Abstract
Vaccines against SARS-CoV-2 have shown remarkable efficacy and thus constitute an important preventive option against coronavirus disease 2019 (COVID-19), especially in fragile patients. We aimed to systematically analyze the outcomes of patients with hematological malignancies who received vaccination and to identify specific groups with differences in outcomes. The primary end point was antibody response after full vaccination (2 doses of mRNA or one dose of vector-based vaccines). We identified 49 studies comprising 11,086 individuals. Overall risk of bias was low. The pooled response for hematological malignancies was 64% (95% confidence interval [CI]: 59-69; I²=93%) versus 96% (95% CI: 92-97; I²=44%) for solid cancer and 98% (95% CI: 96-99; I²=55%) for healthy controls (P<0.001). Outcome was different across hematological malignancies (P<0.001). The pooled response was 50% (95% CI: 43-57; I²=84%) for chronic lymphocytic leukemia, 76% (95% CI: 67-83; I²=92%) for multiple myeloma, 83% (95% CI: 69-91; I²=85%) for myeloproliferative neoplasms, 91% (95% CI: 82-96; I²=12%) for Hodgkin lymphoma, and 58% (95% CI: 44-70; I²=84%) for aggressive and 61% (95% CI: 48-72; I²=85%) for indolent non-Hodgkin lymphoma. The pooled response for allogeneic and autologous hematopoietic cell transplantation was 82% and 83%, respectively. Being in remission and prior COVID-19 showed significantly higher responses. Low pooled response was identified for active treatment (35%), anti-CD20 therapy ≤1 year (15%), Bruton kinase inhibition (23%), venetoclax (26%), ruxolitinib (42%), and chimeric antigen receptor T-cell therapy (42%). Studies on timing, value of boosters, and long-term efficacy are needed. This study is registered with PROSPERO (clinicaltrials gov. Identifier: CRD42021279051).
Introduction
For patients with hematological malignancies, risk of death among adult patients who had coronavirus disease 2019 (COVID-19) was estimated to be 34% in predominantly hospitalized patients, being even higher for patients at older age.1 Similarly, patients undergoing hematopoetic cell transplantation or cellular therapy were found to be at increased risk for lower respiratory tract disease, intensive care admission, and death.2 In addition, COVID-19 elicits an impaired antibody response against SARS-CoV-2 in hematological malignancies.3 Overall, this emphasized the need for stringent surveillance and urgent identification of therapeutic and preventive options.4
Vaccines against SARS-CoV-2 constitute such an important preventive option against COVID-19 in fragile patients, in addition to other non-pharmaceutical measures such as wearing masks, hand-washing, or social distancing.5 Several randomized trials have established the safety and efficacy of several vaccines, using novel messenger RNA (mRNA) or vector-based vaccines.6 For patients undergoing hematopoietic cell transplantation or cellular therapy, current consensus recommends initiation of vaccination approximately between 3 and 6 months after treatment for allogeneic and 2 months for autologous transplants.7– 9 However, the actual effect in patients with hematological malignancies and transplantation/cellular therapy is unclear.
As the COVID-19 pandemic, to date, does not seem to be overcome and the disease still remains a major risk factor for morbidity and mortality in high-risk patients, evidence syntheses are needed facilitating risk group identification and decision-making. Here, we aimed to perform a systematic review and meta-analysis to assess the antibody response, efficacy, and safety after vaccination against SARS-CoV-2 in patients with hematological malignancies.
Methods
Search strategy and selection criteria
We followed the updated Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline and the Meta-analysis of Observational Studies in Epidemiology (MOOSE) checklist.
All studies published since 1 July 2020 on adults with hematological malignancies (myeloid, lymphoid, or plasma cell dyscrasias) after one or two doses of vaccine were considered for inclusion. Full vaccination was defined as two doses of mRNA vaccination or one dose of vector-based vaccines. Case reports/series, or cohort studies with an overall population of less than ten were excluded. The last updated literature search of MEDLINE, the Cochrane Library, and LitCovid was done on 16 September 2021. Additionally, the reference lists of relevant reviews published in 2021 were reviewed. Two authors (NG and NK) independently conducted the search strategy. Differences in opinion were discussed and resolved by consensus with a third author (FP). The full search strategy is available in the Online Supplementary Table S1.
The following key characteristics were extracted: authors, number of patients, type of vaccine, disease, number of patients receiving hematopoietic cell transplantation (allogeneic or autologous) and cellular therapy (chimeric antigen receptor [CAR] T-cell therapy), other therapies, remission status, COVID-19 prior to vaccination, control group, antibody response, safety, infection rate after vaccination, age, antibody assay, and follow-up.
Hematological malignancy subtypes were divided as: multiple myeloma (excluding monoclonal gammopathy of unknown significance), chronic lymphocytic leukemia, myeloproliferative neoplasms (including chronic myeloid leukemia, polycythaemia vera, essential thrombocythaemia, and myelofibrosis), lymphoproliferative disorder (aggressive or indolent non-Hodgkin lymphoma, and Hodgkin lymphoma). For remission status, we categorized patients as in remission at time of COVID-19 vaccination or at stable/progressive disease.
Outcomes
The primary end point was antibody response (serocon-version rate) after full vaccination (1 or 2 doses, depending on the vaccine), as assessed by anti-SARS-CoV-2 spike protein IgG antibody testing. Thresholds for positivity were in accordance with the respective assays used in the studies. The secondary endpoints were efficacy, response after first dose, and safety. Regarding efficacy, COVID-19 diagnosis was based solely on real-time polymerase chain reaction (RT-PCR), as reported by the studies.
Data analysis
The methodological quality of each study was assessed using a tool designed specifically to evaluate non-comparative studies. Domains for potential bias are the following: selection of participants, ascertainment, causality, and reporting.10
We used the Q test to assess between-study heterogeneity, and calculated the I² statistic, which expresses the percentage of the total observed variability caused by study heterogeneity. I² values were defined as low (≤50%), moderate (50-75%), or high heterogeneity (>75%). Publication bias was assessed by visual inspection of the funnel plots, coupled with the Egger’s test.
Event rates and confidence intervals (CI) were pooled for each intervention in a meta-analysis using a random-effects model (DerSimonian and Laird). In order to examine the association of prespecified continuous moderator variables such as age with study effect size, a mixed-effects model was selected for meta-regression. All values showing P<0.05 were considered as statistically significant. All analyses were performed using R version 4.0.3. No informed consent or Institutional Review Boards were needed for this analysis.
This study is registered with PROSPERO (clinicaltrials gov. Identifier: CRD42021279051).
Results
A total of 714 citations were retrieved, after removal of duplicates. Out of those, 89 citations were assessed eligible for full-text screening. Citations were further selected after exclusion of studies reporting on less than ten patients, studies with no extractable antibody response (seroconversion) rate, survey reporting only on qualitative outcomes, and remaining reviews. Subsequently, 49 studies comprising 11,086 patients with hematological malignancies, solid cancer, or healthy controls were eligible (Figure 1).11–59
Most studies investigated response after second dose of mRNA vaccines (either BNT162b2 or mRNA-1273). Twenty-four studies included healthy controls, and seven studies included patients with solid cancer. Median follow-up of studies on full vaccination (second dose of mRNA or first dose of vector-based vaccine) was 52 days (range, 35-107 days) and the median age of hematological patients was 67 years (range of median age, 46-82 years). Main study characteristics are depicted in the Online Supplementary Table S2. The overall risk of bias of included studies was judged to be low, with ten studies showing moderate risk of bias (Online Supplementary Table S3), and no publication bias was identified (P=0.56; Online Supplementary Figure S1).
Figure 1.
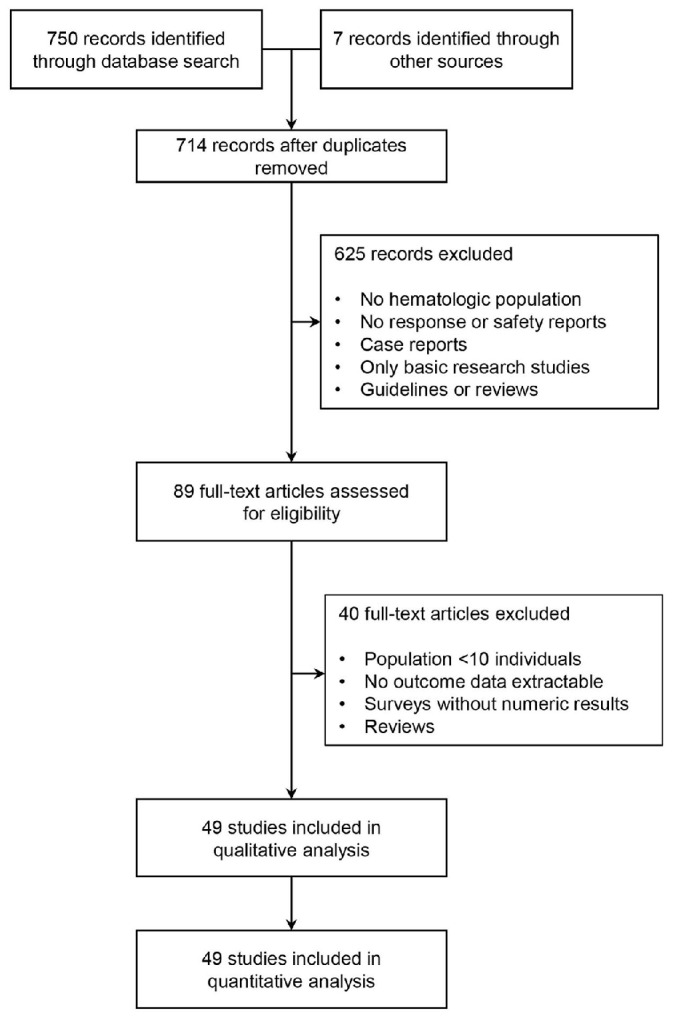
PRISMA flow diagram of study selection process.
Antibody response in hematological malignancies
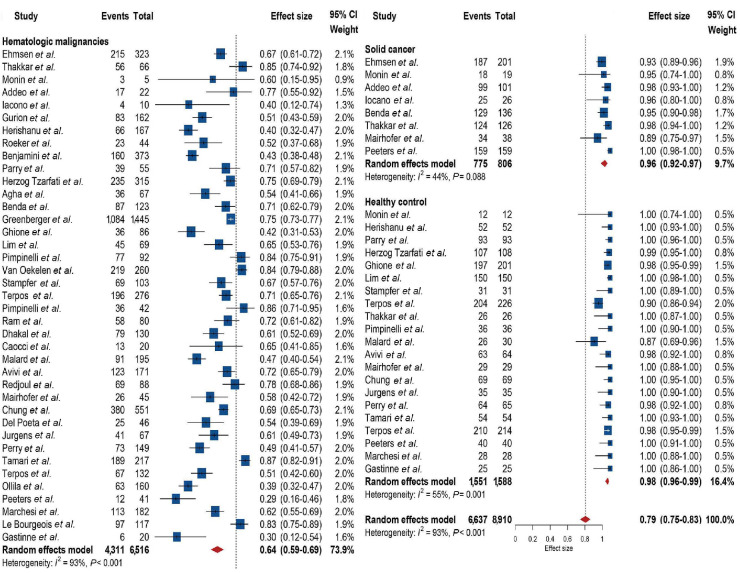
Thirty-nine studies reported on antibody response after full vaccination (mostly second dose of mRNA vaccines). A significant between-group difference in antibody response was identified between hematological malignancies, solid cancer, and healthy control (P<0.001). Four thousand three hundred and eleven of 6,516 patients with hematological malignancies showed an antibody response, and the overall pooled response was 64% (95% CI: 59-69), with high heterogeneity (I²=93%). Seven hundred seventy-five of 806 patients with solid cancer in seven studies showed an antibody response, and the pooled response was 96% (95% CI: 0.92-0.97), with no heterogeneity (I²=44%). One thousand five hundred and fifty-one of 1,588 healthy controls in 21 studies showed an antibody response, and the pooled response was 98% (95% CI: 96-99), and moderate heterogeneity was identified (I²=55%). Detailed results with number of patients and events are depicted in the corresponding forest plot in Figure 2.
Seventeen studies reporting on 1,550 patients with hematological malignancies included early antibody response rates after first inoculum (Online Supplementary Figure S2). A significant difference was identified between hematological malignancies, solid cancer, and healthy controls (P<0.001). The pooled response was 37% (95% CI: 29-45; I²=88%) for hematological malignancies, 62% (95% CI: 37-83; I²=93%) for solid cancer, and 78% (95% CI: 63-89; I²=92%) for healthy controls.
Figure 2.
Forest plot of pooled antibody response rates across included studies.
Sensitivity analysis
In order to minimize potential selection bias, prespecified sensitivity analysis was done according to risk of bias of studies evaluating hematological malignancies (Table 1). The overall pooled response was 64% (95% CI: 58-71; I²=94%) and 63% (95% CI: 52-62; I²=82%) for studies at overall low or moderate risk of bias, respectively (P=0.91). Next, response according to type of mRNA vaccine was evaluated in patients with hematological malignancies. Two thousand five hundred and one of 3,864 patients and 757 of 1,058 who received either BNT162b2 or mRNA-1273 showed an antibody response, and the pooled responses were 63% (95% CI: 57-69) and 72% (95% CI: 60-72), with high heterogeneity (I²= 93% and 96%), respectively. No significant between-group difference according to vaccine was identified (P=0.34; Online Supplementary Figures S3 and S4).
Diseases and treatments
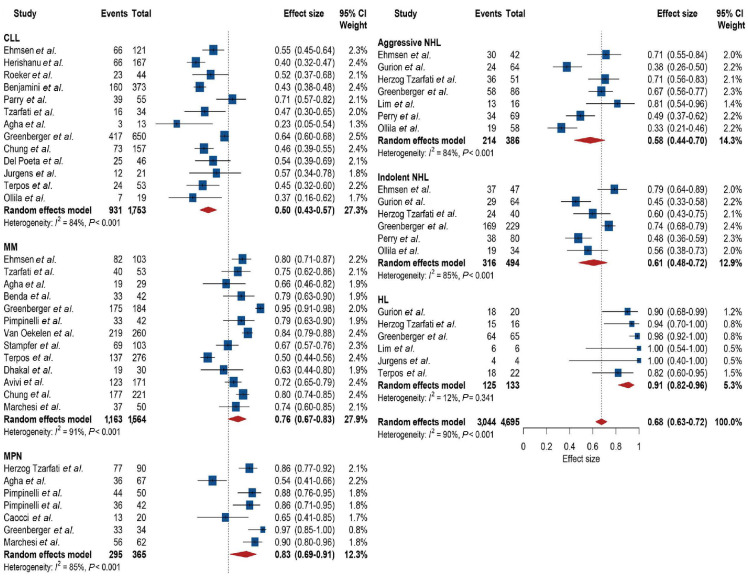
We then stratified the overall population with hematological malignancies according to underlying diagnosis and found a significant difference between the groups (P<0.001), accounting for 25% of the overall heterogeneity (Figure 3). Nine hundred and thirty-one of 1,753 patients with chronic lymphocytic leukemia showed an antibody response, and the pooled response was 50% (95% CI: 43-57), with high heterogeneity (I²=84%). Next, 1,163 of 1564 patients with multiple myeloma showed an antibody response, and the pooled response was 76% (95% CI: 67-83), with high heterogeneity (I²=91%). Two hundred and ninety-five of 365 patients with myeloproliferative neoplasms showed an antibody response, and the pooled response was 83% (95% CI: 69-91), with high heterogeneity (I²=85%). For non-Hodgkin lymphoma, 214 of 386 with aggressive and 316 of 494 with indolent lymphoma showed an antibody response, and the pooled responses were 58% (95% CI: 44-70) and 61% (95% CI: 48-72), with high heterogeneity (I²= 84% and 85%), respectively. For Hodgkin lymphoma, 125 of 133 patients showed antibody response, and the pooled response was 91% (95% CI: 82-96), with no heterogeneity (I²=12%).
Table 1.
Pooled antibody response rates across subgroups.
A significant difference was found for patients in remission compared with patients with stable or progressive disease at time of vaccination (P=0.014). Six hundred and five of 835 patients in remission showed an antibody response compared with 331 of 590 of patients with stable or progressive disease (Online Supplementary Figure S5), and the pooled responses were 72% (95% CI: 64-79) compared with 48% (95% CI: 31-66), with high heterogeneity (I²=80% and 93%), respectively. Antibody response was furthermore affected by history of COVID-19 prior to vaccination (P=0.005). Ninety-seven of 107 patients with hematological malignancies and prior COVID-19 showed an antibody response, and the pooled response was 87% (95% CI: 75-94), with no heterogeneity (I²=26%; Online Supplementary Figure S6).
Thirteen studies evaluated patients who underwent hematopoietic cell transplantation comprising a total of 1,324 patients. No between-group difference for allogeneic and autologous transplants was identified (P=0.60; Online Supplementary Figure S7). For allogeneic transplants, 577 of 697 patients achieved antibody response, and the pooled response was 82% (95% CI: 77-87), with moderate heterogeneity (I²=64%). Most transplantations were received >1 year prior to vaccination and limited data suggested reduced response rates particularly for those receiving allogeneic transplantation <6 or 12 months prior to vaccination.22,41,47,59 For autologous transplants, 466 of 547 patients achieved an antibody response, and the pooled response was 83% (95% CI: 73-90), with high heterogeneity (I²=83%). For patients who received CAR-T therapy, only 35 of 72 patients showed a response, and the pooled response was 42% (95% CI: 27-60), with moderate heterogeneity (I²=54%; Online Supplementary Figure S8).
Next, a significant difference in antibody response was found for active treatment at time of vaccination in comparison with no treatment (P<0.001). Five hundred and fifty-two of 1,228 patients under active treatment and 744 of 1,034 under no treatment showed antibody response (Online Supplementary Figure S9), and the pooled responses were 35% (95% CI: 25-47; I²=93%) compared with 76% (95% CI: 68-82; I²=83%).
Furthermore, targeted treatments were evaluated. The response was significantly affected by the timing of anti-CD20 therapy (P<0.001). Forty-six of 321 patients who Burkitt lymphoma, peripheral T-cell lymphoma, mantle-cell lymphoma, angioimmunoblastic lymphoma, and central nervous system lymphoma. CLL: chronic lymphocytic leukemia; MM: multiple myeloma; MPN: myeloproliferative neoplasms; NHL: non-Hodgkin lymphoma; HL: Hodgkin lymphoma.
Figure 3.
Forest plot of pooled antibody response rates across hematological malignancies. Note: aggressive lymphoma was defined as diffuse large B-cell lymphoma, received anti-CD20 therapy ≤1 year prior to vaccination showed an antibody response compared with 213 of 388 who received anti-CD20 therapy >1 year prior to vaccination (Online Supplementary Figure S10). The pooled responses were 15% (95% CI: 9-24; I²=59%) compared with 59% (95% CI: 46-72; I²=85%). Next, 11 studies comprising 636 patients evaluated outcome for Bruton kinase inhibitor therapy, and the pooled response was 23% (95% CI: 14-35), with high heterogeneity (I²=85%; Online Supplementary Figure S11). Seven studies evaluated the response for patients who received venetoclax therapy, and the pooled response was 26% (95% CI: 14-37), with no heterogeneity (I²=0%; Online Supplementary Figure S12). Four studies included 50 patients with myelofibrosis and ruxolitinib therapy, and the pooled response was 42% (95% CI: 25-61), with no heterogeneity (I²=36%). Seven studies in multiple myeloma patients evaluated response for patients who received anti-CD38 therapy (Online Supplementary Figure S13). Of 351 patients analyzed 211 showed an antibody response, and the pooled response was 55% (95% CI: 40-69), with high heterogeneity (I²=84%). Patients who received chemotherapy showed a pooled response of 69% (Online Supplementary Figure S14).
Efficacy
Fifteen studies comprising 2,719 hematological malignancy patients assessed efficacy of vaccination during follow-up. There were 28 reported COVID-19 cases, with ten and nine reported cases in two studies,14,22 while the remaining nine cases were reported in six studies.15,37,51,53,57,58 The pooled proportion of evaluable patients without COVID-19 during follow-up was 99% (95% CI: 98-99), and no heterogeneity was observed (I²=23%; Online Supplementary Figure S15). Five COVID-19 deaths were observed among fully vaccinated patients with hematological malignancies.
Safety
One-third of patients reported local adverse events such as injection side pain and sore arm. Most frequent systemic adverse events were weakness/fatigue (6-30%) and generalized muscle pain (4-30%). The Online Supplementary Table S4 summarizes the reported safety profiles after first and second dose of vaccination.
Discussion
This meta-analysis of 49 studies and >11,000 patients with hematological malignancies, solid cancer, or healthy controls regarding antibody responses after vaccination against SARS-CoV-2 found markedly reduced response rates for patients with hematological malignancies. The humoral response to vaccination was further affected by type of disease, remission status, history of COVID-19, and treatment.
In terms of disease subgroups, patients with chronic lymphocytic leukemia showed lowest pooled response rates of 50%, followed by patients with aggressive (58%) or indolent non-Hodgkin lymphoma (61%), whereas patients with multiple myeloma and myeloproliferative neoplasms showed responses of 76% and 83%, respectively. Only patients with Hodgkin lymphoma appeared to exhibit comparable responses to healthy controls, showing pooled response of 91%. The underlying causes for lower humoral response to vaccination may be multifactorial, with attributions to disease-related immune dysregulation, treatment-related immunosuppression, as well as to patient-specific factors.60 One identified treatment-related factor across disease was active treatment, suggesting markedly reduced humoral response rates (pooled, 35%) in comparison with patients with no treatment at time of vaccination.
Particularly for patients with chronic lymphocytic leukemia and lymphoma, another important treatment-related factor identified in this analysis may be anti-CD20 antibody (rituximab or obinutuzumab) within 1 year prior to vaccination. This subgroup showed significantly reduced anti–SARS-CoV-2 antibodies in comparison with patients who received anti-CD20 therapy >1 year prior to vaccination who, however, still exhibited overall reduced response. These results are in line with previous findings of reduced response to other vaccines after exposure to B cell–depleting agents.61,62 Furthermore, patients actively treated with Bruton kinase inhibitors exhibited very low response rates. This result is in line with previous studies which found an association between blockaded B-cell receptor signaling and impaired responses to vaccines against influenza and hepatitis B.63,64 Our data synthesis may encourage active discourse and implementation of personalized immunization strategies for patients with respect to their individual treatment.
Regarding weakened responses in multiple myeloma, although we could not further dissect patients into smoldering myeloma, one included study suggested suboptimal response irrespective of smoldering or active multiple myeloma, indicating that the disease itself may have a crucial immunosuppressive role, suppressing normal B-cell expansion and immunoglobulin production and characterized by dysfunctional antigen presentation.65 Myeloma patients were previously found to present sub-optimal seroconversion rates after vaccination against other viruses and, usually, booster doses are needed.66 In terms of treatment-related factors, we found reduced pooled response rates of 55% for myeloma patients receiving anti-CD38 therapy. Mechanistically, depletion of antibody-producing B cells using anti-CD38 monoclonal antibodies directly diminishes immunogenicity.67 Previous findings suggested no interfering effect of this therapy in the setting of other usual vaccination programs.68 However, at least three studies reported ≤50% response rates for patients undergoing anti-CD38 treatment in the present analysis. Other treatment modalities such as immunomodulatory drugs appeared to be associated with higher response rates compared with antibody treatments, showing 81% (95% CI: 71-88; I2=51%).14,19,27,43,46 Whether these results are further affected by other concurrent treatments such as proteasome inhibition cannot be further dissected in this pooled analysis.
Another relevant subgroup consists of patients who underwent hematopoietic cell transplantation or CAR-T therapy. Here, we found slightly higher response rates of 83% for autologous and 82% for allogeneic hematopoietic cell transplantation recipients. Our analysis was unable to differentiate results according to timing of transplantation and thus on the potential effect of ongoing immunosuppression, due to the limited number of studies, while first limited evidence showed higher responses for patients receiving vaccination at least 1 year after transplantation.22,41,47 Another study in myeloma suggested low serological responses for patients who received autologous transplantation within 6 months prior to vaccination, and responses improved significantly afterwards.22 With respect to allografts and although not systematically assessable in our data synthesis, exacerbation of graft-versus-host disease was noted in approximately 3-5% of the patients without serious complications.33,38 Two studies documented development of transient grade 3 or 4 cytopenia.69 For CAR-T patients, we found markedly reduced pooled response of 45%. Whether this is associated with underlying disease, immunosuppression, disease characteristic, previous exposition to B-cell depleting therapy, or cytokine release syndrome needs to be evaluated in larger studies.
In terms of vaccines, mRNA-1273 appeared to induce higher seropositive rates in comparison with BNT162b2, which is in line with previous findings in the setting of solid organ transplantation,70 whereas nearly identical response and efficacy has been shown for healthy participants. Median antibody titers appeared to be higher for mRNA-1273, and whether this can be attributed to different amounts of spike mRNA, or whether this is affected by different compositions altering penetration into host cells remains to be determined.
We acknowledge several shortcomings of our analysis. Considerable heterogeneity was observed in hematological malignancies, while no substantial heterogeneity was observed in solid cancer and healthy controls, suggesting inherent effect of diseases and treatments. In that regard, results in some patient subgroups may be interpreted with caution do due to small sample size, for instance patients who received CAR-T therapy or patients with different myeloproliferative neoplasms (including myelofibrosis or polycythemia vera). Patients with acute leukemia or myelodysplastic syndromes were only reported in three studies comprising a total of 170 patients. The pooled response after full vaccination was 91% (95% CI: 86-95; I²=0%).23,29,47 However, detailed reports on how many of them had acute lymphoblastic leukemia or acute myeloid leukemia, or which treatment (including transplantation) was received by either group were not extractable from all studies, challenging the interpretation of these results. In contrast, when interpreting results of the total cohort of hematological malignancies, some cohorts may confound the results by relative overrepresentation (including chronic lymphocytic leukemia and multiple myeloma), which may have been due to the prevalence as well as the need of rapid recruitment necessary for studies in this evolving pandemic. Therefore, we aimed to stratify patients as adequately as possible to dissect current evidence for each subgroup. Furthermore, we analyzed other patient-specific factors that may have influenced results such as age, which did not seem to affect response between the studies. Meta-regression showed that none of the observed between-study heterogeneity may be accounted to patient age, with a trend toward different outcome for patients >75 years (Online Supplementary Figure S16).
Another limitation might have been introduced through the reliance on anti-spike protein IgG levels as a surrogate for immunity to COVID-19, with a risk of between-study difference due to different assays. Another potential limitation may result from under-estimation of dynamics over time of anti-spike antibodies because cutoffs and follow-up needed to be taken from each study. As with any meta-analysis, the present work depended on time point evaluations, dynamics of outcomes can only be addressed using patient level data.
The anti-spike IgG antibody used in each study might still not necessarily correlate with neutralizing activity against SARS-CoV-2. Not all studies reported homogenously on neutralizing activity which was therefore not included as the main objective to minimize selection bias. However, an explorative pooled analysis of neutralizing antibody response of five studies comprising 856 patients across diseases showed a pooled response of 52% (95% CI: 44-60) after full vaccination, with high heterogeneity (I²=82%).11,23,46,47,52 More studies on the relevance of measuring neutralization assays are needed.
Last, some studies reported on the level of SARS-CoV-2-specific T-cell responses. Recent research indicated that CD8+ T-cell responses may be protective in patients with hematological malignancies in the setting of limited antibody responses after vaccination.71 Analysis of T-cell responses was out of the scope of the present work and will need further evaluation.
Despite all limitations identified, during the COVID-19 pandemic, gathering, analyzing, and reporting outcome data is particularly important for specific risk populations. This large meta-analysis of patients with hematological malignancies who underwent vaccination against SARS-CoV-2 according to current guidance showed significantly reduced response in this cohort. The findings of the presented evidence synthesis may inform decision-making with regards to patient selection and highlights the need for further studies on the best timing of vaccinations as well as the added value of boosters.
Supplementary Material
References
- 1.Vijenthira A, Gong I Y, Fox TA, et al. Outcomes of patients with hematologic malignancies and COVID-19: a systematic review and meta-analysis of 3377 patients. Blood. 2020;136(25):2881-2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ljungman P, La Camara R de, Mikulska M, et al. COVID-19 and stem cell transplantation; results from an EBMT and GETH multicenter prospective survey. Leukemia. 2021;35(10):2885-2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Passamonti F, Romano A, Salvini M, et al. COVID-19 elicits an impaired antibody response against SARS-CoV-2 in patients with haematological malignancies. Br J Haematol. 2021;195(3):371-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ljungman P, Mikulska M, La Camara R de, et al. The challenge of COVID-19 and hematopoietic cell transplantation; EBMT recommendations for management of hematopoietic cell transplant recipients, their donors, and patients undergoing CAR T-cell therapy. Bone Marrow Transplant. 2020;55(11):2071-2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keehner J, Horton LE, Binkin NJ, et al. Resurgence of SARS-CoV-2 Infection in a highly vaccinated health system workforce. N Engl J Med. 2021;385(14):1330-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Creech CB, Walker SC, Samuels RJ. SARS-CoV-2 Vaccines. JAMA. 2021;325(13):1318-1320. [DOI] [PubMed] [Google Scholar]
- 7.Cordonnier C, Einarsdottir S, Cesaro S, et al. Vaccination of haemopoietic stem cell transplant recipients: guidelines of the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis. 2019;19(6):e200-e212. [DOI] [PubMed] [Google Scholar]
- 8.https://www.hematology.org/covid-19/ash-astct-covid-19-vaccination-for-hct-and-car-t-cell-recipients, Accessed 8 November 2021. [Google Scholar]
- 9.https://www.ebmt.org/covid-19-and-bmt, Accessed 8 November 2021. [Google Scholar]
- 10.Murad MH, Sultan S, Haffar S, Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ Evid Based Med. 2018;23(2):60-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Terpos E, Gavriatopoulou M, Ntanasis-Stathopoulos I, et al. The neutralizing antibody response post COVID-19 vaccination in patients with myeloma is highly dependent on the type of anti-myeloma treatment. Blood Cancer J. 2021;11(8):138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Terpos E, Trougakos IP, Gavriatopoulou M, et al. Low neutralizing antibody responses against SARS-CoV-2 in older patients with myeloma after the first BNT162b2 vaccine dose. Blood. 2021;137(26):3674-3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thakkar A, Gonzalez-Lugo JD, Goradia N, et al. Seroconversion rates following COVID-19 vaccination among patients with cancer. Cancer Cell. 2021;39(8):1081-1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Oekelen O, Gleason CR, Agte S, et al. Highly variable SARS-CoV-2 spike antibody responses to two doses of COVID-19 RNA vaccination in patients with multiple myeloma. Cancer Cell. 2021;39(8):1028-1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stampfer SD, Goldwater M-S, Jew S, et al. Response to mRNA vaccination for COVID-19 among patients with multiple myeloma. Leukemia. 2021;35(12):3534-3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roeker LE, Knorr DA, Thompson MC, et al. COVID-19 vaccine efficacy in patients with chronic lymphocytic leukemia. Leukemia. 2021;35(9):2703-2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ram R, Hagin D, Kikozashvilli N, et al. Safety and immunogenicity of the BNT162b2 mRNA COVID-19 vaccine in patients after allogeneic HCT or CD19-based CART therapy - a single-center prospective cohort study. Transplant Cell Ther. 2021;27(9):788-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pimpinelli F, Marchesi F, Piaggio G, et al. Lower response to BNT162b2 vaccine in patients with myelofibrosis compared to polycythemia vera and essential thrombocythemia. J Hematol Oncol. 2021;14(1):119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pimpinelli F, Marchesi F, Piaggio G, et al. Fifth-week immunogenicity and safety of anti-SARS-CoV-2 BNT162b2 vaccine in patients with multiple myeloma and myeloproliferative malignancies on active treatment: preliminary data from a single institution. J Hematol Oncol. 2021;14(1):81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parry H, McIlroy G, Bruton R, et al. Antibody responses after first and second Covid-19 vaccination in patients with chronic lymphocytic leukaemia. Blood Cancer J. 2021;11(7):136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monin L, Laing AG, Muñoz-Ruiz M, et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study. Lancet Oncol. 2021;22(6):765-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maneikis K, Šablauskas K, Ringelevičiūtė U, et al. Immunogenicity of the BNT162b2 COVID-19 mRNA vaccine and early clinical outcomes in patients with haematological malignancies in Lithuania: a national prospective cohort study. Lancet Haematol. 2021;8(8):e583-e592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malard F, Gaugler B, Gozlan J, et al. Weak immunogenicity of SARS-CoV-2 vaccine in patients with hematologic malignancies. Blood Cancer J. 2021;11(8):142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lim SH, Campbell N, Johnson M, et al. Antibody responses after SARS-CoV-2 vaccination in patients with lymphoma. Lancet Haematol. 2021;8(8):e542-e544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kastritis E, Terpos E, Sklirou A, et al. Antibody response after initial vaccination for SARS-CoV-2 in patients with amyloidosis. Hemasphere. 2021;5(8):e614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iacono D, Cerbone L, Palombi L, et al. Serological response to COVID-19 vaccination in patients with cancer older than 80 years. J Geriatr Oncol. 2021;12(8):1253-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herzog Tzarfati K, Gutwein O, Apel A, et al. BNT162b2 COVID-19 vaccine is significantly less effective in patients with hematologic malignancies. Am J Hematol. 2021;96(10):1195-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gurion R, Rozovski U, Itchaki G, et al. Humoral serologic response to the BNT162b2 vaccine is abrogated in lymphoma patients within the first 12 months following treatment with anti-CD2O antibodies. Haematologica. 2022;107(3):715-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greenberger LM, Saltzman LA, Senefeld JW, Johnson PW, DeGennaro LJ, Nichols GL. Antibody response to SARS-CoV-2 vaccines in patients with hematologic malignancies. Cancer Cell. 2021;39(8):1031-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghione P, Gu JJ, Attwood K, et al. Impaired humoral responses to COVID-19 vaccination in patients with lymphoma receiving B-cell directed therapies. Blood. 2021;138(9):811-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gavriatopoulou M, Terpos E, Kastritis E, et al. Low neutralizing antibody responses in WM, CLL and NHL patients after the first dose of the BNT162b2 and AZD1222 vaccine. Clin Exp Med. 2021;1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ehmsen S, Asmussen A, Jeppesen SS, et al. Antibody and T cell immune responses following mRNA COVID-19 vaccination in patients with cancer. Cancer Cell. 2021;39(8):1034-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Easdale S, Shea R, Ellis L, et al. Serologic responses following a single dose of SARS-Cov-2 vaccination in allogeneic stem cell transplantation recipients. Transplant Cell Ther. 2021;27(10):880.e1-880.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caocci G, Mulas O, Mantovani D, et al. Ruxolitinib does not impair humoral immune response to COVID-19 vaccination with BNT162b2 mRNA COVID-19 vaccine in patients with myelofibrosis. Ann Hematol. 2022;101(4):929-931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bird S, Panopoulou A, Shea RL, et al. Response to first vaccination against SARS-CoV-2 in patients with multiple myeloma. Lancet Haematol. 2021;8(6):e389-e392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Benjamini O, Rokach L, Itchaki G, et al. Safety and efficacy of BNT162b mRNA Covid19 vaccine in patients with chronic lymphocytic leukemia. Haematologica. 2022;107(3):625-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Benda M, Mutschlechner B, Ulmer H, et al. Serological SARS-CoV-2 antibody response, potential predictive markers and safety of BNT162b2 mRNA COVID-19 vaccine in haematological and oncological patients. Br J Haematol. 2021;195(4):523-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ali H, Ngo D, Aribi A, et al. Safety and tolerability of SARS-CoV2 emergency-use authorized vaccines for allogeneic hematopoietic stem cell transplant recipients. Transplant Cell Ther. 2021;27(11):938.e1-938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Addeo A, Shah PK, Bordry N, et al. Immunogenicity of SARS-CoV-2 messenger RNA vaccines in patients with cancer. Cancer Cell. 2021;39(8):1091-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Agha ME, Blake M, Chilleo C, Wells A, Haidar G. Suboptimal response to coronavirus disease 2019 messenger RNA vaccines in patients with hematologic malignancies: a need for vigilance in the postmasking era. Open Forum Infect Dis. 2021;8(7):ofab353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dhakal B, Abedin SM, Fenske TS, et al. Response to SARS-CoV-2 vaccination in patients after hematopoietic cell transplantation and CAR-T cell therapy. Blood. 2021;138(14):1278-1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Herishanu Y, Avivi I, Aharon A, et al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic lymphocytic leukemia. Blood. 2021;137(23):3165-3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Avivi I, Balaban R, Shragai T, et al. Humoral response rate and predictors of response to BNT162b2 mRNA COVID19 vaccine in patients with multiple myeloma. Br J Haematol. 2021;195(2):186-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ghandili S, Schönlein M, Lütgehetmann M, et al. Post-vaccination anti-SARS-CoV-2-antibody response in patients with multiple myeloma correlates with low CD19+ B-lymphocyte count and anti-CD38 treatment. Cancers. (Basel) 2021;13(15):3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Redjoul R, Le Bouter A, Beckerich F, Fourati S, Maury S. Antibody response after second BNT162b2 dose in allogeneic HSCT recipients. Lancet. 2021;398(10297):298-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chung DJ, Shah GL, Devlin SM, Lakshmi VR, Doddi S, Pessin MS. Disease and therapy-specific impact on humoral immune responses to COVID-19 vaccination in hematologic malignancies. Blood Cancer Discov. 2021;2(6):568-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tamari R, Politikos I, Knorr DA, et al. Predictors of humoral response to SARS-CoV-2 vaccination after hematopoietic cell transplantation and CAR T cell therapy. Blood Cancer Discov. 2021;2(6):577-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Del Poeta G, Bomben R, Polesel J, et al. COVID-19 vaccination: evaluation of risk for protection failure in chronic lymphocytic leukemia patients. Hematol Oncol. 2021;39(5):712-714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jurgens EM, Ketas TJ, Zhao Z, et al. Serologic response to mRNA COVID-19 vaccination in lymphoma patients. Am J Hematol. 2021;96(11):E410-E413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mairhofer M, Kausche L, Kaltenbrunner S, et al. Humoral and cellular immune responses in SARS-CoV-2 mRNA-vaccinated patients with cancer. Cancer Cell. 2021;39(9):1171-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Perry C, Luttwak E, Balaban R, et al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with B-cell non-Hodgkin lymphoma. Blood Adv. 2021;5(16):3053-3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Terpos E, Gavriatopoulou M, Fotiou D, et al. Poor neutralizing antibody responses in 132 patients with CLL, NHL and HL after vaccination against SARS-CoV-2: a prospective study. Cancers. (Basel) 2021;13(17):4480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ollila TA, Lu S, Masel R, et al. Antibody response to COVID-19 vaccination in adults with hematologic malignant disease. JAMA Oncol. 2021;7(11):1714-1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Harrington P, Lavallade H de, Doores KJ, et al. Single dose of BNT162b2 mRNA vaccine against SARS-CoV-2 induces high frequency of neutralising antibody and polyfunctional T-cell responses in patients with myeloproliferative neoplasms. Leukemia. 2021;35(12):3573-3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Harrington P, Doores KJ, Radia D, et al. Single dose of BNT162b2 mRNA vaccine against severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) induces neutralising antibody and polyfunctional T-cell responses in patients with chronic myeloid leukaemia. Br J Haematol. 2021;194(6):999-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gastinne T, Le Bourgeois A, Coste-Burel M, et al. Safety and antibody response after one and/or two doses of BNT162b2 Anti-SARS-CoV-2 mRNA vaccine in patients treated by CAR T cells therapy. Br J Haematol. 2022;196(2):360-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Peeters M, Verbruggen L, Teuwen L, et al. Reduced humoral immune response after BNT162b2 coronavirus disease 2019 messenger RNA vaccination in cancer patients under antineoplastic treatment. ESMO Open. 2021;6(5):100274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Marchesi F, Pimpinelli F, Sperandio E, et al. The 12-week kinetics of anti-SARS-CoV-2 antibodies in different haematological cancers after vaccination with BNT162b2. Br J Haematol. 2022;196(2):362-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Le Bourgeois A, Coste-Burel M, Guillaume T, et al. Safety and antibody response after 1 and 2 doses of BNT162b2 mRNA vaccine in recipients of allogeneic hematopoietic stem cell transplant. JAMA Netw Open. 2021;4(9):e2126344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Forconi F, Moss P. Perturbation of the normal immune system in patients with CLL. Blood. 2015;126(5):573-581. [DOI] [PubMed] [Google Scholar]
- 61.Nazi I, Kelton JG, Larché M, et al. The effect of rituximab on vaccine responses in patients with immune thrombocytopenia. Blood. 2013;122(11):1946-1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Marasco V, Carniti C, Guidetti A, et al. T-cell immune response after mRNA SARS-CoV-2 vaccines is frequently detected also in the absence of seroconversion in patients with lymphoid malignancies. Br J Haematol. 2022;196(3):548-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sun C, Gao J, Couzens L, et al. Seasonal influenza vaccination in patients with chronic lymphocytic leukemia treated with ibrutinib. JAMA Oncol. 2016;2(12):1656-1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pleyer C, Ali MA, Cohen JI, et al. Effect of Bruton tyrosine kinase inhibitor on efficacy of adjuvanted recombinant hepatitis B and zoster vaccines. Blood. 2021;137(2):185-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Leone P, Solimando AG, Malerba E, et al. Actors on the scene: immune cells in the myeloma niche. Front Oncol. 2020;10:599098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ludwig H, Boccadoro M, Moreau P, et al. Recommendations for vaccination in multiple myeloma: a consensus of the European Myeloma Network. Leukemia. 2021;35(1):31-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Moreno L, Perez C, Zabaleta A, et al. The mechanism of action of the anti-CD38 monoclonal antibody Isatuximab in multiple myeloma. Clin Cancer Res. 2019;25(10):3176-3187. [DOI] [PubMed] [Google Scholar]
- 68.Frerichs KA, Bosman PWC, van Velzen JF, et al. Effect of daratumumab on normal plasma cells, polyclonal immunoglobulin levels, and vaccination responses in extensively pre-treated multiple myeloma patients. Haematologica. 2020;105(6):e302-e306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee E-J, Cines DB, Gernsheimer T, et al. Thrombocytopenia following Pfizer and Moderna SARS-CoV-2 vaccination. Am J Hematol. 2021;96(5):534-537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Boyarsky BJ, Werbel WA, Avery RK, et al. Immunogenicity of a single dose of SARS-CoV-2 messenger RNA vaccine in solid organ transplant recipients. JAMA. 2021;325(17):1784-1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bange EM, Han NA, Wileyto P, et al. CD8+ T cells contribute to survival in patients with COVID-19 and hematologic cancer. Nat Med. 2021;27(7):1280-1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.