Abstract
Central-variant posterior reversible encephalopathy syndrome is an atypical subtype of posterior reversible encephalopathy syndrome that occurs during rapid fluctuations in blood pressure, leading to cerebrovascular autoregulatory failure and endothelial dysfunction. Few reports have described posterior reversible encephalopathy syndrome in infants. A 4-month-old girl, who was diagnosed a month before with hypoxic ischemic encephalopathy due to sudden cardiac arrest, showed persistent renovascular hypertension with a systolic blood pressure of 200 mmHg. Computed tomography of the head revealed a new-onset low-attenuation area in the bilateral basal ganglia, and computed tomography of the trunk revealed severe long-segment narrowing of the abdominal aorta encompassing the bilateral renal arteries. She was treated with antihypertensive drugs and peritoneal dialysis. Follow-up imaging after blood pressure stabilization showed resolution of the low-attenuation area in the bilateral basal ganglia. We diagnosed her basal ganglia lesions as central-variant posterior reversible encephalopathy syndrome. She suffered from neurological sequelae attributable to hypoxic ischemic encephalopathy but showed no evidence of basal ganglia dysfunction. Here, we report a case of infantile central-variant posterior reversible encephalopathy syndrome involving bilateral basal ganglia lesions with mid-aortic syndrome. The differential diagnosis of infantile symmetric bilateral basal ganglia lesions is broad and includes genetic, acquired metabolic or toxic, infectious, inflammatory, vascular, and neoplastic pathologies. Among them, central-variant posterior reversible encephalopathy syndrome is rare but important because neurological prognosis may be favorable, and specific treatment, such as administration of antihypertensive drugs or discontinuation of drugs that induce posterior reversible encephalopathy syndrome, is possible.
Keywords: Hypertensive encephalopathy, Central-variant posterior reversible encephalopathy syndrome, Leigh syndrome, Renovascular hypertension, Mid-aortic syndrome, Basal ganglia
Introduction
Central-variant posterior reversible encephalopathy syndrome (PRES) is an atypical subtype of PRES that occurs during rapid fluctuations in blood pressure (BP), leading to cerebrovascular autoregulatory (CAR) failure and endothelial dysfunction [1,2]. There are few reports of PRES in infants, and PRES in infants under 6 months of age has not been reported to date [3]. Here, we report a 4-month-old infant who exhibited central-variant PRES during refractory renovascular hypertension due to mid-aortic syndrome (MAS). The unique imaging features may offer a rare but important differential diagnosis for bilateral basal ganglia lesions in this age group.
Case report
A 4-month-old previously healthy girl was referred to our institution because of resuscitated cardiac arrest of unknown cause following routine immunization. She was placed on venoarterial extracorporeal membrane oxygenation (VA-ECMO) support for three days. Brain computed tomography (CT) after the withdrawal of VA-ECMO showed cerebral watershed infarction and whole-brain edema (Fig. 1). The patient was diagnosed with hypoxic-ischemic encephalopathy. No evidence of basal ganglia lesions was observed. Moreover, an evaluation revealed heart failure with left ventricular myocardial hypertrophy and end-stage renal failure that required continuous hemodialysis. Renovascular hypertension (systolic BP 100-130 mmHg, exceeding the 99th percentile of age and weight) became apparent at approximately 10 days after resuscitation and persisted despite administering the maximum dose of calcium-channel blockers and α2-adrenoceptor agonists.
Fig. 1.
Brain CT images. Initial CT on day 3 after VA-ECMO withdrawal showed whole brain edema and cerebral watershed infarction. At that time, no abnormalities were observed in the basal ganglia. On day 36, CT at the onset of HE showed a new low-attenuation area in the basal ganglia (white arrow). Follow-up CT was performed after 3 months. The initial low attenuation areas disappeared without atrophy.
Brain magnetic resonance imaging (MRI) taken at 16 days after resuscitation showed mild brain atrophy and T1-weighted image hyperintensities in the bilateral putamen and occipital lobe cortices. These lesions were isointense on T2-weighted images and no diffusion restriction was observed (Fig. 2). Cortical laminar necrosis and reflected hypoxic damage due to the initial cardiac arrest episode were considered.
Fig. 2.
Brain MRI after initial cardiac arrest and before hypertensive crisis. T1-weighted image (T1WI) shows hyperintensities in the bilateral putamen and occipital lobe cortices, watershed infarctions, and whole-brain atrophy. T2-weighted imaging (T2WI), diffusion-weighted imaging (DWI), and the apparent diffusion coefficient (ADC) showed no signal abnormalities in the basal ganglia and occipital lobe cortices.
Hypertension gradually worsened despite treatment, and one month after onset, the systolic BP began to peak at 200 mmHg. After 5 days of persistent severe hypertension, follow-up brain CT revealed new, symmetrical, swollen areas of hypodensity in the putamen, and globus pallidus (Fig. 1). Moreover, contrast-enhanced computed tomography (CT) revealed severe long-segment narrowing of the abdominal aorta encompassing the bilateral renal arteries, establishing a diagnosis of MAS (Fig. 3). After controlling the BP with an angiotensin-converting enzyme inhibitor, the third follow-up CT, taken 2 months after the onset of basal ganglia lesions and 93 days after resuscitation, showed complete resolution of the swelling and hypodensities in the putamen and globus pallidus (Fig. 1).
Fig. 3.
A 3-dimensional reconstruction image of our patient. Diffuse luminal narrowing of the abdominal aorta from the superior mesenteric artery to both femoral arteries (white arrow).
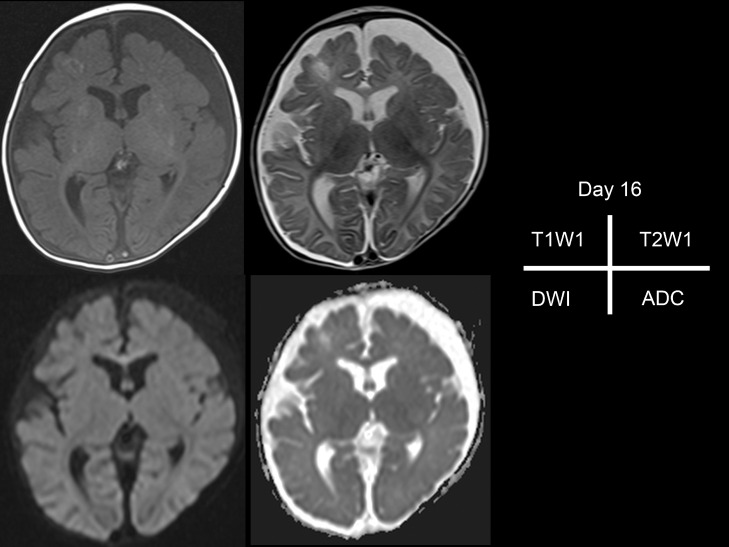
The patient did not exhibit clinical signs suggestive of basal ganglia lesions such as dystonia and dyskinesia. Moreover, MRI performed 1 month after the onset of basal ganglia lesions and at day 51 after resuscitation showed neither gliosis nor atrophy of the bilateral basal ganglia. Susceptibility-weighted imaging of the basal ganglia was negative (Fig. 4). On the other hand, hypoxic ischemic changes of the cerebral hemispheres, attributable to the cardiac arrest, were observed, including cortical laminar necrosis in T1-weighted images and progressive atrophy of the cerebral hemisphere with occipital lobe dominance (Fig. 4). Inborn errors of metabolism, systemic vasculitis, and genetic diseases were ruled out through diagnostic investigations.
Fig. 4.
Follow-up brain MRI taken after 51 days. T1-weighted image (T1WI) remains lamina necrosis from occipital to frontal areas and putamen as in the initial MRI. T2-weighted images (T2WI) show no signal abnormalities in the bilateral globus pallidus or atrophy of the basal ganglia. Susceptibility-weighted imaging (SWI) does not show signal intensity change of the hemorrhage.
Based on the CT and MRI findings, we diagnosed her basal ganglia lesions as central-variant PRES with an underlying etiology of renovascular hypertension caused by MAS. While hypoxic-ischemic encephalopathy complications were observed, there was no evidence of basal ganglia dysfunction. The patient was placed on peritoneal dialysis and discharged at the age of 6 months. She was unable to hold her head up, but could track objects, recognize her mother, and purposefully move her limbs. No critical lower limb ischemia was noted.
Discussion
Our report discusses the first case of bilateral basal ganglia involved in infantile PRES due to MAS. MAS is a rare disease characterized by narrowing of the abdominal aorta and its associated vessels. The pathogenesis of MAS is unknown, and more than half of cases are believed to be idiopathic. Uncontrolled hypertension is the most common symptom in children, which can lead to stroke, hypertensive encephalopathy or PRES, and congestive heart failure. It is also associated with a high mortality rate. Increased severity of MAS is associated with onset at less than 1 year of age, long segment involving more associated vessels, including renal vessels, and degree of stenosis [4]. Patients with hypertension of renal origin are more likely to develop severe hypertension owing to increased fluid retention and CAR hypersensitivity, which may be a high risk for PRES [5].
The pathogenesis of PRES is thought to be the rapid progression of hypertension that exceeds the upper limit of cerebrovascular autoregulation, resulting in increased vascular permeability and vasogenic edema [6]. Disturbed autoregulatory vasoconstriction may also cause hypoperfusion, resulting in an ischemic lesion with imaging features of cytotoxic edema. PRES may occur especially when the pressure rise is rapid and severe, resulting in an insufficient autoregulatory response and hyperperfusion, and may break down the blood-brain barrier.
In pediatric PRES, CAR is immature, and hypertension is more commonly pronounced at the onset than in adults [6,7]. In our patient, the systolic blood pressure reached as high as 200 mmHg for 5 days, which can be sufficient to cause PRES. Central-variant PRES is a subtype defined as predominant involvement of the brainstem and basal ganglia, with sparing of the parieto-occipital and posterior frontal cortico-subcortical regions [2]. This variant has been reported to be more common in certain diseases, such as systemic lupus erythematosus. It is largely unknown why the central variant more frequently affects different brain regions than normal PRES, but it may be related to endothelial cell dysfunction within the perforating vessels supplying the brainstem and basal ganglia, which increases sensitivity in these areas [2]. Furthermore, compared with the older population, frontal lobe or infratentorial involvement was more frequent in younger children [3]. In infants with central-variant PRES, the lack of sympathetic innervation of the posterior circulation causing classic PRES may not be the sole explanation. Reports of imaging abnormalities in infantile PRES are rare, but a few cases of cortical predominance have been reported [2]. To our knowledge, this is the first report of central-variant PRES in an infant. There are only a few reports on the imaging differences between adults and children. More cases are needed to determine whether central-variant PRES is more common in very young brains.
Differential diagnoses of infantile symmetric bilateral basal ganglia lesions are broad and include genetic, acquired metabolic or toxic, infectious, inflammatory, vascular, and neoplastic pathologies [8]. Although the main cause of bilateral basal ganglia involvement in this age group is a genetic etiology, such as Leigh syndrome, we propose central-variant PRES as a rare but important differential diagnosis. This differential diagnosis is important because neurological prognosis may be better than that of Leigh syndrome and specific treatment, such as administration of antihypertensive drugs or discontinuation of drugs that induce PRES, is possible.
Limitations
This study has 2 limitations. First, we could not perform MRI at or around day 36, when basal ganglia lesions were the most prominent. However, swollen hypodensities on CT on day 36 and normalized intensity and volume of basal ganglia on subsequent CT on day 93, as well as MRI on day 51, precludes differential diagnoses other than central-variant PRES. Second, hypoxic ischemic encephalopathy preceding the development of central-variant PRES complicates the imaging findings. We considered the findings of laminar necrosis and putamen lesions on the initial MRI and occipital predominant brain atrophy on follow-up images to be ischemic changes. For occipital lesions, there may be a mixture of irreversible changes due to PRES.
Conclusion
This is the first reported case of infantile central-variant PRES involving bilateral basal ganglia lesions with mid-aortic syndrome. The differential diagnosis of infantile symmetric bilateral basal ganglia lesions is broad but central-variant PRES should not be ruled out. It is a rare subtype but neurological prognosis may be favorable and specific treatment is required to address the symptoms which could progress if not managed immediately.
Patient consent
Written informed consent was obtained from the patient's parents to publish this case report. Ethical approval was not waived for this study in accordance with international and institutional guidelines.
Redundant publication
None.
Acknowledgments
Eri Ohashi and Itaru Hayakawa drafted the manuscript. Yoshiyuki Tsutsumi, Koichi Kamei, Kentaro Ide, and Yuichi Abe were all involved in the clinical care of the patient and revised the manuscript accordingly.
Footnotes
Competing Interests: The authors declare that there is no conflict of interest.
References
- 1.Abraham P, Longardner K, Chen P, Huisa B, Handwerker J. Case 279: central-variant posterior reversible encephalopathy syndrome. Radiology. 2020;296:239–243. doi: 10.1148/radiol.2020181547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McKinney AM, Jagadeesan BD, Truwit CL. Central-variant posterior reversible encephalopathy syndrome: brainstem or basal ganglia involvement lacking cortical or subcortical cerebral edema. AJR Am J Roentgenol. 2013;201:631–638. doi: 10.2214/AJR.12.9677. [DOI] [PubMed] [Google Scholar]
- 3.Cordelli DM, Marra C, Ciampoli L, Barbon D, Toni F, Zama D, et al. Posterior reversible encephalopathy syndrome in infants and young children. Eur J Paediatr Neurol. 2021;30:128–133. doi: 10.1016/j.ejpn.2020.10.009. [DOI] [PubMed] [Google Scholar]
- 4.Forman N, Sinskey J, Shalabi A. A review of middle aortic syndromes in pediatric patients. J Cardiothorac Vasc Anesth. 2020;34:1042–1050. doi: 10.1053/j.jvca.2019.07.130. [DOI] [PubMed] [Google Scholar]
- 5.Ahn CH, Han SA, Kong YH, Kim SJ. Clinical characteristics of hypertensive encephalopathy in pediatric patients. Korean J Pediatr. 2017;60:266–271. doi: 10.3345/kjp.2017.60.8.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fugate JE, Rabinstein AA. Posterior reversible encephalopathy syndrome: clinical and radiological manifestations, pathophysiology, and outstanding questions. Lancet Neurol. 2015;14:914–925. doi: 10.1016/S1474-4422(15)00111-8. [DOI] [PubMed] [Google Scholar]
- 7.Donmez FY, Guleryuz P, Agildere M. MRI findings in childhood PRES: what is different than the adults? Clin Neuroradiol. 2016;26:209–213. doi: 10.1007/s00062-014-0350-2. [DOI] [PubMed] [Google Scholar]
- 8.Van Cauter S, Severino M, Ammendola R, Van Berkel B, Vavro H, van den Hauwe L, et al. Bilateral lesions of the basal ganglia and thalami (central grey matter)-pictorial review. Neuroradiology. 2020;62:1565–1605. doi: 10.1007/s00234-020-02511-y. [DOI] [PMC free article] [PubMed] [Google Scholar]