Key Points
Question
Is treatment with combined polyadenosine diphosphate-ribose polymerase and immune checkpoint inhibition active and safe in patients with recurrent mismatch repair proficient endometrial cancer (MMRP EC)?
Findings
In this single-arm, phase 2, 2-stage, nonrandomized clinical trial of treatment with avelumab and talazoparib in recurrent MMRP EC that included 35 patients, the confirmed objective response rate was 11.4%, and the progression-free survival at 6 months rate was 22.9%. No patients discontinued therapy because of toxic effects, and immunogenomic profiling provided insights into subsets of patients who may derive benefit from this combination.
Meaning
These study findings suggest that treatment with avelumab and talazoparib has a favorable toxic effects profile and support further investigation in certain subsets of patients with recurrent MMRP EC.
Abstract
Importance
Although the activity of pembrolizumab and lenvatinib (the only US Food and Drug Administration–approved immunotherapy for mismatch repair proficient endometrial cancer [MMRP EC]) is compelling, there are no biomarkers of response and most patients do not tolerate, do not respond to, or develop resistance to this regimen, highlighting the need for additional, potentially biomarker-driven therapeutic approaches for patients with recurrent MMRP EC.
Objective
To assess the potential positive outcomes and safety of the combination of the polyadenosine diphosphate-ribose polymerase inhibitor talazoparib and the programmed cell death ligand 1 (PD-L1) inhibitor avelumab in recurrent MMRP EC.
Design, Settings, and Participants
This investigator-initiated, open-label, single-arm, 2-stage, phase 2 study nonrandomized controlled trial patients at 4 institutions in the US. Key eligibility criteria included measurable disease, unlimited prior therapies, and all endometrial cancer histologies.
Interventions
Talazoparib, 1 mg, orally, daily, and avelumab, 10 mg/kg, intravenously, every 2 weeks, were administered until disease progression or unacceptable toxic effects.
Main Outcomes and Measures
Statistical considerations were developed for 2 coprimary objectives of objective response rate and rate of progression-free survival at 6 months, with a 2-stage design that allowed for early discontinuation for futility. Prespecified exploratory objectives included the association of immunogenomic features (determined by targeted-panel next-generation sequencing and immunohistochemistry) with activity.
Results
Thirty-five female patients (mean [SD] age, 67.9 [8.41] years) received protocol therapy; 9 (25.7%) derived clinical benefit after meeting at least 1 of the 2 coprimary end points. Four patients (11.4%) exhibited confirmed objective response rates (4 partial responses), and 8 (22.9%) survived progression free at 6 months. The most common grade 3 and 4 treatment-related toxic effects were anemia (16 [46%]), thrombocytopenia (10 [29%]), and neutropenia (4 [11%]); no patient discontinued receipt of therapy because of toxic effects. Tumors with homologous recombination repair alterations were associated with clinical benefit from treatment with avelumab and talazoparib. Tumor mutational burden, tumor-infiltrating lymphocytes, and PD-L1 status were not associated with clinical benefit.
Conclusions and Relevance
The results of this nonrandomized controlled trial suggest that treatment with avelumab and talazoparib demonstrated a favorable toxic effect profile and met the predetermined criteria to be considered worthy of further evaluation in MMRP EC. Immunogenomic profiling provided insights that may inform ongoing and future studies of polyadenosine diphosphate-ribose polymerase and PD-L1 inhibitor combinations in endometrial cancer.
Trial Registration
ClinicalTrials.gov Identifier: NCT02912572
This nonrandomized clinical trial examines the potential positive outcomes and safety of the combination of talazoparib and avelumab in recurrent mismatch repair proficient endometrial cancer.
Introduction
Unlike hypermutated mismatch repair deficient and polymerase epsilon-mutated endomterial cancer (EC) tumors, mismatch repair proficient (MMRP) tumors respond poorly to immune checkpoint inhibitor monotherapy.1,2,3 Several potential synergistic immune checkpoint inhibitor combinations are being investigated in an effort to promote effective antitumor immunity in MMRP ECs, and one such combination (lenvatinib and pembrolizumab) already has US Food and Drug Administration approval in this setting.4
Preclinical studies have demonstrated synergistic antitumor activity for combinations of polyadenosine diphosphate-ribose polymerase inhibitors (PARPi) with programmed cell death 1 (PD-1)/programmed cell death ligand 1 (PD-L1) inhibitors (PD-1/PD-L1) that is, at least partly, mediated by activation of the stimulator of interferon genes pathway.5 In this article, we evaluated whether the combination of treatment with the PARPi talazoparib and the PD-L1i avelumab would demonstrate promising activity and acceptable toxic effects in recurrent MMRP EC.
Methods
Study Design and Procedures
The primary objective of this investigator-initiated phase 2 study was to evaluate the activity of avelumab and talazoparib as determined by the frequency of patients who survived progression free for 6 months or longer after initiating protocol therapy or had objective response (OR) by Response Evaluation Criteria in Solid Tumours (RECIST), version 1.1 (Supplement 1). Secondary end points included progression-free survival (PFS) and toxic effects. Patients received avelumab, 10 mg/kg, intravenously, every 2 weeks, and talazoparib, 1 mg, orally, daily, continuously until progression or experiencing toxic effects. The study was approved by the institutional review boards of all participating institutions, and participants provided written informed consent. Eligible participants had recurrent or persistent EC of any histology that was MMRP as determined by immunohistochemistry (eMethods in Supplement 2).
Biomarker Evaluation and Statistical Analysis
Immunogenomic profiling was performed on archival specimens from participating patients (eResults in Supplement 2). Statistical considerations were developed for the coprimary objectives of OR rate (ORR) and PFS at a 6-month rate, with a 2-stage design using the method of Sill et al6 that allowed for early discontinuation for futility. A true ORR of 5% or less and PFS at 6 months rate of 10% or less would not be of clinical interest (H0: πOR of ≤5% and πPFS at 6 months of ≤10%), whereas an improvement to a 20% ORR or 30% PFS at 6 months rate would warrant further investigation of treatment with avelumab and talazoparib. Overall, if a response of 4 or more patients with an OR and/or 8 or more patients who survived progression free at 6 months was observed, avelumab and talazoparib would be considered worthy of further investigation.
Binary end points were summarized by count and percentage and the 95% CI; their associations with biomarkers were evaluated by the Fisher exact test. Kaplan-Meier estimates with 95% CIs and the log-rank test were used to summarize time-to-event end points and assess their associations with biomarkers, respectively. Statistical analyses were conducted using R (version 4.0.2; R Foundation).
Results
Antitumor Activity
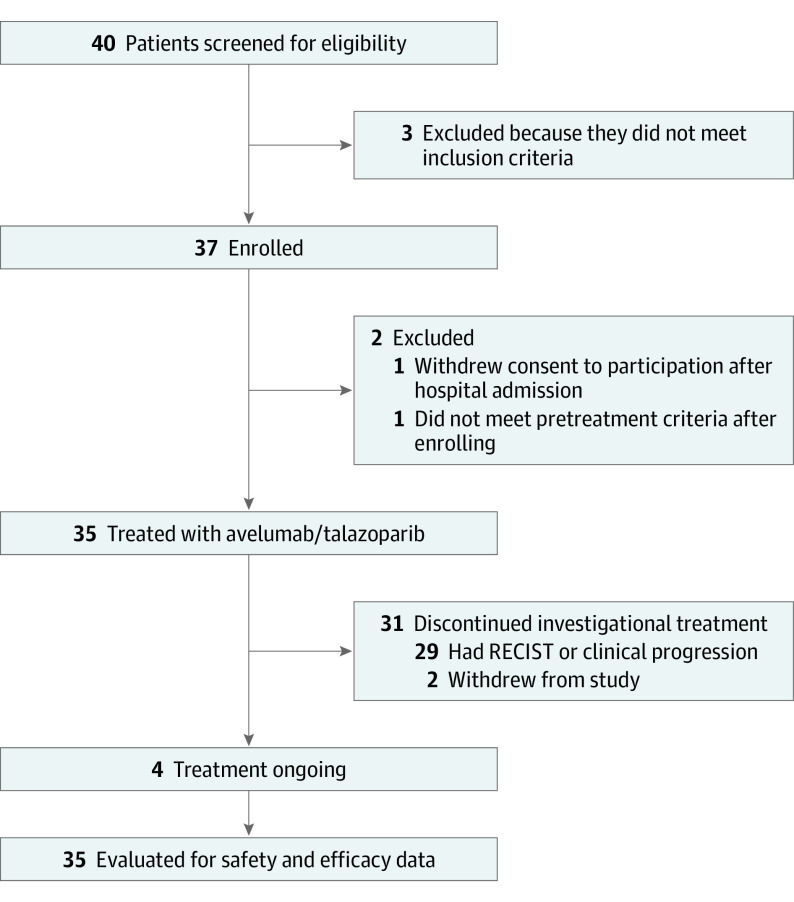
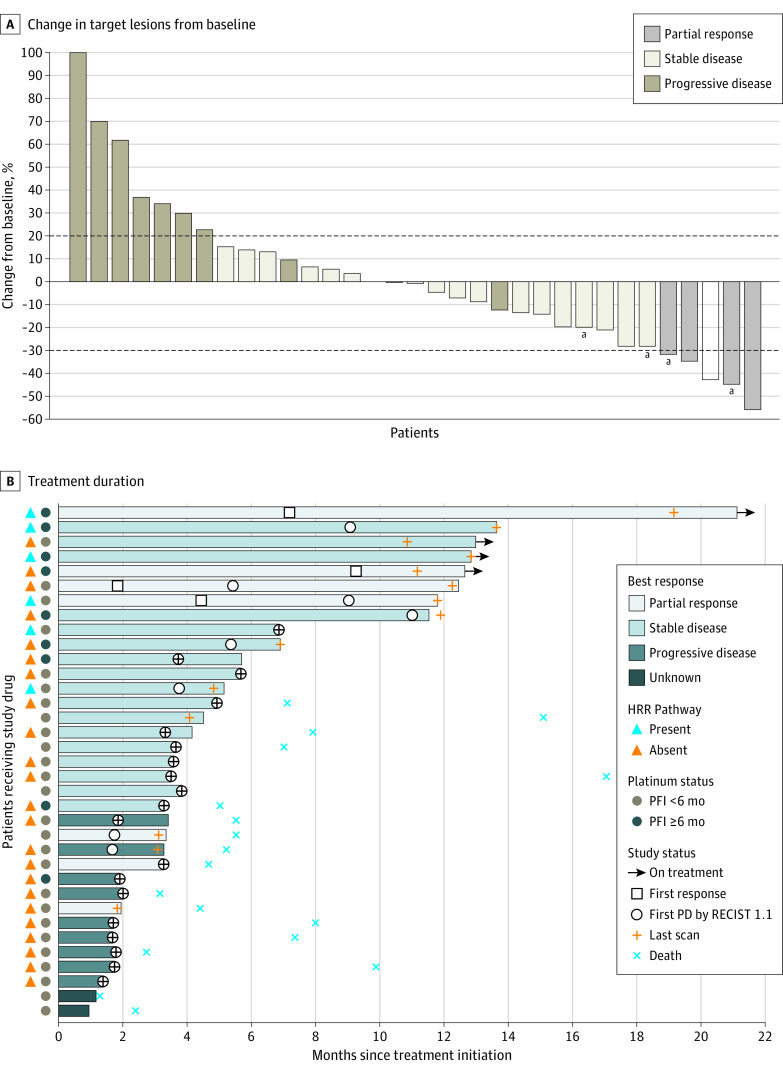
Between February 21, 2019, and December 4, 2019, 35 patients (Figure 1; eTable 1 in Supplement 2) were enrolled and received protocol therapy as the study met criteria to proceed to the second stage. All patients had previously received carboplatin/paclitaxel chemotherapy. Four confirmed ORs (ORR, 11.4%; 95% CI, 3.2%-26.7%) were observed (Figure 2A), all of which were partial responses (2 in grade 2 endometrioid adenocarcinomas and 2 in serous carcinomas). Twenty patients (57.1%) had stable disease of any duration (median duration of stable disease was 3.8 months [range, 1.7-12.8 months]), 9 (25.7%) had progressive disease and 2 were unevaluable, as they discontinued participating in the trial before the first postbaseline imaging because of clinical progression and transitioning to hospice care (eTable 2 in Supplement 2). Eight patients (22.9%; 95% CI, 10.4%-40.1%) survived progression free at 6 months, which was the other coprimary end point of the study (1 carcinosarcoma, 1 clear cell, 3 endometrioid, and 3 serous carcinomas; eTable 2 in Supplement 2). As of the data cutoff date of November 30, 2020, the median PFS was 3.6 months (95% CI, 2.4-5.4 months) with a median follow-up of 12.9 months (range, 1.3-20.9 months) (eFigure 1 in Supplement 2). Overall, 9 patients (25.7%) derived clinical benefit from treatment with avelumab and talazoparib after meeting 1 or more of the 2 coprimary end points (OR PFS at 6 months). Four patients continued to receive the protocol treatment at the data cutoff date, all after 12 months (Figure 2B).
Figure 1. Study Overview.
RECIST indicates Response Evaluation Criteria in Solid Tumours.
Figure 2. Antitumor Activity of Avelumab and Talazoparib.
A, Best change in target lesions from baseline in all patients evaluable for Response Evaluation Criteria in Solid Tumours (RECIST), version 1.1 response (data cutoff date, November 30, 2020). The dotted lines at −30% and 20% indicate partial response and progression per RECIST, respectively. B, Avelumab and talazoparib treatment duration. Presence or absence of homologous recombination repair (HRR) alterations and platinum-free interval (PFI) of 6 months or longer vs PFI of less than 6 months is indicated for each patient. PD indicates programmed cell death.
aPatients still receiving protocol therapy as of the data cutoff date.
Safety
Treatment-related adverse events (TRAEs) in 10% or more of patients, and all grade 3 or higher TRAEs are presented in eTable 3 in Supplement 2. The most common grade 3 TRAEs were anemia (16 [46%]), thrombocytopenia (10 [29%]), and neutropenia (4 [11%]). Thrombocytopenia was the only treatment-related grade 4 toxic effect (3 [9%]); there were no grade 5 TRAEs. Six patients (17%) had dose reductions owing to toxic effects; no patients discontinued receipt of therapy because of toxic effects. Serious adverse events were observed in 9 of 35 participants (25.7%); the 2 most frequent were decreased platelet counts (3 patients) and small intestinal obstruction (3 patients), which were not associated with treatment with avelumab and talazoparib.
Biomarker Analyses
Biomarker analyses are presented in the eResults in Supplement 2. In prespecified exploratory analyses, there was no association between PD-L1 status, tumor-infiltrating lymphocytes (TILs), and tumor mutational burden with outcomes (eTable 5 in Supplement 2). No tumor was polymerase epsilon mutated. Furthermore, patients with homologous recombination repair (HRR)–altered tumors were more likely to derive clinical benefit compared with non–HRR-altered tumors (83.3% vs 17.4%, respectively; P = .01; eTable 4 in Supplement 2) and exhibited better PFS (P = .03; supporting data available in Figure 3A).
Figure 3. Progression-Free Survival.
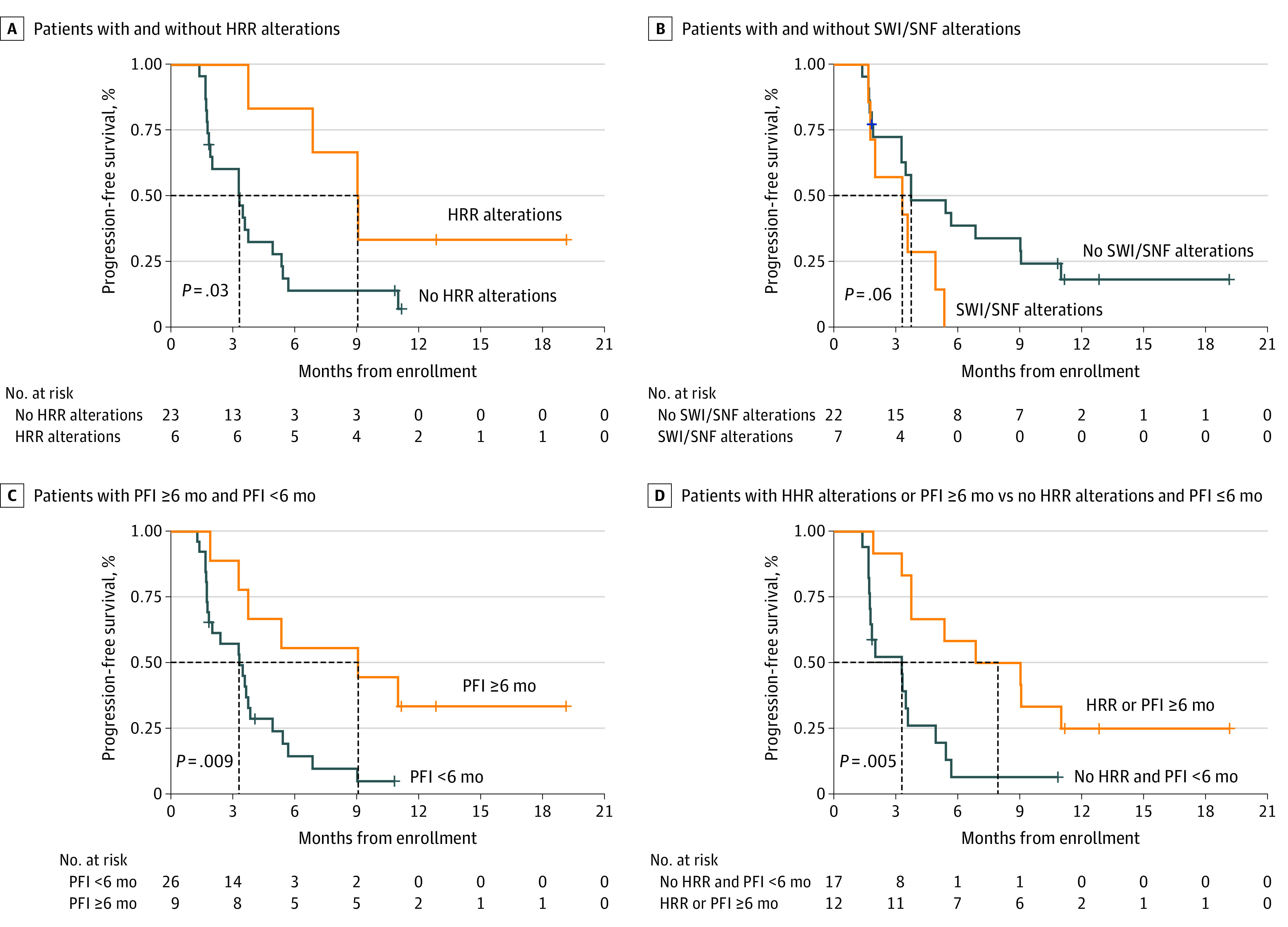
A, Patients with and without homologous recombination repair (HRR) alterations. B, Patients with and without switch/sucrose nonfermentable complex (SWI/SNF) alterations. C, Patients with a platinum-free interval (PFI) of 6 months or longer and PFI of less than 6 months. D, Patients with HRR-altered tumors or PFI of 6 months or longer vs those with no HRR alterations and a PFI of less than 6 months.
In post hoc analyses, a platinum-free interval (PFI, defined as the time between the last cycle of platinum and disease progression) of 6 months or longer was associated with clinical benefit from treatment with avelumab and talazoparib (55.6% vs 15.4%; P = .03; eTable 4 in Supplement 2) and better PFS (Figure 3C). Conversely, presence of ARID1A and other switch/sucrose nonfermentable complex alterations was associated with a trend toward absence of clinical benefit (0% vs 40.9%; P = .07; eTable 4 in Supplement 2) and worse PFS (Figure 3B).
Overall, 7 of the 9 patients who derived benefit from treatment with avelumab and talazoparib exhibited a PFI of 6 months or longer and/or HRR alterations. Of the 2 remaining patients, 1 patient harbored a hotspot U2AF1 spliceosome gene variation; this tumor had an immune-inflamed phenotype with high TILs and positive PD-L1 (combined positive score, 30) (eFigure 2 in Supplement 2).
Discussion
Although the activity of pembrolizumab and lenvatinib (the only US Food and Drug Administration–approved immunotherapy for MMRP EC) is compelling, there are no biomarkers of response and most patients do not tolerate, do not respond to, or develop resistance to this regimen, highlighting the need for additional, potentially biomarker-driven therapeutic approaches for patients with recurrent MMRP EC. In this investigator-initiated phase 2 study, treatment with avelumab and talazoparib demonstrated overall modest activity compared with pembrolizumab and lenvatinib in a biomarker-unselected patient population with recurrent MMRP EC.4 However, immunogenomic profiling provided useful insights in terms of potentially defining a subset of patients who were most likely to benefit from treatment with avelumab and talazoparib. To that end, HRR alterations were identified in approximately 20% of MMRP ECs (6 of 29 tumors profiled by targeted next-generation sequencing) and were associated with clinical benefit from treatment with avelumab and talazoparib (in 5 of 6 patients) and a median PFS of 9.1 months. Although other studies have reported HRR alterations in EC,7,8,9 to our knowledge, this is the first study prospectively evaluating a PARPi-containing regimen specifically in EC and demonstrating activity in ECs with HRR alterations. Furthermore, HRR alterations, together with the mechanistically relevant biomarker of a PFI of 6 months or longer (in a post hoc analysis), defined a sizeable subset of patients who benefited from treatment with avelumab and talazoparib with an ORR of 25%, PFS at 6 months of 58.3%, and median PFS of 8 months (Figure 3D), which was comparable with that of pembrolizumab and lenvatinib.4 This subset accounted for 3 of 4 ORs, 3 of 4 patients who continued to receive protocol therapy as of the cutoff date (all for >12 months) and 7 of 9 patients who derived clinical benefit. Conversely, tumor mutational burden, TILs, and PD-L1 status were not associated with clinical benefit. Finally, the absence of benefit among patients with ARID1A-variated ECs and the durable response of 1 patient with a hotspot U2AF1 spliceosome gene variant (which may have led to induction of a T-cell–mediated antitumor response owing to enhanced abnormal alternative splicing as reflected by the immune-inflamed phenotype of this tumor10,11,12) warrant further investigation.
Limitations
We acknowledge certain limitations of this study, including the small number of patients and the fact that the biomarker analyses were exploratory. We also acknowledge the hypothesis-generating nature of the biomarker analyses that would require independent prospective validation in larger confirmatory studies to be adequately powered.
Conclusions
In this nonrandomized clinical trial, treatment with avelumab and talazoparib met the prespecified criteria to be considered worthy of further investigation in patients with recurrent MMRP EC. Tumor genomic profiling identified a sizeable subset of patients (those with HRR alterations and/or a PFI of ≥6 months) who may benefit from treatment with avelumab and talazoparib and supports prioritization of further development of this combination among this subset of patients.
Trial protocol
eMethods.
eResults.
eTable 1. Demographic and baseline characteristics of patients
eTable 2. Objective response (confirmed) and PFS6
eTable 3. Treatment-related adverse events (TEAEs) of any grade in ≥10% of patients and G3+ TEAEs in any patient
eTable 4. Associations between molecular biomarkers and clinical activity,
as measured by objective response or clinical benefit
eTable 5. Associations between immune biomarkers and clinical activity,
as measured by objective response or clinical benefit
eFigure 1. Progression-free survival in all patients
eFigure 2. IHC evaluation of the tumor with a hotspot mutation in the U2AF1 spliceosome gene
eReferences.
Data sharing statement
References
- 1.Konstantinopoulos PA, Luo W, Liu JF, et al. Phase II study of avelumab in patients with mismatch repair deficient and mismatch repair proficient recurrent/persistent endometrial cancer. J Clin Oncol. 2019;37(30):2786-2794. doi: 10.1200/JCO.19.01021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ott PA, Bang YJ, Berton-Rigaud D, et al. Safety and antitumor activity of pembrolizumab in advanced programmed death ligand 1-positive endometrial cancer: results from the KEYNOTE-028 study. J Clin Oncol. 2017;35(22):2535-2541. doi: 10.1200/JCO.2017.72.5952 [DOI] [PubMed] [Google Scholar]
- 3.Yoland Catherine A, Peey Sei K, Kristy R, et al. Activity of durvalumab in advanced endometrial cancer (AEC) according to mismatch repair (MMR) status: the phase II PHAEDRA trial (ANZGOG1601). J Clin Oncol. 2019;37(15):5501-5501. https://ascopubs.org/doi/10.1200/JCO.2019.37.15_suppl.5501 [Google Scholar]
- 4.Makker V, Rasco D, Vogelzang NJ, et al. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer: an interim analysis of a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 2019;20(5):711-718. doi: 10.1016/S1470-2045(19)30020-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee EK, Konstantinopoulos PA. PARP inhibition and immune modulation: scientific rationale and perspectives for the treatment of gynecologic cancers. Ther Adv Med Oncol. 2020;12:1758835920944116. doi: 10.1177/1758835920944116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sill MW, Rubinstein L, Litwin S, Yothers G. A method for utilizing co-primary efficacy outcome measures to screen regimens for activity in two-stage Phase II clinical trials. Clin Trials. 2012;9(4):385-395. doi: 10.1177/1740774512450101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cilento MA, Poplawski NK, Paramasivam S, Thomas DM, Kichenadasse G. Germline PALB2 variants and PARP inhibitors in endometrial cancer. J Natl Compr Canc Netw. 2021;19(11):1212-1217. doi: 10.6004/jnccn.2021.7067 [DOI] [PubMed] [Google Scholar]
- 8.Kadan Y, Raviv O, Segev Y, et al. Impact of BRCA mutations on outcomes among patients with serous endometrial cancer. Int J Gynaecol Obstet. 2018;142(1):91-96. doi: 10.1002/ijgo.12486 [DOI] [PubMed] [Google Scholar]
- 9.Kwon JS, Lenehan J, Carey M, Ainsworth P. Prolonged survival among women with BRCA germline mutations and advanced endometrial cancer: a case series. Int J Gynecol Cancer. 2008;18(3):546-549. doi: 10.1111/j.1525-1438.2007.01030.x [DOI] [PubMed] [Google Scholar]
- 10.Ding L, Odunsi K. RNA splicing and immune-checkpoint inhibition. N Engl J Med. 2021;385(19):1807-1809. doi: 10.1056/NEJMcibr2110736 [DOI] [PubMed] [Google Scholar]
- 11.Esfahani MS, Lee LJ, Jeon YJ, et al. Functional significance of U2AF1 S34F mutations in lung adenocarcinomas. Nat Commun. 2019;10(1):5712. doi: 10.1038/s41467-019-13392-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y, Qian J, Gu C, Yang Y. Alternative splicing and cancer: a systematic review. Signal Transduct Target Ther. 2021;6(1):78. doi: 10.1038/s41392-021-00486-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
eMethods.
eResults.
eTable 1. Demographic and baseline characteristics of patients
eTable 2. Objective response (confirmed) and PFS6
eTable 3. Treatment-related adverse events (TEAEs) of any grade in ≥10% of patients and G3+ TEAEs in any patient
eTable 4. Associations between molecular biomarkers and clinical activity,
as measured by objective response or clinical benefit
eTable 5. Associations between immune biomarkers and clinical activity,
as measured by objective response or clinical benefit
eFigure 1. Progression-free survival in all patients
eFigure 2. IHC evaluation of the tumor with a hotspot mutation in the U2AF1 spliceosome gene
eReferences.
Data sharing statement