Abstract
Acute exercise has been shown to improve memory in humans. Potential mechanisms include increased Bdnf expression, noradrenergic activity, and modification of glutamate receptors. Because mice are commonly used to study exercise and brain plasticity, it is important to explore how acute exercise impacts behavior in this model. C57BL/6J mice were assigned to 3 groups: control, moderate-intensity running, and high-intensity running. Control mice were placed on a stationary treadmill for 30 min and moderate- and high-intensity mice ran for 30 min at 12 m/min and 15–17 m/min, respectively. Mice were sacrificed immediately after running and the hippocampus removed. Total Bdnf, Bdnf exon IV, and glutamate receptor subunits were quantified with qPCR. Total and phosphorylated GluR1 (Ser845 and Ser831) protein was quantified following immunoblotting. Utilizing the same protocol for control and high-intensity running, object location memory was examined in a separate cohort of mice. Anxiety-like behavior was assessed in the open field task (OFT) in a third cohort of mice that were separated into 4 groups: control–saline, control–DSP-4, acute exercise–saline, and acute exercise–DSP-4. DSP-4 was used to lesion the central noradrenergic system. We observed higher Bdnf IV mRNA in high-intensity runners compared to controls, but no effects of acute exercise on memory. In the OFT, runners traveled less distance and spent more time grooming than controls. DSP-4 did not attenuate the effects of exercise. CONCLUSION: A single bout of exercise increases Bdnf IV mRNA in an intensity-dependent manner; however, high-intensity running reduces exploratory behavior in C57BL/6J mice.
Keywords: Brain-derived neurotrophic factor, Acute Exercise, Anxiety, AMPA, Hippocampus
Introduction
A preponderance of evidence supports that exercise training and chronic physical activity are beneficial for brain health (for reviews, see 1–3). The hippocampus is thought to be particularly sensitive to physical activity, as both functional and structural adaptations have been reported following periods of physical activity, including enhanced adult neurogenesis 4–7 (for review, see 8), increased branching of neuronal dendrites 9,10, and an increase in the amplitude and a reduction in the threshold of long-term potentiation (LTP) 6,11. Physical activity also improves hippocampus-dependent learning 12–17. The underlying mechanisms of the response to chronic physical activity has received much attention, however, little is known about the response to a single acute bout of exercise. As chronic physical activity is an accumulation of single acute bouts of exercise, we posit that if exercise is to be used as a strategy to enhance learning and memory, it is advantageous to understand the response to each individual bout of acute exercise.
Meta-analyses report that acute exercise is effective at improving performance on memory tasks 18–20, though the mechanisms remain largely unknown. One possibility is that acute exercise-induced elevations in circulating catecholamines act to facilitate plasticity (reviewed in 21), as it has been shown that norepinephrine (NE) promotes spike-timing dependent synaptic plasticity 22. Indeed, exercise of sufficient intensity and duration increases circulating catecholamines (epinephrine and NE) and central NE 23,24 (for review, see 21). Norepinephrine can activate Gs-dependent signaling cascades that mediate post-translational modifications to the GluR1 subunit of the AMPA-type glutamate receptor (AMPAR) 25, the ionotropic glutamate receptor at excitatory synapses. The threshold for the induction of LTP is lowered following phosphorylation of specific sites on the C-terminal tail of the GluR1 subunit, which can regulate the concentration and conductance of synaptic AMPARs 26–28 (for reviews, see 29,30). Two such residues in the C-terminal tail of GluR1, serine 845 (Ser845), and to a lesser extent, serine 831 (Ser831), are phosphorylated in the hippocampus following NE signaling, which reduces the threshold for LTP and learning 25. Phosphorylation of Ser831 on GluR1 by CAMKII increases ion channel open probability and channel conductance 31,32, while phosphorylation of Ser845 by PKA increases perisynaptic insertion and decreases AMPAR internalization 28,33. Similarly, stimulation that reduces synaptic strength is associated with a rapid dephosphorylation of Ser845 34. We have previously shown that continuous exposure to a voluntary running wheel for one month resulted in an increase in the expression of GluR1 and phosphorylation of Ser845 35. However, we observed no change in Ser845 phosphorylation 15 minutes after a single 45-minute bout of treadmill exercise 35. Hu et al. 25 found elevated Ser845 phosphorylation 15 minutes following a peripheral injection of epinephrine, suggesting that the phosphorylation of Ser845 is induced rapidly.
Alternatively, exercise training may induce structural and functional plasticity in the hippocampus by increased transcription and translation of brain derived neurotrophic factor (BDNF) 8. BDNF is critical for the maintenance of optimal brain health and plays an integral role in functional and structural plasticity in the hippocampus throughout the lifespan (for review, see 36). BDNF is important for structural plasticity, including hippocampal neurogenesis 37–40 and dendritic/synaptic development 41–43, as well as functional adaptations at the synaptic 44–51 and behavioral 51–57 levels. BDNF has been shown to be necessary for the benefits of exercise training on structural and functional plasticity 16,58,59, however, little is known about the influence of acute exercise on BDNF transcription.
Alternative splicing of the nine exons of the BDNF gene can produce 22 possible mRNA transcripts 60. Each transcript has a different subcellular localization and transport capability 61,62 and the large number of transcripts allow for differential temporal and regional control of translation, mRNA longevity, and distribution. BDNF transcript IV (Bdnf IV) is of particular interest, as it appears to be uniquely sensitive to neuronal stimuli in vitro 63,64 and in vivo 65,66. We previously demonstrated an increase in total Bdnf and Bdnf IV mRNA expression 15 minutes following a 45-minute bout of treadmill exercise 35 - an effect that was independent of exercise intensity. The exercise protocol utilized in our previous investigation was long (45 minutes) and employed a rest period (15 minutes) prior to determination of Bdnf expression, so the threshold of exercise duration and intensity is yet to be determined.
Although research in humans suggests acute exercise can improve memory 18,20, this has not been adequately investigated in rodents. Since rodents are commonly used as models to understand the mechanisms that drive behavioral adaptations in humans, it is important to understand the behavioral responses to acute exercise in this model. Several studies have reported that chronic exercise improves both spatial 14,58,67 and non-spatial 13,67 memory in rodents, yet the influence of acute exercise has been mostly neglected. In addition, the novelty of an acute bout of exercise may induce unique responses related to both the physical and psychological stress of the exercise. Beyond the novelty of exercise, there is evidence that exploratory behavior may be reduced after a night with access to a voluntary running wheel, even after three weeks of running wheel exposure 68. This suggests that behavioral tests performed immediately after acute exercise may be associated with reduced exploratory behavior, which could impact performance on several tests of learning and memory. Here we investigate the influence of a single, 30-minute bout of acute treadmill exercise on phosphorylation and abundance of GluR1 protein and Bdnf mRNA and spatial memory. Furthermore, exercise is known to stimulate the release of central and peripheral catecholamines 23,69,70, which influence anxiety-like behavior 71. We therefore also used a pharmacological approach to reduce the output of the central noradrenergic system prior to acute exercise, to examine the impact on anxiety-like behavior.
Materials and Methods
Mouse model.
Three-month-old male C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME, USA) were used in this investigation. This mouse strain is commonly used to study the impact of exercise on brain phenotypes and, in our lab, displays adequate treadmill running ability 35,72. All mice were group housed and cared for by University of Maryland veterinary staff. Mice were kept on a 12hr light/12hr dark cycle and provided standard rodent chow. The University of Maryland Institutional Animal Care and Use Committee approved all protocols.
Overview and Treadmill Protocol:
Mice (N=35) were randomly separated into three groups: 1) treadmill without exercise (CON; n=12), 2) moderate-intensity acute treadmill exercise (MOD; n=11), 3) high-intensity acute treadmill exercise (HI; n=12). All procedures were performed 4 hours into the light phase, beginning at approximately 10:00am. For three days leading up to the experiment, mice were placed on the stationary treadmill for five minutes per day, during which the electrical stimulus grid at the end of the treadmill belt was activated and mice were allowed to explore the treadmill-testing environment 35. During active treadmill running, the stimulus grid provides a weak foot shock, which causes an involuntary muscle contraction that encourages running. Tactile stimulation to the tail was used to encourage mice to run prior to touching the stimulus grid. On day four, mice underwent their group-determined intervention, one at a time. This was performed in a counterbalanced order so that the initial animal for each day was from a different group than the previous day. Mice in the CON group were placed on the stationary treadmill for 36 minutes with the electrical stimulus grid activated. MOD and HI group mice were placed on the treadmill and underwent a six-minute warm up, where the first minute was a no-exercise treadmill exposure; thereafter the treadmill belt began to move at 5 m/min, increasing 1 m/min every minute for five minutes35. The treadmill speed was then incrementally increased to the group-appropriate speed and the mouse ran for 30 minutes at this pace. The MOD group ran for 30 minutes at 12 m/min at 0% grade; this stimulus has been reported to be ~75% of VO2max in adult C57BL/6J mice 73. The HI group ran for 30 minutes at a speed ranging from 15–17 m/min at 0% grade, depending on running ability; this speed has been reported to be ~80% of VO2max in adult C57BL/6J mice 73.
Tissue Processing:
Mice were sacrificed by decapitation under isoflurane anesthesia immediately following group-specific treadmill exposure. The hippocampus from each hemisphere was removed and immediately frozen in liquid nitrogen. For western blot analysis, the hippocampus was sonicated in 1% SDS, boiled for 10 minutes 25,35, and stored at −80°C. Protein concentration was determined by spectrophotometry using the BCA Protein Assay (Pierce®, Rockford, IL, USA). For RNA extraction, samples were homogenized in a glass Dounce tissue homogenizer followed by RNA isolation using TRI Reagent (Life Technologies, Grand Island, NY, USA). RNA quantity and purity were assessed by UV spectroscopy.
Western Blotting:
Twenty-five μg of protein was loaded onto 7.5% SDS polyacrylamide gels and electrophoresed, followed by transfer to nitrocellulose membranes and immunoblotting. Nitrocellulose membranes were incubated with a monoclonal rabbit anti-phospho-GluR1 (Ser845: 1:5000, Millipore, Temecula, CA, USA; or Ser831: 1:1000, Millipore, Temecula, CA, USA) antibody, stripped with a 100 mM glycine-HCl (pH 3.0) solution, and re-probed with a polyclonal rabbit anti-GluR1 antibody (1:1000, Millipore, Temecula, CA, USA; 35). Although the short time between the initiation of the exercise bout and sacrifice (30 minutes) is unlikely to produce changes in total GluR1 translation, we also immunoblotted for total GluR1 protein followed by stripping and re-probing for the neuronal nuclear marker NeuN with a polyclonal rabbit anti-NeuN antibody (1:1000, Millipore, Temecula, CA, USA). Appropriate fluorescent secondary antibodies were used for detection [goat anti-rabbit IgG Cyanine3 (Invitrogen, Waltham, MA, USA) and goat anti-rabbit IgG Alexafluor488 (Invitrogen, Waltham, MA, USA)]. Proteins were visualized with a Typhoon TRIO Variable Imager (GE Healthcare, Pittsburgh, PA, USA) using fluorescence acquisition mode with an emission filter for Alexa 488. Band intensities were quantified with ImageQuant TL (GE Healthcare, Pittsburgh, PA, USA) utilizing Rubber Band function for background subtraction. Levels of phosphorylation, expressed as the ratio of phospho-GluR1 divided by total GluR1 intensity from the same lane, were used for statistical analysis. Total GluR1 divided by NeuN intensity from the same lane was used for statistical analysis to determine total GluR1 protein expression.
To confirm that peripheral epinephrine (via stimulating central NE release) increases hippocampal GluR1 Ser845 phosphorylation, a subsample of mice were injected with saline (10ml/kg; n=3) or epinephrine (0.5mg/kg at 10 ml/kg; n=4) and sacrificed by decapitation under isoflurane anesthesia, 15 minutes post-injection. Hippocampi were dissected, sonicated in lysis buffer [50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1% NP-40, protease inhibitor cocktail], and immunoblotted as described above.
Gene Expression:
One μg of total RNA was reverse transcribed into cDNA using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA). Real-time quantitative PCR (qPCR) was used to assess mRNA expression of total Bdnf (exon IX), Bdnf transcript IV (Bdnf IV; exon IV), GluR1, NR2A, NR2B, Gapdh, and ActB (Gapdh & ActB; expression controls; primer sequences listed in supplemental table 1). Primer:probe assays were purchased pre-made [GluR1 (Gria1), NR2A (Grin2a), NR2B (Grin2b), Gapdh, ActB] or designed (Bdnf IX, Bdnf IV) for the mRNA sequence of each gene using PrimeTime qPCR Assay designer (Integrated DNA Technology, Coralville, IA, USA). All primer pairs, except Bdnf total, spanned exons to prevent amplification of genomic DNA. Because Bdnf total is represented by amplification of only exon IX, this primer pair did not span exons. Efficiency for each primer:probe assay was confirmed prior to use. qPCR data were normalized to the geometric mean of Gapdh and ActB using the −ΔΔCt method 74,75 and expressed as fold induction (2−ΔΔCt) of mRNA expression compared to the control group (1.0-fold induction).
Object Location Memory Task:
A subset of three-month-old male C57BL/6J mice (N=30) were tested on the object location memory task immediately following the acute bout of exercise or no-exercise treadmill exposure. The three-day treadmill familiarization approach was the same as described above. Mice in the treadmill control group (n=15) sat on the stationary treadmill for 36 minutes while mice in the exercise group (n=15) ran on the treadmill at 15 to 17 m/min for 30 minutes following a six-minute warm-up. The procedures for the object location task were adapted from Barker and Warburton 76. On the two days immediately prior to testing, mice were exposed to the testing arena (43×43×21.5 cm open field box) for five minutes/day to acclimate them to the procedure and environment. On the test day, immediately following the treadmill exposure, mice were placed in the testing apparatus for the first phase of the task (familiarization), in which they were allowed to explore the arena containing two identical objects (small, Duplo blocks®) for five minutes followed by a fifteen-minute inter-trial interval in their home cages. Subsequently, mice were returned to the testing box for the second phase (test) in which they were allowed to explore the arena containing one object from the first exposure and a third object that was identical to the initial objects, but moved to a different location for five minutes. The left/right position of the new object was counterbalanced between mice. Behavior during the familiarization and test phases was monitored using EthoVision XT 11 Behavioral Tracking Software (Noldus, Leesburg, VA, USA), which provides automatic tracking, analysis, and storage of animal activity and behavior. Object interaction (time spent interacting with objects and number of interactions; defined as nose within 2 cm of object) and total distance traveled were recorded for each mouse.
Open Field Behavior Task:
An additional subset of three-month-old male C57BL/6J mice (N=36) were used to assess anxiety-like behavior and the influence of noradrenergic signaling on behavior following acute exercise. Mice were separated into four groups: 1) Control – Saline (CON-SAL; n=9), 2) Control – DSP-4 (CON-DSP-4; n=10), Acute Exercise – Saline (EX-SAL; n=8), and Acute Exercise – DSP-4 (EX-DSP-4; n=9). Mice underwent the same treadmill familiarization and running as described above and ran at a treadmill speed between 15 and 17 m/min depending on running ability. Immediately after the acute bout of exercise or no-exercise treadmill exposure, performance on the open field task was assessed. Mice were placed in the testing apparatus (43×43×21.5 cm field box) and allowed to explore for 15 minutes while behavior was monitored using the EthoVision XT 11 Behavioral Tracking Software. Total distance traveled, time spent in central and peripheral zones, and time spent grooming were recorded and separated into five-minute blocks (0–5 minutes, 5–10 minutes, 10–15 minutes).
N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine (DSP-4):
DSP-4 is a neurotoxin that specifically lesions the locus coeruleus (LC) noradrenergic (NA) system and reduces tissue levels of NE in regions innervated by the LC 77–85. DSP-4 easily crosses the blood brain barrier and irreversibly disrupts central NA signaling while leaving non-LC NA neurons and serotonergic and dopaminergic systems unaffected (for review, see 86). We have previously demonstrated that DSP-4 does not influence treadmill-running performance 35. DSP-4 (Sigma Aldrich) was prepared in 0.9% saline and a single 50 mg/kg dose was delivered by IP injection in a volume of 10 ml/kg; this dose is frequently used in both rats and mice and is effective in depleting hippocampal NE 86. Control mice received a single IP injection of 0.9% saline in a volume of 10 ml/kg. Solutions were prepared for five animals, were protected from light exposure and injected within 15 minutes of preparation. Mice received injections seven days prior to treadmill familiarization (i.e., 10 days prior to experimental treadmill day). This dose of DSP-4 and the interval between injection and task performance results in >90% reduction in hippocampal NE in C57BL/6J mice 85.
Statistical Analysis:
To determine differences in GluR1 protein expression/phosphorylation and mRNA expression among groups, we used a one-way analysis of variance with Tukey’s post hoc comparisons when p<0.05. Object location memory performance was analyzed with a repeated measures ANOVA and Sidak’s multiple comparison test when appropriate. Open field behavior data were analyzed using a three-way repeated measures ANOVA (treadmill exposure x drug treatment x time). Upon no significant main or interaction effects of treadmill exposure and/or drug treatment, data were collapsed across the drug condition and a two-way repeated measures ANOVA (treadmill condition x time) was performed followed by Sidak’s multiple comparison tests when appropriate. P values for post-hoc contrasts are presented as “adjusted p values” to indicate that they have been corrected for multiple comparisons.
Results
To confirm that peripheral epinephrine (via stimulating central NE release) increases hippocampal GluR1 Ser845 phosphorylation, a subsample of mice (N=7) were injected with saline (10ml/kg; n=3) or epinephrine (0.5mg/kg at 10 ml/kg; n=4) and sacrificed 15 minutes post-injection. The IP injection of epinephrine was sufficient to induce phosphorylation of Ser845 (t(5)= 3.048; p=0.03) but did not influence Ser831 phosphorylation or GluR1 protein expression (Fig. 1).
Figure 1. Intraperitoneal injection of epinephrine induces Ser845 phosphorylation of GluR1 in the hippocampus.
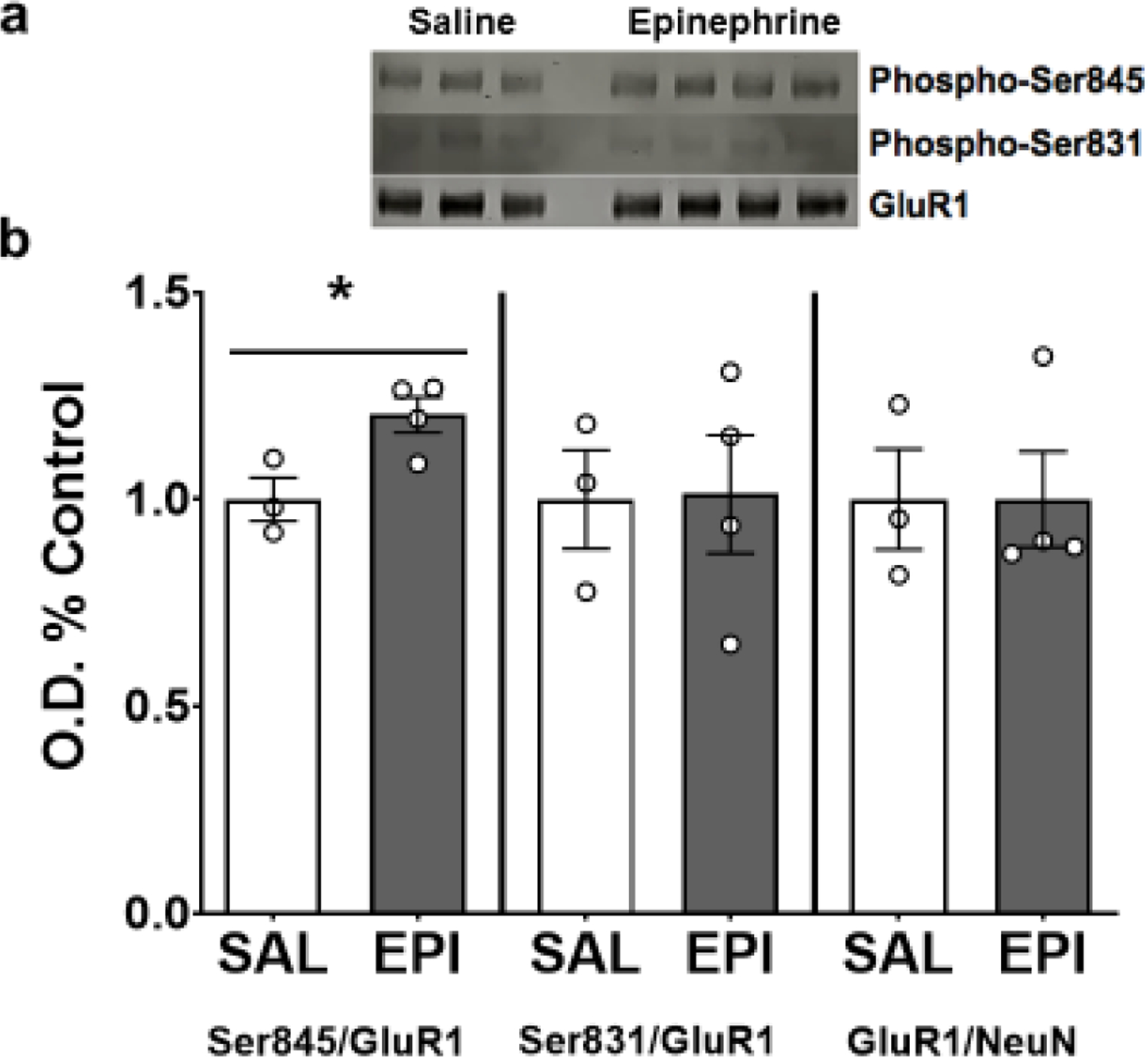
(A) Immunoblot of 3 saline controls (lanes 1–3) and 4 epinephrine experimental subjects (lanes 5–8). (B) Intraperitoneal injection of epinephrine increased the ratio of Ser845 phosphorylated over total GluR1 protein but had no effect on Ser831 phosphorylation or total GluR1. Data depicted as average +/− SEM. * indicates p<0.05.
To determine how acute exercise influences GluR1 phosphorylation in the hippocampus, mice (N=35) were randomly separated into three groups: 1) treadmill without exercise (CON; n=12), 2) moderate-intensity acute treadmill exercise (MOD; n=11), or 3) high-intensity acute treadmill exercise (HI; n=12). One protein sample from the CON group and one protein sample from the HI group were compromised during protein isolation. We found no significant difference in the phosphorylation of Ser845, Ser831, or the total level of GluR1 protein following acute high- or moderate-intensity exercise (Fig. 2). Similarly, we found no effect of acute high- or moderate-intensity exercise on GluR1, NR2A, or NR2B mRNA levels (Fig. 3). In contrast, we observed an effect of acute exercise intensity on the levels of Bdnf IV mRNA (F(2, 32)=3.79; p=0.03; Fig. 4a). High-intensity acute exercise induced higher mRNA levels compared to controls (adjusted p=0.03), but no effect on total Bdnf mRNA (Fig. 4b). Together, this demonstrates a modest impact of acute exercise on markers for synaptic plasticity.
Figure 2. Acute exercise does not affect GluR1 phosphorylation.
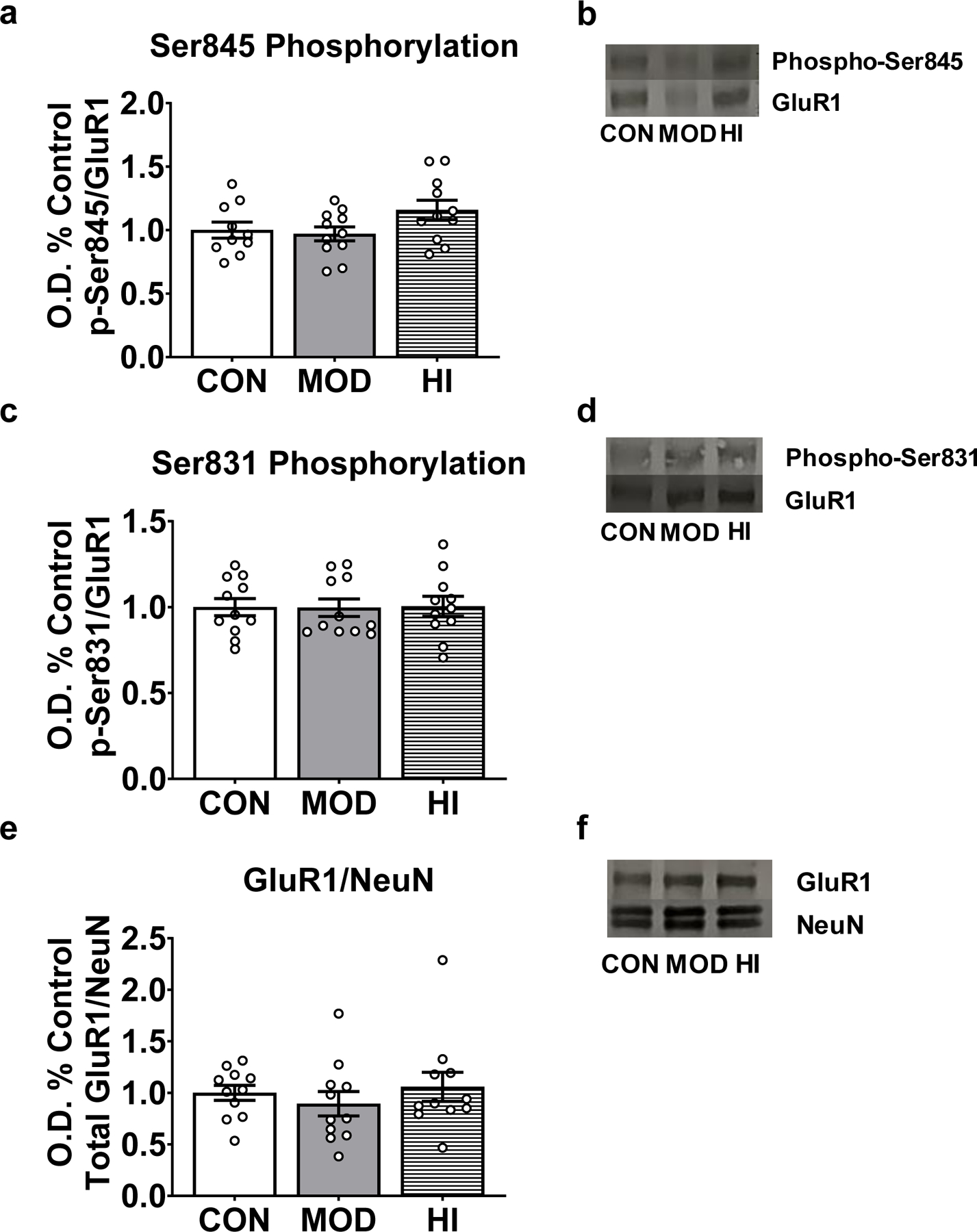
No significant effect of acute exercise on Ser845 phosphorylation (A), Ser831 phosphorylation (B) or total GluR1 protein level (C). (D-F): Representative immunblots for each condition. Data depicted as average +/− SEM.
Figure 3. Acute exercise does not affect glutamate receptor subunit mRNA levels in the mouse hippocampus.
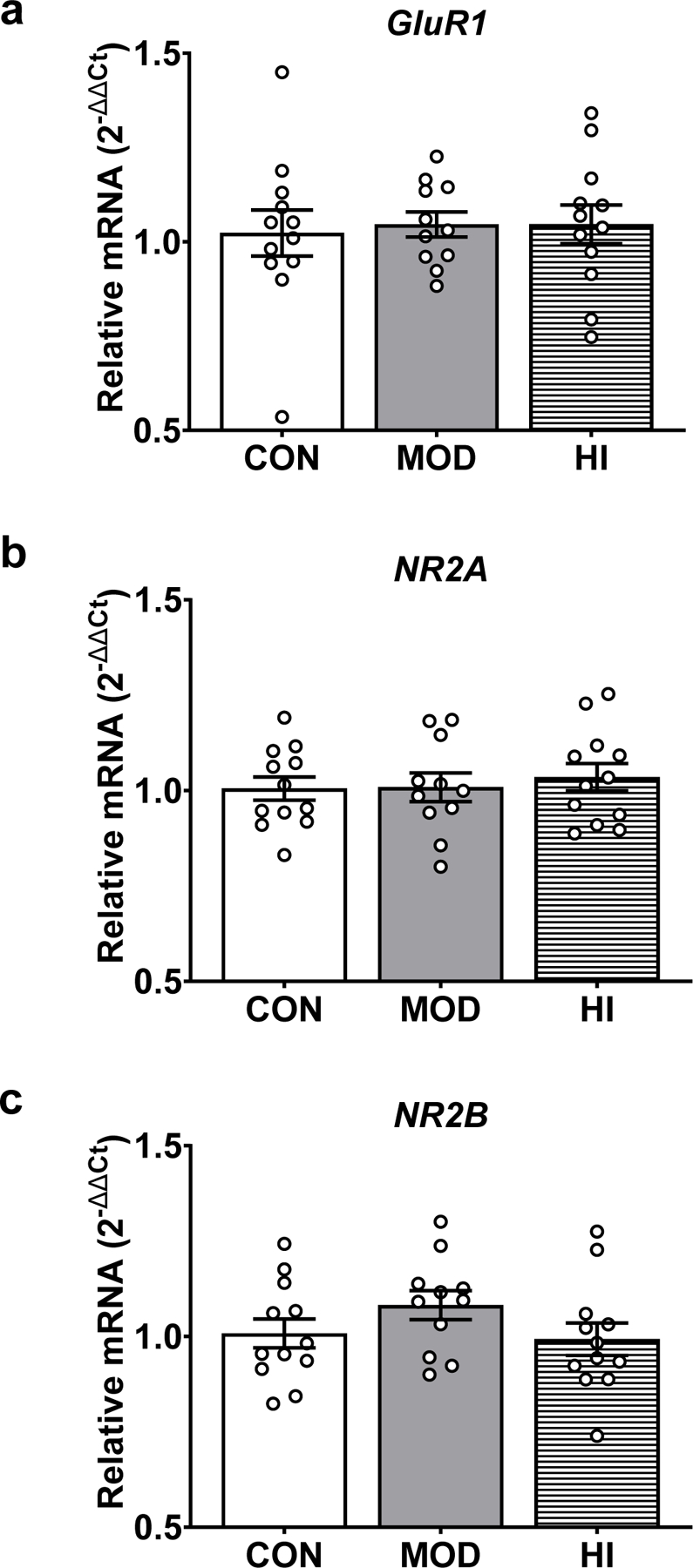
qPCR revealed no significant effect of acute exercise on GluR1 (A), NR2A (B), or NR2B (C) mRNA levels. Target mRNA levels are presented as 2−ΔΔCt relative to the geometric mean of ActB and Gapdh mRNA. Data depicted as average +/− SEM.
Figure 4. High-intensity exercise increases transcript-specific Bdnf expression.
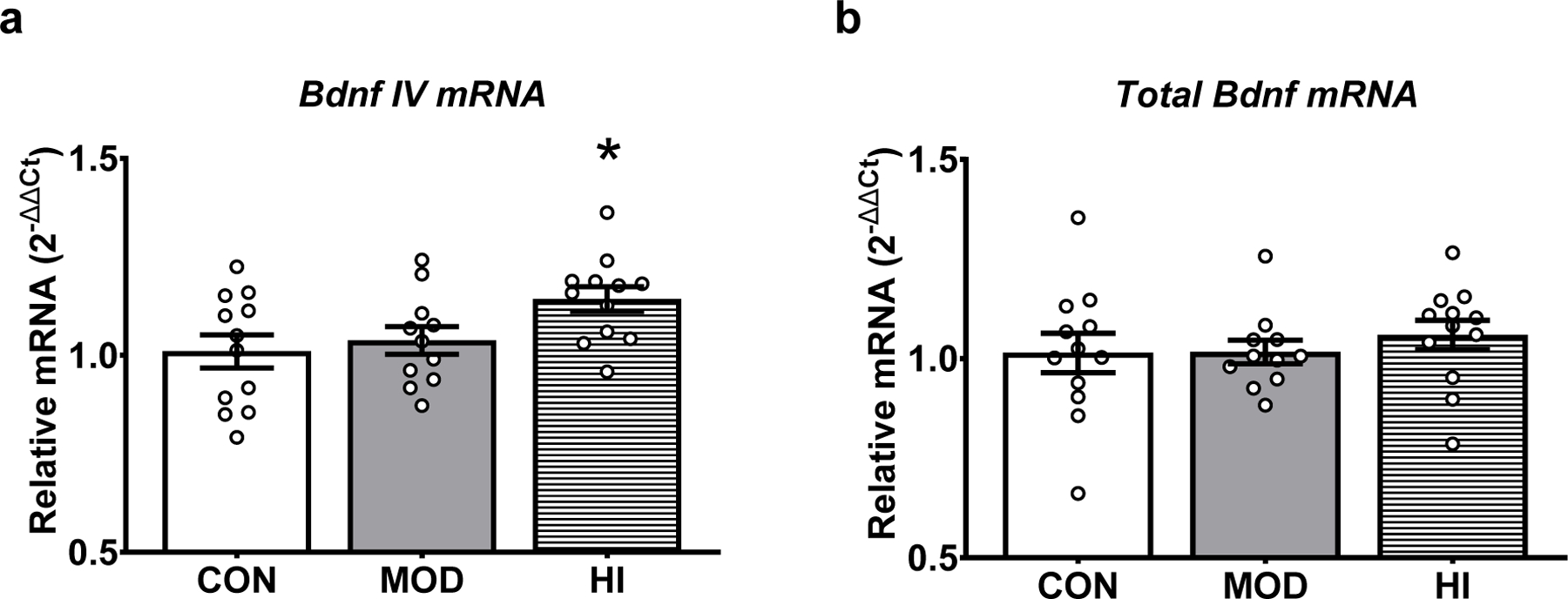
(A) Acute exercise significantly affects Bdnf IV mRNA levels in hippocampus. High-intensity exercise resulted in greater Bdnf IV mRNA relative to controls. (B) No significant effect of acute exercise on total Bdnf mRNA expression. Target mRNA levels are presented as 2−ΔΔCt relative to the geometric mean of ActB and Gapdh. Data depicted as average +/− SEM. * indicates p<0.05 versus control.
To ask if acute exercise affects memory, a cohort of three-month-old male C57BL/6J mice (N=30) were tested on a novel object location task immediately following acute exercise. We found no significant effect of acute exercise on total time spent exploring the two objects during either the familiarization or test phase of the task (Fig. 5a). During the test phase, there was no significant difference between controls and exercisers in time spent with the newly moved object relative to time spent with either of the other objects (i.e., discrimination ratio; Fig. 5b). However, we observed a significant main effect of acute exercise on total distance moved (F(1,28)=14.06; p=0.008; Fig. 5c). There was a tendency for an interaction between acute exercise and phase of test on total distance moved (F(1,28)=3.684; p=0.07). Mice exposed to high-intensity treadmill running moved significantly less (total distance traveled) during the familiarization phase compared to controls (Fig. 5c; p=0.0003). This difference was not observed during the test phase. We observed a significant main effect of acute exercise on the number of interactions with the objects (F(1,28)=4.553; p=0.04; Fig. 5d) and an interaction effect between exercise and phase of test (F(1,28)=6.938; p=0.01; Fig. 5d). The exercisers interacted with the two objects less frequently than controls during the familiarization phase (Fig. 5D; p=0.003). Again, the difference was not observed during the test phase.
Figure 5. Acute exercise does not influence object recognition, but reduces exploratory behavior.
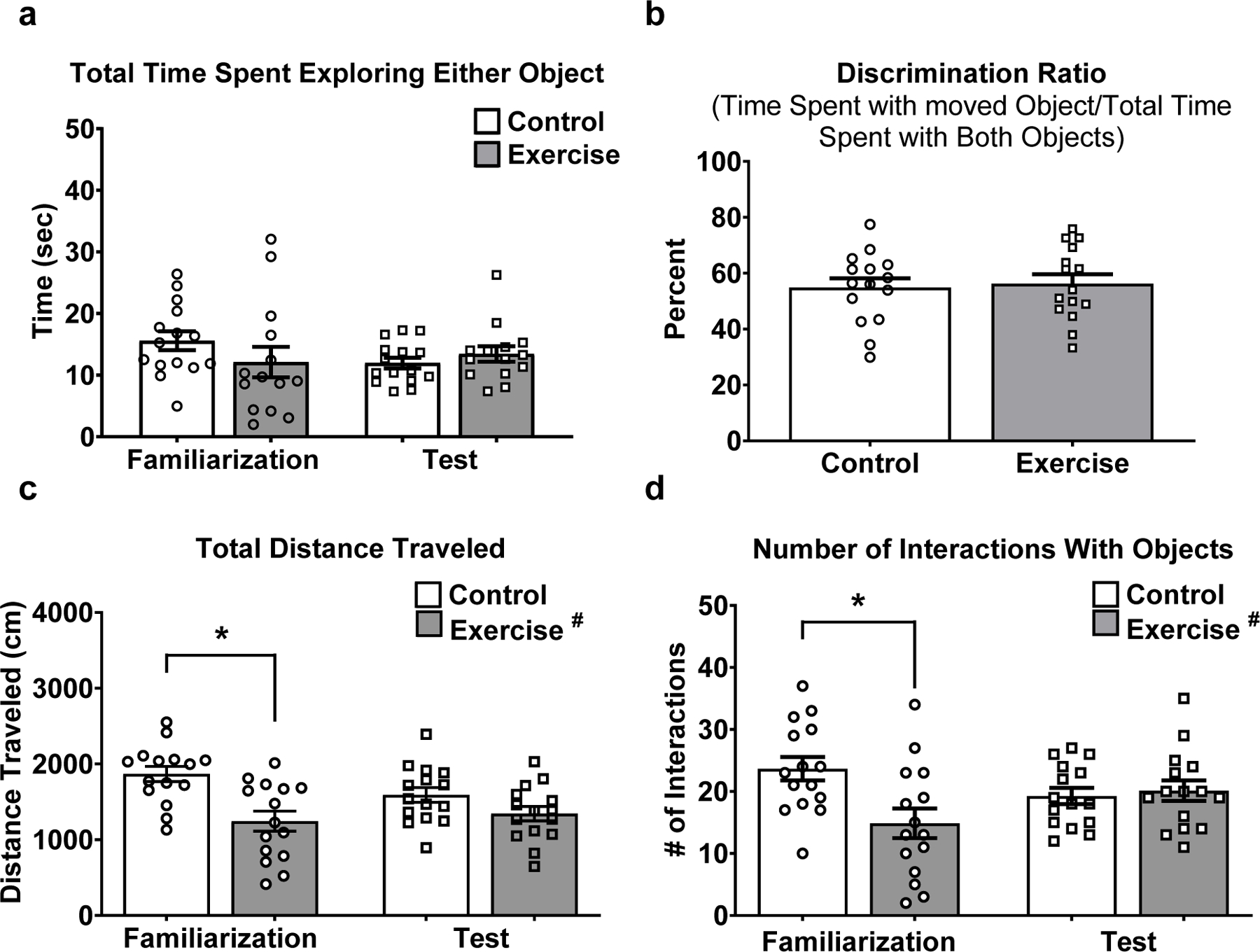
(A) No significant effect of acute exercise, phase of test, or interaction on the time spent exploring the objects. (B) No significant effect of acute exercise on % time spent exploring the moved object relative to the time spent exploring both objects during the test phase. (C) Mice that performed high-intensity treadmill running traveled less distance than treadmill controls during the familiarization phase. (D) A significant effect of acute exercise and an interaction between acute exercise and test phase on the number of interactions with the objects. Mice that performed high-intensity treadmill running had fewer interactions with the objects during the familiarization phase relative to the treadmill controls. Data depicted as average +/− SEM. * indicates p<0.05 after Sidak correction for multiple comparisons
Because the influence of acute exercise on activity during the object location task was observed during the familiarization phase (immediately following exercise), but not the test phase (15–20 minutes following exercise), we used an additional cohort of three-month-old male C57BL/6J mice (N=36) in the open field task to analyze behavior in five minute intervals. Utilizing a three-way repeated measures ANOVA [treadmill condition (acute exercise vs. stationary treadmill) X drug (DSP-4 vs. saline) X time (0–5 minutes vs. 5–10 minutes vs. 10–15 minutes], we observed no main effect of drug and no interaction effects for drug x time, drug x treadmill condition, or drug x treadmill condition x time for any dependent variable of interest. We therefore collapsed across the drug condition. All statistical data presented for the open field task were analyzed by a two-way repeated measures ANOVA (treadmill condition x five-minute time interval) with Sidak’s multiple comparisons when appropriate.
We observed a significant main effect of treadmill condition (F(1,34) = 25.12; p <0.0001), a main effect of time (F(2,68) = 29.91; p <0.0001) and an interaction between treadmill condition and time (F(2,68) = 12.81; p <0.0001) on total distance traveled (Fig. 6a). Post hoc analysis revealed that exercise mice traveled less distance during the first five-minute interval (adjusted p<0.0001), the second five-minute interval (adjusted p=0.04), and the final five minute interval (adjusted p=0.03) compared to control mice (Fig. 6a). Concerning the effect of time, post hoc analysis revealed that control animals moved significantly less distance during the second (adjusted p<0.0001) and third (adjusted p<0.0001) five-minute time intervals compared to the first five minutes of the task. There were no significant differences between time intervals for total distance traveled in mice that performed 30 minutes of exercise (Fig. 6a). We observed a significant main effect of treadmill condition on time spent self-grooming during the open field task (F(1,34) = 53.93; p<0.0001) but no main effect of time or interaction effect (Fig. 6B). There was a main effect of time (F(2,68) = 4.755; p =0.01) and an interaction between treadmill condition and time (F(2,68) = 4.478; p =0.01) for frequency of entries into the center of the testing arena, but no main effect of treadmill condition (Fig. 6C). Post hoc analysis revealed that in exercise mice, the number of entries was lower during the second (adjusted p=0.005) and third (adjusted p=0.009) five-minute time intervals compared to the first five minutes of the task. Further, during the 10–15-minute time interval, exercise mice had fewer entries into the center of the arena compared to control mice (adjusted p=0.03). There were no main effects of treadmill condition or time on the percent of total time spent in the center of the testing arena. There was an interaction between treadmill condition and time (F(2,68) = 3.556; p =0.03; Fig. 6D). Post hoc analysis revealed that in exercise mice, less time was spent in the center of the testing arena during the final five-minute time interval compared to the first time interval (adjusted p=0.03).
Figure 6. High-intensity acute exercise induces anxiety-like behavior in the initial 5 minutes of open field task.
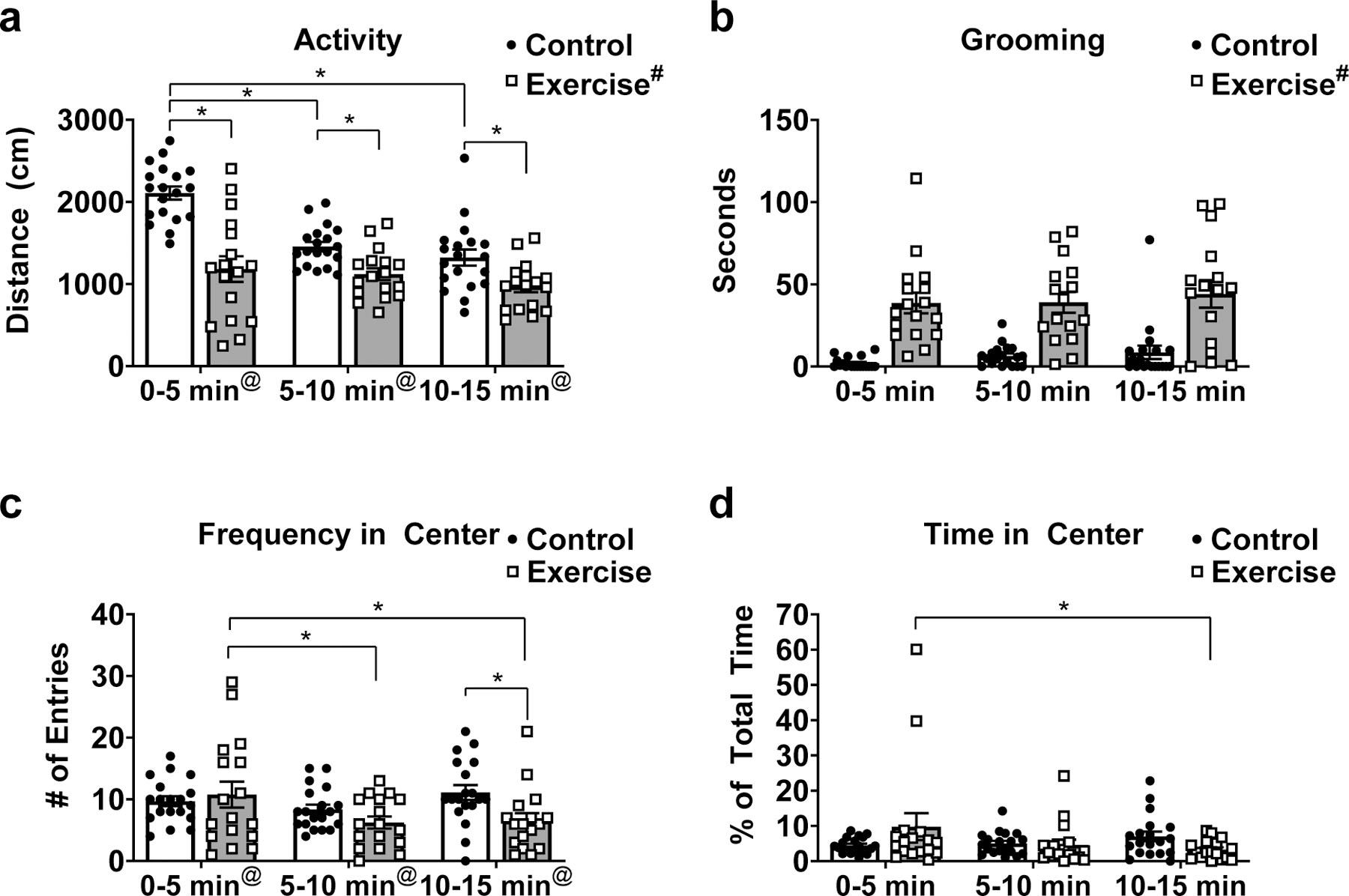
(A) Mice that performed high-intensity treadmill running traveled less total distance compared to sedentary controls at each time point. There was an interaction between treadmill condition and time, which demonstrated that total distance traveled was lower at 5–10 minutes and 10–15 minutes compared to 0–5 minutes in sedentary controls. (B) Mice that performed high-intensity treadmill running spent significantly more time self-grooming compared to sedentary controls during each time interval. (C) There was a significant effect of time on number of entries into the center of the testing arena and an interaction between time and treadmill condition. Fewer numbers of entries were observed at 5–10 minutes and 10–15 minutes compared to 0–5 minutes in the exercised mice. Mice that performed high-intensity treadmill running had fewer entries into the center of the arena during the 10–15 minute time interval compared to controls. (D) There was a significant interaction between time and treadmill condition for time spent in the center of the arena. In mice exposed to high-intensity treadmill running, there was less time spent in the center of the arena during the 10–15 minute time interval compared to the 0–5 minute time interval. Data depicted as average +/− SEM. # indicates significant main effect of acute exercise. @ indicates significant main effect of time. * indicates p<0.05 after Sidak correction for multiple comparisons.
We observed no significant difference between EX-SAL and EX-DSP-4 in running performance, indicated by number of stimulus grid touches (Fig. 7a). However, we did observe a significant negative correlation between the total number of stimulus grid touches and distance traveled during the first five-minute block of the open field task (p=0.005; Fig. 7b). Better running performance, indicated by fewer stimulus grid touches, was associated with higher activity in the open field task during the first five-minute block. This correlation was no longer observed during the second and third five-minute blocks (data not shown). There was no correlation between running performance and time spent self-grooming or entries into the center of the testing arena during any five-minute time block (data not shown).
Figure 7. Negative correlation between number of stimulus grid touches and distance travelled in open field task.
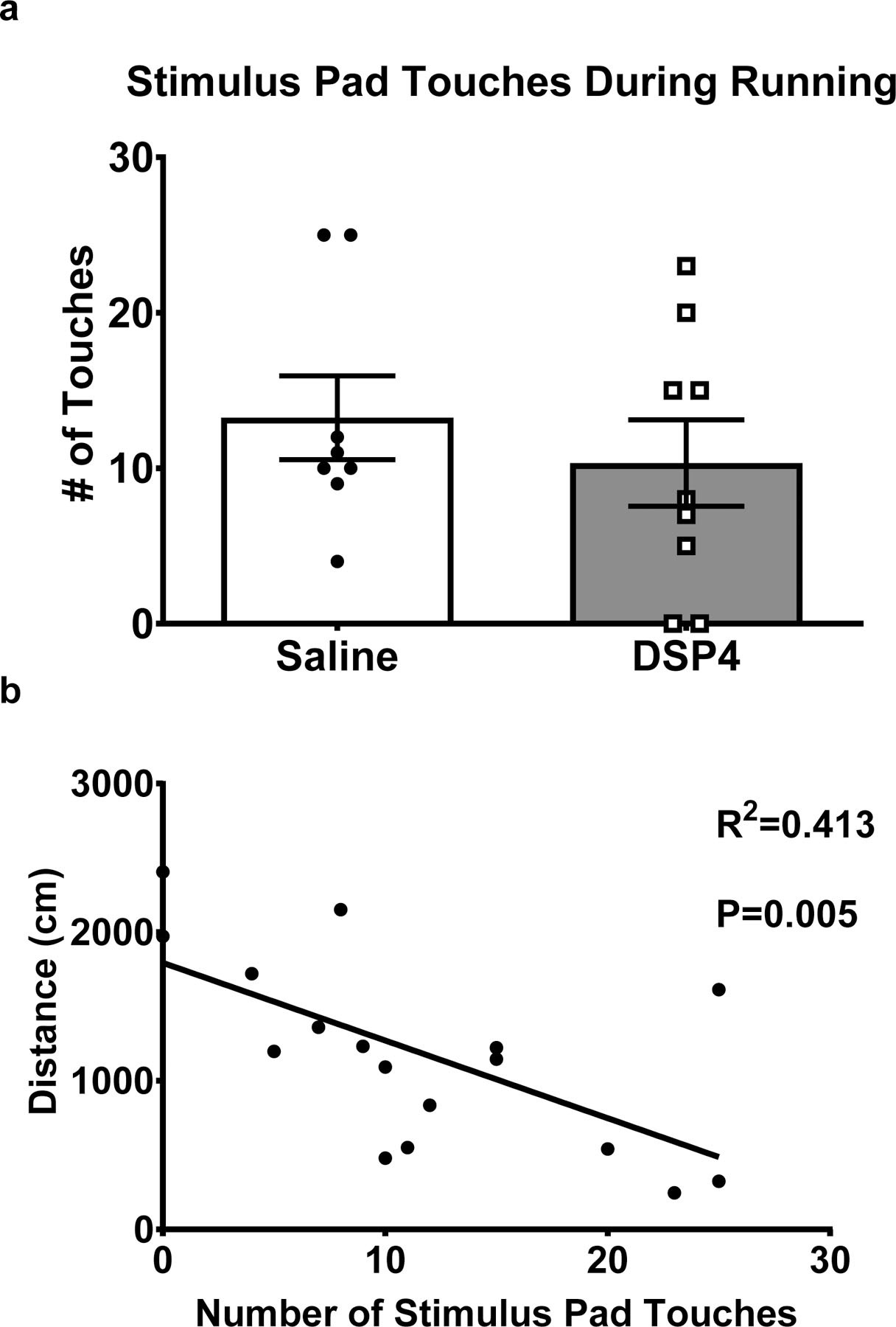
(A) No significant difference between DSP-4 treated and saline treated mice in stimulus grid touches during running. (B) A significant correlation between the total number of stimulus grid touches and distance traveled during the first five-minute block of the open field task.
Discussion
A single 30-minute bout of high-intensity treadmill exercise was sufficient to increase mRNA expression of Bdnf transcript IV, suggesting that signaling pathways known to be engaged by chronic exercise 3,8 can be initiated by single exercise bouts. However, a single 30-minute bout of acute exercise did not influence the phosphorylation status of the GluR1 subunit of the AMPAR, and therefore differs significantly from the response to peripheral injection of catecholamines 25. To ask if acute exercise improves memory performance in mice, as observed in humans 18,20, mice performed a low stress, one-trial memory task immediately following an acute bout of exercise. However, instead of revealing the expected effect on memory retention, following an acute bout of exercise, the behavior of mice appeared to reflect enhanced anxiety. Indeed, acute exercise significantly reduced locomotor activity and significantly increased time spent self-grooming in the open field task, measures often used as indicators of anxiety-like behavior.
We hypothesized that, like acute psychological stress and peripheral injections of epinephrine 25, acute forced treadmill exercise would increase phosphorylation of GluR1 at Ser845 in the hippocampus, potentially via the release of catecholamines and central noradrenergic signaling. However, while an IP injection of epinephrine induced the expected increase in the phosphorylation of Ser845 on GluR1 25, 30 minutes of high- or moderate-intensity acute exercise did not. Thus the effect of acute exercise may differ from the effects of acute psychological stress and chronic exercise, which has been shown to increase phosphorylation of Ser845 35,87. Alternatively, exposure to the treadmill environment alone may have elevated Ser845 phosphorylation in controls, and therefore masked the effect of acute exercise. Without a cage-control group that did not undergo the acclimation or experimental day treadmill exposure, we cannot rule out the possibility that the three days of acclimation to the treadmill environment or the 36-minute treadmill exposure in the stationary controls produced novelty-induced arousal. This arousal may have activated the noradrenergic system and stimulated hippocampal β2 adrenergic receptors 88. Indeed, novelty exploration increases neuronal activity in the LC and increases the release of NE in the hippocampus 89.
The lack of an effect of acute exercise on GluR1 phosphorylation may also reflect that 30 minutes of exercise was not sufficient to elevate peripheral epinephrine/central NE and/or engage the signaling pathways that target GluR1 phosphorylation. Pagliari and Peyrin 23 found that cortical NE increases in response to treadmill running after ~40 minutes of exercise in the rat, while Goekint et al. 90 observed no influence of 60 minutes of treadmill running on extracellular hippocampal NE. In contrast, Dishman et al. 91 reported that 15 minutes of treadmill running or immobilization stress decreased NE levels in the LC and hippocampus, likely through release and metabolism of the neurotransmitter.
Our data demonstrate that an acute bout of treadmill exercise does not stimulate rapid transcription of glutamate receptor subunits in mouse hippocampus. Potentially, multi-day treadmill exposures, or chronic voluntary wheel running, may be necessary to increase mRNA expression of GluR1, NR2A, or NR2B. Alternatively, it is possible that there was insufficient time for activity-dependent transcription of glutamate receptors between the start of exercise and sacrifice. The literature reporting the effects of exercise training on glutamate receptor expression is inconsistent. Prior research has reported an increase in NR2B mRNA expression following short-term exposure to a voluntary running wheel 11,92. Molteni et al. 92 reported that three days of voluntary running increased both NR2B, NR2A, and to a much lesser extent, GluR1 expression in rat hippocampus. NR2A remained significantly different than controls after seven days of wheel running but was no longer significantly different after one-month of exposure. Ni et al. 93 found that GluR1 mRNA expression was not influenced by six days of daily treadmill running in healthy Sprague-Dawley rats.
Numerous studies have demonstrated that exercise increases protein and mRNA expression of Bdnf in rodent hippocampus 58,92,94–99 and we previously demonstrated that a single 45-minute bout (followed by 15 minutes of rest) of high-intensity exercise increased expression of total Bdnf, while both high- and moderate-intensity exercise increased Bdnf IV 35. Here we show that 30 minutes of high-intensity, but not moderate-intensity, treadmill running increased only Bdnf IV. Bdnf stimulates hippocampal neurogenesis 37–40,100, synaptic plasticity 44–51, and promotes memory 51–57 – all effects that have also been observed following exercise training 8,101,102. Blocking Bdnf activity prevents exercise-induced improvements in spatial memory and expression of plasticity-associated genes 58. The finding that an acute 30-minute bout of exercise increased the transcription of Bdnf IV gene suggests that these pathways can be induced with short bouts of acute exercise, albeit forced and highly stressful in our investigation.
Acute exercise caused an increase in Bdnf IV but not total Bdnf expression, suggesting a compensatory decrease in another Bdnf transcript may compensate for the rapid activation of Bdnf IV transcription. We previously demonstrated that 45 minutes of exercise did not influence the expression of Bdnf transcripts I, II, III or VI 35. Indeed, expression of Bdnf IV is rapidly initiated in response to exercise, similar to an immediate early gene, while the other Bdnf transcripts have a slower pattern of transcription 63,64,103. Hippocampal Bdnf IV expression is also known to increase in response to acute immobilization stress 104, fear conditioning 65, and exercise training 16,105. It is important to note that our exercise protocol was likely stressful, as aspects of the protocol were unpredictable and uncontrollable. In this sense, our protocol may mimic aspects of fear conditioning and immobilization stress.
There is not yet a consensus, either in research approach or experimental findings, concerning the influence of acute exercise on Bdnf mRNA expression. For example, Oliff et al. 106 reported that six hours of voluntary wheel running increased total Bdnf but not Bdnf IV mRNA expression in rat hippocampus (i.e., hilus, CA1, and CA3). Importantly, these investigators allowed acclimation of mice to the running wheel for three nights followed by a 10-day washout period. However, mice that underwent the three days of wheel acclimation, but no acute wheel exposure after the washout, had significantly elevated Bdnf IV in all hippocampal regions examined. This demonstrates that acclimation protocols, commonly used in acute exercise studies, can influence hippocampal Bdnf expression. Rasmussen et al. 107 reported significantly greater total Bdnf mRNA in the hippocampus two- and six-hours post-treadmill running to exhaustion but not immediately after exercise. Similar to Oliff et al. 106, Rasmussen et al. 107 used an acclimation protocol that included running on the treadmill for multiple days before the acute treadmill running.
We hypothesized that an acute bout of high-intensity exercise would lower the threshold for memory formation and/or improve memory performance, as has been shown with epinephrine injections 25 and three weeks of voluntary wheel running 16. However, mice exposed to high-intensity acute exercise showed significantly less exploratory behavior (e.g., frequency of interaction with objects and significantly less distance traveled) during the familiarization phase of the novel object location task, a memory task known to be dependent on the hippocampus 76. The absence of active exploration of the novel objects likely prevented learning during the task and interfered with our ability to assess memory. Potentially, catecholamines elevated by our acute exercise protocol contributed to the reduced exploratory behavior and negatively impacted performance in the novel object location task. We speculate that the elevation in catecholamines was transient and returned to baseline by the test phase when exploratory behavior was similar between runners and controls. Another possibility is that the mice were fatigued following the bout of treadmill exercise. Although the treadmill exercise was not exhaustive, even moderate fatigue induced by exercise may cause a reduction in activity 108.
A similar behavioral profile was observed in the open field task, a task commonly used to examine anxiety-like behavior in rodents. Mice that performed an acute bout of exercise showed significantly less activity, indicated by total distance travelled, during the task and spent significantly more time self-grooming. Moreover, exercise mice had a pattern of reduced entries during the final five minutes of the open field task. These behaviors are often indicative of anxiety-like behavior 109,110, yet we are cautious in our interpretation of these behaviors as anxiety.
Self-grooming is a complex behavior that has been shown to be increased with high levels of anxiety and stressful situations, and is reduced by benzodiazepines 110. However, grooming behavior is controlled by many brain regions/circuits and influenced by numerous pharmacological manipulations 110. Alternatively to increased anxiety, increased grooming could represent reduced vigilance and more internally-directed behavior 111, and so we are cautious in our interpretation of increased grooming following forced exercise. Percent of time spent in the center of the testing arena and number of entries into the testing arena, both common indicators of anxiety-like behavior 109, were not impacted by exercise other than a small yet significant difference in number of entries into the center of the arena during the 10–15 minute interval.
The majority of published research on exercise and anxiety has investigated the influence of chronic exercise. This research has reported primarily anxiolytic effects of exercise (reviewed in 113); however, a few studies have reported anxiogenic effects 114–116. Salam et al. 117 provided C57BL/6J mice with access to a voluntary running wheel for two weeks prior to exposure to the open field task and found behaviors suggestive of both reduced and increased anxiety. They reported that runners spent significantly more time in the center of the testing box and entered the center more frequently than sedentary mice. These behaviors are indicative of less anxiety; however, they also observed significantly less activity and more grooming behavior in runners, which is indicative of higher levels of anxiety and is similar to what we observed. Some investigations on the effects of chronic exercise on anxiety offer insight into rodent behavior immediately following a bout of exercise. For example, Duman et al. 68 reported that three weeks of voluntary wheel running in C57BL/6J mice increased anxiety-like behavior (i.e., reduced activity) in the open field task if the task was initiated the morning after a night of voluntary wheel running. In contrast, if the task was initiated 24 hours after the last exposure to the voluntary running wheel, they observed anxiolytic-like behavior. Further, Fuss et al. 108 reported reduced anxiety-like behavior in the dark-light box immediately after an acute five-hour bout of voluntary wheel running. Although mice exposed to running spent more time in the bright area, they showed reduced activity indicated by the number of exits from the dark compartment to the light compartment. These results are consistent with our findings and suggest that tests dependent on exploratory and/or locomotor activity are compromised when performed immediately after an acute bout of exercise. Fuss et al. 108 further observed that when mice were returned to cages with running wheels following behavioral testing, mice that underwent five hours of acute exercise immediately before behavioral testing were less active compared to mice that did not undergo the acute bout of exercise before behavioral testing. This further suggests that the reduction in activity following acute exercise may be due to fatigue. Fatigue following the acute exercise could explain the reduction in exploratory behavior observed in both the object location task and the open field task. Indeed, there was a significant negative correlation between number of stimulus pad touches during treadmill running, our indirect measure of running ability, and total distance travelled during the first 5 minutes of the open field task. Mice that experienced more stimulus pad touches presumably struggled more with the intensity of running and may have experienced more fatigue following the exercise.
An alternative explanation for the observed anxiety-like behaviors during the novel object location and open field tasks may be in response to the release of adrenal stress hormones and central noradrenergic signaling. The reduced locomotor behavior observed in the object location and open field tasks is similar to what we observed in the home cage following an IP injection of epinephrine (unpublished observation). Administration of selective NE reuptake inhibitors used as antidepressants (e.g., reboxetine) are initially anxiogenic 118, but become anxiolytic after chronic administration by reducing stress-induced cortical NE release 119. Potentially, acute and chronic exercise mimic acute and chronic treatment with NE reuptake inhibitors by increasing extrasynaptic NE, which is acutely anxiogenic but becomes anxiolytic with chronic exposure. The role of NE in anxiety is complex and is associated with both anxiolytic and anxiogenic behavior depending on the type of acute stress stimulating the NE release 71. It is important to recognize that our treadmill protocol was forced and likely highly stressful. Forced treadmill running, in contrast to voluntary wheel running, is uncontrollable and associated with higher levels of stress hormones, such as corticosterone and NE, compared to voluntary wheel running 120–123. It is possible that we would have observed a different behavioral profile if voluntary wheel running was utilized.
We hypothesized that the anxiogenic-like behaviors (e.g., reduced exploratory behavior, increased self-grooming) observed following acute exercise would be attenuated with pre-treatment with the selective neurotoxin for the LC- noradrenergic system, DSP-4. However, we did not observe an effect of the drug on any measure of behavior in the open field task. DSP-4 treatment has been previously shown to reduce overall activity in the open field task, which can be attenuated with chronic mild stress 124. Potentially, three days of treadmill acclimation and the 36 minutes of stationary treadmill exposure was the optimal level of stress to attenuate the effect of DSP-4 treatment on exploratory behavior in the control mice and may explain why we did not observe a significant reduction in activity in mice that received DSP-4 alone. This would suggest that an inverted-U effect of LC-derived NE may exist, with both low and high levels resulting in reduced exploratory activity. Other stress hormones (e.g., corticosterone) and amygdala activity, independent of LC innervation, may be sufficient to cause the behavioral profile that we observed. Moreover, high levels of exogenous catecholamines can bypass the LC to exert behavioral effects. Bennett et al. 83 reported that peripheral injections of epinephrine following DSP-4 treatment can attenuate impairments in active avoidance induced by DSP-4. Our exercise protocol may have been psychologically and physically stressful enough to raise peripheral catecholamines and bypass the LC noradrenergic system; however, peripheral catecholamines were not assayed.
We were primarily interested in the influence of forced exercise, and not the influence of the novel treadmill environment. We hypothesized that the novel environment alone may induce plasticity and designed our study to observe the effects of the treadmill exercise beyond the effects of novelty. We did not envision an occlusion of plasticity markers by the novelty; however, not having a home-cage control prevents us from being able to determine if our lack of observed effect on GluR1 phosphorylation and total Bdnf was due to the novel environment masking the effects of acute exercise. In addition, a limitation of this investigation is that we were unable to measure the tissue and extracellular content of NE in response to exercise and DSP-4. Although there is ample evidence to support that DSP-4 reduces tissue content of NE 77–85, the possibility of an ineffective drug treatment or increased extracellular content of NE 86 following treatment allows for uncertainty.
Summary:
We show that a single acute bout of exercise does not increase GluR1 phosphorylation but increases the level of mRNA of the important plasticity-promoting gene, Bdnf, in a transcript- and intensity-dependent manner. Furthermore, following acute exercise, locomotor and exploratory behavior are reduced and self-grooming behavior increased. Exercise does not increase anxiety or incidents of anxiety attacks in humans and generally appears to be anxiolytic in rodents 113,125,126, so we are hesitant to conclude that the acute bout of exercise is actually anxiogenic. Our data suggest that tasks with low intrinsic motivation and/or dependent on locomotor or exploratory behavior may give results that can be interpreted as an anxious phenotype. Careful consideration should be used when selecting the appropriate behavioral task to assess memory or anxiety following acute exercise exposures.
Supplementary Material
Acknowledgements
This work was supported by NIH F31 MJ103951-01A1 to ACV.
Footnotes
Competing interest
Non declared
References
- 1.Rendeiro C, Rhodes JS. A new perspective of the hippocampus in the origin of exercise–brain interactions. Brain Structure and Function. 2018;223(6):2527–2545. [DOI] [PubMed] [Google Scholar]
- 2.Erickson KI, Hillman C, Stillman CM, Ballard RM, Bloodgood B, Conroy DE, Macko R, Marquez DX, Petruzzello SJ, Powell KE. Physical activity, cognition, and brain outcomes: a review of the 2018 Physical Activity Guidelines. Medicine & Science in Sports & Exercise. 2019;51(6):1242–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Voss MW, Soto C, Yoo S, Sodoma M, Vivar C, van Praag H. Exercise and hippocampal memory systems. Trends in Cognitive Sciences. 2019;23(4):318–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pereira AC, Huddleston DE, Brickman AM, Sosunov AA, Hen R, McKhann GM, Sloan R, Gage FH, Brown TR, Small SA. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proceedings of the National Academy of Sciences. 2007;104(13):5638–5643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nature Neuroscience. 1999;2(3):266–270. [DOI] [PubMed] [Google Scholar]
- 6.van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proceedings of the National Academy of Sciences. 1999;96(23):13427–13431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Redila VA, Christie BR. Exercise-induced changes in dendritic structure and complexity in the adult hippocampal dentate gyrus. Neuroscience. 2006;137(4):1299–1307. [DOI] [PubMed] [Google Scholar]
- 8.Voss MW, Vivar C, Kramer AF, van Praag H. Bridging animal and human models of exercise-induced brain plasticity. Trends in Cognitive Sciences. 2013;17(10):525–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stranahan AM, Khalil D, Gould E. Running induces widespread structural alterations in the hippocampus and entorhinal cortex. Hippocampus. 2007;17(11):1017–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin T-W, Chen S-J, Huang T-Y, Chang CY, Chuang JI, Wu FS, Kuo YM, Jen CJ. Different types of exercise induce differential effects on neuronal adaptations and memory performance. Neurobiology of Learning and Memory. 2012;97(1):140–147. [DOI] [PubMed] [Google Scholar]
- 11.Farmer J, Zhao X, van Praag H, Wodtke K, Gage FH, Christie BR. Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male sprague–dawley rats in vivo. Neuroscience. 2004;124(1):71–79. [DOI] [PubMed] [Google Scholar]
- 12.Cassilhas RC, Tufik S, de Mello MT. Physical exercise, neuroplasticity, spatial learning and memory. Cellular and Molecular Life Sciences. 2015;73(5):975–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Callaghan R, Ohle R, Kelly A. The effects of forced exercise on hippocampal plasticity in the rat: A comparison of LTP, spatial- and non-spatial learning. Behavioural Brain Research. 2007;176(2):362–366. [DOI] [PubMed] [Google Scholar]
- 14.Creer DJ, Romberg C, Saksida LM, van Praag H, Bussey TJ. Running enhances spatial pattern separation in mice. Proceedings of the National Academy of Sciences. 2010;107(5):2367–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kohman RA, Clark PJ, DeYoung EK, Bhattacharya TK, Venghaus CE, Rhodes JS. Voluntary wheel running enhances contextual but not trace fear conditioning. Behavioural Brain Research. 2012;226(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Intlekofer KA, Berchtold NC, Malvaez M, Carlos AJ, McQuown SC, Cunningham MJ, Wood MA, Cotman CW. Exercise and sodium butyrate transform a subthreshold learning event into long-term memory via a brain-derived neurotrophic factor-dependent mechanism. Neuropsychopharmacology. 2013;38(10):2027–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, Wojcicki TR, Mailey E, Vieira VJ, Martin SA, Pence BD, Woods JA, McAuley E, Kramer AF. Exercise training increases size of hippocampus and improves memory. Proceedings of the National Academy of Sciences. 2011;108(7):3017–3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lambourne K, Tomporowski P. The effect of exercise-induced arousal on cognitive task performance: A meta-regression analysis. Brain Research. 2010;1341:12–24. [DOI] [PubMed] [Google Scholar]
- 19.Chang YK, Labban JD, Gapin JI, Etnier JL. The effects of acute exercise on cognitive performance: A meta-analysis. Brain Research. 2012;1453:87–101. [DOI] [PubMed] [Google Scholar]
- 20.Roig M, Nordbrandt S, Geertsen SS, Nielsen JB. The effects of cardiovascular exercise on human memory: A review with meta-analysis. Neuroscience & Biobehavioral Reviews. 2013;37(8):1645–1666. [DOI] [PubMed] [Google Scholar]
- 21.McMorris T, Turner A, Hale BJ. Beyond the catecholamines hypothesis for an acute exercise-cognition interaction: a neurochemical perspective. In Exercise-Cognition Interaction, Edition: 1st, Chapter: 4, Publisher: Elsevier, Editors: McMorris Terry, 2016. pp.65–103. [Google Scholar]
- 22.Huang S, Huganir RL, Kirkwood A. Adrenergic gating of hebbian spike-timing-dependent plasticity in cortical interneurons. Journal of Neuroscience. 2013;33(32):13171–13178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pagliari R, Peyrin L. Norepinephrine release in the rat frontal cortex under treadmill exercise: a study with microdialysis. Journal of Applied Physiology. 1995;78(6):2121–2130. [DOI] [PubMed] [Google Scholar]
- 24.Pagliari R, Peyrin L. Physical conditioning in rats influences the central and peripheral catecholamine responses to sustained exercise. European Journal of Applied Physiology and Occupational Physiology. 1995;71(1):41–52. [DOI] [PubMed] [Google Scholar]
- 25.Hu H, Real E, Takamiya K, Kang MG, Ledoux J, Huganir RL, Malinow R. Emotion Enhances Learning via Norepinephrine Regulation of AMPA-Receptor Trafficking. Cell. 2007;131(1):160–173. [DOI] [PubMed] [Google Scholar]
- 26.Makino Y, Johnson RC, Yu Y, Takamiya K, Huganir RL. Enhanced synaptic plasticity in mice with phosphomimetic mutation of the GluA1 AMPA receptor. Proceedings of the National Academy of Sciences. 2011;108(20):8450–8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang Y, Wang X-B, Frerking M, Zhou Q. Delivery of AMPA receptors to perisynaptic sites precedes the full expression of long-term potentiation. Proceedings of the National Academy of Sciences. 2008;105(32):11388–11393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oh MC, Derkach VA, Guire ES, Soderling TR. Extrasynaptic membrane trafficking regulated by GluR1 serine 845 phosphorylation primes AMPA receptors for long-term potentiation. The Journal of Biological Chemistry. 2006;281(2):752–758. [DOI] [PubMed] [Google Scholar]
- 29.Huganir RL, Nicoll RA. AMPARs and synaptic plasticity: the last 25 years. Neuron. 2013;80(3):704–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anggono V, Huganir RL. Regulation of AMPA receptor trafficking and synaptic plasticity. Current Opinion in Neurobiology. 2012;22(3):461–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Derkach V, Barria A, Soderling TR. Ca2+/calmodulin-kinase II enhances channel conductance of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate type glutamate receptors. Proceedings of the National Academy of Sciences. 1999;96(6):3269–3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kristensen AS, Jenkins MA, Banke TG, Schousboe A, Makino Y, Johnson RC, Huganir R, Traynelis SF. Mechanism of Ca2+/calmodulin-dependent kinase II regulation of AMPA receptor gating. Nature Neuroscience. 2011;14(6):727–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Santos SD, Carvalho AL, Caldeira MV, Duarte CB. Regulation of AMPA receptors and synaptic plasticity. Neuroscience. 2009;158(1):105–125. [DOI] [PubMed] [Google Scholar]
- 34.Lee H-K, Kameyama K, Huganir RL, Bear MF. NMDA induces long-term synaptic depression and dephosphorylation of the GluR1 subunit of AMPA receptors in hippocampus. Neuron. 1998;21(5):1151–1162. [DOI] [PubMed] [Google Scholar]
- 35.Venezia AC, Quinlan E, Roth SM. A single bout of exercise increases hippocampal Bdnf: influence of chronic exercise and noradrenaline. Genes, Brain and Behavior. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park H, Poo M-M. Neurotrophin regulation of neural circuit development and function. Nature Reviews Neuroscience. 2013;14(1):7–23. [DOI] [PubMed] [Google Scholar]
- 37.Taliaz D, Stall N, Dar DE, Zangen A. Knockdown of brain-derived neurotrophic factor in specific brain sites precipitates behaviors associated with depression and reduces neurogenesis. Molecular Psychiatry. 2009;15(1):80–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scharfman H, Goodman J, Macleod A, Phani S, Antonelli C, Croll S. Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats. Experimental Neurology. 2005;192(2):348–356. [DOI] [PubMed] [Google Scholar]
- 39.Lee J, Duan W, Mattson MP. Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. Journal of Neurochemistry. 2002;82(6):1367–1375. [DOI] [PubMed] [Google Scholar]
- 40.Sairanen M, Lucas J, Ernfors P,Castren M, Castren E. Brain-derived neurotrophic factor and antidepressant drugs have different but coordinated effects on neuronal turnover, proliferation, and survival in the adult dentate gyrus. Journal of Neuroscience. 2005;25(5):1089–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bergami M, Rimondini R, Santi S, Blum R, Götz M, Canossa M. Deletion of TrkB in adult progenitors alters newborn neuron integration into hippocampal circuits and increases anxiety-like behavior. Proceedings of the National Academy of Sciences. 2008;105(40):15570–15575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alonso M, Medina JH, Pozzo-Miller L. ERK1/2 activation is necessary for BDNF to increase dendritic spine density in hippocampal CA1 pyramidal neurons. Learning & Memory. 2004;11(2):172–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bohlen und Halbach von O, Minichiello L, Unsicker K. TrkB but not trkC receptors are necessary for postnatal maintenance of hippocampal spines. Neurobiology of Aging. 2008;29(8):1247–1255. [DOI] [PubMed] [Google Scholar]
- 44.Korte M, Carroll P, Wolf E, Brem G, Thoenen H, Bonhoeffer T. Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proceedings of the National Academy of Sciences. 1995;92(19):8856–8860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Korte M, Griesbeck O, Gravel C, et al. Virus-mediated gene transfer into hippocampal CA1 region restores long-term potentiation in brain-derived neurotrophic factor mutant mice. Proceedings of the National Academy of Sciences. 1996;93(22):12547–12552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Patterson SL, Abel T, Deuel TA, Martin KC, Rose JC, Kandel ER. Recombinant BDNF rescues deficits in basal synaptic transmission and hippocampal LTP in BDNF knockout mice. Neuron. 1996;16(6):1137–1145. [DOI] [PubMed] [Google Scholar]
- 47.Zakharenko SS, Patterson SL, Dragatsis I, Zeitlin SO, Siegelbaum SA, Kandel ER, Morozov A. Presynaptic BDNF required for a presynaptic but not postsynaptic component of LTP at hippocampal CA1-CA3 synapses. Neuron. 2003;39(6):975–990. [DOI] [PubMed] [Google Scholar]
- 48.Kang H, Welcher AA, Shelton D, Schuman EM. Neurotrophins and time: different roles for TrkB signaling in hippocampal long-term potentiation. Neuron. 1997. [DOI] [PubMed] [Google Scholar]
- 49.Chen G, Kolbeck R, Barde YA, Bonhoeffer T, Kossel A. Relative contribution of endogenous neurotrophins in hippocampal long-term potentiation. Journal of Neuroscience. 1999;19(18):7983–7990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Figurov A, Pozzo-Miller LD, Olafsson P, Wang T, Lu B. Regulation of synaptic responses to high-frequency stimulation and LTP by neurotrophins in the hippocampus. Nature. 1996;381(6584):706–709. [DOI] [PubMed] [Google Scholar]
- 51.Ma YL, Wang HL, Wu HC, Wei CL, Lee EH. Brain-derived neurotrophic factor antisense oligonucleotide impairs memory retention and inhibits long-term potentiation in rats. Neuroscience. 1998;82(4):957–967. [DOI] [PubMed] [Google Scholar]
- 52.Bekinschtein P, Cammarota M, Katche C, Slipczuk L, Rossato JI, Goldin A, Izquierdo I, Medina JH. BDNF is essential to promote persistence of long-term memory storage. Proceedings of the National Academy of Sciences. 2008;105(7):2711–2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Heldt SA, Stanek L, Chhatwal JP, Ressler KJ. Hippocampus-specific deletion of BDNF in adult mice impairs spatial memory and extinction of aversive memories. Molecular Psychiatry. 2007;12(7):656–670. doi: 10.1038/sj.mp.4001957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Linnarsson S, Björklund A, Ernfors P. Learning deficit in BDNF mutant mice. European Journal of Neuroscience. 1997;9(12):2581–2587. [DOI] [PubMed] [Google Scholar]
- 55.Mu JS, Li WP, Yao ZB, Zhou XF. Deprivation of endogenous brain-derived neurotrophic factor results in impairment of spatial learning and memory in adult rats. Brain Research. 1999;835(2):259–265. [DOI] [PubMed] [Google Scholar]
- 56.Mizuno M, Yamada K, Olariu A, Nawa H, Nabeshima T. Involvement of brain-derived neurotrophic factor in spatial memory formation and maintenance in a radial arm maze test in rats. Journal of Neuroscience. 2000;20(18):7116–7121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alonso M, Vianna MRM, Depino AM, Mello e Souza T, Pereira P, Szapiro G, Viola H, Pitossi F, Izquierdo I, Medina JH. BDNF-triggered events in the rat hippocampus are required for both short- and long-term memory formation. Hippocampus. 2002;12(4):551–560. [DOI] [PubMed] [Google Scholar]
- 58.Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. European Journal of Neuroscience. 2004;20(10):2580–2590. [DOI] [PubMed] [Google Scholar]
- 59.Gomez-Pinilla F, Vaynman S, Ying Z. Brain-derived neurotrophic factor functions as a metabotrophin to mediate the effects of exercise on cognition. European Journal of Neuroscience. 2008;28(11):2278–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zheng F, Zhou X, Moon C, Wang H. Regulation of brain-derived neurotrophic factor expression in neurons. International Journal of Physiology, Pathophysiology, and Pharmacology. 2012;4(4):188–200. [PMC free article] [PubMed] [Google Scholar]
- 61.Baj G, Del Turco D, Schlaudraff J, Torelli L, Deller T, Tongiorgi E. Regulation of the spatial code for BDNF mRNA isoforms in the rat hippocampus following pilocarpine-treatment: A systematic analysis using laser microdissection and quantitative real-time PCR. Hippocampus. 2013;23(5):413–423. [DOI] [PubMed] [Google Scholar]
- 62.Baj G, Leone E, Chao MV, Tongiorgi E. Spatial segregation of BDNF transcripts enables BDNF to differentially shape distinct dendritic compartments. Proceedings of the National Academy of Sciences. 2011;108(40):16813–16818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tao X, Finkbeiner S, Arnold DB, Shaywitz AJ, Greenberg ME. Ca2+ influx regulates BDNF transcription by a CREB family transcription factor-dependent mechanism. Neuron. 1998;20(4):709–726. [DOI] [PubMed] [Google Scholar]
- 64.Martinowich K, Hattori D, Wu H, Fouse S, He F, Hu Y, Fan G, Sun YE. DNA methylation-related chromatin remodeling in activity-dependent BDNF gene regulation. Science. 2003;302(5646):890–893. [DOI] [PubMed] [Google Scholar]
- 65.Lubin FD, Roth TL, Sweatt JD. Epigenetic regulation of bdnf gene transcription in the consolidation of fear memory. Journal of Neuroscience. 2008;28(42):10576–10586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fuchikami M, Morinobu S, Kurata A, Yamamoto S, Yamawaki S. Single immobilization stress differentially alters the expression profile of transcripts of the brain-derived neurotrophic factor ( BDNF) gene and histone acetylation at its promoters in the rat hippocampus. International Journal of Neuropsychopharmacology. 2008;12(01):73. [DOI] [PubMed] [Google Scholar]
- 67.Liu YF, Chen HI, Wu CL, Kuo YM, Yu L, Huang AM, Wu FS, Chuang JI, Jen CJ. Differential effects of treadmill running and wheel running on spatial or aversive learning and memory: roles of amygdalar brain-derived neurotrophic factor and synaptotagmin I. The Journal of Physiology. 2009;587(13):3221–3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Duman CH, Schlesinger L, Russell DS, Duman RS. Voluntary exercise produces antidepressant and anxiolytic behavioral effects in mice. Brain Research. 2008;1199:148–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zouhal H, Jacob C, Delamarche P, Gratas-Delamarche A. Catecholamines and the effects of exercise, training and gender. Sports Medicine. 2008;38(5):401–423. [DOI] [PubMed] [Google Scholar]
- 70.Chatterton RT, Vogelsong KM, Lu YC, Ellman AB, Hudgens GA. Salivary alpha-amylase as a measure of endogenous adrenergic activity. Clinical Physiology. 1996;16(4):433–448. [DOI] [PubMed] [Google Scholar]
- 71.Goddard AW, Ball SG, Martinez J, Robinson MJ, Yang CR, Russell JM, Shekhar A. Current perspectives of the roles of the central norepinephrine system in anxiety and depression. Depression and Anxiety. 2010;27(4):339–350. [DOI] [PubMed] [Google Scholar]
- 72.Ludlow AT, Lima LCJ, Wang J, Hanson ED, Guth LM, Spangenburg EE, Roth SM. Exercise alters mRNA expression of telomere-repeat binding factor 1 in skeletal muscle via p38 MAPK. Journal of Applied Physiology. 2012;113(11):1737–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schefer V, Talan MI. Oxygen consumption in adult and AGED C57BL/6J mice during acute treadmill exercise of different intensity. Experimental Gerontology. 1996;31(3):387–392. [DOI] [PubMed] [Google Scholar]
- 74.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biology. 2002;3(7):RESEARCH0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nature Protocols. 2008;3(6):1101–1108. [DOI] [PubMed] [Google Scholar]
- 76.Barker GRI, Warburton EC. When is the hippocampus involved in recognition memory? Journal of Neuroscience. 2011;31(29):10721–10731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Archer T, Ögren SO, Johansson G, Ross SB. DSP4-induced two-way active avoidance impairment in rats: involvement of central and not peripheral noradrenaline depletion. Psychopharmacology. 1982. [DOI] [PubMed] [Google Scholar]
- 78.Jonsson G, Hallman H, Ponzio F, Ross S. DSP4 (N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine)—A useful denervation tool for central and peripheral noradrenaline neurons. European Journal of Pharmacology. 1981;72(2–3):173–188. [DOI] [PubMed] [Google Scholar]
- 79.Ross SB. Long-term effects of N-2-chlorethyl-N-ethyl-2-bromobenzylamine hydrochloride on noradrenergic neurones in the rat brain and heart. British Journal of Pharmacology. 1976;58(4):521–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zahniser NR, Weiner GR, Worth T, Philpott K, Yasuda RP, Jonsson G, Dunwiddie TV. DSP4-induced noradrenergic lesions increase beta-adrenergic receptors and hippocampal electrophysiological responsiveness. Pharmacology Biochemistry and Behavior. 1986;24(5):1397–1402. [DOI] [PubMed] [Google Scholar]
- 81.Szot P, Miguelez C, White SS, et al. A comprehensive analysis of the effect of DSP4 on the locus coeruleus noradrenergic system in the rat. Neuroscience. 2010;166(1):279–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Anisman H, Beauchamp C, Zacharko RM. Effects of inescapable shock and norepinephrine depletion induced by DSP4 on escape performance. Psychopharmacology. 1984;83(1):56–61. [DOI] [PubMed] [Google Scholar]
- 83.Bennett MC, Kaleta-Michaels S, Arnold M, McGaugh JL. Impairment of active avoidance by the noradrenergic neurotoxin, DSP4: attenuation by post-training epinephrine. Psychopharmacology. 1990;101(4):505–510. [DOI] [PubMed] [Google Scholar]
- 84.Ögren SO, Archer T, Ross SB. Evidence for a role of the locus coeruleus noradrenaline system in learning. Neuroscience Letters. 1980;20(3):351–356. [DOI] [PubMed] [Google Scholar]
- 85.Scullion GA, Kendall DA, Sunter D, Marsden CA, Pardon MC. Central noradrenergic depletion by DSP-4 prevents stress-induced memory impairments in the object recognition task. Neuroscience. 2009;164(2):415–423. [DOI] [PubMed] [Google Scholar]
- 86.Ross SB, Stenfors C. DSP4, a selective neurotoxin for the locus coeruleus noradrenergic system. A review of its mode of action. Neurotoxicity Research. 2014;27(1):15–30. [DOI] [PubMed] [Google Scholar]
- 87.Mizutani K, Sonoda S, Wakita H, Shimpo K. Protein Kinase C activator, Bryostatin-1, promotes exercise-dependent functional recovery in rats with cerebral infarction. American Journal of Physical Medicine & Rehabilitation. 2015;94(3):239–243. [DOI] [PubMed] [Google Scholar]
- 88.King SO, Williams CL. Novelty-induced arousal enhances memory for cued classical fear conditioning: Interactions between peripheral adrenergic and brainstem glutamatergic systems. Learning & Memory. 2009;16(10):625–634. doi: 10.1101/lm.1513109. [DOI] [PubMed] [Google Scholar]
- 89.Sara SJ, Vankov A, Hervé A. Locus coeruleus-evoked responses in behaving rats: a clue to the role of noradrenaline in memory. Brain Research Bulletin. 1994;35(5–6):457–465. [DOI] [PubMed] [Google Scholar]
- 90.Goekint M, Bos I, Heyman E, Meeusen R, Michotte Y, Sarre S. Acute running stimulates hippocampal dopaminergic neurotransmission in rats, but has no influence on brain-derived neurotrophic factor. Journal of Applied Physiology. 2012;112(4):535–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dishman RK, Renner KJ, White-Welkley JE, Burke KA, Bunnell BN. Treadmill exercise training augments brain norepinephrine response to familiar and novel stress. Brain Research Bulletin. 2000;52(5):337–342. [DOI] [PubMed] [Google Scholar]
- 92.Molteni R, Ying Z, Gomez-Pinilla F. Differential effects of acute and chronic exercise on plasticity-related genes in the rat hippocampus revealed by microarray. European Journal of Neuroscience. 2002;16(6):1107–1116. [DOI] [PubMed] [Google Scholar]
- 93.Ni H, Li C, Tao L-Y, Cen J-N. Physical exercise improves learning by modulating hippocampal mossy fiber sprouting and related gene expression in a developmental rat model of penicillin-induced recurrent epilepticus. Toxicology Letters. 2009;191(1):26–32. [DOI] [PubMed] [Google Scholar]
- 94.Venezia AC, Guth LM, Sapp RM, Spangenburg EE, Roth SM. Sex-dependent and independent effects of long-term voluntary wheel running on Bdnf mRNA and protein expression. Physiology & Behavior. 2016;156:8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Berchtold N, Chinn G, Chou M, Kesslak J, Cotman C. Exercise primes a molecular memory for brain-derived neurotrophic factor protein induction in the rat hippocampus. Neuroscience. 2005;133(3):853–861. [DOI] [PubMed] [Google Scholar]
- 96.Ding Q, Ying Z, Gómez-Pinilla F. Exercise influences hippocampal plasticity by modulating brain-derived neurotrophic factor processing. Neuroscience. 2011;192:773–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Neeper SA, Gomez-Pinilla F, Choi J, Cotman CW. Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain Research. 1996;726(1–2):49–56. [PubMed] [Google Scholar]
- 98.Sartori CR, Vieira AS, Ferrari EM, Langone F, Tongiorgi E, Parada CA. The antidepressive effect of the physical exercise correlates with increased levels of mature BDNF, and proBDNF proteolytic cleavage-related genes, p11 and tPA. Neuroscience. 2011;180:9–18. [DOI] [PubMed] [Google Scholar]
- 99.Vaynman S, Ying Z, Gómez-Pinilla F. Interplay between brain-derived neurotrophic factor and signal transduction modulators in the regulation of the effects of exercise on synaptic-plasticity. Neuroscience. 2003;122(3):647–657. [DOI] [PubMed] [Google Scholar]
- 100.Rossi C, Angelucci A, Costantin L, Braschi C, Mazzantini M, Babbini F, Fabbri ME, Tessarollo L, Maffei L, Berardi N, Caleo M. Brain-derived neurotrophic factor (BDNF) is required for the enhancement of hippocampal neurogenesis following environmental enrichment. European Journal of Neuroscience. 2006;24(7):1850–1856. [DOI] [PubMed] [Google Scholar]
- 101.Vivar C, Potter MC, van Praag H. All about running: synaptic plasticity, growth factors and adult hippocampal neurogenesis. Current Topics in Behavioral Neurosciences. 2012; 15:189–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cotman CW, Berchtold NC, Christie L-A. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends in Neurosciences. 2007;30(9):464–472. [DOI] [PubMed] [Google Scholar]
- 103.Tao X, West AE, Chen WG, Corfas G, Greenberg ME. A calcium-responsive transcription factor, CaRF, that regulates neuronal activity-dependent expression of BDNF. Neuron. 2002;33(3):383–395. [DOI] [PubMed] [Google Scholar]
- 104.Marmigère F, Givalois L, Rage F, Arancibia S, Tapia-Arancibia L. Rapid induction of BDNF expression in the hippocampus during immobilization stress challenge in adult rats. Hippocampus. 2003;13(5):646–655. [DOI] [PubMed] [Google Scholar]
- 105.Zajac MS, Pang TYC, Wong N, Weinrich B, Leang LS, Craig JM, Saffery R, Hannan AJ. Wheel running and environmental enrichment differentially modify exon-specific BDNF expression in the hippocampus of wild-type and pre-motor symptomatic male and female Huntington’s disease mice. Hippocampus. 2009; 20(5): 621–36. [DOI] [PubMed] [Google Scholar]
- 106.Oliff HS, Berchtold NC, Isackson P, Cotman CW. Exercise-induced regulation of brain-derived neurotrophic factor (BDNF) transcripts in the rat hippocampus. Molecular Brain Research. 1998;61(1):147–153. [DOI] [PubMed] [Google Scholar]
- 107.Rasmussen P, Brassard P, Adser H, Pedersen MV, Leick L, Hart E, Secher NH,Pedersen BK, Pilegaard H. Evidence for a release of brain-derived neurotrophic factor from the brain during exercise. Experimental Physiology. 2009;94(10):1062–1069. [DOI] [PubMed] [Google Scholar]
- 108.Fuss J, Steinle J, Bindila L, Auer MK, Kirchherr H, Lutz B, Gass P. A runner’s high depends on cannabinoid receptors in mice. Proceedings of the National Academy of Sciences, 2015; 112(42): 13105–13108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Crawley JN. Exploratory behavior models of anxiety in mice. Neuroscience & Biobehavioral Reviews. 1985;9(1):37–44. [DOI] [PubMed] [Google Scholar]
- 110.Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. European Journal of Pharmacology. 2003;463(1–3):3–33. [DOI] [PubMed] [Google Scholar]
- 111.Kalueff AV, Stewart AM, Song C, Berridge KC, Graybiel AM, Fentress JC. Neurobiology of rodent self-grooming and its value for translational neuroscience. Nature Reviews Neuroscience. 2015;17(1):45–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sothmann MS, Buckworth J, Claytor RP, Cox RH, White-Welkley JE, Dishman RK. Exercise training and the cross-stressor adaptation hypothesis. Exercice and Sport Sciences Reviews. 1996;24:267–287. [PubMed] [Google Scholar]
- 113.Sciolino NR, Holmes PV. Exercise offers anxiolytic potential: A role for stress and brain noradrenergic-galaninergic mechanisms. Neuroscience & Biobehavioral Reviews. 2012;36(9):1965–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fuss J, Ben Abdallah NMB, Hensley FW, Weber K-J, Hellweg R, Gass P. Deletion of running-induced hippocampal neurogenesis by irradiation prevents development of an anxious phenotype in mice. PLoS ONE. 2010;5(9):e12769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fuss J, Ben Abdallah NMB, Vogt MA, Touma C,Pacifici PG, Palme R, Witzemann V, Hellweg R, Gass P. Voluntary exercise induces anxiety-like behavior in adult C57BL/6J mice correlating with hippocampal neurogenesis. Hippocampus. 2009; 20(3):364–76. [DOI] [PubMed] [Google Scholar]
- 116.Onksen JL, Briand LA, Galante RJ, Pack AI, Blendy JA. Running-induced anxiety is dependent on increases in hippocampal neurogenesis. Genes Brain Behav. 2012;11(5):529–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Salam JN, Fox JH, Detroy EM, Guignon MH, Wohl DF, Falls WA. Voluntary exercise in C57 mice is anxiolytic across several measures of anxiety. Behavioural Brain Research. 2009;197(1):31–40. [DOI] [PubMed] [Google Scholar]
- 118.Inoue T, Nakagawa S, Izumi T, Kitaichi Y, Koyama T. Effect of combined treatment with noradrenaline and serotonin reuptake inhibitors on conditioned freezing. European Journal of Pharmacology. 2006;540(1–3):91–95. [DOI] [PubMed] [Google Scholar]
- 119.Dazzi L, Seu E, Cherchi G, Biggio G. Antagonism of the stress-induced increase in cortical norepinephrine output by the selective norepinephrine reuptake inhibitor reboxetine. European Journal of Pharmacology. 2003;476(1–2):55–61. [DOI] [PubMed] [Google Scholar]
- 120.Hayes K, Sprague S, Guo M, Davis W, Friedman A, Kumar A, Jimenez DF, Ding Y. Forced, not voluntary, exercise effectively induces neuroprotection in stroke. Acta Neuropathologica. 2008; 115, 289–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ke Z, Yip SP, Li L, Zheng X-X, Tong K-Y. The effects of voluntary, involuntary, and forced exercises on brain-derived neurotrophic factor and motor function recovery: a rat brain ischemia model. PLoS ONE 2011; 6, e16643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Liu Y-F, Chen H-I, Wu C-L, Kuo Y-M, Yu L, Huang A-M, Wu F-S, Chuang J-I, Jen CJ. Differential effects of treadmill running and wheel running on spatial or aversive learning and memory: roles of amygdalar brain-derived neurotrophic factor and synaptotagmin I. Journal of Physiology. 2009; 587. 3221–3231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ploughman M, Granter-Button S, Chernenko G, Tucker BA, Mearow KM, Corbett D. Endurance exercise regimens induce differential effects on brain-derived neurotrophic factor, synapsin-I and insulin-like growth factor I after focal ischemia. Neuroscience 2005;136. 991–1001 [DOI] [PubMed] [Google Scholar]
- 124.Harro J, Häidkind R, Harro M, Modiri AR, Gillberg PG, Pähkla R, Matto V, Oreland L. Chronic mild unpredictable stress after noradrenergic denervation: attenuation of behavioural and biochemical effects of DSP-4 treatment. European Neuropsychopharmacology. 1999;10(1):5–16. [DOI] [PubMed] [Google Scholar]
- 125.O’connor PJ, Smith JC, Morgan WP. Physical activity does not provoke panic attacks in patients with panic disorder: A review of the evidence. Anxiety, Stress, and Coping. 2000; 13(4):333–53. [Google Scholar]
- 126.Ensari I, Greenlee TA, Motl RW, Petruzzello SJ. Meta-analysis of acute exercise effects on state anxiety: an update of randomized controlled trials over the past 25 years. Depression and Anxiety. 2015;32(8):624–634. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


