Abstract
The European Alliance of Associations for Rheumatology recently defined difficult to treat (D2T) rheumatoid arthritis (RA) and provided points to consider in its management. This review summarises the key concepts of D2T-RA that underpinned this recent guidance. D2T-RA is primarily characterised by failure of at least two different mechanism of action biologic/targeted synthetic disease-modifying antirheumatic drug (DMARDs) with evidence of active/progressive disease. The basis for progressive disease, however, is not limited to clear inflammatory joint pathology, capturing wider contributors to treatment cycling such as comorbidity, obesity and fibromyalgia. This means D2T-RA comprises a heterogeneous population, with a proportion within this exhibiting bona fide treatment-refractory disease. The management points to consider, however, emphasise the importance of checking for the presence of inflammatory pathology before further treatment change. This review suggests additional considerations in the definition of D2T-RA, the potential value in identifying D2T traits and intervening before the development of D2T-RA state and the need for real world evidence of targeted synthetic DMARD in this population to compare to recent trial data. Finally, the review asks whether the presence of D2T-RA implies a failure to treat effectively from the outset, and the need for pharmacological and non-pharmacological management approaches to address the wider D2T-RA population effectively.
Keywords: rheumatoid arthritis, biological therapy, therapeutics
Key messages.
The European Alliance of Associations for Rheumatology definition of difficult to treat (D2T) rheumatoid arthritis (RA) captures a heterogeneous population that have failed multiple mechanism of action targeted therapies,
Management of D2T-RA requires careful characterisation for the presence or absence of inflammation to support pharmacological and non-pharmacological strategies,
Further understanding of this population may identify new therapeutic targets and offer the opportunity to intervene earlier in the disease course to mitigate the development of D2T-RA.
Introduction
Significant therapeutic advances in the treatment of people with rheumatoid arthritis (RA) have improved their quality of life and outcomes. The treat-to-target (T2T) strategy promotes prompt diagnosis and efficient initiation and titration of disease-modifying antirheumatic drugs (DMARDs) including progression to biologic (b) and targeted synthetic (ts) DMARDs in line with the European Alliance of Associations for Rheumatology (EULAR) RA management guidelines.1
Despite the availability of advanced therapies that target different cytokines and molecular pathways, a sizeable proportion of patients with RA sequence through multiple therapies and remain symptomatic. Management of this group, recently termed ‘Difficult to Treat’ (D2T) RA, is challenging, has a limited evidence base, and is associated with significant economic health burden.2 A recent survey in the Netherlands demonstrated that D2T-RA patients incurred almost twice the annual cost of direct healthcare utilisation compared with non-D2T RA.3 Indirect costs such as informal help from family and friends and reduced work productivity was also reportedly higher.
The unmet need of this patient group is increasingly recognised. Here, we review the current definition and understanding of D2T-RA, the management strategies that can be employed to address this group of patients and provide thoughts to advance this field further.
Current concept of D2T RA
An international survey of rheumatologists was undertaken to capture the clinical perspectives of D2T RA and to characterise it further. This highlighted multiple components for consideration, including number of DMARDs failed, disease activity state and persistence of patient symptoms.4
In 2021, a EULAR task force undertook an initiative to define this group for greater consistency in clinical and research settings. The task force confirmed D2T-RA as the formal term to describe the patient population of interest and established a EULAR definition comprising three elements: history of failed treatments, features of active disease and the perception of challenging RA by the clinician and/or patient (figure 1).2
Figure 1.
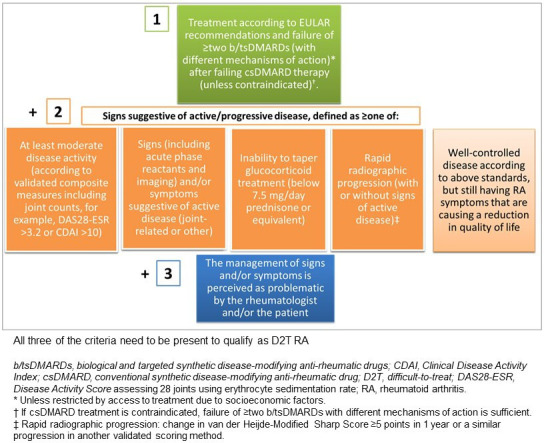
EULAR definition of difficult-to-treat rheumatoid arthritis (D2T-RA). EULAR, European Alliance of Associations for Rheumatology.
The first criterion of the EULAR definition of D2T-RA refers to the fundamental concept of treatment-resistant disease as evidenced by a history of multiple b/tsDMARD failures. All patients must fail at least two b/ts DMARDs of different mechanisms of action to qualify as D2T-RA, equivalent to having reached phase 3 of the 2019 EULAR management of RA.1 The cut-off of ≥two b/tsDMARDs is somewhat arbitrary but stipulating two different mechanisms of action confers a meaningful drug-resistant state. This is also consistent with trials and registry studies that have illustrated diminished response from most treatments following initial bDMARD failure.4–6 In real-world practice, however, a refractory state typically follows exhaustion of all available therapeutic options. The absence of predictive treatment biomarkers and trial and error approach to prescribing has clearly contributed to this state.7
Active or progressive disease is the second key criterion of the EULAR definition, which may be evidenced by one or more of a clinically meaningful indicators (a–d) of suboptimal disease control with existing DMARD therapy. Criterion 2e describing well-controlled disease, but still having RA symptoms that are causing a reduction in quality of life diverges from the conventional description of active RA. This frames D2T-RA within a wider construct that may not be contingent on the central tenet of inflammatory pathology. By including patients that have sequenced therapies (criterion 1) and satisfy an active D2T RA state characterised by criterion 2e, the EULAR definition for D2T-RA does not confine itself to the specific notion of refractory RA. Similarly, the final criterion 3 is deliberately non-specific and seeks to capture challenging features in the wider management of RA.8
A subsequent study that employed this EULAR definition and compared D2T to non-D2T RA patients reflected the breadth introduced by the definition and identified several, diverse factors associated with D2T-RA; namely, limited drug options due to adverse events, comorbidities, a mismatch between rheumatologist and patient and the need for escalation of treatment, coexistent fibromyalgia and poor coping mechanisms. In addition, three subgroups were described—(1) ‘non-adherent dissatisfied patients’; (2) patients with ‘pain syndromes and obesity’ and (3) patients closest to the concept of ‘true refractory RA’. This study emphasised the marked heterogeneity of patients that are identified under the EULAR D2T-RA definition.9
Management of D2T RA
The EULAR definition of D2T-RA provided an effective framework to develop the EULAR points to consider (PtC) in the management of D2T-RA. Indeed, while the definition lends itself towards including a heterogeneous patient group, the PtC advise DMARD strategies based on clear demonstration of persistent active inflammatory pathology, an overarching principle being ‘The presence or absence of inflammation should be established to guide pharmacological and non-pharmacological interventions’.10 This clarity is crucial in the management of D2T RA to mitigate unnecessary cycling of further DMARD therapies. One of the specific PtC highlights that musculoskeletal ultrasound may be an effective tool where evaluation of inflammation proves challenging. This may serve to reassure the rheumatologist and the patient if a decision is made not to change DMARD despite ongoing symptoms and measured disease activity.11 In this regard, acceptance of a low disease activity state as stated in the treat to target recommendations may be more appropriate.
This overarching principle also supports consideration of other factors that may complicate accurate assessment of disease activity state. Obesity and fibromyalgia are highlighted as two prevalent conditions in people with RA that should be accommodated when evaluating disease activity and progression to D2T-RA. Secondary fibromyalgia and osteoarthritis may overestimate patient global health scores and tender joint count assessments. Obesity may make accurate detection of swollen joint count challenging and/or overestimate acute phase reactants and contribute to an excess inflammatory drive and phenotype.12 A reappraisal of the diagnosis and the need to identify coexistent disease and/or mimics that may interfere with the assessment of disease activity were also recommended. Modern RA paradigms encourage us to seek out new and early diagnoses of RA, risking misdiagnosis at the earliest stages of diseases when often still evolving. The hazards of mimics and coexistent pathology is relevant across a patient’s disease course including in the older demographic when conditions such as polymyalgia rheumatica and/or osteoarthritis may complicate the clinical assessment.13
Subsequent PtC focus on pharmacological management. A limited evidence base was available for the EULAR defined D2T-RA population specifically. Nevertheless, where third/fourth line targeted disease modifying pharmacotherapy is indicated, tocilizumab and emergent data on Janus kinase (JAK) inhibitors (JAKi) have been shown to be more effective than placebo. This contrasts with other bDMARDs (TNFi, abatacept and rituximab) that lack such evidence.14 A specific PtC also advises on the benefits of selecting higher dose drug (where such dose choice is an option and not precluded by comorbidity and safety concerns). This applies currently only to intravenous tocilizumab and baricitinib (with higher dose tofacitinib not an approved dose).
The other key factor that influences treatment decision in RA generally and this group in particular, is co-morbidity and specific safety concerns around certain therapeutics. While the PtC emphasise the limited evidence base, they highlight clinical scenarios where we have existing guidelines to inform drug selection—such as hepatitis B and C, and pregnancy.10 In line with the EULAR RA management guidelines, caution of tsDMARD JAKi use and risk of VTE was emphasised.1 The draft 2022 update of the EULAR Recommendations on the management of Rheumatoid Arthritis advise evaluation of pertinent risk factors for cardiovascular (CV) disease when conisdering JAKi in individual treatment decision.15
Finally, non-pharmacological management strategies are included, particularly for patients where an absence of inflammation, residual pain and secondary fibromyalgia are key drivers. Education, self-management and psychological interventions are advised to enable (1) better shared decision making between the patient and rheumatologist on the appropriate target of treatment, thus minimising risk of ‘mismatch’ in expectations (2) improved patient symptom profile and (3) effective coping strategies.10
The PtC, therefore, advise a systematic approach to diagnose and assess D2T-RA more accurately before cycling and intensifying DMARD.
What next?
Identifying D2T/challenging traits early and preventing progression to D2T-RA
The D2T-RA definition is being applied increasingly in clinical practice to identify a population of specific concern and to consider tailored management strategies.
The current concept and definition of D2T-RA focuses on established RA.2 However, the D2T traits of individuals with RA that have been associated with reaching D2T-RA state compared with non-D2T-RA (smoking status, obesity, adherence, fibromyalgia, comorbidity limiting treatment options and so on) are likely to be present prior to progressing to failure of at least 2 b/tsDMARDs.16 17 Although an evidence base is not available, it would seem intuitive that identifying these and intervening at time of diagnosis, in the early stages of disease could arguably, in a proportion of patients, limit progression to a multidrug failure D2T-RA state (figure 2).
Figure 2.
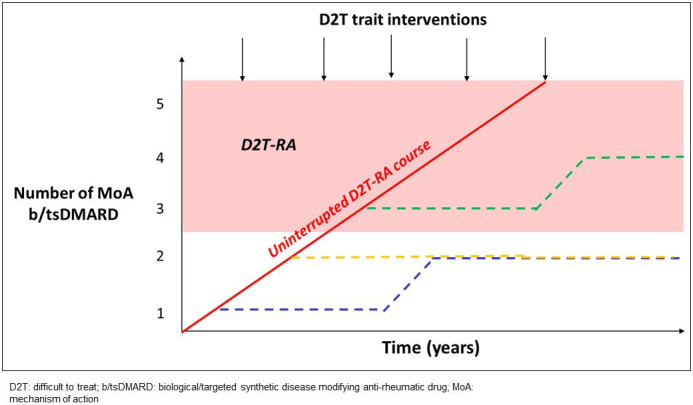
Postulated opportunity for interventions to address difficult to treat traits early to modify a D2T-RA course. RA, rheumatoid arthritis.
Monitoring those with D2T (or perhaps more appropriate to term challenging) traits from time of diagnosis, as opposed to focusing only on those reaching a D2T-RA state following failure of several effective therapeutics would have greater reach of our patient population. This would encourage the clinical and research communities to focus efforts to intervene and prevent progression to D2T-RA and not only manage once progressed to a D2T-RA state (figure 2).
Key factors to consider with the definition of D2T-RA
The suggestion that D2T-RA be viewed as a broader framework within which refractory RA is a subgroup has been articulated previously.18 D2T-RA with clear inflammatory involvement (as per the overarching principle #2 of the EULAR PtC) represents a refractory group at highest risk of poor outcomes such as CV burden, interstitial lung disease, erosive damage and osteoporosis.18 Distinguishing D2T-RA from refractory RA, terms that currently, are used interchangeably would allow more accurate characterisation, with refractory RA, a distinct state that in the majority of cases, may stem from a D2T disease course (figure 3). Confirming persistent inflammatory disease remains key to be able to identify bona fide refractory RA. Admittedly, D2T-RA and refractory RA, may develop in individuals without clear D2T traits, although likely to a lesser extent.
Figure 3.
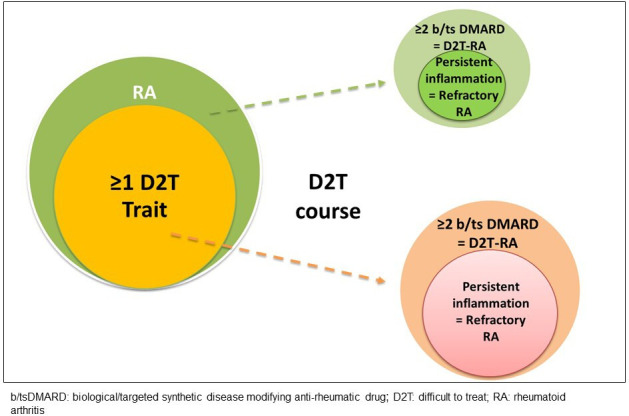
D2T (or challenging) traits, development of a D2T course and the presence of refractory RA.
Importantly, in those with coexistent comorbidity such as grade III/IV heart failure, interstitial lung disease, cardiovascular disease and diabetes, an optimal therapeutic approach may not be pursued initially due to safety concerns.19–21 A refractory population may thus emerge due to adverse risk:benefit assessment and prescribing limitations (see later).
The current definition raises additional points of importance. The entry criterion cut-off of failure of 2 different mechanism of action b/ts DMARDs is arbitrary, and groups together patients that may have failed between 2 and 5 classes (±multiple within-class therapies). Evaluation of response and how (and whether) to accommodate primary failure vs acquired failure and duration on prior treatments also merits discussion.
In addition, the different levels of disease activity that trigger treatment between and even within individuals over time are all factors that add to the complex make up of this population (figure 4). A British Society for Rheumatology Biologics Register for RA identified several factors associated with refractory RA disease. Of 13 502 patients recruited to the registry, 6% of patients had received more than three different bDMARDs with a median time to third bDMARD of 8 years. Interestingly, patients diagnosed from 2011 were 15 times more likely to be identified as being refractory, possibly reflecting a stronger treat to target approach and increased availability of different DMARDs rather than evidence for greater risk of refractory disease in the modern era.5 The cut-off in glucocorticoid dose greater than 7.5 mg daily as a reflection of ineffective b/tsDMARD is debatable, and doses of 7.5 mg daily and lower risk being an ongoing contributor to comorbidities and/or D2T trajectory.22 Incorporating intermittent use of oral and/or intramuscular glucocorticoid in the assessment is challenging, and our still liberal use is likely to be masking the true extent of refractory RA.23
Figure 4.
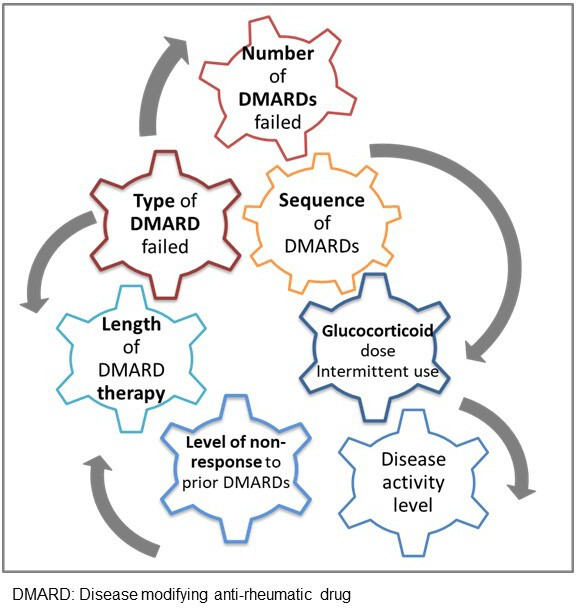
Factors to consider in the definition of difficult to treat rheumatoid arthritis.
It is not feasible and not necessary perhaps to accommodate all these factors into a definition. Nevertheless, the definition may benefit from refining in the future and these issues may help in our understanding of this group of patients.
Key factors to consider in the management of D2T-RA
While data on current therapeutics in a D2T-RA equivalent cohort have been reported, there remains a need to confirm in real-world practice and identify new targets for this population. The emergence of JAKi trials in more refractory populations is providing a basis for pursuing this therapeutic strategy in our current clinical practice.24–27 A fundamental question is whether ‘true’ refractory RA exists (that requires a biological understanding to answer fully) and the pharmacotherapeutic approach. The presumption is that other forms of D2T-RA may be characterised predominantly by confounders that jeopardise otherwise potentially effective treatment responses; whereas true refractory RA, persistent inflammatory RA in the face of such treatments, may comprise tractable targets that have evaded current therapeutic strategies. The stratification of D2T-RA into a persistent inflammatory refractory subgroup vs non-inflammatory state would reduce the ‘noise’ of other confounders and help identify relevant pathways for future intervention studies towards stronger confirmation of drug success. More effective implementation of the overarching principle #2 of the EULAR PtC and use of ultrasound or similar where needed to confirm the presence of inflammatory activity before cycling b/tsDMARDs, would be anticipated to reduce the overall refractory RA population and/or at least the non-inflammatory subgroup.
Greater comorbidity exhibited in D2T-RA, however, complicates treatment decision even when options may exist. This has been brought into focus with the results from the post-authorisation safety study, Oral Surveillance (ORALSURV). In a patient population enriched for CV disease (CVD), ORALSURV showed tofacitinib, a JAK1 and JAK3 inhibitor, not to be non-inferior to TNF-inhibitor for the coprimary endpoints of major adverse cardiovascular events (MACE), and malignancy.28 Subsequent post hoc analyses indicate the risk for MACE and malignancy appears to be mainly associated with those with a past history of CVD and/or at high risk of CVD, clinical features that may be more prevalent in a D2T-RA population.29 The need for disease control to minimise adverse consequences of RA, including CV comorbidity, against a potentially greater risk associated with a therapeutic agent is a delicate balance that needs careful discussion with the patient.
It is also important to maintain monitoring of patients who at some point qualified as being in an (active) D2T-RA state but now have effective disease control. This group remains at risk of reverting to D2T-RA—for example, the patient who has achieved controlled disease on their fourth or fifth b/tsDMARD and would perhaps not be captured in the current definition compared with a patient having just failed a second targeted therapy with moderate or high disease activity. Although it is likely that the former individual would have a poorer quality of life (reflected in criteria 2e and 3), a management approach that is not contingent on a single time point disease activity state and takes a longitudinal view would be useful going forwards. This population is also important to investigate to understand the immunopathogenic basis of reaching a refractory state.
Re-evaluating outcomes of early RA studies
Finally, the treat to target management paradigm and the use of highly effective therapies in a timelier fashion do not appear to have removed the undesirable trajectory to D2T and refractory RA. This raises the question of whether we are failing to treat effectively from the outset. Pragmatic studies with longer-term outcomes to identify patients that reach a D2T/refractory state need to be conducted alongside the conventional short-term early RA intervention studies to evaluate for progression to refractory RA.
Recognising the multifactorial basis for progression to D2T-RA, a multipronged management approach is needed to address the risk of developing D2T-RA and its management. The PtC and algorithm present pharmacotherapy (through immunosuppression) and non-pharmacological interventions as dichotomous management strategies of choice—in reality, a large proportion of patients would benefit from both.10 Recognising the different components that contribute to a D2T-RA state (figure 5) is essential for tailored care. Testing management strategies that combine pharmacological and non-pharmacological intervention would represent a shift in pragmatic clinical trial design that reflects the needs of the real-world population. If shown to be successful, it would provide a stronger basis to lever the necessary multidisciplinary resources for implementation into clinical pathways and services. This approach aligns with a ‘dual target’ strategy suggested by Ferreira et al in response to the risks of overtreatment with immunosuppression in pursuit of a treatment target that may not be achievable with DMARD alone. The authors recommended a strategy for control of inflammation and another for control of disease impact to deliver more complete benefit to individual patients.
Figure 5.
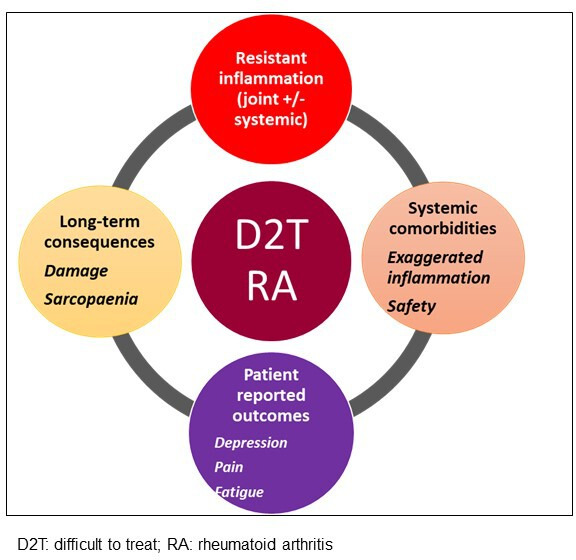
The multiple contributors to a difficult to treat rheumatoid arthritis state.
Identifying the basis for D2T-RA
We do not have a handle yet of the immunopathogenic characterisation of recalcitrant disease although data are emerging.30 It is beyond the scope of this article to discuss the potential biological basis of D2T-RA and within this, refractory RA, which has been discussed previously.18 Stratification according to the presence and absence of inflammation would be an important first step and align with the EULAR PtC. Conversely, defining highly curated disease cohorts in this context may lead to oversplitting of patient groups with limited validity in the scientific investigation as this risks (dis)missing the potential interactions between environmental and biological traits that convergemay contribute to D2T-RA and refractory states.
Conclusion
Despite treating to target and the availability of a range of advanced therapies, D2T-RA remains a relevant clinical problem in 2022. The EULAR definition of D2T-RA provides an effective basis to identify this group, closely monitor and intervene in clinical practice. Recalcitrant inflammatory disease that associates conventionally with the term refractory RA can be seen to exist within the wider context of D2T factors. The definition of D2T-RA will likely evolve to describe D2T and/or refractoriness more clearly.
An individual’s ‘true’ disease state is a culmination and interplay of a whole host of factors including social determinants of health, coexistence of comorbidities, compliance and wider health literacy. Research is needed in the field to refine the definition and understand the risks and predictors and underlying mechanisms. It is also vital to not only recognise those in an ‘active’ disease state but those who have been refractory to multiple therapies. One would anticipate that more effective implementation of the overarching principle (#2) of EULAR PtC into clinical practice, would ensure more accurate basis for DMARD cycling and less progression to D2T-RA. This would enable clearer demonstration of an inflammatory drug-resistant group that warrant new therapeutics vs those that require more intensive non-pharmacological strategies.
Clinical trials confirm the benefit of some of the currently available b/ts DMARDs but further interrogation, especially in real-world populations is needed. The incidence of comorbidities that may already identify D2T traits and the impact of comorbidities, particularly where particular risks with therapies have been identified, would benefit from further investigation. It is unclear whether the specific sequence of b/tsDMARDs and/or resistance to specific drugs influence progression to a D2T-RA and refractory state. Combined pharmacological and non-pharmacological strategies if appropriate should be tested based on the drivers of persistent disease activity and symptoms.
Finally, more effective strategies early in disease and sequencing may change the trajectory and prevent development of future D2T-RA. Nevertheless, the presence of recalcitrant disease highlights the need for continued target discovery and therapeutic pipeline.
Footnotes
Contributors: YT drafted the review, MHB edited and refined this and developed the figures to provide a final manuscript. Both authors approve the submitted version.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Disclaimer: The views expressed in this article are those of the authors and not necessarily those of the NHS, the National Institute for Health and Care Research or the Department of Health.
Competing interests: MHB is supported by the National Institute for Health and Care Research (NIHR) Manchester Biomedical Research Centre and is in receipt of an NIHR Senior Investigator award.
Provenance and peer review: Commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Smolen JS, Landewé RBM, Bijlsma JWJ, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis 2020;79:685–99. 10.1136/annrheumdis-2019-216655 [DOI] [PubMed] [Google Scholar]
- 2.Nagy G, Roodenrijs NM, Welsing PM, et al. EULAR definition of difficult-to-treat rheumatoid arthritis. Ann Rheum Dis 2021;80:31–5. 10.1136/annrheumdis-2020-217344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roodenrijs NMT, Welsing PMJ, van der Goes MC, et al. Healthcare utilization and economic burden of difficult-to-treat rheumatoid arthritis: a cost-of-illness study. Rheumatology 2021;60:4681–90. 10.1093/rheumatology/keab078 [DOI] [PubMed] [Google Scholar]
- 4.Roodenrijs NMT, de Hair MJH, van der Goes MC, et al. Characteristics of difficult-to-treat rheumatoid arthritis: results of an international survey. Ann Rheum Dis 2018;77:77. 10.1136/annrheumdis-2018-213687 [DOI] [PubMed] [Google Scholar]
- 5.Kearsley-Fleet L, Davies R, De Cock D, et al. Biologic refractory disease in rheumatoid arthritis: results from the British Society for rheumatology biologics register for rheumatoid arthritis. Ann Rheum Dis 2018;77:1405–12. 10.1136/annrheumdis-2018-213378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li H, Zhu H, Xu L, Xue J, et al. The characteristics and its contributing factors of refractory rheumatoid arthritis, view of the rheumatologists of China: results of a nationwide cross-sectional survey. Clin Rheumatol 2021;40:4029–38. 10.1007/s10067-021-05687-7 [DOI] [PubMed] [Google Scholar]
- 7.Cuppen BVJ, Welsing PMJ, Sprengers JJ, et al. Personalized biological treatment for rheumatoid arthritis: a systematic review with a focus on clinical applicability. Rheumatology 2016;55:826–39. 10.1093/rheumatology/kev421 [DOI] [PubMed] [Google Scholar]
- 8.de Hair MJH, Jacobs JWG, Schoneveld JLM, et al. Difficult-To-Treat rheumatoid arthritis: an area of unmet clinical need. Rheumatology 2018;57:1135–44. 10.1093/rheumatology/kex349 [DOI] [PubMed] [Google Scholar]
- 9.Roodenrijs NMT, van der Goes MC, Welsing PMJ, et al. Difficult-To-Treat rheumatoid arthritis: contributing factors and burden of disease. Rheumatology 2021;60:3778–88. 10.1093/rheumatology/keaa860 [DOI] [PubMed] [Google Scholar]
- 10.Nagy G, Roodenrijs NMT, Welsing PMJ, et al. EULAR points to consider for the management of difficult-to-treat rheumatoid arthritis. Ann Rheum Dis 2022;81:20–33. 10.1136/annrheumdis-2021-220973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ciurtin C, Jones A, Brown G, et al. Real benefits of ultrasound evaluation of hand and foot synovitis for better characterisation of the disease activity in rheumatoid arthritis. Eur Radiol 2019;29:6345–54. 10.1007/s00330-019-06187-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levitsky A, Brismar K, Hafström I, et al. Obesity is a strong predictor of worse clinical outcomes and treatment responses in early rheumatoid arthritis: results from the SWEFOT trial. RMD Open 2017;3:e000458. 10.1136/rmdopen-2017-000458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roodenrijs NMT, Kedves M, Hamar A, et al. Diagnostic issues in difficult-to-treat rheumatoid arthritis: a systematic literature review Informing the EULAR recommendations for the management of difficult-to-treat rheumatoid arthritis. RMD Open 2021;7:e001511. 10.1136/rmdopen-2020-001511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roodenrijs NMT, Hamar A, Kedves M, et al. Pharmacological and non-pharmacological therapeutic strategies in difficult-to-treat rheumatoid arthritis: a systematic literature review Informing the EULAR recommendations for the management of difficult-to-treat rheumatoid arthritis. RMD Open 2021;7:e001512. 10.1136/rmdopen-2020-001512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smolen JS. Update of the EULAR recommendations on the management of rheumatoid arthritis. EULAR congress 2022; 01/06/2022, 2022. [Google Scholar]
- 16.Takanashi S, Kaneko Y, Takeuchi T. Characteristics of patients with difficult-to-treat rheumatoid arthritis in clinical practice. Rheumatology 2021;60:5247–56. 10.1093/rheumatology/keab209 [DOI] [PubMed] [Google Scholar]
- 17.Batko B, Urbański K, Świerkot J, et al. Comorbidity burden and clinical characteristics of patients with difficult-to-control rheumatoid arthritis. Clin Rheumatol 2019;38:2473–81. 10.1007/s10067-019-04579-1 [DOI] [PubMed] [Google Scholar]
- 18.Buch MH, Eyre S, McGonagle D. Persistent inflammatory and non-inflammatory mechanisms in refractory rheumatoid arthritis. Nat Rev Rheumatol 2021;17:17–33. 10.1038/s41584-020-00541-7 [DOI] [PubMed] [Google Scholar]
- 19.Avina-Zubieta JA, Thomas J, Sadatsafavi M, et al. Risk of incident cardiovascular events in patients with rheumatoid arthritis: a meta-analysis of observational studies. Ann Rheum Dis 2012;71:1524–9. 10.1136/annrheumdis-2011-200726 [DOI] [PubMed] [Google Scholar]
- 20.Tian Z, Mclaughlin J, Verma A, et al. The relationship between rheumatoid arthritis and diabetes mellitus: a systematic review and meta-analysis. Cardiovasc Endocrinol Metab 2021;10:125–31. 10.1097/XCE.0000000000000244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Antin-Ozerkis D, Evans J, Rubinowitz A, et al. Pulmonary manifestations of rheumatoid arthritis. Clin Chest Med 2010;31:451–78. 10.1016/j.ccm.2010.04.003 [DOI] [PubMed] [Google Scholar]
- 22.Hua C, Buttgereit F, Combe B. Glucocorticoids in rheumatoid arthritis: current status and future studies. RMD Open 2020;6:e000536. 10.1136/rmdopen-2017-000536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crossfield SSR, Buch MH, Baxter P, et al. Changes in the pharmacological management of rheumatoid arthritis over two decades. Rheumatology 2021;60:4141–51. 10.1093/rheumatology/keaa892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Genovese MC, Kremer J, Zamani O, et al. Baricitinib in patients with refractory rheumatoid arthritis. N Engl J Med 2016;374:1243–52. 10.1056/NEJMoa1507247 [DOI] [PubMed] [Google Scholar]
- 25.Genovese MC, Kalunian K, Gottenberg J-E, et al. Effect of Filgotinib vs placebo on clinical response in patients with moderate to severe rheumatoid arthritis refractory to disease-modifying antirheumatic drug therapy: the finch 2 randomized clinical trial. JAMA 2019;322:315–25. 10.1001/jama.2019.9055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee YH, Bae S-C. Comparative efficacy and safety of tocilizumab, rituximab, abatacept and tofacitinib in patients with active rheumatoid arthritis that inadequately responds to tumor necrosis factor inhibitors: a Bayesian network meta-analysis of randomized controlled trials. Int J Rheum Dis 2016;19:1103–11. 10.1111/1756-185X.12822 [DOI] [PubMed] [Google Scholar]
- 27.Singh JA, Hossain A, Tanjong Ghogomu E, et al. Biologics or tofacitinib for people with rheumatoid arthritis unsuccessfully treated with biologics: a systematic review and network meta-analysis. Cochrane Database Syst Rev 2017;3:CD012591. 10.1002/14651858.CD012591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ytterberg SR, Bhatt DL, Mikuls TR, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med 2022;386:316–26. 10.1056/NEJMoa2109927 [DOI] [PubMed] [Google Scholar]
- 29.Dougados M, Charles-Schoeman C, Szekanecz Z. OP0264 impact of baseline cardiovascular risk on the incidence of major adverse cardiovascular events in the tofacitinib rheumatoid arthritis clinical programme. Annals of the Rheumatic Diseases 2022;81:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rivellese F, Surace AEA, Goldmann K, et al. Rituximab versus tocilizumab in rheumatoid arthritis: synovial biopsy-based biomarker analysis of the phase 4 R4RA randomized trial. Nat Med 2022. 10.1038/s41591-022-01789-0. [Epub ahead of print: 19 May 2022]. [DOI] [PMC free article] [PubMed] [Google Scholar]


