. Association of patient care with ventilator-associated conditions in critically ill patients: risk factor analysis. PLoS One 2016;11:e0153060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kamada T, Satoh K, Itoh T, Ito M, Iwamoto J, Okimoto T. et al. Evidence-based clinical practice guidelines for peptic ulcer disease 2020. J Gastroenterol 2021;56:303–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. MacLaren R, Reynolds PM, Allen RR.. Histamine-2 receptor antagonists vs proton pump inhibitors on gastrointestinal tract hemorrhage and infectious complications in the intensive care unit. JAMA Intern Med 2014;174:564–74. [DOI] [PubMed] [Google Scholar]
- 30. Howell MD, Novack V, Grgurich P, Soulliard D, Novack L, Pencina M. et al. Iatrogenic gastric acid suppression and the risk of nosocomial Clostridium difficile infection. Arch Intern Med 2010;170:784–90. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This article’s data cannot be shared publicly given the authors do not have ethics approval to share the primary trial’s raw data. The corresponding author will consider reasonable requests to share the data for peer review.