Accumulating data suggest that hemostatic dysfunction contributes to Plasmodium falciparum malaria pathogenesis.1 In addition, specific mechanisms through which the protein C pathway modulates P. falciparum pathogenesis have been described.1 We hypothesized that the anticoagulant and anti-inflammatory activities of recombinant activated protein C (APC) may possess therapeutic value in the setting of cerebral malaria (CM). In order to address this hypothesis, we assessed hemostatic parameters in an established murine model of experimental cerebral malaria (ECM), and using the same model, investigated the ability of recombinant APC to ameliorate ECM. In keeping with findings in patients with severe P. falciparum malaria, we observed that dysregulated thrombin generation and protein C pathway dysfunction were both late features of ECM. Furthermore, pretreatment with a monoclonal anti-EPCR antibody that blocks protein C/APC binding prior to P. berghei inoculation significantly reduced overall survival. Conversely, mice treated with recombinant APC exhibited a marked attenuation in clinical ECM progression and parasitemia, in parallel with a significant increase in overall survival. All together, these findings confirm that hemostatic and protein C pathway dysfunction are both consistent features in human and ECM, and demonstrate for the first time a role for recombinant APC in reducing clinical progression and mortality in ECM.
CM is a life-threatening complication of P. falciparum infection characterized by ataxia, seizures, altered consciousness and coma. Although CM is associated with significant mortality, the biological mechanisms underlying its pathogenesis remain poorly defined. Significant coagulation cascade activation including elevations in levels of fibrin degradation products (FDP) and thrombin-antithrombin (TAT) complexes are common in patients with P. falciparum infection.2,3 Furthermore, Moxon et al. recently described overt DIC in 19% of children with retinopathy-positive CM.3 Together, these findings suggest that hemostatic dysfunction may contribute to malaria pathogenesis. This hypothesis is supported by the observation that coagulation activation correlates with peripheral blood parasitemia levels, and is inversely-related to overall survival.4,5 Moreover, microvascular fibrin deposition has also been reported in postmortem studies of patients with fatal CM.6 A number of molecular mechanisms through which P . falciparum infection triggers coagulation activation have been described.1 Recent studies have also highlighted specific mechanisms through which the protein C pathway influences P. falciparum pathogenesis.6,7 Importantly, both hrombomodulin (TM) and the endothelial protein C receptor (EPCR) can bind to PfEMP1 expressed on P. falciparum to infected erythrocyte (IE) surfaces,7,8 thereby facilitating IE cytoadhesion to endothelial cells (EC). PfEMP1 binding to EPCR also limits generation of anticoagulant activated protein C (APC), and inhibits EPCR-dependent PAR1-mediated protection of EC barrier integrity.9,10 The typical late clinical presentation of patients with CM makes it difficult to determine whether hemostatic dysfunction directly contributes to the pathogenesis of CM, or whether coagulation activation merely constitutes a secondary epiphenomenon. Therefore, in this study we sought to further investigate the role of coagulation activation and the protein C pathway in malaria pathogenesis using an established murine model of ECM, in which C57BL/6J mice were infected with P. berghei ANKA.11
We have previously described in detail the murine experimental cerebral malaria (ECM) model. All mouse experiments were performed in compliance with the Irish Medicines Board and approved by the Trinity College Dublin BioResource Ethics Committee. Mice aged 8 to 10 weeks were infected by intraperitoneal injection of 2x106 P. berghei ANKA. Following inoculation, malaria progression was monitored using a previously validated ECM clinical scoring system.11 P. berghei parasitemia levels were monitored by Giemsa-stained thin blood smears. Platelet counts were measured using a Sysmex haematology analyser (KX-21N). In order to prepare platelet-poor plasma, blood samples were centrifuged at 1,500 g for 15 minutes at 20°C, aliquoted and stored at -80°C until use. Murine plasma activated partial thromboplastin time (APTT) was determined using a commercial kit (C.K. Prest, Stago) and time for clot formation was determined using Amelung KC4 Micro Clinical Coagulation Analyzer (Amelung, Trinity Biotech, Ireland). Similarly, PT was determined using Hemosil recombiplastin 2G according to the manufacturer’s guidelines. Plasma levels of thrombin-antithrombin (TAT) complexes, soluble thrombomodulin (sTM) and soluble endothelial cell protein C receptor (EPCR) were quantified using specific commercial enzyme-linked immunsorbant assays (ELISA) (Abcam, Cambridge, UK and R&D Systems, UK) according to the manufacturer’s instructions. In order to study the role of EPCR, ECM progression was assessed in mice pretreated with 50 µg of an EPCR blocking antibody RCR-16,12 or isotype control antibody, immediately prior to P. berghei infection. Recombinant murine APC was generated, expressed, and purified as previously described.13 Consistent with previous studies assessing the anti-inflammatory properties of recombinant APC in murine endotoxemia models,14 mice were treated with 10 µg mAPC administered intravenously immediately prior to P. berghei infection. Subsequently another 10 µg of mAPC was administered 4 hours post-infection. Alternatively, mice were treated with two 10 µg mAPC boluses administered 4 hours apart on day +3 following P. berghei inoculation.
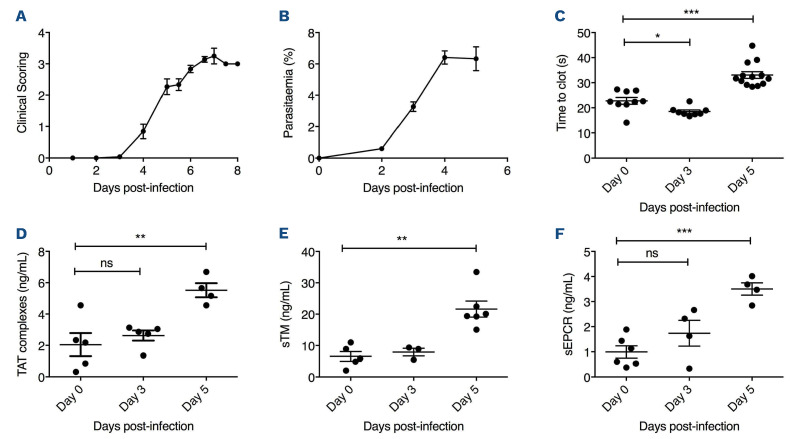
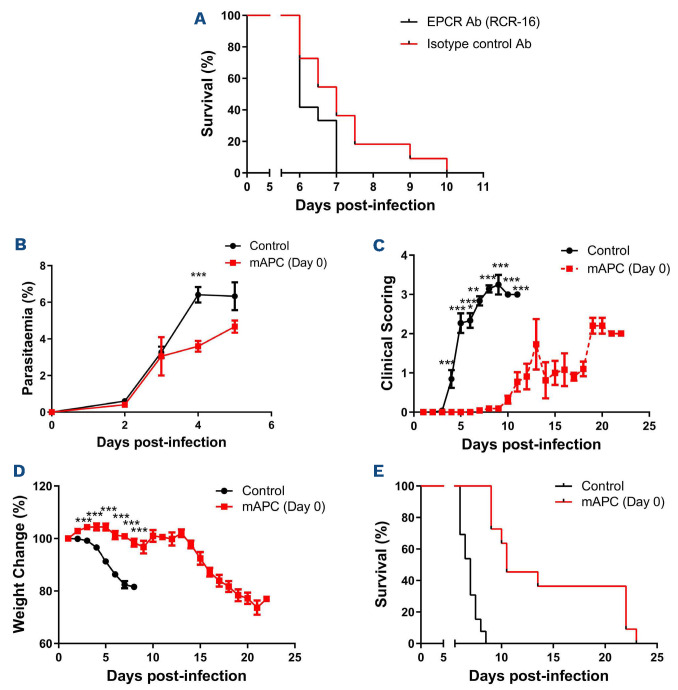
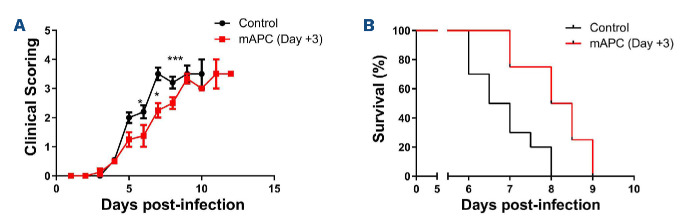
Progressive clinical features similar to those observed in human CM were evident in mice from day +3 following infection (Figure 1A). Peripheral P. berghei parasitemia levels increased progressively (Figure 1B), and mice typically died within 7-10 days. In keeping with previous findings in patients with severe P. falciparum malaria, a significant increase in APTT was observed by day +5 following P. berghei inoculation (Figure 1C).2 Again in keeping with human studies,3 no significant changes in either PT or murine fibrinogen levels were seen through the course of P. berghei infection (data not shown). Nevertheless, by day +5 after inoculation, median plasma TAT levels were increased approximately 2.5 fold (2.0 ng/mL at day 0 vs. 5.5 ng/mL at day +5; P<0.01) (Figure 1D), which was similar in magnitude to the increase in plasma TAT levels observed in patients with CM.2,4,6 In contrast to the early increase in plasma VWF levels that occurs within 24 hours following P. berghei infection,11 plasma TAT levels remained normal for 72 hours post-infection. Murine plasma protein C and antithrombin levels were not significantly reduced during P. berghei infection (data not shown). However, again as reported in previous findings in children infected with P. falciparum,3,6 plasma levels of both sTM and sEPCR were significantly elevated in C57BL/6J mice following P. berghei infection (sTM 6.5 ng/mL at day 0 vs. 21.6 ng/mL at day +5; P<0.001 and sEPCR 0.99 ng/mL at day 0 vs. 3.50 ng/mL at day +5; P<0.001) (Figure 1E and F). In keeping with the hypothesis that hemostastic dysfunction is a relatively late feature in ECM, the increases in plasma sTM and sEPCR were not observed until day +5 following innoculation. Collectively, these findings demonstrate that dysregulated thrombin generation represents a consistent feature of both human and experimental murine CM. Critically however, unlike the acute EC activation that represents an early hallmark in both murine and human malaria, hemostatic and protein C pathway dysfunction both develop at a much later stage. In order to investigate whether protein C pathway dysfunction contributes to ECM pathogenesis, mice were pre- treated with a monoclonal anti-EPCR antibody (RCR-16) previously shown to block protein C/APC binding,12 or an isotype control antibody, immediately prior to P. berghei inoculation. We observed significantly reduced overall survival (P<0.05) in the cohort of mice treated with RCR-16 (Figure 2A), suggesting that EPCR-dependent APC generation and/or signaling is important for controlling ECM development. Case studies involving a small number of patients with severe P. falciparum malaria treated with APC have reported variable effects.15 Consequently, we further investigated whether administration of recombinant mAPC influenced ECM pathogenesis. Mice were treated with 10 µg mAPC immediately prior to P. berghei infection, and a subsequent second 10 µg mAPC dose was administered 4 hour later. Mice treated with recombinant mAPC exhibited a mild but significant reduction in parasitemia at day +4 (Figure 2B; P<0.001). In contrast however, the APC-treated mice demonstrated significantly attenuated clinical ECM progression (Figure 2C; P<0.001) and weight loss (Figure 2D; P<0.001). Furthermore, mAPC administration also caused a significant increase in overall survival (Figure 2E; P<0.001). In order to investigate whether mAPC administered later in the course of ECM can still influence clinical progression, the effect of administering mAPC on day +3 was investigated. Once again, clinical progression (Figure 3A) and overall survival (Figure 3B) were both marginally improved in mice treated with mAPC compared to controls (P<0.05). Interestingly, however, the magnitude of this effect was markedly less than that observed in mice treated with mAPC on day 0. This attenuated efficacy of APC administered later in the disease course is in keeping with the concept that CM is associated with progressive shedding of EPCR and TM from EC surfaces.10
Figure 1.
Hemostatic and protein C pathway dysfunction constitute late features of experimental cerebral malaria. (A) Following intraperitoneal inoculation with 2x106 Plasmodium berghei ANKA parasites, experimental cerebral malaria (ECM) progression was followed in wild-type (WT) C57BL/6J mice (n=12) using a validated clinical scoring algorithm. Results presented represent the mean values ± standard error of the mean (SEM) unless otherwise stated. (B) Following P. berghei infection, peripheral blood parasitemia levels were determined each day using Giemsa-stained smears (n=12). By day +5 following P. berghei infection, murine activated partial thromboplastin time (APTT) levels (C) and plasma thrombin-antithrombin (TAT) complex levels (D) were both significantly increased (*P<0.05, **P<0.01, ***P<0.0001 respectively). Similarly, plasma levels of soluble endothelial cell protein C receptor (sEPCR) (E) and soluble thrombomodulin (sTM) (F) were also both significantly elevated by day +5. Data are expressed as mean values ± SEM. In order to assess statistical differences, data were analyzed using Student’s unpaired 2-tailed t-test. ECM clinical scoring data were assessed by two-way ANOVA analysis.
Figure 2.
Figure 2. Recombinant anticoagulant activated protein C significantly attenuates clinical progression and markedly improves overall survival in experimental cerebral malaria. (A) In order to determine if endothelial protein C receptor (EPCR) plays a role in experimental cerebral malaria (ECM) pathogenesis, mice were pretreated with the EPCR blocking antibody RCR-16 or an isotype control antibody prior to infection with 2x106 Plasmodium berghei ANKA parasites. Twelve mice were treated in the RCR-16 and 11 in the isotype control groups. Overall survival was significantly reduced in mice treated with RCR-16. In order to investigate whether recombinant murine anticoagulant activated protein C (mAPC) administration influences ECM progression, mice were pretreated with 10 �g mAPC immediately prior to P. berghei infection and a second 10 �g mAPC dose was administered 4 hour later. Twelve mice were treated in the mAPC and control groups. Mice treated with recombinant mAPC exhibited (B) mildly reduced parasitemia at day +4, (C) attenuated clinical ECM progression (D) reduced weight loss and (E) significantly increased overall survival (*P<0.05, **P<0.01, ***P<0.001).
Figure 3.
Recombinant anticoagulant activated protein C later in the course of experimental cerebral malaria has only mild effects on clinical progression and survival. In order to investigate whether murine anticoagulant activated protein C (mAPC) later in the course of experimental cerebral malaria (ECM) can still influence clinical progression, 2 doses of mAPC were administered on day +3 following Plasmodium berghei innoculation. Once again, (A) clinical progression and (B) overall survival were both mildly improved in mice treated with mAPC compared to controls. Mouse survival data were compared using a log-rank (Mantel-Cox) Test.
In conclusion, our findings demonstrate that hemostatic and protein C pathway dysfunction are both consistent features in human and experimental murine CM. We also show for the first time that recombinant mAPC markedly reduces clinical progression and overall mortality in ECM. Further studies will be required to elucidate the molecular mechanisms through which APC modulates ECM pathogenesis, together with the optimal APC dosing and treatment regimen. Nevertheless, given the significant morbidity and mortality that are still associated with CM, novel adjunctive therapies to limit vascular dysfunction and slow disease progression are urgently required.
Funding Statement
Funding: This work was supported by funds from the NIH for the Zimmerman Program (HL081588); a Science Foundation Ireland Principal Frontiers for the Future (FFP) award (20/FFP-A/8952), a Health Research Board Investigator Lead Project Award (ILP-POR-2017-008) and a National Children’s Research Center Project Award (C/18/1).
References
- 1.O'Sullivan JM, Preston RJ, O'Regan N, O'Donnell JS. Emerging roles for hemostatic dysfunction in malaria pathogenesis. Blood. 2016;127(19):2281-2288. [DOI] [PubMed] [Google Scholar]
- 2.Clemens R, Pramoolsinsap C, Lorenz R, Pukrittayakamee S, Bock HL, White NJ. Activation of the coagulation cascade in severe falciparum malaria through the intrinsic pathway. Br J Haematol. 1994;87(1):100-105. [DOI] [PubMed] [Google Scholar]
- 3.Moxon CA, Chisala NV, Mzikamanda R, et al. Laboratory evidence of disseminated intravascular coagulation is associated with a fatal outcome in children with cerebral malaria despite an absence of clinically evident thrombosis or bleeding. J Thromb Haemost. 2015;13(9):1653-1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holst FG, Hemmer CJ, Foth C, Seitz R, Egbring R, Dietrich M. Low levels of fibrin-stabilizing factor (factor XIII) in human Plasmodium falciparum malaria: correlation with clinical severity. Am J Trop Med Hyg. 1999;60(1):99-104. [DOI] [PubMed] [Google Scholar]
- 5.Horstmann RD, Dietrich M. Haemostatic alterations in malaria correlate to parasitaemia. Blut. 1985;51(5):329-335. [DOI] [PubMed] [Google Scholar]
- 6.Moxon CA, Wassmer SC, Milner DA, et al. Loss of endothelial protein C receptors links coagulation and inflammation to parasite sequestration in cerebral malaria in African children. Blood. 2013;122(5):842-851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turner L, Lavstsen T, Berger SS, et al. Severe malaria is associated with parasite binding to endothelial protein C receptor. Nature. 2013;498(7455):502-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gysin J, Pouvelle B, Le Tonqueze M, Edelman L, Boffa MC. Chondroitin sulfate of thrombomodulin is an adhesion receptor for Plasmodium falciparum-infected erythrocytes. Mol Biochem Parasitol. 1997;88(1-2):267-271. [DOI] [PubMed] [Google Scholar]
- 9.Gillrie MR, Avril M, Brazier AJ, et al. Diverse functional outcomes of Plasmodium falciparum ligation of EPCR: potential implications for malarial pathogenesis. Cell Microbiol. 2015;17(12):1883-1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gleeson EM, O'Donnell JS, Preston RJ. The endothelial cell protein C receptor: cell surface conductor of cytoprotective coagulation factor signaling. Cell Mol Life Sci. 2012;69(5):717-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O'Regan N, Gegenbauer K, O'Sullivan JM, et al. A novel role for von Willebrand factor in the pathogenesis of experimental cerebral malaria. Blood. 2016;127(9):1192-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ye X, Fukudome K, Tsuneyoshi N, et al. The endothelial cell protein C receptor (EPCR) functions as a primary receptor for protein C activation on endothelial cells in arteries, veins, and capillaries. Biochem Biophys Res Commun. 1999;259(3):671-677. [DOI] [PubMed] [Google Scholar]
- 13.Harmon S, Preston RJ, Ni Ainle F, et al. Dissociation of activated protein C functions by elimination of protein S cofactor enhancement. J Biol Chem. 2008;283(45):30531-30539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kerschen EJ, Fernandez JA, Cooley BC, et al. Endotoxemia and sepsis mortality reduction by non-anticoagulant activated protein C. J Exp Med. 2007;204(10):2439-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rankin LG, Austin DL. The use of activated protein C in severe Plasmodium falciparum malaria. Anaesth Intensive Care. 2007;35(3):428-432. [DOI] [PubMed] [Google Scholar]