Abstract
This cohort study examines the incidence of cancer diagnosis and risk factors among patients on a daily regimen of insulin.
Previous studies among persons with type 1 diabetes (T1D) found a higher incidence of certain cancers in this population compared with the general population.1 However, no studies have evaluated the risk factors of cancer incidence in T1D. In the present study, using data from the Diabetes Control and Complications Trial (DCCT) and the Epidemiology of Diabetes Interventions and Complications (EDIC) study, we explored the associations of risk factors with cancer incidence in patients with T1D over a 28-year follow-up period.
Methods
Detailed descriptions of the DCCT/EDIC study have been published.2 Briefly, 1441 patients from 29 centers in North America were enrolled in the DCCT between 1983 and 1989. At the end of the DCCT in 1993, 1375 surviving participants volunteered to continue in the EDIC follow-up study. By year 18 of the EDIC study in 2012, 1304 patients had completed the annual cancer history update, and 1 patient with a cancer diagnosis before enrollment was excluded. This cohort study was approved by The Ohio University Office of Research Compliance, which waived the informed consent requirement because publicly available data were used. We followed the STROBE reporting guideline.
Cancer incidence was calculated with 95% CIs. Associations of risk factors (eMethods in the Supplement) with cancer incidence were evaluated individually using Cox proportional hazards regression models adjusted for age and sex. Race and ethnicity were collected but not analyzed because most participants were White individuals. Two-sided P < .05 was considered significant, and statistically significant variables were included in multivariable models for further assessment. Because daily insulin dose (time-dependent variable) remained significant in the multivariable model, it was also examined as a fixed variable (mean dose during follow-up). We examined cancer incidence by 3 categories of mean daily insulin dose: low (<0.5 units/kg), medium (≥0.5 and <0.8 units/kg), and high (≥0.8 units/kg).
Results
Of the 1303 patients in the cohort, 93 (7%) had cancer diagnoses after a total of 33 813 person-years follow-up, and the incidence rate was 2.8 (95% CI, 2.2-3.3) per 1000 person-years. At first diagnosis, the mean age was 50 years, and the mean duration of diabetes was 25 years. Among 93 patients, 57 were female individuals (61%), 36 were male individuals (39%), 8 (9%) developed cancer within 10 years, 31 (33%) developed cancer between 11 and 20 years, and 54 (58%) developed cancer between 21 and 28 years.
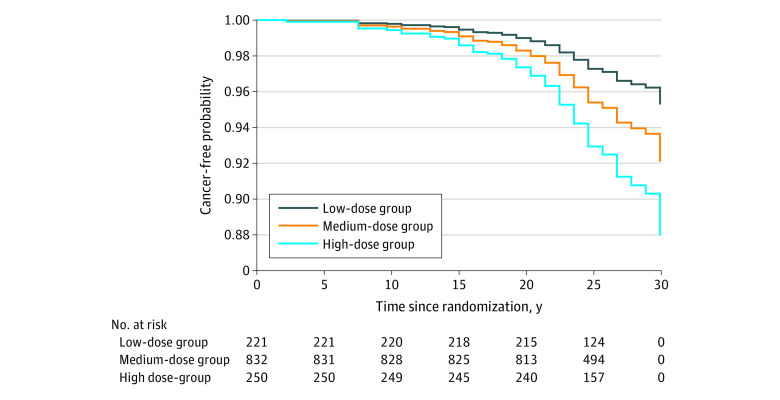
Age and sex were associated with cancer incidence (hazard ratio [HR] for age, 1.08 [95% CI, 1.05-1.12]; for female sex, 1.74 [95% CI, 1.15-2.64]). Among other variables, exercising habit and high-density lipoprotein cholesterol were inversely associated and daily insulin dose was associated with cancer incidence after adjusting for age and sex. Daily insulin dose remained associated with cancer incidence in the multivariable model 1 (HR, 5.93; 95% CI, 1.21-29.06) and model 2 (HR, 4.13; 95% CI, 1.13-15.17) (Table). Cancer incidence was 2.11, 2.87, and 2.91 per 1000 person-years in the low-, medium-, and high-dose groups, respectively (Figure).
Table. Daily Insulin Dose and Cancer Incidence in Multivariable Models.
| Variable | Model 1a | Model 2b | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age, y | 1.11 (1.06-1.15) | <.001 | 1.09 (1.06-1.13) | <.001 |
| Female vs male sex | 2.39 (1.30-4.42) | .005 | 2.02 (1.28-3.19) | .003 |
| Exercise (moderate or strenuous vs sedentary) | 0.63 (0.36-1.10) | .10 | 0.31 (0.16-0.59) | .001 |
| HDL-C, mg/dL | 0.98 (0.95-1.00) | .10 | 1.00 (0.98-1.02) | .84 |
| Daily insulin dose, units/kg | 5.93 (1.21-29.06) | .03 | 4.13 (1.13-15.17) | .03 |
Abbreviations: HDL-C, high-density lipoprotein cholesterol; HR, hazard ratio.
SI conversion factor: To convert HDL-C to millimoles per liter, multiply by 0.0259.
Model 1 included time-dependent variables.
Model 2 included time-fixed variables (mean daily insulin dose and HDL-C level during follow-up, and proportion of having exercise during follow-up).
Figure. Cancer-Free Probability by Daily Insulin Dose Over 28 Years of Follow-up.
Probability was adjusted for age and sex. Among 1303 patients, 93 had cancer diagnoses, including skin (n = 27), other (n = 21), breast (n = 15), reproductive (n = 8), digestive (n = 6), head and neck (n = 5), bone or blood (n = 4), prostate (n = 4), urinary (n = 2), thoracic (n = 2), and unknown (n = 2).
Discussion
Hyperinsulinemia is a recognized risk factor for cancer.3 However, clinical data in type 2 or unspecified diabetes cohorts showed conflicting results; the association was suggested in several studies.4,5 A meta-analysis reported no association between exogenous insulin treatment and cancer risk in 13 of 16 studies, although 4 studies found an association between glargine and breast cancer.6 Daily insulin doses in those cohorts were low (usually <0.3 units/kg), and many patients discontinued insulin temporarily or permanently during follow-up.6
This study showed that daily insulin dose was associated with cancer risk in T1D. The HRs were significantly higher in the high-dose vs low-dose group.
A limitation of this study is the relatively small sample size, which precluded analyses with specific cancer types and resulted in a wide CI for daily insulin dose. Moreover, the association found may be subject to residual confounding and was not necessarily causal. Furthermore, larger studies in T1D are needed to validate this association.
eMethods.
References
- 1.Carstensen B, Read SH, Friis S, et al. ; Diabetes and Cancer Research Consortium . Cancer incidence in persons with type 1 diabetes: a five-country study of 9,000 cancers in type 1 diabetic individuals. Diabetologia. 2016;59(5):980-988. doi: 10.1007/s00125-016-3884-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Epidemiology of Diabetes Interventions and Complications (EDIC) . Epidemiology of Diabetes Interventions and Complications (EDIC). Design, implementation, and preliminary results of a long-term follow-up of the Diabetes Control and Complications Trial cohort. Diabetes Care. 1999;22(1):99-111. doi: 10.2337/diacare.22.1.99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sciacca L, Vella V, Frittitta L, et al. Long-acting insulin analogs and cancer. Nutr Metab Cardiovasc Dis. 2018;28(5):436-443. doi: 10.1016/j.numecd.2018.02.010 [DOI] [PubMed] [Google Scholar]
- 4.Holden SE, Jenkins-Jones S, Morgan CL, Schernthaner G, Currie CJ. Glucose-lowering with exogenous insulin monotherapy in type 2 diabetes: dose association with all-cause mortality, cardiovascular events and cancer. Diabetes Obes Metab. 2015;17(4):350-362. doi: 10.1111/dom.12412 [DOI] [PubMed] [Google Scholar]
- 5.Hemkens LG, Grouven U, Bender R, et al. Risk of malignancies in patients with diabetes treated with human insulin or insulin analogues: a cohort study. Diabetologia. 2009;52(9):1732-1744. doi: 10.1007/s00125-009-1418-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu JW, Filion KB, Azoulay L, Doll MK, Suissa S. Effect of long-acting insulin analogs on the risk of cancer: a systematic review of observational studies. Diabetes Care. 2016;39(3):486-494. doi: 10.2337/dc15-1816 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods.