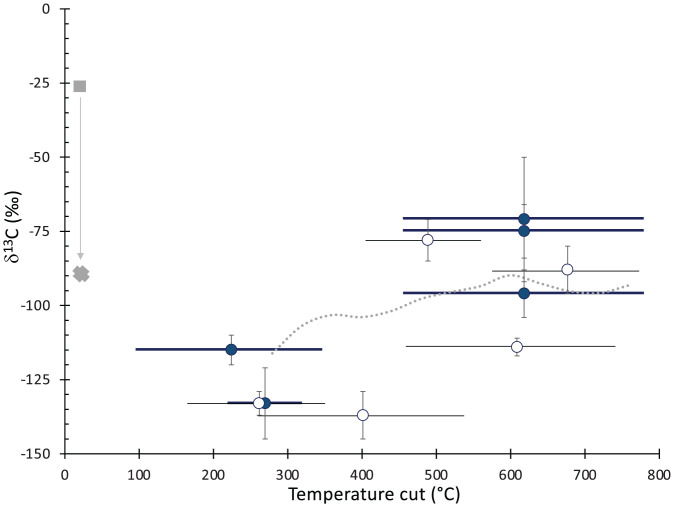
In response to our reporting the carbon isotopic compositions of CH4 released during pyrolysis of solid Mars sediments (1), Schoell (2) states that our full dataset does not follow a trend with temperature observed for the pyrolysis of Earth kerogen-rich sediments. We appreciate Schoell’s highlighting that the changing isotopic composition is an important constraint useful for understanding the origin of CH4 released during pyrolysis. Our published dataset (1) includes analyses of five aliquots from the Cumberland drill hole, one of the locations where highly 13C-depleted carbon was observed by the tunable laser spectrometer (TLS). A different temperature cut was sent to TLS for each aliquot, but, collectively, CH4 released from Cumberland during pyrolysis follows a trend with temperature similar to that observed in the West Siberian hydrocarbon source rocks (3) mentioned in Schoell’s (2) critique, once the terrestrial data are adjusted to reflect a more 13C-depleted source carbon (Fig. 1). The other samples shown in Fig. 1, single aliquots from multiple other drill holes, are interpreted to have similar carbon isotopic values for their CH4 sources. These samples, when taken together, also show a trend with temperature similar to that observed during pyrolysis of kerogen-rich rocks on Earth.
Fig. 1.
TLS CH4 δ13C results (±1 SE) from evolved gas analyses of samples of the Cumberland drill hole material (filled circles) as a function of the temperature cut used for TLS (1). Horizontal lines show the complete range of each temperature cut. The gray dashed curve represents the experimental results reported in ref. 3 but adjusted to approximate the isotopic values for methane released from the Cumberland samples. To generate such strong depletions under this scenario, the original δ13C value for the Cumberland carbon source would need to be approximately −90‰ (gray cross); the gray square shows, for reference, the δ13C of the West Siberian hydrocarbon source rocks studied in ref. 3. The open circles are other Gale crater CH4 TLS isotope results (GB2, HF, RH, HU, and EB) that, while each from separate experiments, were interpreted to have evolved CH4 δ13C values in family with the Cumberland set, due to the strong 13C depletions observed.
Notably, unlike West Siberia’s hydrocarbon source rocks, the Gale crater samples represent multiple geologic assemblages (Yellowknife Bay clastic sediments, mudstone-rich Murray lacustrine sediments, Vera Rubin ridge geochemically altered sediments, sandstone-rich Carolyn Shoemaker sediments, and the Stimson sandstone deposited after a disconformity). The observed geologies (including geochemical alteration, veining, and an erosional disconformity) imply that our full suite of samples represents significant geological time, so it is not surprising that Curiosity’s 25-km traverse encountered a variety of organics with differing isotopic compositions. Additionally, the Gale crater samples release mineral-bound carbon at a variety of temperatures (4), indicating that the full dataset reported includes pyrolysis CH4 released from a variety of different carbon sources, as previously discussed (1).
Schoell (2) ends his critique with a claim that our paper “infer[s] methane as a proven biosignature on Mars.” We do not. Our report exhaustively discusses mechanisms (abiotic and biotic) by which isotopic fractionations can occur [including serpentinization (5, 6)] and how these processes could apply to Gale crater sediments (1). The discussion narrows the options to a few working hypotheses, each requiring further exploration before being confirmed or refuted. Naturally, one hypothesis invokes large carbon isotopic fractionations mediated by methanogens under certain conditions (7), which result in 13C-depleted subsurface CH4 on Earth (8). The other working hypotheses utilize large isotopic fractionations observed between CO and dust in interstellar giant molecular clouds or suspected for UV-mediated CO2 reduction in the Martian atmosphere (1). By presenting distinct working hypotheses, we intentionally aimed to remain cautious about biosignature implications of the most 13C-depleted values and provide the community with optimistic guidance as to how progress can be made in understanding the origin of the specific carbon we reported.
Footnotes
The authors declare no competing interest.
References
- 1.House C. H., Wong G. M., Webster C. R., Mahaffy P. R., Depleted carbon isotope compositions observed at Gale crater, Mars. Proc. Natl. Acad. Sci. U.S.A. 119, 10.1073/pnas.2115651119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schoell M., Methane 13C/12C isotope analyses with the SAM-EGA pyrolysis instrument suite on Mars Curiosity rover: A critical assessment. Proc. Natl. Acad. Sci. U.S.A., 10.1073/pnas.2205344119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cramer B., Krooss B. M., Littke R.. Modelling isotope fractionation during primary cracking of natural gas: A reaction kinetic approach. Chem Geol. 149, 235–250 (1998). [Google Scholar]
- 4.Sutter B., et al. , Evolved gas analyses of sedimentary rocks and eolian sediment in Gale Crater, Mars: Results of the Curiosity rover’s sample analysis at Mars instrument from Yellowknife Bay to the Namib Dune. J. Geophys. Res. Planets 122, 2574–2609 (2017). [Google Scholar]
- 5.Etiope G., Ehlmann B. L., Schoell M., Low temperature production and exhalation of methane from serpentinized rocks on Earth: A potential analog for methane production on Mars. Icarus 224, 276–285 (2013). [Google Scholar]
- 6.Steele A., et al. , Organic synthesis associated with serpentinization and carbonation on early Mars. Science 375, 172–177 (2022). [DOI] [PubMed] [Google Scholar]
- 7.Okumura T., et al. , Hydrogen and carbon isotope systematics in hydrogenotrophic methanogenesis under H2-limited and H2-enriched conditions: Implications for the origin of methane and its isotopic diagnosis. Prog. Earth Planet. Sci. 3, 14 (2016). [Google Scholar]
- 8.Schoell M., Multiple origins of methane in the Earth. Chem. Geol. 71, 1–10 (1988). [Google Scholar]