Abstract
This study analyzed whole blood samples (n = 56) retrieved from 30 patients at 1 to 21 (median 9) mo after verified COVID-19 to determine the polarity and duration of antigen-specific T cell reactivity against severe acute respiratory syndrome coronavirus 2–derived antigens. Multimeric peptides spanning the entire nucleocapsid protein triggered strikingly synchronous formation of interleukin (IL)-4, IL-12, IL-13, and IL-17 ex vivo until ∼70 d after confirmed infection, whereafter this reactivity was no longer inducible. In contrast, levels of nucleocapsid-induced IL-2 and interferon-γ remained stable and highly correlated at 3 to 21 mo after infection. Similar cytokine dynamics were observed in unvaccinated, convalescent patients using whole-blood samples stimulated with peptides spanning the N-terminal portion of the spike 1 protein. These results unravel two phases of T cell reactivity following natural COVID-19: an early, synchronous response indicating transient presence of multipolar, antigen-specific T helper (TH) cells followed by an equally synchronous and durable TH1-like reactivity reflecting long-lasting T cell memory.
Keywords: SARS-CoV-2, COVID-19, T cell cytokines, longevity, T helper cells
After encountering antigen, naïve CD4+ T cells follow trajectories into subsets of antigen-specific memory and effector T helper (TH) cells that may be identified by their patterns of cytokine formation (1). TH1-polarized cells thus generate interleukin (IL)-2 and interferon-γ (IFN-γ) to induce cell-mediated elimination of intracellular pathogens, including viruses (2). TH2 cells produce IL-4 and IL-13 that facilitate B cell function and promote tissue repair after infection (3, 4). Additional TH cell subsets include TH17 cells producing IL-17 that attracts neutrophils to infected tissue (5) and T follicular helper (TFH) cells that produce IL-21 to promote B cell function in germinal centers (6). In a parallel pathway, naïve CD8+ T cells differentiate into central memory, effector memory, and effector cells endowed with cytotoxicity against virus-infected cells alongside the ability to generate IL-2 and IFN-γ (7).
This study aimed to determine severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-specific T cell reactivity in whole-blood samples from healthy volunteers available 1 to 21 mo after recovery from verified COVID-19. We analyzed antigen-specific TH cell cytokines induced in these blood samples ex vivo after exposure to peptides spanning the entire nucleocapsid (NC) protein (amino acids [aa] 1 to 419, 102 peptides) or the N-terminal portion of the spike 1 (S1) protein (aa 1 to 692, 170 peptides).
Results
Transient and Multipolar TH-Type Reactivity after COVID-19.
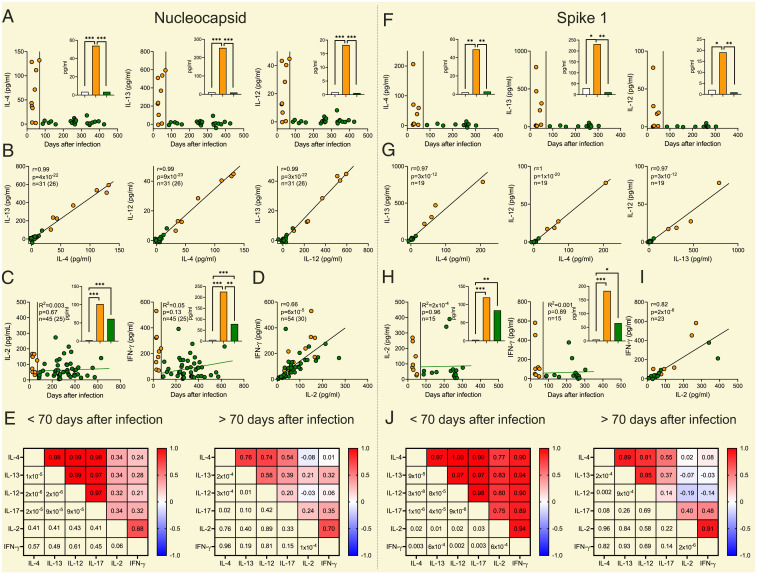
Whole-blood samples from previously infected patients (n = 30) produced IL-4 and IL-13 in response to NC peptides until ∼70 d from confirmed COVID-19. Thereafter, these TH2-type cytokines were not inducible over levels in parallel unstimulated samples or in NC peptide-stimulated samples from uninfected controls. The initial TH2-type reactivity occurred concomitantly with a TH17-type response. Levels of NC-induced TH2 and TH17 cytokines were remarkably correlated in the initial phase after infection with intercytokine Pearson r approaching 1. Similar results were obtained by the addition of multimeric S1 peptides to whole blood recovered from previously infected patients who had not been vaccinated (Fig. 1).
Fig. 1.
Two phases of antigen-specific T cell reactivity to SARS-CoV-2–derived peptides after COVID-19. The left (Nucleocapsid) and the right (Spike 1) panels show NC and S1 peptide-induced formation of (A and F) IL-4, IL-12, and IL-13 and (C and H) IL-2 and IFN-γ in whole-blood samples from patients with previously confirmed COVID-19 vs. day after infection. The inset bars show mean levels of induced formation of each cytokine in uninfected controls (n = 24 samples from 17 subjects for NC and n = 11 for S1, white bars) and convalescent patients sampled early (n = 9 samples from 8 subjects for NC and n = 8 for S1, orange bars) or late (n = 22 samples from 19 subjects [A], n = 45 samples from 25 subjects [C] for NC and n = 11 [F] and n = 15 [H] for S1, green bars) after infection. All data points are shown in each graph. For each donor a mean of all samples within each phase was used for statistical analysis as calculated by permutation. In C and H the green regression lines show NC- and S1-induced cytokines in the late convalescent phase and were estimated using linear mixed-effects models. B, D, G, and I are scatter plots of NC- and S1 peptide-induced formation of (B and G) IL-4, IL-12, and IL-13 and (D and I) IL-2 and IFN-γ in patients with previous COVID-19. The number of observations is indicated by n with the unique number of donors in brackets if serial samples from the same subject were available. All data points are shown in each graph and the regression line was estimated by linear mixed-effects models. A mean of all samples within the early (<70 d after infection) or the late (>70 d after infection) phase from each donor was used to estimate Pearson correlation for unique donors. E and J are a heat maps showing the correlation (Pearson r, above the diagonal) between NC- and S1-induced formation of cytokines in the early (Left) and late (Right) phase after confirmed COVID-19. P values corresponding to each correlation are shown below the diagonal. For each donor a mean of all samples within each phase was used for statistical estimation of correlation. Orange dots show results at early (<70 d) and green dots at later (>70 d) convalescence after infection. *P < 0.05, **P < 0.01, ***P < 0.001.
Unexpectedly, IL-12, a prototype mediator of TH1-type immunity (8), was induced by NC and S1 peptides synchronously with IL-4, IL-13, and IL-17 in the first months after infection (Fig. 1). The stark correlation between antigen-induced levels of these early-phase cytokines suggests that IL-4, IL-12, IL-13, and IL-17 were generated by the same T cell. In search for a unifying T cell phenotype that may produce these cytokines, we considered TFH cells that transcribe multiple cytokine genes and are induced early after COVID-19 (9). The TFH signature cytokine IL-21 was, however, weakly induced by NC or S1 peptides in whole-blood cultures (induction observed in one of five analyzed samples at <70 d after infection), which argues against IL-21+ TFH cells as the source of the observed multipolar cytokine response.
Long-Lasting TH1-Type Immunity.
NC- and S1-specific IL-2 and IFN-γ were induced early (<70 d) after confirmed infection and correlated weaker, or not at all, with IL-4, IL-12, IL-13, or IL-17 in the early convalescent phase. However, antigen-induced IL-2 and IFN-γ persisted without discernable waning over time and were strikingly correlated, suggesting that the SARS-CoV-2–specific T cell reactivity observed early and late after COVID-19 derived from separate clones of antigen-specific T cells (Fig. 1).
Discussion
The observed durability of IL-2 and IFN-γ responses imply the existence of long-lasting T cell memory following COVID-19, congruous with recent findings demonstrating presence of virus-reactive CD4+ and CD8+ memory-type T cells after infection (10, 11). In previous studies, however, the half-life of T cell memory after COVID-19 was estimated at 3 to 5 mo (10). Our results suggest that IL-2– and IFN-γ–producing T cells reactive with SARS-CoV-2 prevail longer than anticipated from previous reports. However, our results do not exclude that fewer but more efficient antigen-specific T cells contributed IL-2 and IFN-γ in the late convalescence phase.
This study has weaknesses, including a small sample size and an inherently short follow-up after infection. Also, our study did not identify the detailed phenotypes of cytokine-producing T cells. Although IL-4, IL-13, and, in particular, IL-17 responses to antigen are likely reflecting TH cell function, the finding of highly synchronous formation of IL-12, which is produced mainly by dendritic cells and macrophages (12), implies that antigen-presenting cells may have contributed IL-12 in the first months after COVID-19. The strengths embrace the identification of two distinct phases of antigen-specific T cell responses among patients recovering from COVID-19. The persistent TH1-type reactivity is consistent with reports of low incidence of severe COVID-19 in reinfected patients (13). In particular, the observed long-term T cell reactivity against NC is compatible with the reportedly reduced incidence of reinfection despite the emergence of strains of SARS-CoV-2 carrying mutations in the spike region (14).
Materials and Methods
The study included 81 blood samples from 44 otherwise healthy hospital workers. Ethics approval was granted by the Swedish Ethical Review Authority and by the Swedish Medical Products Agency (for more details, see SI Appendix, Extended Methods). All participants gave written informed consent before enrolment. Freshly recovered whole-blood samples were stimulated with 15-mer peptides spanning the NC protein (aa 1 to 419, 102 peptides; 130-126-699; Miltenyi Biotec), the N-terminal portion of the S1 protein (aa 1 to 692, 170 peptides; 130-127-041; Miltenyi Biotec), or were unstimulated as described in ref. 15. Levels of IL-2, IL-4, IL-12, IL-13, IL-17, and IFN-γ were measured in plasma supernatant from whole-blood samples using FirePlex-96 Key Cytokines Immunoassay panel (ab243549 and ab285173; Abcam), and levels of IL-21 in these plasma supernatants were measured by enzyme-linked immunosorbent assay (IL-21 DuoSet ELISA, DY8879-05; R&D Systems). Results are presented as peptide-induced cytokine responses with cytokine levels in unstimulated samples subtracted. Participants, samples, and methods are further described in SI Appendix, Extended Methods. Additional details and deidentified primary data are available in Dataset S1.
Supplementary Material
Acknowledgments
This work was supported by the Swedish Research Council (Grants 2020-01437, 2017-00855, and 2021-04779) and the AFA Foundation (Grant 20045).
Footnotes
The authors declare no competing interest.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2203659119/-/DCSupplemental.
Data Availability
All study data are included in the article and/or supporting information.
References
- 1.Tuzlak S., et al. , Repositioning TH cell polarization from single cytokines to complex help. Nat. Immunol. 22, 1210–1217 (2021). [DOI] [PubMed] [Google Scholar]
- 2.Lee J., et al. , The multifaceted role of Th1, Th9, and Th17 cells in immune checkpoint inhibition therapy. Front. Immunol. 12, 625667 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bosurgi L., et al. , Macrophage function in tissue repair and remodeling requires IL-4 or IL-13 with apoptotic cells. Science 356, 1072–1076 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walker J. A., McKenzie A. N. J., TH2 cell development and function. Nat. Rev. Immunol. 18, 121–133 (2018). [DOI] [PubMed] [Google Scholar]
- 5.Weaver C. T., Elson C. O., Fouser L. A., Kolls J. K., The Th17 pathway and inflammatory diseases of the intestines, lungs, and skin. Annu. Rev. Pathol. 8, 477–512 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vogelzang A., et al. , A fundamental role for interleukin-21 in the generation of T follicular helper cells. Immunity 29, 127–137 (2008). [DOI] [PubMed] [Google Scholar]
- 7.Kalia V., Sarkar S., Regulation of effector and memory CD8 T cell differentiation by IL-2-A balancing act. Front. Immunol. 9, 2987 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trinchieri G., Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 3, 133–146 (2003). [DOI] [PubMed] [Google Scholar]
- 9.Rajamanickam A., et al. , Characterization of memory T cell subsets and common γ-chain cytokines in convalescent COVID-19 individuals. J. Leukoc. Biol., 10.1002/JLB.5COVA0721-392RR (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dan J. M., et al. , Immunological memory to SARS-CoV-2 assessed for up to eight months after infection. Science 371, 6529 (2021). [DOI] [PMC free article] [PubMed]
- 11.Gao Y., et al. Ancestral SARS-CoV-2-specific T cells cross-recognize the Omicron variant. Nat. Med. 28, 472–476 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chouaib S., et al. , Interleukin 12 induces the differentiation of major histocompatibility complex class I-primed cytotoxic T-lymphocyte precursors into allospecific cytotoxic effectors. Proc. Natl. Acad. Sci. U.S.A. 91, 12659–12663 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abu-Raddad L. J., Chemaitelly H., Bertollini R.; National Study Group for COVID-19 Epidemiology, Severity of SARS-CoV-2 reinfections as compared with primary infections. N. Engl. J. Med. 385, 2487–2489 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Altarawneh H. N., et al. , Protection against the omicron variant from previous SARS-CoV-2 infection. N. Engl. J. Med. 386, 1288–1290 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tornell A., et al. , Rapid cytokine release assays for analysis of SARS-CoV-2-specific T cells in whole blood. J. Infect. Dis., 10.1093/infdis/jiac005 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or supporting information.