Abstract
Background
Detecting Breast Cancer (BC) at earlier stages comes with a better prognosis, while diagnosis at late stages has poor outcomes and escalating mortality rates from the disease. The study aims to understand the factors associated with the late-stage diagnosis of BC in Egypt.
Design and Methods
A sample of 400 women with a pathologically confirmed BC were enrolled from one of the main tertiary cancer hospitals in Egypt. A cross-sectional study design was conducted. The collected data included: clinical characteristics of the tumor, socio-demographic characteristics of the studied women, reproductive and medical history, screening practices, and the time from symptom onset to definite diagnosis as suspected predictors to the stage of BC at diagnosis. Data was analyzed by crude odds ratios (95% confidence interval) and multivariate logistic regression analysis.
Results
The study revealed that 47.5% were diagnosed at late stages (40% at stage III/ 7.5% at stage IV), while (52.5%) were diagnosed at early stages (6.5% at stage I/46% at stage II). A binary logistic regression model showed that unmarried females (p=0.012), had non-luminal molecular subtype of BC including HER2 enriched and triple-negative tumors (p<0.001), presentation with breast changes and a non-palpable lump (p=0.024) or non-breast symptoms (P=0.002), a delay longer than 3 months to the first presentation by patients (p<0.001), and a delay to definite diagnosis longer than 1 month by providers (p<0.001) were significant risk factors of late-stage diagnosis of BC.
Conclusions
Late-stage diagnosis of BC in Egypt is associated with the aggressiveness of some molecular subtypes and other important modifiable factors that should be addressed.
Keywords: Stage at diagnosis, late-stage breast cancer, tumor aggressiveness, delayed diagnosis
Significance for public health
Breast Cancer (BC) is a major public health problem worldwide. Late-stage diagnosis of breast cancer is a common public health threat in Egypt and similar developing countries. While the high incidence rates of breast cancer are reported by the developed countries, the highest mortality rates are reported by low-resources countries. These discrepancies are largely stem from variations in the pattern of the disease stage at presentation. Detecting BC at earlier stages comes with better prognosis, better response to treatment, and thereby higher survival rates, while diagnosis at late-stages has poor outcomes despite the extensive and costly treatments. As a result, promoting early detection of BC can largely improve patient survival in limited-resource countries. The aim of our study to provide a comprehensive understanding of the factors associated with late-stage diagnosis of breast cancer in Egypt to better inform evidence-based tailored policies and adaptive early detection strategies based on the country context.
Introduction
Breast Cancer (BC) in women is a major public health problem worldwide. The burden of the disease, in terms of incidence, mortality, and economic costs, is substantially on the rise globally. 1 Breast cancer is the most prevalent cancer and the leading cause of cancer death among Egyptian females with a more progressive increase in mortality rates over the last decades.2,3 The tumor Stage of BC at the time of initial Diagnosis crucially determines its prognosis as BC is a curable disease when detected early. 4 According to the American Joint Committee on Cancer (AJCC), the TNM staging system defines the anatomic stage of BC based on the primary tumor size (T), the extent of spread to the lymph nodes (N), and the presence or absence of metastases (M). The earliest stage is stage 0, which refers to non-invasive breast cancers, and then stage I to IV describes invasive tumors with the latest of the worst prognosis. 5
Late-stage diagnosis of BC means that the tumor has already progressed to late stages (III & IV) at the time of initial diagnosis. Late-stage tumors have lower treatment options and minimal chances of successful therapy resulting in poor outcomes and elevated mortality rates. Furthermore, detecting BC at an advanced stage presents a challenge to many countries, particularly in lower resources settings as the cost of treatment increases with the severity of the disease. It adds economic burden and government expenditures on cancer care in these already resources constrained regions. Consequently, early detection of BC is much more cost-effective than curing late stages of the disease.6,7
Nevertheless, the majority of women with BC in Low- and Middle-Income Countries (LMICs) are found at late stages, and the downstaging of BC is not even in their public health priorities. Notably, most of the resources in these countries are devoted to curative services rather than early detection strategies. 8
In Egypt, several studies reported that 60% to 70% of females with BC firstly presented with late-stage. 9 Based on Gharbiah population-based cancer registry, over 60% of the Egyptian women with BC were initially diagnosed at late stages; 45.93% were diagnosed at stages III and 15.95% at stage I V. 10 Consequently, the downstaging of BC in Egypt is an important public health goal and it can be done by understanding the underlying causes for the late-stage diagnosis of BC.
Many researchers investigated the time lag between symptom discovery by the patient and definite diagnosis, which is called Diagnosis Delay (DD), as a major contributor to the advanced stage at diagnosis. Therefore, controlling for DD can help BC patients to be managed at an earlier stage, resulting in a better prognosis. 11
Diagnosis delay longer than three months was cited as a clinically significant delay. Factors associated with late-stage BC due to DD in women with BC symptoms are reported for two intervals: “the patient interval” that defined as the time from the first symptom to the first presentation for medical consultation, in which late-stage disease may be attributed to delay by the patient, and “diagnostic interval” that defined as the time from the first presentation to definite diagnosis, in which the late-stage diagnosis may be attributed to delay by healthcare providers or health system, commonly known as “provider or system delay”. 12
On the other hand, the time needed for the tumor to advance to later stages may be different across different subtypes of BC. Accordingly, the role of the inherent adversity and aggressiveness of the tumor is crucial to be studied for a more comprehensive understanding of the late-stage diagnosis.13,14
Consequently, there is a need for more integral research to provide a comprehensive understanding of the late-stage diagnosis of BC within the setting of each country to inform evidence-based tailored policies and adaptive strategies to improve BC attention according to each context. The purpose of the current study is to analyze a wide range of variables in relation to the tumor stage at diagnosis among women with BC who were diagnosed at one of the tertiary healthcare facilities in Alexandria, Egypt.
Design and Methods
Settings
The present study adopted a cross-sectional study design. The target population was Egyptian women with a pathologically confirmed diagnosis of breast cancer who were newly diagnosed within one year prior to the interview excluding the recurrent cases. A total of 400 cases were enrolled in this study from one of the largest specialized cancer hospitals in Egypt. The hospital is located in Alexandria and provides medical services to more than 800 cancer patients daily from Alexandria and the surrounding governorates. The study was approved by the Ethics Committee of the High Institute of Public Health in Alexandria University and compiled with the International Guidelines for Research Ethics. Informed consent was obtained from the study participants after explaining the purpose of the study. Anonymity, confidentiality, and voluntary participation were ensured throughout the study.
Sampling and data collection
All consecutive eligible cases who were admitted to the hospital during the study period were included in the study until the required sample size was fulfilled. Data on the clinical characteristics of the tumor including tumor stage at initial diagnosis, histological type, hormonal status, and molecular subtype were collected from the medical records. A structured interviewing question- naire was used to collect data on sociodemographic characteristics, reproductive and medical history, screening practices and barriers to screening, symptom detection, and the time intervals from the first discovered symptom until the definite diagnosis of BC.
The tumor stage was either directly abstracted from the medical records or was calculated based on the TNM classification rules of the American Joint Committee on Cancer (AJCC) by using data on tumor size, lymph node invasion, and metastasis 15 from the medical records, then transformed into a dichotomous variable: i) Early stages: stages I and II were grouped; ii) Late stages: stages III and IV were grouped; iii) Molecular subtype was either directly abstracted from the medical records or calculated based on the AJCC using data from medical records on hormonal status (Estrogen Receptors (ER) & Progesterone Receptors (PR), Human Epidermal growth factor Receptor-2 (HER2), and tumor grade, then transformed into a dichotomous variable: i) Luminal Types (LT): Including Luminal A and B types; ii) Non-Luminal Type (NLT): Including HER2 enriched and Triple Negative/Basal type.
Data on the date of the first symptom, the date of the first medical consultation, and the date of definite pathologically confirmed diagnosis were collected through the patient interview and confirmed by the dates in medical records. Then, it was used to calculate the diagnosis delay with both time intervals of patient delay and diagnostic delay.
“Patient delay” was calculated as the number of days between the date of the first noticed symptoms by the patient and the date of the first medical consultation.
“Provider or system delay” was calculated as the number of days between the date of the first medical consultation and the date of the pathologically confirmed diagnosis.
“Diagnosis Delay (DD)” is the total number of days between the first symptom till definite diagnosis.
Statistical analysis
Data entry and statistical analysis were done using the IBM SPSS software (Statistical Packages for Social Sciences) version 23, two-tailed tests were used for the whole analysis, and alpha error 0.05. Additionally, Microsoft Excel version 2016 was used to design some charts for graphical representation of the data. Crude Odds Ratio (OR) with a 95% Confidence Interval (CI) was used to assess the association between the outcome (tumor stage at diagnosis) and the study variables including socio-demographics, medical and reproductive history, clinical characteristics, screening practices, symptom detection, and diagnosis delay.
Results
A total of 400 newly diagnosed women with BC are enrolled in this study, (47.5%) were diagnosed at late stages (40% at stage III/ 7.5% at stage IV), while (52.5%) were diagnosed at early stages (6.5% at stage I/ 46% at stage II) as shown in Figure 1. Table 1 shows the distribution of participants according to socio-demographic characteristics and the tumor stage at diagnosis. The risk of late-stage disease among unmarried was 1.68 times relative to married females and this was statistically significant (p=0.028).
Figure 1.
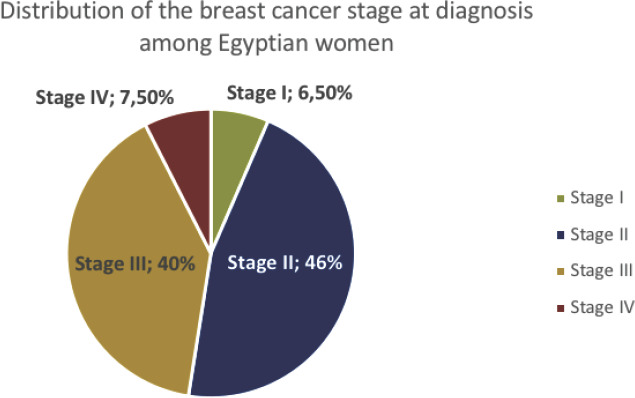
Distribution of the studied cases with breast cancer by tumor stage at initial diagnosis.
Table 1.
The association between socio-demographic characteristics of the studied cases with breast cancer and the tumor stage at diagnosis.
| Variables | Early Stage (N=210) Number (%) | Late Stage (N=190) Number (%) | Crude OR | 95% CI | P |
|---|---|---|---|---|---|
| Age (Years) | |||||
| < 35 | 9 (40.9) | 13 (59.1) | 1.44 | 0.52 – 4.04 | 0.483 |
| 35 - | 48 (52.2) | 44 (47.8) | 0.92 | 0.45 – 1.86 | 0.810 |
| 45 - | 80 (57.6) | 59 (42.4) | 0.74 | 0.38 – 1.44 | 0.372 |
| 55- | 50 (49.5) | 51 (50.5) | 1.02 | 0.51 – 2.05 | 0.956 |
| 65+ <sup>R</sup> | 23 (50.0) | 23 (50.0) | 1 | ||
| Marital Status | |||||
| Married R | 169 (55.6) | 135 (44.4) | 1 | ||
| Unmarried | 41(42.7) | 55 (57.3) | 1.68 | 1.06 – 2.67 | 0.028* |
| Place of residence | 1 | ||||
| Urban R | 172 (54.1) | 146 (45.9) | 1.36 | ||
| Rural | 38 (46.3) | 44 (53.7) | 0.84 – 2.22 | 0.211 | |
| Level of Education | |||||
| UniversityR | 35 (61.4) | 22 (38.6) | 1 | ||
| High school | 67 (58.8) | 47 (41.2) | 1.12 | 0.58 – 2.14 | 0.741 |
| Middle school | 21 (44.7) | 26 (55.3) | 1.97 | 0.89 – 4.32 | 0.090 |
| Primary | 27 (50.9) | 26 (49.1) | 1.53 | 0.72 – 3.27 | 0.270 |
| Illiterate | 60 (46.5) | 69 (53.5) | 1.83 | 0.97 – 3.46 | 0.063 |
| Occupation | |||||
| UnemployedR | 189 (53.2) | 166 (46.8) | 1 | ||
| Employed | 21 (46.7) | 24 (53.3) | 1.30 | 0.69 – 2.42 | 0.406 |
| Income | |||||
| Moderate to High R | 162 (54.9) | 133 (45.1) | 1 | ||
| Low | 48 (45.7) | 57 (54.3) | 1.45 | 0.93 – 2.26 | 0.106 |
Significant (p<0.05), OR :Odds Ratio, CI: Confidence Interval.
The association between the clinical characteristics of the participants and the tumor stage at diagnosis is shown in Table 2. Regarding the reproductive and medical history, there was no statistically significant association with the tumor stage at diagnosis in both groups (p>0.05). However, more women with non-luminal types of BC are found at a late stage (66.7% and 55.7% with the HER-2 enriched subtype and triple-negative subtype respectively) than women with luminal types of BC (42.5%). The risk of the late-stage diagnosis of BC among women with non-luminal subtypes was 2.707 times in case of the HER-2 enriched subtype and 1.7 times in case of the triple-negative subtype compared with women with the luminal subtypes, and this was statistically significant (P= 0.004, 0.049).
Table 2.
The association between the clinical characteristics of the studied cases with breast cancer and the tumor stage at diagnosis.
| Variables | Early Stage (N=210) Number (%) | Late Stage (N=190) Number (%) | Crude OR | 95% CI | P |
|---|---|---|---|---|---|
| Parity | |||||
| Parous R | 192 (53.8) | 165 (46.2) | 1 | ||
| Nulliparity | 18 (41.9) | 25 (58.1) | 1.62 | 0.85 – 3.07 | 0.142 |
| Menopausal Status | |||||
| Pre-menopause R | 133 (55.6) | 106 (44.4) | 1 | ||
| Post-menopause | 77 (47.8) | 84 (52.2) | 1.37 | 0.92 – 2.04 | 0.125 |
| Breastfeeding | |||||
| Stopped breastfeeding R | 174 (54.7) | 144 (45.3) | 1 | ||
| On recent breastfeeding | 11 (45.8) | 13 (54.2) | 1.43 | 0.62 – 3.28 | 0.402 |
| Never did breastfeeding | 25 (43.1) | 33 (56.9) | 1.59 | 0.91 – 2.81 | 0.105 |
| History of breast problems | |||||
| No R | 166 (50.3) | 164 (49.7) | 1 | ||
| Yes | 44 (62.9) | 26 (37.1) | 0.59 | 0.35 – 1.02 | 0.058 |
| Family history of breast cancer | |||||
| No R | 149 (53.0) | 132 (47.0) | 1 | ||
| Yes | 61 (51.3) | 58 (48.7) | 1.07 | 0.69 – 1.65 | 0.747 |
| Other chronic diseases | |||||
| No R | 128 (56.4) | 99 (43.6) | 1 | 0 | |
| Yes | 82 (47.4) | 91 (52.6) | 1.44 | 0.96 – 2.14 | .075 |
| Smoking practice | |||||
| Non smoker | 199 (52.5) | 180 (47.5) | 1 | ||
| Smoker | 5 (41.7) | 7 (58.3) | 1.55 | 0.48 – 4.96 | 0.462 |
| Ex-smoker | 6 (66.7) | 3 (33.3) | 0.55 | 0.14 – 2.24 | 0.407 |
| Molecular Subtype | |||||
| Luminal Types (LT) R | 157 (57.5) | 116 (42.5) | 1 | ||
| Non- Luminal Types (NLT) | |||||
| Her-2 Enriched | 14 (33.3) | 28 (66.7) | 2.707 | 1.365 – 5.370 | 0.004* |
| Triple Negative | 31 (44.3) | 39 (55.7) | 1.703 | 1.003 – 2.890 | 0.049 |
Statistically Significant.
Table 3 shows that the vast the majority had never sought breast screening, 83.2% had never performed BSE, 97.2% had never sought CBE, and 96% had never been screened by mammogram before their condition. For females who were not performing BSE, the main reason for the most of respondents (78.1%) was the lack of awareness. Only 11 participants representing 2.8% of the total studied females were visiting the physician for CBE before their condition. As well, only 16 participants (4%) were compliant to mammogram screening before their condition. Barriers to mammogram screening were; unawareness which was the most common barrier among 86.7% of the studied females. Other barriers to mammogram screening included ignoring the risk that was declared by 27 females (7%), fear from diagnosis was a barrier for 10 females (2.6%), competing life priorities constituted barrier for 6 females (1.6%), in addition to the cost of mammogram was a constraint to screening for 8 participants (2.1%).
Table 3.
Distribution of the studied cases with breast cancer according to their health behavior towards early detection methods and barriers to screening before their condition.
| Screening methods (n=400) | BSE (n=400) | CBE | Mammogram (n=400) |
|---|---|---|---|
| Practice | |||
| Yes | 67 (16.8) | 11 (2.8) | 16 (4.0) |
| No | 333 (83.2) | 389 (97.2) | 384 (96.0) |
| Barriers | (n=333)* | (n=389)* | (n=384)* |
| Unawareness of this method | 260 (78.1) | 322 (82.8) | 333 (86.7) |
| Not expect the risk having BC | 46 (13.8) | 35 (9.0) | 27 (7.0) |
| Afraid from discovering any problems | 9 (2.7) | 12 (3.1) | 10 (2.6) |
| Competing life priorities (No time to do it) | 14 (4.2) | 14 (3.6) | 6 (1.6) |
| Don't know how to self-examine my breasts | 4 (1.2) | NA | NA |
| Financial constraints (not afford the cost) | NA | 6 (1.5) | 8 (2.1) |
BSE; Breast Self-Examination, CBE; Clinical Breast Examination.
Barriers were presented for women were not performing screening.
Table 4 illustrates that the risk of the late-stage diagnosis of BC among women who were not performing BSE was 2.91 times compared with women who were used to practicing BSE before their condition, and this was very highly statistically significant (p<0.001). The risk of late-stage diagnosis among women who had never been screened by mammogram was 4.11 times relative to women who were compliant with mammogram screening, and this was statistically significant (p=0.029). Regarding the relationship between symptom detection and the tumor stage at diagnosis, the risk of the late-stage diagnosis of BC among women who firstly noticed breast changes without a palpable lump was almost 3 times relative to women who firstly had a breast lump, and this was statistically significant (p=0.01), while women who had firstly breast pain or nipple changes had equal probabilities of having early or late stage at diagnosis, but this was not statistically significant (p>0.05). Most of the women who firstly noticed non-breast symptoms rather than breast mass are found at a late stage (77.1%). The risk of late-stage diagnosis among women who firstly noticed non-breast symptoms was 4.74 times compared with women who firstly noticed breast lump, and this was very high statistically significant (p<0.001).
Table 4.
The association between participants' behavior for breast cancer screening, the first symp-tom detected and diagnosis delay, and the tumor stage at diagnosis.
| Characteristic | Early Stage (N=210) Number (%) | Late Stage (N=190) Number (%) | Crude OR | 95% CI | P |
|---|---|---|---|---|---|
| Screening methods | |||||
| BSE | |||||
| Yes R | 49 (73.1) | 18 (26.9) | 1 | ||
| No | 161 (48.3) | 172 (51.7) | 2.91 | 1.63 – 5.21 | P<0.001* |
| CBE | |||||
| Yes R | 9 (81.8) | 2 (18.2) | 1 | ||
| No | 201 (51.7) | 188 (48.3) | 4.21 | 0.89 – 19.73 | 0.068 |
| Mammogram | |||||
| Yes R | 13 (81.3) | 3 (18.8) | 1 | ||
| No | 197 (51.3) | 187 (48.7) | 4.11 | 1.15 – 14.66 | 0.029* |
| The first symptom** | |||||
| Breast lump | 160 (58.4) | 114 (41.6) 1 | |||
| Breast changes | 9 (32.1) | 19 (67.9) | 2.96 | 1.29-6.79 | 0.01* |
| Breast Pain | 8 (50.0) | 8 (50.0) | 1.4 | 0.51-3.85 | 0.51 |
| Nipples changes or discharge | 20 (50.0) | 20 (50.0) | 1.4 | 0.72-2.73 | 0.32 |
| Non breast symptoms | 8 (22.9) | 27 (77.1) | 4.74 | 2.08-10.81 | P<0.001* |
| Patient Delay(months)** | |||||
| ≤3 R | 164 (69.2) | 73 (30.8) | 1 | ||
| 3- 11 (47.8) | 12 (52.2) | 2.45 | 1.03-5.81 | 0.04* | |
| 6- 16 (27.1) | 43 (72.9) | 6.04 | 3.19-11.41 | <0.001* | |
| 12+ | 14 (18.9) | 60 (81.1) | 9.63 | 5.06-18.33 | <0.001* |
| System Delay (months)** | |||||
| ≤1 R | 189 (63.2) | 110 (36.8) | 1 | ||
| 1- 9 (15.5) | 49 (84.5) | 9.35 | 4.43-19.78 | <0.001* | |
| 3+7 (19.4) | 29 (80.6) | 7.12 | 3.02-16.79 | <0.001* | |
| Total Diagnosis Delay** | |||||
| ≤3R | 151 (76.3) | 47 (23.7) | 1 | ||
| >354 (27.7) | 141 (72.3) | 8.39 | 5.33-13.20 | P<0.001* | |
Statistically Significant
Missed cases were asymptomatic or did not recognize their symptoms.
In order to study the detailed relationship between the diagnosis delay intervals and the tumor stage at diagnosis, we further binned the delay (in months) into more groups as shown in Figure 2. As regards the delay by patients to the first presentation, a longer delay has progressively increased the odds of a more advanced stage at diagnosis to a peak of 9.63 in delay equal to 12 months or longer compared with women presented within 3 months (p<0.001). Concerning total diagnosis delay, 72.3% of the studied females with total DD longer than 3 months had late-stage BC at diagnosis. The risk of late-stage diagnosis due to longer delay than 3 months is 8 times compared with women who were diagnosed within 3 months (p<0.001).
Figure 2.
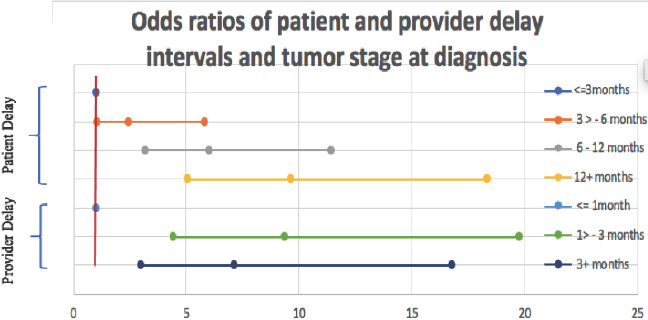
Odds ratios and confidence intervals of delay by patients and providers, and tumor stage at diagnosis.
Multivariate regression analysis was used to control simultaneously for the possible confounding effect of the different variables. Based on the binary outcome, a binary Logistic regression model was utilized. 16 Table 5 shows the results of the binary logistic regression model for factors associated with the late-stage diagnosis of BC using the enter method. Variables were selected based on the significance of the results of the univariate and multi-collinearity analyses. The overall goodness of fit of the model is indicated by Hosmer – Leme show test that showed a non-significant difference between the observed and the predicted probabilities (p=0.815) indicating a good model fit. The significant predictors of late-stage diagnosis are unmarried women, non-luminal subtype, no mammogram screening, the first symptom is breast changes without lump, the first symptom is non-breast symptoms, patient delay longer than 3 months, and provider or system delay longer than 1 month.
Table 5.
Logistic regression model for factors associated with late-stage diagnosis of breast cancer.
| Variables | B | Adjusted OR | 95% CI for AORP | ||
|---|---|---|---|---|---|
| LL | UL | ||||
| Unmarried | 0.76 | 2.14 | 1.18 | 3.88 | 0.012* |
| Non- Luminal Types (NLT) | 1.34 | 3.82 | 2.07 | 7.05 | < 0.001* |
| No Mammogram screening | 0.59 | 1.81 | 0.39 | 8.38 | 0.446 |
| First Symptom | |||||
| Breast lump (ref) | |||||
| Breast changes | 1.28 | 3.60 | 1.18 | 10.98 | 0.024* |
| Breast pain | 0.45 | 1.57 | 0.39 | 6.33 | 0.528 |
| Nipple changes or discharge | -0.11 | 0.89 | 0.37 | 2.15 | 0.803 |
| Non-breast symptoms | 1.68 | 5.35 | 1.89 | 15.16 | 0.002* |
| Patient delay > 3 months | 2.22 | 9.22 | 5.22 | 16.30 | < 0.001* |
| Provider delay > 1 months | 2.45 | 11.54 | 5.59 | 23.79 | < 0.001* |
| Constant | -2.85 | 0.06 | < 0.001* | ||
Statistically significant, AOR; Adjusted Odds Ratio. The Cox and Snell R square was 0.357, and the Nagelkerke R square was 0.477, Hosmer – Leme show test (p=0.815).
Discussion
Women's health is of a particular public health concern as it has an important impact on the health of their children, family, and community. Breast cancer is one of the major causes of morbidity and mortality for women, especially when detected at late stages, it has a heavy clinical, social, and psychological burden on affected women and their families. In the present study, 47.5% of the studied females with BC were diagnosed at late stages (40% at stage III/ 7.5% at stage IV). This percentage is close to the results of another study from two of the largest cancer centers in Egypt (the National Cancer Institute of Cairo University and Tanta Cancer Center in the Nile delta), which reported 46% of the participants had presented at late stages. 17 However, the previous studies in Egypt in the past decades reported higher percentages of late-stage diagnosis of BC ranged from 60% to 70%.9,10
This finding reflects the significant improvement in the down-staging of BC in Egypt. However, it is still considered a high rate compared with rates of late-stage diagnosis of BC in other countries. In the US, late-stage diagnosis of BC was 10.3%, in Sweden was 7% (5 % at stage III, and 2% at stage IV), and in South Korea was 5.1% of patients diagnosed at stage III.18,19
The current study revealed that the factors associated with late-stage diagnosis among the studied females with BC are having non-luminal types of the disease, non-compliance to mammogram screening before their condition, having non-lump or non-breast symptoms as their first symptom, unmarried females, a delay longer than 3 months to the first presentation by patients, and a delay to definite diagnosis longer than 1 month by providers or because of health system barriers (Table 5).
The molecular subtype of BC has been shown to have a vital role in the stage of the disease at diagnosis. Breast cancer is a heterogeneous disease of various molecular subtypes comprising different growth rates, clinical course, and metastatic behavior. Therefore, in patients with biologically indolent BC, tumors with a slow growth rate may take long time to progress to advanced stages. Other patients may have a fast-growing tumor due to its intrinsic aggressive nature, spreading rapidly and presenting at a late stage of disease in a short time. 5
From this perspective, some studies hypothesized that longer diagnosis delay may result in a later stage at diagnosis suggesting a worse prognosis and diminished survival. Alternatively, other studies showed a better prognosis and increased survival with a longer delay which may be attributed to the hypothesis that slowly growing and asymptomatic tumors have a better survival pattern than those fast-growing with apparent symptoms which easily suggesting cancer and can be diagnosed shortly but underlying more aggressive tumor with worse prognosis. 11
In the current study, women who presented with the aggressive non-luminal types (including HER2 enriched and triple-negative subtypes) were found at a higher risk to late stage of the disease (Table 2). In accordance with this finding, Khokher et al. reported a statistically significant association of the non-luminal molecular subtypes with late-stage at diagnosis among the early presented women with BC. 13
On the other hand, the present study showed that 42.5% of the females who were presented with non-aggressive luminal tumors were found at late stages. This result may suggest that the longer time to diagnosis allows non-aggressive tumors as well as the aggressive types to grow and progress to late stages (Table 2).
Many previous studies reported three months or longer between the first symptom and the pathologically confirmed diagnosis of BC in symptomatic women as the clinically significant risk factor associated with later stages of the disease.12,20,21
Our results were in coherence with these studies and showed that women with total diagnosis delay longer than 3 months had 8 times increased risk of late-stage diagnosis compared with women who were diagnosed within 3 months (p<0.001).
Further, we investigated both intervals of time to diagnosis. Results showed a strong statistically significant association between increasing the length of time to diagnosis and the disease stage at diagnosis. For instance, women with longer patient delay are more likely to have a later stage of the disease with odds increasing with the longer delay to a peak of 9.63 in delay of 12 months or longer (Table 4, Figure 2).
In the current study, sociodemographic characteristics of the patients are studied in relation to the stage of tumor at diagnosis. The marital status showed an independent and statistically significant association with the tumor stage at diagnosis. Unmarried women had an increased risk of late-stage diagnosis by 2.14 times relative to married women (Table 5). In agreement with this finding, Ali et al., reported that widowed/divorced/unmarried females were more likely diagnosed at a later stage of BC, and explained that this trend may be attributed to the lack of support from the family of the patient which would discourage them from seeking treatment. 22 Additionally, our results showed women with non-breast symptoms in the present study were found strongly associated with late stages of the disease. They had an increased risk of late-stage diagnosis by almost 5 times compared with women with a breast lump (Table 5). We can explain that when women firstly presented with non-breast symptoms, it means that the disease has been already progressed to late stages and reached distant organs before being discovered. In this case, the real length of the patient delay is not known and the time interval between the first noticed (non-breast symptom) to the first presentation is underestimated. This result also implies the lack of attention of females to their body changes as they failed to recognize any changes before complications of the disease seriously appeared. These observations were in accordance with previous studies that suggested breast cancer symptoms other than lumps are more likely to be ignored.17,23
In the same context, our study revealed that the vast majority had never sought screening services and the main barrier was their unawareness (Table 3). In accordance with our findings, barriers to screening were investigated by Mamdouh et al., study in Alexandria which reported that the majority of women would not seek care until they were seriously ill, and unawareness of the importance of early detection were among the significant barriers to screening. 24
Conclusions and Recommendations
Late-stage diagnosis of breast cancer is still at a high rate in Egypt, therefore downstaging should be assigned as a public health priority. Women with non-luminal subtypes of breast cancer are at a higher risk of late-stage disease at diagnosis. Consequently, our results call for more focus of attention on these molecular subtypes that should be early detected and managed carefully. Diagnosis delay is a significant mediator for an advanced stage at diagnosis. As a result, promoting early diagnosis is a key and cost-effective approach to control for the tumor stage at diagnosis. Underlying factors for delay by patients to seek medical consultation, or delay by health providers or health system barriers should be further studied. Finally, our findings emphasize the importance of implementing tailored interventions to address the barriers to early detection of breast cancer in Egypt and similar developing countries.
References
- 1.Coughlin SS, Ekwueme DU. Breast cancer as a global health concern. Cancer Epidemiol 2009;33:315–8. [DOI] [PubMed] [Google Scholar]
- 2.Ibrahim AS, Khaled HM, Mikhail NN. et al. Cancer incidence in Egypt: Results of the national population-based cancer registry program. J Cancer Epidemiol 2014;2014:437971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA2021;71:209–49. [DOI] [PubMed] [Google Scholar]
- 4.Saadatmand S, Bretveld R, Siesling S, et al. Influence of tumour stage at breast cancer de-tection on survival in modern times: Population based study in 173 797 patients. BMJ 2015;351:h4901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giuliano AE, Edge SB, Hortobagyi GN. Eighth Edition of the AJCC Cancer Staging Man-ual: Breast Cancer. Ann Surg Oncol 2018;25:1783–5. [DOI] [PubMed] [Google Scholar]
- 6.Smith RA, Caleffi M, Albert US, et al. Breast cancer in limited-resource countries: early detection and access to care. Breast J 2006;12:S16–S26. [DOI] [PubMed] [Google Scholar]
- 7.Skrundevskiy AN, Omar OS, Kim J, et al. Return on Investment Analysis of Breast Cancer Screening and Downstaging in Egypt: Implications for Developing Countries. Value Health Regional Issues 2018;16:22–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dey S. Preventing breast cancer in LMICs via screening and/or early detection: The real and the surreal. World J Of Clin Oncol 2014;5:509–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Omar S, Khaled H, Gaafar R, et al. Breast cancer in Egypt: a review of disease presentation and detection strategies. Eastern Mediterranean Health J 2003;9:448–463. [PubMed] [Google Scholar]
- 10.Dey S, Soliman AS, Hablas A, et al. Urban-rural differences in breast cancer incidence in Egypt (1999-2006). Breast 2010;19:417–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caplan L. Delay in breast cancer: Implications for stage at diagnosis and survival. Front Public Health 2014;2:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dianatinasa M, Fararouei M, Mohammadianpanah M, et al. Impact of social and clinical factors on diagnostic delay of breast cancer A Cross-sectional Study. Medicine 2016;95:e4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khokher S, Qureshi MU, Mahmood S, et al. Determinants of Advanced Stage at Initial Di-agnosis of Breast Cancer in Pakistan: Adverse Tumor Biology vs Delay in Diagnosis. Asian Pacif-ic J Cancer Prev 2016;17:759–65. [DOI] [PubMed] [Google Scholar]
- 14.Howlader N, Cronin KA, Kurian A, et al. Differences in Breast Cancer Survival by Molec-ular Subtypes in the United States. Cancer Epidemiol Biomarkers Prev 2018;27:619–26. [DOI] [PubMed] [Google Scholar]
- 15.Hortobagyi GN, Connolly JL, D'Orsi CJ, et al. American Joint Committee on Cancer (AJCC) Cancer Staging Manual, 8th ed 589-636, Springer 2017. [Google Scholar]
- 16.Wilson, Lorenz KA. Standard Binary Logistic Regression Model. In Wilson J. R. & Lorenz K.A. (Eds.), Modeling Binary Correlated Responses using SAS, SPSS and R, 25-54, Springer 2015. [Google Scholar]
- 17.Stapleton JM, Mullan PB, Dey S, et al. Patient-mediated factors predicting early- and late-stage presentation of breast cancer in Egypt. Psycho-oncol 2011;20:532–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sariego J. Patterns of breast cancer presentation in the United States: Does geography mat-ter? Am Surgeon 2009;75:545–9. [DOI] [PubMed] [Google Scholar]
- 19.Leong SPL, Shen ZZ, Liu TJ, et al. Is Breast cancer the same disease in Asian and Western countries? World J Surg 2010;34:2308–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abdel-Fattah MM, Anwar MA, Mari E, et al. Patient- and system-related diagnostic delay in breast cancer: Evidence from Alexandria, Egypt. Eur J Public Health 1999;9:15–9. [Google Scholar]
- 21.Pace LE, Mpunga T, Hategekimana V, et al. Delays in Breast Cancer Presentation and Di-agnosis at Two Rural Cancer Referral Centers in Rwanda. Oncologist 2015;20:780–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ali R, Mathew A, Rajan B. Effects of socio-economic and demographic factors in delayed reporting and late-stage presentation among patients with breast cancer in a major cancer Hospital in South India. Asian Pacific J Cancer Prev 2008;9:703–7. [PubMed] [Google Scholar]
- 23.Bish A, Ramirez A, Burgess C, et al. Understanding why women delay in seeking help for breast cancer symptoms. J Psychosomatic Res 2005;58:321–6. [DOI] [PubMed] [Google Scholar]
- 24.Mamdouh H, El-Mansy H, Kharboush I, et al. Barriers to breast cancer screening among a sample of Egyptian females. J Family Comm Med 2014;21:119. [DOI] [PMC free article] [PubMed] [Google Scholar]


