Significance
The establishment of a carbon-negative bioeconomy that eliminates the need for crude oil will require a range of bioproducts. Accumulating value-added bioproducts directly in bioenergy crops can be an important strategy for enabling economically competitive biorefineries that produce a range of renewable fuels and replacements for petrochemicals. However, microbial chassis may have advantages over plants for some products. To date, there has been no systematic analysis aimed at comparing microbial production routes with in planta accumulation to establish breakeven targets for yields and accumulation rates. In this study, we provide generalizable insights into these breakeven points by exploring four bioproducts (4-hydroxybenzoic acid [4-HBA], 2-pyrone-4,6-dicarboxylic acid [PDC], muconic acid, and catechol) currently produced both in plants and by microbial hosts.
Keywords: in planta accumulation, microbial production, value-added bioproduct, technoeconomic analysis (TEA), bioeconomy
Abstract
Plants and microbes share common metabolic pathways for producing a range of bioproducts that are potentially foundational to the future bioeconomy. However, in planta accumulation and microbial production of bioproducts have never been systematically compared on an economic basis to identify optimal routes of production. A detailed technoeconomic analysis of four exemplar compounds (4-hydroxybenzoic acid [4-HBA], catechol, muconic acid, and 2-pyrone-4,6-dicarboxylic acid [PDC]) is conducted with the highest reported yields and accumulation rates to identify economically advantaged platforms and breakeven targets for plants and microbes. The results indicate that in planta mass accumulation ranging from 0.1 to 0.3 dry weight % (dwt%) can achieve costs comparable to microbial routes operating at 40 to 55% of maximum theoretical yields. These yields and accumulation rates are sufficient to be cost competitive if the products are sold at market prices consistent with specialty chemicals ($20 to $50/kg). Prices consistent with commodity chemicals will require an order-of-magnitude-greater accumulation rate for plants and/or yields nearing theoretical maxima for microbial production platforms. This comparative analysis revealed that the demonstrated accumulation rates of 4-HBA (3.2 dwt%) and PDC (3.0 dwt%) in engineered plants vastly outperform microbial routes, even if microbial platforms were to reach theoretical maximum yields. Their recovery and sale as part of a lignocellulosic biorefinery could enable biofuel prices to be competitive with petroleum. Muconic acid and catechol, in contrast, are currently more attractive when produced microbially using a sugar feedstock. Ultimately, both platforms can play an important role in replacing fossil-derived products.
Reducing the use of fossil carbon sources is central to the global efforts to mitigate the most catastrophic effects of climate change (1). Although consumption of some fossil fuels, such as coal, has flattened globally and declined in many developed nations (2), global petroleum demand has continued to grow steadily, averaging an increase of around 1 to 2 million barrels per day prior to the pandemic (3). Substantially reducing the use of crude oil requires that renewable replacements for both fuel and other petrochemical products be scaled up in parallel. Building biorefineries that mimic current petroleum refineries by coproducing a suite of fuels and products that are difficult to decarbonize via other means is a potentially attractive approach. A prior study explored the degree to which increasing in planta accumulation of value-added products could enable economically competitive lignocellulosic biorefineries, finding that compounds such as latex, limonene, and polyhydroxybutyrate must be accumulated at 0.3 to 1.2% of total plant dry weight % (dwt%) to justify the costs of extraction, while higher-value cannabidiol and artemisinin need only reach 0.01 to 0.02 dwt% (4). However, in planta accumulation is not the only option for producing renewable alternatives to petrochemical products; rapid developments in metabolic engineering and synthetic biology have enabled host microbes to serve as microbial cell factories that can also produce a diverse range of products (5). The question of how in planta accumulation and microbial production of bioproducts compare on a cost basis remains unanswered. Through detailed process simulation and economic analysis, we seek to better understand how microbial and in planta production routes compare based on currently demonstrated and maximum theoretical performance metrics, using four industrially relevant molecules as exemplars: 4-hydroxybenzoic acid (4-HBA), catechol, cis,cis-muconic acid (muconic acid), and 2-pyrone-4,6-dicarboxylic acid (PDC).
In planta accumulation of some bioproducts can be cost competitive even if the remaining biomass is discarded after extraction, particularly for pharmaceuticals, personal-care products, and other high-value applications such as medical cannabinoids and high-value proteins (4, 6). Integrating this strategy with the conversion of residual biomass to renewable liquid fuels provides an additional revenue stream, thus making lower levels of accumulation and/or lower-value products viable (4). However, tying bioproduct extraction from plants with large-scale biorefineries has drawbacks. Commercial-scale lignocellulosic biorefineries are likely to operate at scales much larger than typical production facilities for high-value specialty chemicals (4). If artemisinin were accumulated in planta and extracted prior to biofuel production, global demand would be met with fewer than 10 commercial-scale biorefineries, and if the chemotherapy medication vinblastine were produced instead, its global demand would be fully supplied many times over by just a single facility (4). In contrast, standalone microbial production of these chemicals using simple sugars is less capital intensive relative to a fuel-producing lignocellulosic biorefinery (7, 8), so simple sugars can be sized more appropriately for the scale of the target market.
A variety of model and nonmodel microbial hosts have been modified to produce industrially relevant chemicals. Metabolic engineering of Corynebacterium glutamicum has led to the microbial conversion of monomeric sugars to several valuable chemicals—such as shikimate, 4-HBA, and 4-aminobenzoate—at high titers suitable for commercialization (9). Escherichia coli is a versatile host that has been engineered to produce a variety of bioproducts including terpenoids, alkaloids, and indigo at high productivities due to its high growth rate and cell density (10, 11). The oleaginous and carotenogenic yeast Rhodosporidium toruloides has demonstrated an ability to metabolize a variety of biomass-derived substrates to produce bisabolene and pharmaceutical products (12). These microbial production routes do have drawbacks; some have not yet reached titers, rates, and yields (TRYs) that allow them to be competitive with currently available petrochemicals. At lower TRYs, the sugar feedstock costs and capital-intensive recovery and purification processes can dominate the overall production costs (13, 14).
In this study, we aim to demonstrate how in planta and microbial routes can be compared through technoeconomic modeling and explore the relative viability of both routes to four chemicals (4-HBA, catechol, muconic acid, and PDC). Each of these four molecules has established biosynthetic routes both in microorganisms and in bioenergy crops or model plants, and their successful production has been demonstrated in the literature (15–18). The selection of these bioproducts is based on their broad applications, market values, documentation in peer-reviewed literature, and potential as a chemical platform (19). 4-HBA is an intermediate for the production of food, cosmetic, and pharmaceutical products (20). Catechol is a precursor to pesticides, flavors, and fragrances (21). Muconic acid can be used as a precursor to producing bio-based nylon-6,6 and polyethylene terephthalate (22). PDC, owing to its structural similarity to terephthalic acid, can serve as a precursor to diverse bioplastics with novel functionalities (23). Notably, a recent study also indicated that producing PDC in plants led to a reduction in lignin content and, thus, improved biomass saccharification efficiency (16). Although these exemplar compounds represent a small fraction of potential in planta and microbially produced products, they provide a representative range of options through which we are able to gauge the competitiveness of each strategy and establish yield/accumulation thresholds needed to reach cost parity.
Results
Technoeconomic Results.
In this study, we developed separate process simulation and cash flow models for the selected bioproducts (4-HBA, catechol, muconic acid, and PDC) using SuperPro Designer v12 and Python. Separate scenario models captured the two alternatives: 1) accumulation of the product in planta followed by extraction prior to the deconstruction and conversion of remaining biomass to ethanol; and 2) dedicated microbial production of the product using glucose as a feedstock. The rationale for modeling microbial conversion of a glucose feedstock rather than lignocellulosic sugars is that currently available literature has not demonstrated the ability to convert pentose sugars to the four products analyzed in this study, nor have prior studies explored the use of mixed hydrolysates. In each case, we use the simulations to calculate a minimum selling price (MSP) for the purified bioproduct. In the plant system, the mass accumulation rate (dwt%) of each bioproduct in biomass sorghum (a proxy bioenergy feedstock crop) is based on the highest reported yield in the literature (3.2 dwt% 4-HBA [18], 3.0 dwt% PDC [16], 0.06 dwt% muconic acid [15], and 0.045 dwt% catechol [17]). This represents the average mass accumulation rate in all of the harvestable sorghum biomass, including both stems and leaves. Once the biomass is delivered to the biorefinery, bioproducts are extracted by solvents. The remaining biomass is routed for downstream conversion to ethanol, consisting of processes including one-pot high-gravity ionic liquid (IL) pretreatment, enzymatic hydrolysis, fermentation, ethanol recovery and purification, wastewater treatment, and onsite energy generation. To determine the selling price for lignocellulosic ethanol, a base case lignocellulosic biorefinery was modeled first where no bioproduct is extracted from the biomass sorghum, and only ethanol is produced as the product. In the base case model, the minimum ethanol selling price (MESP) is $1.44/liter of gasoline equivalent (LGE), and this biorefinery generates 248 L ethanol per bone-dry tonne of biomass and requires a capital investment of ∼$420 million. In the microbial systems, there is no analogous base case biorefinery because each bioproduct is modeled as the single main product from a dedicated facility using glucose as the feedstock.
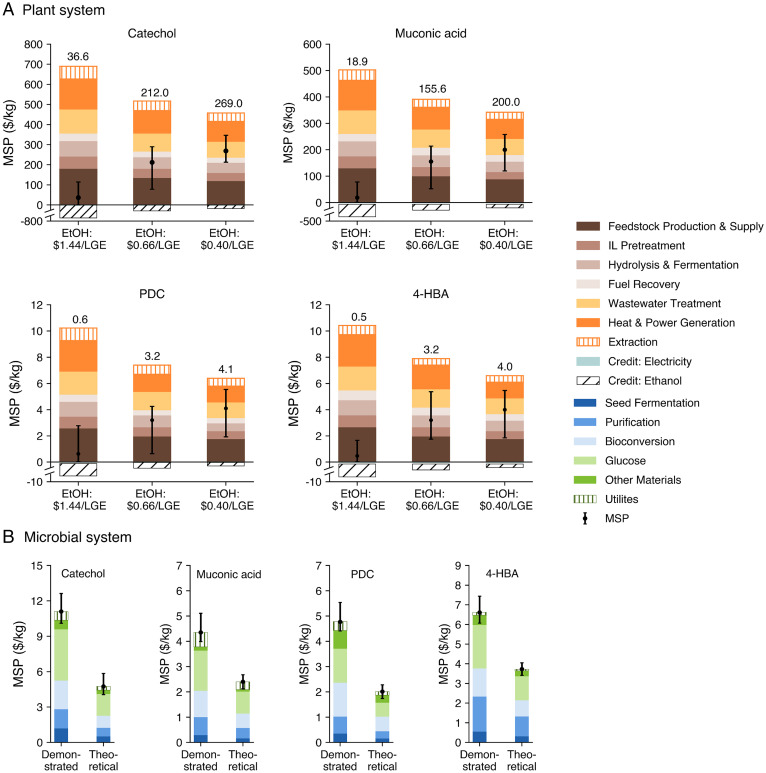
In scenarios where bioproducts are accumulated in planta and extracted prior to converting biomass to ethanol, the revenue collected from the sale of ethanol impacts the minimum viable threshold for accumulation (see Fig. 1). We quantified the bioproduct MSP across three possible ethanol selling prices: 1) a base case price ($1.44/LGE) as calculated in our base case ethanol biorefinery model, 2) a target fuel price ($0.66/LGE or $2.50/gasoline gallon equivalent) as set by the U.S. Department of Energy (DOE) (24), and 3) a historical average gasoline price ($0.40/LGE or $1.53/gal of gasoline) based on 1940 to 2020 average U.S. gasoline rack prices (25). A higher ethanol selling price translates to a lower bioproduct MSP. Even in the base case ethanol price scenario ($1.44/LGE), the relatively low accumulation rates (<0.1 dwt%) in planta reported for catechol and muconic acid result in challenging economics. Biorefineries that extract catechol from biomass sorghum must sell the product for $36.6/kg, which is approximately triple the MSP that can be achieved through microbial conversion of glucose based on the best reported TRY. This MSP for catechol produced in planta is also approximately 7 times its current market price. Based on the ethanol selling price corresponding to the base case scenario ($1.44/LGE), muconic acid could be sold at a minimum price of $18.9/kg, which is ∼4 times the achievable MSP if muconic acid is produced microbially from glucose. Increasing accumulation rates for in planta production of muconic acid and catechol will be crucial to making this option competitive. In contrast, the best-reported accumulation rates in plants are considerably higher for 4-HBA (3.2 dwt%) and PDC (3.0 dwt%). As a result, 4-HBA and PDC accumulated in biomass can be sold for $0.5 and $0.6/kg, respectively, which are well below their current market prices. If fuel ethanol must be sold at the DOE target price ($0.66/LGE), the MSPs for 4-HBA and PDC increase to ∼$3.0/kg, and these MSPs increase further to ∼$4.0/kg if ethanol sells for the long-term historical average U.S. gasoline rack price ($0.40/LGE).
Fig. 1.
MSPs obtained from plant systems (highest demonstrated accumulation) and microbial routes (demonstrated and maximum theoretical yield scenarios). In the plant systems, three ethanol selling prices are assumed: 1) ethanol produced in the integrated biorefinery is sold at $1.44/LGE, as quantified in the base case model; 2) ethanol is sold at the target fuel selling price of $0.66/LGE ($2.50/gal gasoline equivalent), as set by the U.S. DOE (24); and 3) ethanol selling price is equivalent to the 1940 to 2020 historical average U.S. gasoline rack sales price ($0.40/LGE or $1.53/gal of gasoline) (25). In the microbial system, the demonstrated case is developed based on the state-of-the-art reported yield for these bioproducts, and the theoretical case is built assuming the maximum theoretical yield. Detailed input parameters used for technoeconomic modeling are documented in SI Appendix, Table S1. Numerical results are listed in SI Appendix, Table S2.
Notably, under all three ethanol price scenarios, 4-HBA and PDC accumulated in planta can be sold for a value below their current market prices, suggesting that engineering bioenergy feedstocks to accumulate these commodity chemicals in all or most of the plant tissue may give the resulting biorefineries an advantage over competing production routes. Extraction costs do vary across each bioproduct. Among the selected bioproducts, we find that catechol extraction at the integrated lignocellulosic biorefinery results in the highest capital investment (∼$40 million) mainly due to the energy-intensive extraction processes and comparatively low accumulation rate, while 4-HBA extraction results in capital costs totaling ∼$30 million (see SI Appendix, Fig. S1 for process flow diagrams).
The strengths and weaknesses of microbial production across the four products explored here are different from the plant systems. Catechol and muconic acid are less costly to produce microbially, based on demonstrated yields. Using the best-reported microbial production yield of 0.26 mol catechol/mol glucose, or 0.16 g catechol/g glucose (42% of the theoretical maximum yield, as determined by elementary mode flux analysis), with a titer of 4.47 g/L (26), we found that catechol must sell for an MSP of $11.1/kg (Fig. 1), which is double its current market price. Muconic acid production via microbial conversion of glucose, on the other hand, has been demonstrated at a much higher yield (0.378 mol muconic acid/mol glucose, or 0.298 g muconic acid/g glucose, with a production rate of 0.1 g/L/h, corresponding to 50% of the theoretical yield [27]); thus, it achieves a lower MSP of $4.4/kg. The price of pure glucose ($0.59/kg [28]) is the largest cost contributor for all microbially produced bioproducts, as shown in Fig. 1. For 4-HBA, its product recovery process is more capital intensive due to the extra acidification and the purification processes relative to other processes. For other bioproducts, the capital cost of the bioconversion process is higher than the final product purification process. In an optimal scenario, where yields are increased to the theoretical maxima and residence times are reduced (theoretical yield scenario in Fig. 1; details in SI Appendix, Table S1), the MSPs of all four bioproducts could be reduced to prices approaching those of commodity chemicals, ranging from $2 to $5/kg. The MSPs of PDC and 4-HBA in the theoretical yield scenario are $2 and $3.7/kg, respectively, which are comparable with their market selling prices but still higher than the achievable MSPs in the plant systems. For catechol, 4-HBA, and muconic acid, the theoretical maximum yield is obtained using flux analysis. However, no theoretical maximum yield has been reported for PDC using flux analysis, so we use reaction stoichiometry to estimate the theoretical maximum yield. The results of our technoeconomic analysis (TEA) of microbial production are aligned with prior studies. For example, Krömer et al. found that 90% of theoretical yield (predicted by flux analysis) and a titer of 100 g/L of 4-HBA produced in C. glutamicum results in selling prices in the range of $1.61 to $3.59/kg with water recycling and/or biomass recycling scenarios (29). When producing catechol from lignin, Mabrouk et al. predicted the selling price to be $1,100/t with 2,544 kg per day of biomass from olive tree residue (30). For muconic acid production using first-generation sugar at the price of $0.27/kg (considerably lower than our assumed price of $0.59/kg) and assumed production yield of 0.4 mol/mol glucose and rate of 1 g/L/h, the previously predicted MSP was $1.95/kg (27).
Cost Comparison between Plant and Microbial Systems.
To better understand the potential economic advantages of one expression system over the other, we compared the production costs of these four bioproducts in both plant and microbial systems by varying mass accumulation rates (dwt%) in bioenergy crops and yields from glucose (% of theoretical maximum) in microbial routes. Specifically, we modeled feedstock input amounts of 2,000 bone-dry tonnes of biomass sorghum per day in the plant systems and a bioethanol selling price of $1.44/LGE as calculated in our base case biorefinery. We only altered the mass accumulation rates of bioproducts in the plant system to calculate the final MSPs for each bioproduct. In microbial systems, the daily glucose input is fixed at 1,000 bone-dry tonnes, and we varied the production yields to quantify the final MSPs for each bioproduct. Bioconversion times range from 24 to 144 h, depending on the reported titers and yields for each specific product (SI Appendix, Table S1).
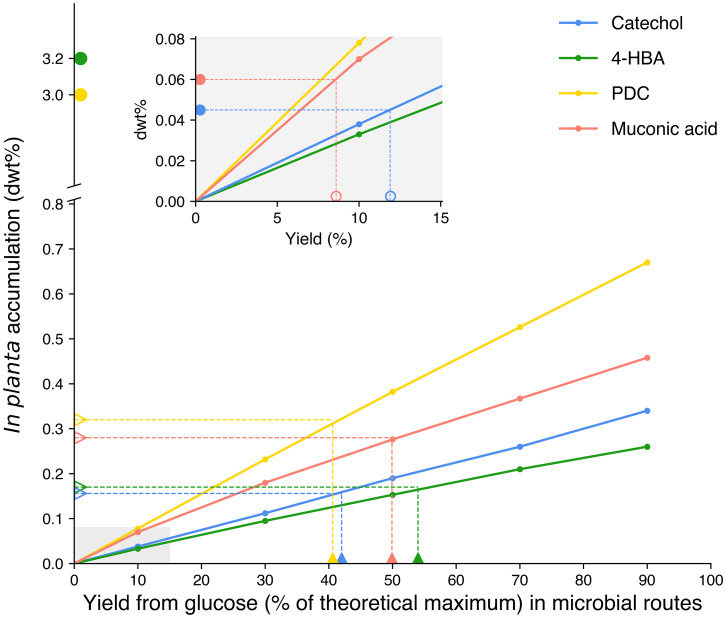
The cost comparison results for plant and microbial systems are shown in Fig. 2. Solid icons denote the highest reported yield in either engineered plants or microbial routes; the corresponding empty (outline only) icons show the mass accumulation rate or production yield required in the other system to reach cost parity when producing the same bioproduct. We find that the breakeven accumulation rate for plant systems required to compete with microbial production across the four products explored here ranges from 0.1 to 0.3 dwt% of all plant tissue in a bioenergy crop (biomass sorghum, in this case). The highest reported yields of 4-HBA and PDC (shown in Fig. 2) are well beyond the threshold needed to compete with microbial routes, at 3.2 (18) and 3.0 dwt% (16), respectively. The breakeven yield for microbial production of these compounds from glucose, given the 3.2 and 3.0 dwt% accumulation rates, far exceeds the theoretical maxima for both compounds. However, it is conceivable that bioenergy crops might achieve more modest accumulation rates in some cases, particularly if high accumulation rates can be achieved only in particular components of the plants (e.g., stems or leaves). Based on the reported yields of 4-HBA and PDC in microbial routes (solid green and yellow triangles in Fig. 2), the minimum required in planta mass accumulation rates are ∼0.2 dwt% for 4-HBA (empty green triangle) and ∼0.3 dwt% for PDC (empty yellow triangle) to reach cost parity with microbial production from glucose. This finding provides a clear indication that it may be more advantageous to produce PDC and 4-HBA in planta, and if engineering efforts aimed specifically at high-yielding bioenergy crops result in lower accumulation rates, this may still be competitive as long as those rates exceed 0.2 to 0.3 dwt%.
Fig. 2.
Comparison of MSPs ($/kg) between in planta accumulation and microbial routes to bioproducts, using the base case ethanol selling price ($1.44/LGE) for lignocellulosic biorefineries that convert residual biomass after bioproduct extraction from plants. The solid circles represent the highest reported mass accumulation rates in plants, and the empty circles represent the corresponding yields required to reach cost parity using microbial routes for the same products. The solid triangles indicate the highest reported yields as a fraction of maximum theoretical yields in microbial systems. For catechol, 4-HBA, and muconic acid, the theoretical maximum yield is determined by flux analysis; no theoretical maximum yield has been reported for PDC using flux analysis, so we use reaction stoichiometry to determine its theoretical maximum. The empty triangles represent in planta mass accumulation rates needed to reach cost parity with the best-reported microbial yields for each bioproduct. Breakeven microbial production yields for 4-HBA and PDC exceed the theoretical maximum and, therefore, are not shown. (Inset) The in planta accumulation rates for muconic acid and catechol, which are less than 0.08 dwt%.
Unlike 4-HBA and PDC, production of catechol and muconic acid so far proves to be more advantageous in microbes when compared to the best-demonstrated accumulation in plants. With the current highest reported microbial yield of catechol (0.26 mol/mol glucose or 0.16 g/g glucose [26], corresponding to 42% of theoretical yield) and muconic acid (0.378 mol/mol glucose or 0.298 g/g glucose [27], corresponding to 50% of theoretical yield), the mass accumulation rates required in plants are ∼0.15 and ∼0.3 dwt%, respectively. The highest reported mass accumulation rates of catechol and muconic acid in engineered plants are considerably lower than these thresholds: catechol has been accumulated at 0.045 dwt% (17), and muconic acid was accumulated at 0.06 dwt% (15) (Fig. 2, Inset). Based on these best-demonstrated accumulation rates, microbial production routes must only exceed 8 and 12% of theoretical maxima for muconic acid and catechol, respectively, to outcompete the in planta production route.
Uncertainty Analysis.
The scenarios presented—specifically, the scenarios in which bioproducts are coproduced alongside lignocellulosic ethanol—carry considerable uncertainty. An important source of long-term uncertainty is the expected selling price for lignocellulosic ethanol, which will depend on the behavior of energy markets and any policy incentives applied to low-carbon fuels in the future. The bioproduct selling prices are also uncertain because these products are not well established as commodity chemicals. We investigated the impact of these factors by varying selling prices ($/kg) of these four bioproducts and mass accumulation rates (dwt%) in biomass sorghum needed to reach a range of MESPs ($/LGE) in lignocellulosic biorefineries.
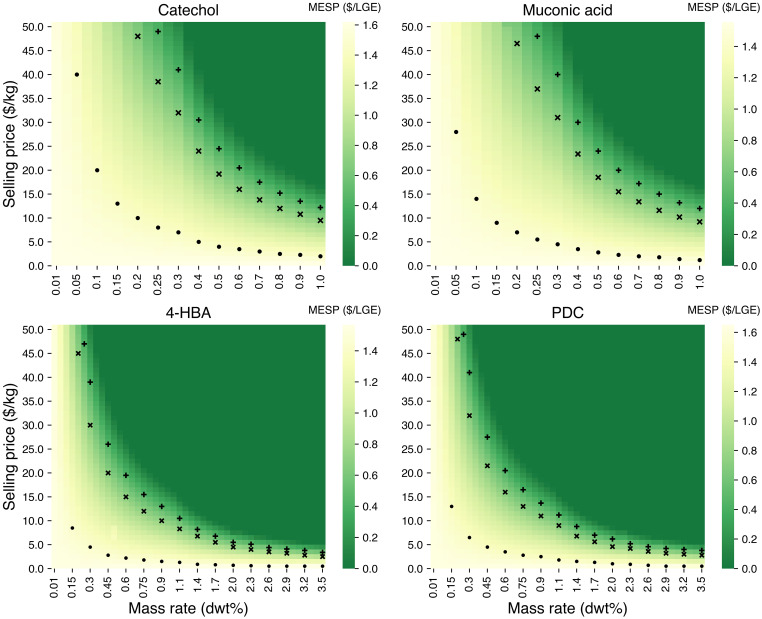
As expected, higher accumulation rates of bioproducts in planta increase revenue and result in a reduced MSP for fuel ethanol (Fig. 3). When selling ethanol at a fixed price, the minimum required bioproduct selling price decreases as the bioproduct accumulation rate increases. To reach cost parity with a base case biorefinery in which no bioproduct is extracted ($1.44/LGE; see dot symbol [“•”] in Fig. 3) under the lower mass accumulation rate scenario (0.05 dwt%), the required selling price of catechol is the highest ($40/kg) among four bioproducts due to its more capital-intensive extraction process, followed by PDC ($36/kg), muconic acid ($28/kg), and 4-HBA ($22/kg). For comparable selling prices (<$50/kg), increasing the accumulation rate to ∼0.2 dwt% enables the biorefinery to reach the target ethanol selling price of $0.66/LGE (see “x” in Fig. 3). To compete with long-term historical gasoline rack price ($0.40/LGE; see plus sign [“+”] in Fig. 3), a bioproduct selling price in the range of $30 to $50/kg combined with 0.2 to 0.4 dwt% accumulation would be required.
Fig. 3.
Bioproduct selling price ($/kg) versus mass accumulation rate (dwt%) in engineered biomass sorghum utilized as feedstock in lignocellulosic biorefineries. The bar on the right side of each subplot shows the MESP ($/LGE) obtained in lignocellulosic biorefineries. The dot symbol in each subplot is the bioproduct selling price at each mass accumulation rate for reaching cost parity with the base case biorefineries developed in this study ($1.44/LGE, as calculated in our base case model). The “x” in each subplot refers to the product selling price and mass accumulation rate required to reach a target ethanol selling price ($0.66/LGE [24]). The plus symbol in each plot corresponds to a product selling price and mass accumulation rate needed for ethanol to reach parity with the U.S. long-term historical gasoline rack price from 1940 to 2020 ($0.40/LGE [25]).
Ultimately, the competitiveness of these in planta accumulation routes to bioproducts depends on their intended market. If PDC, 4-HBA, catechol, and muconic acid are to be sold into commodity markets, their selling prices must be at least an order of magnitude lower than the selling prices previously discussed. Both 4-HBA and PDC have already been accumulated in planta at levels higher than what is needed to reach cost parity with ethanol using the base case price (target rates of ∼2.0 and ∼1.0 dwt%, respectively). Even if ethanol must sell at a price equal to the long-term historical gasoline rack price, the required accumulation rates for PDC and 4-HBA (∼3.0 dwt%) are comparable with the current state of the art. These results suggest that if similar accumulation rates can be achieved in the harvestable biomass for sorghum or any other high-yielding biomass crop, extraction of these bioproducts can benefit biorefineries in the immediate future. Catechol (0.045 dwt%) and muconic acid (0.06 dwt%), however, require dramatic improvements in accumulation rates to become viable. These results are consistent with previous findings that established minimum in planta accumulation rates needed to offset the cost of extraction (a breakeven price for a facility producing ethanol and not recovering a bioproduct) (4).
Regardless of which bioproduct is produced, the biomass sorghum price has the greatest impact on costs for the plant production systems. In microbial production systems, product yield and glucose feedstock price are the two leading sources of variation (see results in SI Appendix, Figs. S5 and S6). If the biomass sorghum price is increased from $95 to $120/bone-dry tonne in the plant systems, the bioproduct MSPs can increase by as much as ∼80%, assuming the ethanol selling price must remain constant. A common theme in the sensitivity analysis for the plant systems is that if the ethanol selling price is held constant, even small changes to capital and operating costs result in large changes in the bioproduct MSP because those changes in the facility economics must be compensated for by altering the price of a comparatively small-volume product. For microbial production, there may be opportunities to source cheaper sugars, assuming pure glucose is not required. Shifting from pure glucose ($0.59/kg) to first-generation sugar ($0.27/kg) will decrease the bioproduct MSP by $1.0 to $4.0/kg.
Process parameters also impact the MSP of bioproducts under both the microbial and the plant systems. We find extraction efficiency (measured as a percentage of maximum recovery) for plant systems can vary the MSP by ∼25%, followed by extraction time and temperature. This is because longer extraction times and higher temperatures increase the facility’s energy use. Improving parameters related to lignocellulosic ethanol production in the plant system—such as glucan-to-glucose conversion rate, xylan-to-xylose conversion rate, and glucose utilization—can decrease bioproduct MSPs by up to $20/kg since these minimize total costs and increase revenue at the facility. Additionally, decreasing IL (the biomass pretreatment solvent) cost in lignocellulosic biorefineries from $2 to $1/kg can also decrease the bioproduct MSP by up to $10/kg. For microbial production systems, improving production yield relative to the best currently demonstrated values can reduce the bioproduct MSP by 40 to 74% (see SI Appendix, Fig. S6 for detailed results).
Discussion
In this study, we aimed to elucidate the economic competitiveness of two different paths to producing bioproducts: directly accumulating the products in bioenergy crops for eventual recovery at biorefineries and dedicated microbial production of those same bioproducts using glucose as a feedstock. Developing detailed technoeconomic models for both sets of scenarios across four bioproducts that can be produced microbially and in planta allowed us to explore breakeven yields and accumulation rates. Our results suggest that with the exception of bioproducts accumulated at very high rates (>1 dwt%), microbial and plant systems are likely to be competitive, and product-specific performance in both systems will be the deciding factor in which platform is most attractive. In plant systems, accumulating low-to-moderate value bioproducts in bioenergy crops can improve the economics of biorefineries only when a high mass fraction of bioproducts can be achieved. For example, with a 3.2 dwt% of 4-HBA accumulated in biomass sorghum, 19,000 tonnes per year of 4-HBA could be produced alongside ethanol at a commercial biorefinery processing 2,000 bone-dry tonnes per day of sorghum, and this will result in a market-competitive MSP of the bioproduct. PDC production in planta is similarly attractive, given the 3.0 dwt% accumulation. However, due to the lower mass accumulation rates of catechol and muconic acid, biorefineries coproducing catechol or muconic acid cannot compete with microbial production. To reach cost parity with microbial routes, a large improvement (up to fourfold) in the mass accumulation rates of catechol and muconic acid in engineered plants is required. The approximate relationships between target accumulation rates and microbial yields can be used to evaluate other compounds that are outside the scope of this study. For example, cannabidiol for medical applications can be extracted from Cannabis sativa L., which can accumulate up to 7.5 dwt% in planta (31). Although cannabidiol can also be produced from Saccharomyces cerevisiae using galactose (32), our results would suggest that microbial production may have challenges competing with the plant system, barring any other potential advantages of microbial production.
Environmental impacts, while not incorporated into our cost analysis, are important to consider and may directly impact the economics in cases where facilities are eligible for policy incentives tied to greenhouse gas (GHG) emissions mitigation. The life-cycle GHG footprint of producing biochemicals microbially from glucose is driven by the type of feedstock from which glucose is sourced (e.g., corn, sugarcane, potato, wheat) and the energy footprint of the bioconversion process itself (33, 34). Because PDC, 4-HBA, catechol, and muconic acid are all produced aerobically, which involves sparging the bioreactor with air, there will be no pure CO2 stream available for capture and sequestration. If, however, some other bioproduct of interest can be produced anaerobically, this would present an opportunity for very low-cost capture and sequestration of the pure CO2 stream from fermentation (35). Additionally, sourcing sugars from alternative sources, such as lignocellulosic biomass, can reduce the GHG footprint of the feedstock (36). The in planta accumulation route involves downstream conversion of biomass to ethanol as a renewable fuel blendstock, so the potential for GHG benefits are twofold: ethanol can displace the use of petroleum fuels, and the pure CO2 stream from ethanol fermentation can be captured and sequestered (35). Such a strategy can become economically favorable after accounting for policy incentives tied to low carbon fuels (35).
Although the analysis presented here is focused on in planta accumulation and microbial production as two competing alternatives, one could reasonably suggest that the two strategies should be combined in a single biorefinery to maximize bioproduct yield and avoid additional separation and purification steps. In this integrated case, the bioproduct could be retained throughout pretreatment and saccharification of the biomass, assuming it remains stable, and loaded into the bioconversion reactor, where lignocellulosic sugars are microbially converted to produce additional bioproduct. However, this strategy would require additional information regarding the potential losses of bioproduct during pretreatment, saccharification, and intermediate separations required prior to bioconversion. To produce each bioproduct in a lignocellulosic biorefinery, the production must be carried out in microbial hosts capable of coutilizing pentose and hexose sugars, which has not yet been demonstrated for the best-available yields used in our analysis. During bioconversion, retained bioproduct would also contribute to the overall titer in the reactor. The higher titers could require additional dilution to avoid toxicity issues, although such limitations will be product and host dependent.
As an exploratory analysis, we evaluated the potential for PDC production in this integrated configuration where in planta accumulation and microbial production are combined (see SI Appendix, Fig. S3 for the process flow diagram). We analyzed the differences between two scenarios: one in which PDC is extracted from the biomass prior to deconstruction and plant-derived sugar is converted to PDC downstream, and a second scenario in which the PDC accumulated in planta is carried through bioconversion and recovered alongside microbially produced PDC. The goal of this exercise was to gauge the importance of eliminating upfront recovery of PDC accumulated in planta. Both scenarios relied on the same demonstrated TRYs used previously for glucose and do not incorporate hypothetical production from xylose. In an optimistic case where only 5% of PDC from the plant is lost during upstream processing, the savings achieved in the integrated configuration are relatively modest (<10%) when compared to a facility that extracts and recovers PDC prior to pretreatment and then later converts plant-derived glucose to additional PDC (see SI Appendix, Fig. S4 for results).
In summary, accumulation of bioproducts in high-yielding crops and production of bioproducts using microbial hosts both have important roles to play in the expansion of the volume and diversity of bioderived compounds needed to replace fossil-derived products. Our results suggest that accumulation rates exceeding ∼1.0 dwt% in plants are likely to outcompete microbial production of bioproducts, assuming the remaining biomass can be valorized through conversion to a liquid fuel (although conversion of biomass to other nonfuel products may achieve similar results). For microbial routes that have achieved yields at or above half of the theoretical maximum, this option is likely to be competitive with, or superior to, in planta accumulation, assuming realistic accumulation rates fall in the range of 0.1 to 0.3 dwt%. These guidelines can help the research community take a more systematic approach in setting realistic performance targets, prioritizing research and commercialization efforts in different chassis based on their relative advantages, and evaluating progress toward economically viable bioproduct production at scale.
Materials and Methods
Scenario Description.
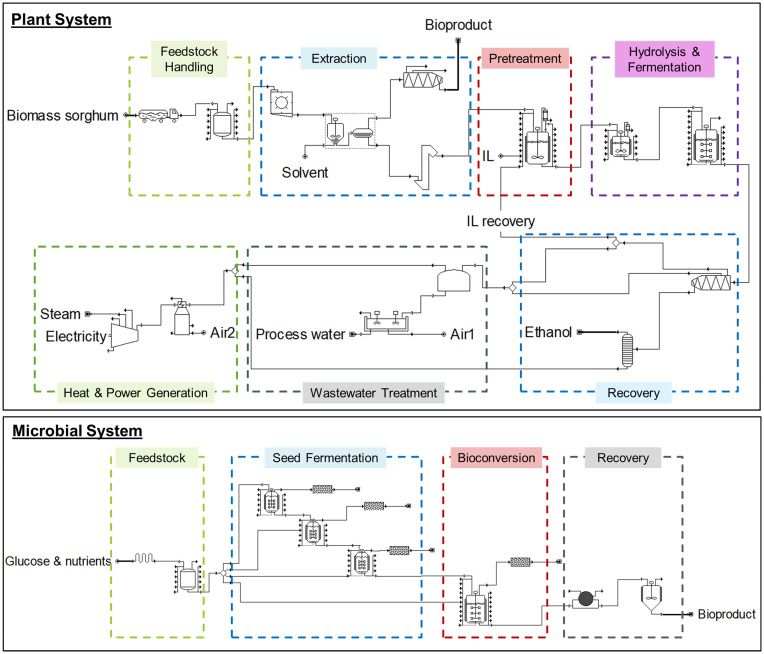
In this study, SuperPro Designer v12 was used to develop technoeconomic models of four bioproducts (4-HBA, PDC, catechol, and muconic acid) that can be produced from both plant and microbial systems. The simplified process flow diagram for both systems is shown in Fig. 4. In the plant systems, we adopted integrated lignocellulosic biorefineries to maximize the utilization of bioenergy crops to produce biofuels and bioproducts simultaneously. We assume the selected bioproducts can be accumulated in engineered biomass sorghum at a range of mass fractions (dwt%). Biomass sorghum was selected as a representative bioenergy crop because of its high biomass yield, its natural drought tolerance and water-use efficiency, and the relative ease with which sorghum can be engineered when compared to other bioenergy crops such as Miscanthus (37–40). In our simulated biorefineries, the desired bioproduct accumulated in the engineered biomass sorghum is extracted up front (before downstream deconstruction and bioconversion). We assume that the bioproduct is accumulated in the whole plant, meaning that the dwt% indicates an average accumulation in all harvestable biomass (accumulation levels in some types of tissue may be higher than others in practice). We also assume that the bioproduct will remain stable during biomass harvest, drying, and storage. During bioproduct extraction, we model extraction processes intended to avoid further breakage down the cell wall. Such premature deconstruction of the cell wall can release impurities such as sugars or lignin to complicate the bioproduct recovery process. The simulated lignocellulosic biorefineries include the following: 1) feedstock handling section to remove foreign materials and to store feedstock onsite for a short time; 2) extraction processes tailored to each bioproduct; 3) integrated one-pot high gravity IL (cholinium lysinate) pretreatment to deconstruct the lignin-cellulose-complex structure of biomass cell wall; 4) enzymatic hydrolysis and fermentation processes to break down polysaccharides to monosaccharides and then convert glucose and xylose to ethanol; 5) ethanol recovery and purification; 6) wastewater treatment to clean wastewater for reuse; and 7) onsite energy generation to fulfill the heat and electricity demands of the facility where the excess electricity is sold to the grid at a price of $0.068/kWh (41).
Fig. 4.
Simplified process diagrams for bioproduct production in plant systems and microbial systems. In plant systems, the bioproduct is accumulated in the engineered biomass sorghum and then extracted onsite before the remaining biomass sorghum is used to produce ethanol.
In microbial systems, sugar (pure glucose) is used as the carbon source to produce bioproducts. Other nutrients are provided based on medium requirements of host organisms for each bioproduct. Unlike plant systems, in microbial systems, only desired bioproducts will be produced using the established production pathways, and no coproduct is produced. The microbial production facility starts with a feedstock section to receive raw materials. After mixing, 10% of the glucose is sent to three-stage onsite seed fermentation processes, and the rest is routed to the aerobic bioconversion process to produce bioproducts under specific conditions. After the bioproduct is synthesized in the bioconversion process, it goes through a recovery and purification process designed for each bioproduct, and wastewater is treated offsite.
Selection of Bioproducts.
In this study, we selected four bioproducts (4-HBA, PDC, catechol, and muconic acid) that can be accumulated in engineered plants and also produced from microbial hosts. These products were selected because of their diverse set of potential applications; we deliberately did not focus on compounds whose sole (or primary) use is in pharmaceuticals, such as artemisinin or morphine, because of the risk that coproduction at lignocellulosic biorefineries would overwhelm their limited market volumes (4,42). However, the results could be generalized to pharmaceuticals, with the caveat that the purification processes may be more extensive in some cases.
4-HBA is regarded as a valuable intermediate to produce a diverse range of final products that can be used in food, cosmetics, and pharmaceuticals (20). Its market size is projected to reach US$80 million by 2026 at a growth rate of 5.2% from 2021 to 2026 (43). High accumulation of 4-HBA was reported in transgenic tobacco (26.5 dwt% of 4-HBA glucose conjugates in the leaves, equivalent to 12.2 dwt% of free 4-HBA) (44) and sugarcane (7.3 dwt% of 4-HBA glucose conjugates in the leaves, equivalent to 3.2 dwt% of free 4-HBA) (18). In the microbial system, C. glutamicum was reported to have a higher tolerance to 4-HBA toxicity, and the maximum titer of 4-HBA produced was 36.6 g/L, with a yield of 0.41 mol/mol glucose or 0.314 g/g glucose (45).
Given the similar structure between PDC and terephthalic acid, PDC is a valuable industrial chemical to make diverse polyesters with novel functionalities (23). By engineering E. coli, the highest titer of PDC reported to date was 16.72 g/L from glucose (23). In P. putida, the highest reported yield was 0.341 mol/mol produced from glucose or 0.348 g PDC/g glucose (27). In the plant system, Lin et al. introduced PDC biosynthetic genes into the Arabidopsis and reported ∼3 dwt% PDC yield in plant biomass with a 40 to 45% reduction in lignin content in the stems of transgenic lines relative to the wild-type plants (16).
Catechol is mainly produced as a precursor to pesticides, flavors, and fragrances (21). The total pesticides export value increased from US$38.5 billion in 2017 to US$40.5 billion in 2019, according to the Food and Agriculture Organization of the United Nations (46). Bio-based catechol using lignin as the starting material has been estimated to achieve a 2% reduction in greenhouse gas emissions, 7% reduction in ecotoxicity, and 59% reduction in fossil fuel use relative to fossil-based catechol (47). In the microbial system, a catechol yield of 0.26 mol/mol glucose (or 0.16 g/g glucose) was achieved in recombinant E. coli, which corresponds to 42% of the maximum theoretical yield determined by elementary node flux analysis (26).
The production of muconic acid attracts much attention due to its end use in the production of several polymers, such as nylon-6,6 and polyethylene terephthalate (22). The muconic acid market size is expected to be US$111.8 million by 2023 at an expanding growth rate of >7% from 2019 to 2024 (48). Muconic acid has been successfully produced in Arabidopsis at 637 μg/g in the best transgenic line (15). In the microbial system, muconic acid produced in E. coli reached a titer of 36.8 g/L in a 2-L fed-batch bioconversion reactor (49), and the final yield of muconic acid from glucose has been reported at 0.378 mol/mol glucose or 0.298 g/g glucose in engineered P. putida (27). Choi et al. recently reviewed the bio-based muconic acid pathway in several microorganisms and found that using engineered C. glutamicum in muconic acid biosynthesis resulted in a high titer of 85 g/L (50).
Extraction Processes in Plants.
4-HBA is extracted by 50% (vol/vol) methanol with a loading rate of 150 mg to 1 mL at 70 °C for 6 h (18). After extraction, bead milling is used to further separate the product from the mixture; then, the wastewater is sent to centrifugation to remove the debris. Methanol is distilled and recycled back to the extractor, with a 95% recovery rate. 4-HBA is further dried, crystallized, and stored onsite. Catechol is extracted by 80% (vol/vol) ethanol, which is produced onsite in our modeled biorefinery configuration. The extraction process takes 8 h at 80 °C and is repeated twice (17). Then, the extractant is sent to the evaporator and subsequently sent to the centrifuge. After centrifugation, the catechol is dried, crystallized, and stored onsite. PDC is extracted by 80% (vol/vol) methanol with a PDC loading rate of 50 mg to 1 mL for three times at 70 °C for 8 h (16, 51). Then, the PDC-containing slurry is washed with NaCl and dehydrated to a higher concentration. Later, PDC is filtered, crystallized, and stored onsite. Muconic acid is directly extracted by 80% (vol/vol) methanol with a loading rate of 50 mg to 1 mL at 70 °C for 6 h (15). This extraction process is repeated twice. Then, the biomass tissue is centrifuged, filtered, evaporated, and stored onsite. Methanol is evaporated, condensed, and recycled at 95%. The process flow diagrams for these four bioproducts extraction processes in the plant system are shown in SI Appendix, Fig. S1.
Production Processes in Microbial Hosts.
4-HBA is produced in engineered C. glutamicum from glucose at 33 °C for 24 h. The yield reported in C. glutamicum was 0.41 mol/mol glucose (or 0.31 g/g glucose), and the maximum theoretical yield of 0.58 g/g glucose (or 0.76 mol/mol glucose) was obtained by metabolic network analysis (52). 4-HBA is accumulated extracellularly in fermentation broth (53). Its purification and separation process follows the procedure published by Krömer et al., and the final purity of 4-HBA is ∼99% (29). Catechol is produced from glucose in engineered E. coli at 32 °C for 72 h (26). The yield of catechol was 0.16 g/g glucose (or 0.26 mol/mol glucose) in a fed-batch fermenter at 37 °C, corresponding to 42% of the theoretical maximum determined by elementary node flux analysis (26). The maximum theoretical yield is 0.376 g/g glucose (or 0.61 mol/mol glucose) (26). After the bioconversion process, catechol is separated from the mixture stream and purified to the final purity of ∼99%. PDC is produced from glucose using P. putida in fed-batch fermenters at 30 °C for 144 h that produces 12.9 g/L PDC with a yield of 0.341 mol/mol glucose (27). The purification process follows the description by Mase et al., and the final purity is ∼99% (54). Muconic acid is produced from glucose using engineered P. putida at 35 °C for 89 h. The final yield was 0.378 mol/mol glucose (or 0.298 g/g glucose), and the maximum theoretical yield was 0.739 mol/mol glucose (or 0.583 g/g glucose) using flux balance analysis (27). The purification and recovery process follows the description previously published by the National Renewable Energy Laboratory (NREL), and the purity of muconic acid is ∼99% (55). The process flow diagrams are shown in SI Appendix, Fig. S2.
TEA.
We first developed a mass and energy balance of each process and then conducted the discounted cash flow analysis to obtain the final MSP of each bioproduct produced either from plant systems or from microbial systems. In the plant system, the lignocellulosic biorefinery is assumed to operate 24 h per day and 330 d per year (7,920 h per year) for 30 y. Biomass sorghum is used as the feedstock, and the unit price of biomass sorghum is $95 per bone-dry tonne (4, 35), with a range of $60 to $120 per bone-dry tonne (56). The biorefinery takes in 2,000 bone-dry tonnes per day of biomass sorghum feedstock, which is milled and routed to pretreatment. The IL pretreatment takes place at 140 °C for 3 h (57). The IL is recycled after fermentation, and the recycle rate is assumed to be 97%. The unit price of IL is $2/kg, with a range of $1 to $5/kg (58). In enzymatic hydrolysis, 10 mg protein per g of glucan is used, and the hydrolysis time is 3 d (57). In bioconversion, glucose-to-ethanol and xylose-to-ethanol conversion rates are assumed at 95 and 85%, respectively (59). Remaining lignin, solids, and biogas produced in the wastewater treatment section are sent to the heat and power generation section to produce onsite heat and power. Excess electricity produced in the heat and power generation section is sold to the grid as a coproduct. In microbial systems, glucose is used as the feedstock with a price of $0.59/kg, as reported in 2020 (28). The microbial production facilities take in 1,000 bone-dry tonnes of glucose per day. The demonstrated scenario is based on the highest reported yields from experiments, and the theoretical scenario is based on the maximum theoretical yields. The current market prices for 4-HBA, PDC, catechol, and muconic acid are $2.6, $5.5, $5.0, and $1.5/kg, respectively (29, 60–62). To better understand the parameters that determine the final cost, we conducted a sensitivity analysis by exploring the maximum and minimum values of the input parameters. The data are documented in SI Appendix, Table S1.
Supplementary Material
Acknowledgments
This work was part of the DOE Joint BioEnergy Institute (https://www.jbei.org), supported by the U.S. DOE, Office of Science, Office of Biological and Environmental Research, through contract DE-AC02-05CH11231 between Lawrence Berkeley National Laboratory and the U.S. DOE. This study was also supported by the U.S. DOE, Energy Efficiency and Renewable Energy, Bioenergy Technologies Office. The U.S. Government retains, and the publisher, by accepting the article for publication, acknowledges that the U.S. Government retains a nonexclusive, paid-up, irrevocable, worldwide license to publish or reproduce the published form of this manuscript, or allow others to do so, for U.S. Government purposes.
Footnotes
Competing interest statement: C.-Y.L. and A.E. are co-inventors on the U.S. patent application entitled “Novel plants and methods for producing 2-pyrone-4, 6-dicarboxylic acid (PDC),” number US63/091,82. A.E. is an inventor on the U.S. patent entitled “Novel plants and methods for producing muconic acid,” number US16/796,790.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2122309119/-/DCSupplemental.
Data Availability
All input parameters and study data are included in the article and/or SI Appendix. The code and SuperPro Designer process models used to generate results are available upon reasonable request.
References
- 1.IPCC, Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change (IPCC, 2014). [Google Scholar]
- 2.IEA, Coal 2020 (IEA, 2020). [Google Scholar]
- 3.IEA, Oil 2020 (IEA, 2020). [Google Scholar]
- 4.Yang M., et al. , Accumulation of high-value bioproducts in planta can improve the economics of advanced biofuels. Proc. Natl. Acad. Sci. U.S.A. 117, 8639–8648 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park S. Y., Yang D., Ha S. H., Lee S. Y., Metabolic engineering of microorganisms for the production of natural compounds. Adv. Biosys. 2, 1700190 (2017). [Google Scholar]
- 6.Daniell H., et al. , Green giant-a tiny chloroplast genome with mighty power to produce high-value proteins: History and phylogeny. Plant Biotechnol. J. 19, 430–447 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scown C. D., Baral N. R., Yang M., Vora N., Huntington T., Technoeconomic analysis for biofuels and bioproducts. Curr. Opin. Biotechnol. 67, 58–64 (2021). [DOI] [PubMed] [Google Scholar]
- 8.Baral N. R., et al. , Approaches for more efficient biological conversion of lignocellulosic feedstocks to biofuels and bioproducts. ACS Sustain. Chem. Eng. 7, 9062–9079 (2019). [Google Scholar]
- 9.Kogure T., Inui M., Recent advances in metabolic engineering of Corynebacterium glutamicum for bioproduction of value-added aromatic chemicals and natural products. Appl. Microbiol. Biotechnol. 102, 8685–8705 (2018). [DOI] [PubMed] [Google Scholar]
- 10.Yang D., Park S. Y., Park Y. S., Eun H., Lee S. Y., Metabolic engineering of Escherichia coli for natural product biosynthesis. Trends Biotechnol. 38, 745–765 (2020). [DOI] [PubMed] [Google Scholar]
- 11.Zhang H., Pereira B., Li Z., Stephanopoulos G., Engineering Escherichia coli coculture systems for the production of biochemical products. Proc. Natl. Acad. Sci. U.S.A. 112, 8266–8271 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yaegashi J., et al. , Rhodosporidium toruloides: A new platform organism for conversion of lignocellulose into terpene biofuels and bioproducts. Biotechnol. Biofuels 10, 241 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weber C., et al. , Biosynthesis of cis,cis-muconic acid and its aromatic precursors, catechol and protocatechuic acid, from renewable feedstocks by Saccharomyces cerevisiae. Appl. Environ. Microbiol. 78, 8421–8430 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aramvash A., Gholami-Banadkuki N., Moazzeni-Zavareh F., Hajizadeh-Turchi S., An environmentally friendly and efficient method for extraction of PHB biopolymer with non-halogenated solvents. J. Microbiol. Biotechnol. 25, 1936–1943 (2015). [DOI] [PubMed] [Google Scholar]
- 15.Eudes A., et al. , Production of muconic acid in plants. Metab. Eng. 46, 13–19 (2018). [DOI] [PubMed] [Google Scholar]
- 16.Lin C.-Y., et al. , In-planta production of the biodegradable polyester precursor 2-pyrone-4,6-dicarboxylic acid (PDC): Stacking reduced biomass recalcitrance with value-added co-product. Metab. Eng. 66, 148–156 (2021). [DOI] [PubMed] [Google Scholar]
- 17.Morse A. M., et al. , Salicylate and catechol levels are maintained in nahG transgenic poplar. Phytochemistry 68, 2043–2052 (2007). [DOI] [PubMed] [Google Scholar]
- 18.McQualter R. B., et al. , Initial evaluation of sugarcane as a production platform for p-hydroxybenzoic acid. Plant Biotechnol. J. 3, 29–41 (2005). [DOI] [PubMed] [Google Scholar]
- 19.Bozell J. J., Petersen G. R., Technology development for the production of biobased products from biorefinery carbohydrates—The US Department of Energy’s “Top 10” revisited. Green Chem. 12, 539 (2010). [Google Scholar]
- 20.Wang S., Bilal M., Hu H., Wang W., Zhang X., 4-Hydroxybenzoic acid-a versatile platform intermediate for value-added compounds. Appl. Microbiol. Biotechnol. 102, 3561–3571 (2018). [DOI] [PubMed] [Google Scholar]
- 21.Lin C.-Y., Eudes A., Strategies for the production of biochemicals in bioenergy crops. Biotechnol. Biofuels 13, 71 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khalil I., Quintens G., Junkers T., Dusselier M., Muconic acid isomers as platform chemicals and monomers in the biobased economy. Green Chem. 22, 1517–1541 (2020). [Google Scholar]
- 23.Luo Z. W., Kim W. J., Lee S. Y., Metabolic engineering of Escherichia coli for efficient production of 2-pyrone-4,6-dicarboxylic acid from glucose. ACS Synth. Biol. 7, 2296–2307 (2018). [DOI] [PubMed] [Google Scholar]
- 24.Bioenergy Technologies Office, Multi-Year Program Plan (U.S. Department of Energy, 2016). [Google Scholar]
- 25.U.S. EIA, Data from “U.S. Regular Gasoline Rack Sales Price by Refiners (Dollars per Gallon).” U.S. Energy Information Administration. https://www.eia.gov/dnav/pet/hist/LeafHandler.ashx?n=PET&s=EMA_EPMR_PRG_NUS_DPG&f=M. Accessed July 20, 2020.
- 26.Balderas-Hernández V. E., et al. , Catechol biosynthesis from glucose in Escherichia coli anthranilate-overproducer strains by heterologous expression of anthranilate 1,2-dioxygenase from Pseudomonas aeruginosa PAO1. Microb. Cell Fact. 13, 136 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson C. W., et al. , Innovative chemicals and materials from bacterial aromatic catabolic pathways. Joule 3, 1523–1527 (2019). [Google Scholar]
- 28.USDA, Data from “USDA ERS - Sugar and Sweeteners Yearbook Tables.” U.S. Department of Agriculture, Economic Research Service. https://www.ers.usda.gov/data-products/sugar-and-sweeteners-yearbook-tables/. Accessed April 5, 2021.
- 29.Krömer J. O., Ferreira R. G., Petrides D., Kohlheb N., Economic process evaluation and environmental life-cycle assessment of bio-aromatics production. Front. Bioeng. Biotechnol. 8, 403 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mabrouk A., Erdocia X., Alriols M. G., Labidi J., Economic analysis of a biorefinery process for catechol production from lignin. J. Clean. Prod. 198, 133–142 (2018). [Google Scholar]
- 31.Pacifico D., Miselli F., Carboni A., Moschella A., Mandolino G., Time course of cannabinoid accumulation and chemotype development during the growth of Cannabis sativa L. Euphytica 160, 231–240 (2008). [Google Scholar]
- 32.Luo X., et al. , Complete biosynthesis of cannabinoids and their unnatural analogues in yeast. Nature 567, 123–126 (2019). [DOI] [PubMed] [Google Scholar]
- 33.Montazeri M., Zaimes G. G., Khanna V., Eckelman M. J., Meta-analysis of life cycle energy and greenhouse gas emissions for priority biobased chemicals. ACS Sustain. Chem.& Eng. 4, 6443–6545 (2016). [Google Scholar]
- 34.Hermann B. G., Blok K., Patel M. K., Producing bio-based bulk chemicals using industrial biotechnology saves energy and combats climate change. Environ. Sci. Technol. 41, 7915–7921 (2007). [DOI] [PubMed] [Google Scholar]
- 35.Yang M., Baral N. R., Anastasopoulou A., Breunig H. M., Scown C. D., Cost and life-cycle greenhouse gas implications of integrating biogas upgrading and carbon capture technologies in cellulosic biorefineries. Environ. Sci. Technol. 54, 12810–12819 (2020). [DOI] [PubMed] [Google Scholar]
- 36.Jin V. L., et al. , Management controls the net greenhouse gas outcomes of growing bioenergy feedstocks on marginally productive croplands. Sci. Adv. 5, eaav9318 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cui X., Kavvada O., Huntington T., Scown C. D., Strategies for near-term scale-up of cellulosic biofuel production using sorghum and crop residues in the US. Environ. Res. Lett. 13, 124002 (2018). [Google Scholar]
- 38.Rooney W. L., Blumenthal J., Bean B., Mullet J. E., Designing sorghum as a dedicated bioenergy feedstock. Biofuels Bioprod. Biorefin. 1, 147–157 (2007). [Google Scholar]
- 39.Aregawi K., et al. , Morphogene-assisted transformation of sorghum bicolor allows more efficient genome editing. Plant Biotechnol. J. 20, 748–760 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang M., Dahlberg J., Baral N. R., Putnam D., Scown C. D., Identifying forage sorghum ideotypes for advanced biorefineries. ACS Sustain. Chem.& Eng. 9, 7873–7881 (2021). [Google Scholar]
- 41.U.S. EIA, Electricity Power Annual 2019 (U.S. EIA, 2021). [Google Scholar]
- 42.Budzianowski W. M., High-value low-volume bioproducts coupled to bioenergies with potential to enhance business development of sustainable biorefineries. Renewable and Sustainable Energy Reviews 70, 793–804 (2017). [Google Scholar]
- 43.360 ResearchReports, Global P-hydroxybenzoic acid market report, history and forecast 2015-2026, breakdown data by manufacturers, key regions, types and application. https://www.360researchreports.com/global-p-hydroxybenzoic-acid-market-15956171. Accessed 15 February, 2022. [Google Scholar]
- 44.Viitanen P. V., Devine A. L., Khan M. S., Deuel D. L., Van Dyk D. E., Daniell H., Metabolic Engineering of the Chloroplast Genome Using the Echerichia coli ubi C Gene Reveals That Chorismate Is a Readily Abundant Plant Precursor for p-Hydroxybenzoic Acid Biosynthesis. Plant Physiology 136, 4048–4060 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kitade Y., Hashimoto R., Suda M., Hiraga K., Inui M., Production of 4-Hydroxybenzoic Acid by an Aerobic Growth-Arrested Bioprocess Using Metabolically Engineered Corynebacterium glutamicum. Appl. Environ. Microbiol. 84, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.FAO, Data from “FAOSTAT - Pesticides trade.” Food and Agriculture Organization of the United Nations. https://www.fao.org/publications/card/en/c/CB0488EN/. Accessed December 5, 2021. [Google Scholar]
- 47.Montazeri M., Eckelman M. J., Life Cycle Assessment of Catechols from Lignin Depolymerization. ACS Sustainable Chem. Eng. 4, 708–718 (2016). [Google Scholar]
- 48.ResearchAndMarkets, Muconic Acid Market: Global Industry Trends, Share, Size, Growth, Opportunity and Forecast 2019-2024. ResearchAndMarkets.com. https://www.researchandmarkets.com/reports/5562422/muconic-acid-market-global-industry-trends. Accessed October 26, 2020. [Google Scholar]
- 49.Niu W., Draths K. M., Frost J. W., Benzene-free synthesis of adipic acid. Biotechnol. Prog. 18, 201–211 (2002). [DOI] [PubMed] [Google Scholar]
- 50.Choi K. R., Jiao S., Lee S. Y., Metabolic engineering strategies toward production of biofuels. Curr. Opin. Chem. Biol. 59, 1–14 (2020). [DOI] [PubMed] [Google Scholar]
- 51.Kang M. J., et al. , A chemo-microbial hybrid process for the production of 2-pyrone-4,6-dicarboxylic acid as a promising bioplastic monomer from PET waste. Green Chem. 22, 3461–3469 (2020). [Google Scholar]
- 52.Krömer J. O., et al. , Production of aromatics in Saccharomyces cerevisiae—A feasibility study. J. Biotechnol. 163, 184–193 (2013). [DOI] [PubMed] [Google Scholar]
- 53.Barker J. L., Frost J. W., Microbial synthesis of p-hydroxybenzoic acid from glucose. Biotechnol. Bioeng. 76, 376–390 (2001). [DOI] [PubMed] [Google Scholar]
- 54.Mase K., Shimo T., Ohara N., Katayama Y., Shigehara K., Yamamoto Y., Murase H., “Large scale purification of 2-pyrone-4,6-dicarboxylic acid.” European Patent EP2175033A4 (2011).
- 55.NREL, “Process design and economics for the conversion of lignocellulosic biomass to hydrocarbon fuels and coproducts: 2018 biochemical design case update” (Tech. Rep. NREL/TP-5100-71949, National Renewable Energy Laboratory, 2018).
- 56.Baral N. R., et al. , Techno-economic analysis and life-cycle greenhouse gas mitigation cost of five routes to bio-jet fuel blendstocks. Energy Environ. Sci. 12, 807–824 (2019). [Google Scholar]
- 57.Xu F., et al. , Transforming biomass conversion with ionic liquids: Process intensification and the development of a high-gravity, one-pot process for the production of cellulosic ethanol. Energy Environ. Sci. 9, 1042–1049 (2016). [Google Scholar]
- 58.Klein-Marcuschamer D., Simmons B. A., Blanch H. W., Techno-economic analysis of a lignocellulosic ethanol biorefinery with ionic liquid pre-treatment. Biofuels Bioprod. Biorefin. 5, 562–569 (2011). [Google Scholar]
- 59.Humbird D., et al. , Process Design and Economics for Biochemical Conversion of Lignocellulosic Biomass to Ethanol: Dilute-Acid Pretreatment and Enzymatic Hydrolysis of Corn Stover (National Renewable Energy Laboratory (NREL), 2011). [Google Scholar]
- 60.Roland-Holst D., Triolo R., Heft-Neal S., Bayrami B., Bioplastics in California: Economic Assessment of Market Conditions for PHA/PHB Bioplastics Produced from Waste Methane (California Department of Resources Recycling and Recovery; ) 2013). [Google Scholar]
- 61.Wagemann K., Tippkötter N., Eds., Biorefineries (Springer International Publishing, 2019). [Google Scholar]
- 62.Kumar R., “Valorization of lignocellulosic biomass” in A Biorefinery: From Logistics To Environmental And Performance Impact (biochemistry Research Trends) (Nova Science Publishers, Inc., 2016). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All input parameters and study data are included in the article and/or SI Appendix. The code and SuperPro Designer process models used to generate results are available upon reasonable request.