Significance
The C-natriuretic peptide (CNP) analog vosoritide has recently been approved for the treatment of achondroplasia in children. However, the dosing regimen is burdensome, requiring daily subcutaneous injections in pediatric patients over multiple years. We developed a hydrogel microsphere drug delivery system for a CNP analog that allows for once-weekly to once-monthly subcutaneous administration. Mouse pharmacokinetic studies of theses conjugates demonstrate terminal half-lives of 200 and 600 h, while pharmacodynamic studies show that growth of the mice with weekly to monthly dosing was comparable to or exceeded growth with daily vosoritide injections.
Keywords: C-natriuretic peptide, achondroplasia, half-life extension, hydrogel microsphere
Abstract
The C-natriuretic peptide (CNP) analog vosoritide has recently been approved for treatment of achondroplasia in children. However, the regimen requires daily subcutaneous injections in pediatric patients over multiple years. The present work sought to develop a long-acting CNP that would provide efficacy equal to or greater than that of vosoritide but require less frequent injections. We used a technology for half-life extension, whereby a drug is attached to tetra-polyethylene glycol hydrogels (tetra-PEG) by β-eliminative linkers that cleave at predetermined rates. These hydrogels—fabricated as uniform ∼60-μm microspheres—are injected subcutaneously, where they serve as a stationary depot to slowly release the drug into the systemic circulation. We prepared a highly active, stable CNP analog—[Gln6,14]CNP-38—composed of the 38 C-terminal amino acids of human CNP-53 containing Asn to Gln substitutions to preclude degradative deamidation. Two microsphere [Gln6,14]CNP-38 conjugates were prepared, with release rates designed to allow once-weekly and once-monthly administration. After subcutaneous injection of the conjugates in mice, [Gln6,14]CNP-38 was slowly released into the systemic circulation and showed biphasic elimination pharmacokinetics with terminal half-lives of ∼200 and ∼600 h. Both preparations increased growth of mice comparable to or exceeding that produced by daily vosoritide. Simulations of the pharmacokinetics in humans indicated that plasma [Gln6,14]CNP-38 levels should be maintained within a therapeutic window over weekly, biweekly, and likely, monthly dosing intervals. Compared with vosoritide, which requires ∼30 injections per month, microsphere [Gln6,14]CNP-38 conjugates—especially the biweekly and monthly dosing—could provide an alternative that would be well accepted by physicians, patients, and patient caregivers.
Achondroplasia (ACH) is a form of dwarfism in which conversion of cartilage to bone is impaired, resulting in short stature and disordered architecture in the long bones, spine, face, and base of the skull (recent reviews are in refs. 1 and 2). The condition occurs with a worldwide pooled birth prevalence of 4.6 cases per 100,000 births or 1 in 22,000 births (3). ACH arises as a result of a spontaneous genetic mutation in the fibroblast growth factor receptor 3 (FGFR3) gene in ∼80% of patients and is inherited in an autosomal dominant pattern in the remaining 20%. Almost all ACH cases are due to a G380R mutation in the transmembrane domain of FGFR3 that confers prolonged activation.
Therapeutic approaches for ACH directly or indirectly target the overactive FGFR3 (2, 4) and include 1) inhibition of FGFR1 to -3 tyrosine kinase (Infigratinib), 2) neutralization of excess fibroblast growth factor by the FGFR3 extracellular domain (Recifercept; TA-46), and 3) agonism of natriuretic peptide receptor B (NPR-B) that inhibits the mitogen-activated protein kinase (MAPK) pathway to counteract FGFR3 overactivation and restore chondrogenesis. The most advanced candidates are 17–amino acid cyclic disulfide peptide analogs of C-type natriuretic peptide (CNP)—vosoritide and Transcon CNP—that are agonists of NPR-B.
CNP is first formed as a propeptide, proCNP(1 to 103), that is sequentially converted to bioactive CNP-53 and then, to the active C-terminal 22–amino acid fragment CNP-22 (5). CNP bioactivity is tightly regulated by natriuretic peptide clearance receptor (NPR-C), which promotes internalization and lysosomal degradation, and neutral endopeptidase (NEP), which cleaves and inactivates CNP (6).
Vosoritide (BMN 111) is a 39–amino acid peptide analog that has a Pro-Gly appended to the N terminus of the 37 C-terminal amino acids of human CNP-53. Vosoritide was the culmination of an extensive structure-activity study of CNP aimed to maximize its half-life and NPR-B activity while minimizing degradation by NEP (7). A phase 3 trial of once daily 15 μg/kg subcutaneous (SC) vosoritide in children with ACH showed a sustained increase in the annualized growth velocity and a mild side effect profile (8). More recently, vosoritide has been approved by the European Commission and the US Food and Drug Administration and is marketed as VOXZOGO. Nevertheless, the short t1/2 of ∼30 min requiring daily injections, the high Cmax of ∼5 nM, and the high daily peak-to-trough excursions encouraged development of longer-acting CNP agonists. The importance of long-acting CNP agonists was strengthened by preclinical observations that continuous CNP exposure results in higher efficacy and minimizes risks of hypotension (9, 10).
Transcon CNP is a conjugate of 40-kDa polyethylene glycol (PEG) and CNP-38—the 38 C-terminal amino acids of human CNP-53—connected by a releasable linker that upon cleavage, provides CNP-38 (9). The CNP-38 released from Transcon CNP showed a t1/2 of ∼90 h in the cynomolgus monkey (9), indicating suitability for weekly (QWk) administration. While QWk Transcon CNP may provide benefits over daily (QD) vosoritide, since the renal elimination half-life of the 40-kDa PEG carrier is ∼6 d in humans, it is unlikely that the prodrug can be improved to further extend the in vivo lifetime of CNP-38.
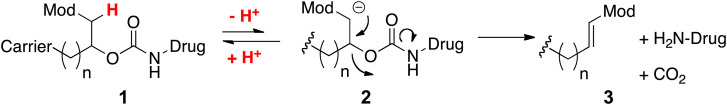
We have developed a general approach for half-life extension of therapeutics that is applicable for short-lived peptides, such as CNP, and can be used to achieve half-lives of the released peptide of 1 mo or more (11, 12). Here, a drug is covalently tethered to a long-lived carrier by a linker that slowly cleaves by β-elimination to release the native drug (Scheme 1) (11). The cleavage rate of the linker is controlled by the nature of an electron-withdrawing “modulator,” which regulates the acidity of an adjacent carbon–hydrogen bond. These linkers are not affected by enzymes and are extraordinarily stable when stored at low pH and temperature (11, 13). One carrier we use is a mesoporous tetra-PEG hydrogel (14, 15). These hydrogels—fabricated as uniform ∼60-μm microspheres (MS) (16, 17)—are injected SC through a small-bore needle, where they serve as a stationary depot to slowly release the drug to the systemic circulation. We also incorporate slower-cleaving β-eliminative linkers in cross-links of these polymers, so gel degradation occurs in vivo after drug release (18).
Scheme 1.
β-eliminative drug release mechanism. Mod, modulator.
The primary objective of this work was to develop a very long-acting CNP. First, we describe a 38–amino acid CNP analog—[Gln6,14]CNP-38—that is equipotent with vosoritide or CNP-38 but sufficiently stable to allow long-term residence in the SC interstitium. Next, we report the synthesis and characterization of MS [Gln6,14]CNP-38 (MS∼[Gln6,14]CNP-38) conjugates designed for once weekly and once monthly administration. Then, we describe the pharmacokinetics of the conjugate in mice and show long half-lives of released [Gln6,14]CNP-38. Finally, we demonstrate the remarkable growth-promoting effects of these long-acting CNPs on normal juvenile mice and posit that they should show similar effects on achondroplastic children.
Results
Selection of [Gln6,14]CNP-38.
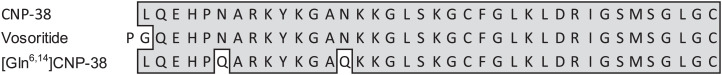
The CNP analogs vosoritide and the CNP-38 component of Transcon CNP have Asn residues at positions 6 and 14 of CNP-38 that are potentially susceptible to deamidation (Fig. 1). Asn deamidation is a well-studied reaction that involves formation, hydrolysis, and rearrangements of a succinimide intermediate to produce isomers of isoaspartic acid (isoAsp) and Asp in an ∼3:1 ratio (19–21). We initially observed by high-performance liquid chromatography (HPLC) that at pH 7.4 and 37 °C, vosoritide converted to several products with a degradation t1/2 of ∼7 d (SI Appendix, Fig. S1). Using protein l-isoaspartyl methyltransferase (PIMT), we demonstrated the formation of one isoAsp/peptide from CNP-38 or vosoritide with a t1/2 of ∼120 h.
Fig. 1.
Sequences of CNP-38 (the natural sequence in Transcon CNP), vosoritide, and stabilized [Gln6,14]CNP-38. The cysteine residues in all sequences form a 17–amino acid intramolecular disulfide bond. Shaded areas show amino acids in natural CNP.
While the most rapid asparagine deamidation reactions have half-lives of ∼1 d, corresponding Gln deamidations take much longer to occur (22, 23), and model reactions indicate that Asn to Gln substitutions in the sequence context of CNP-38—PN6A and AN14K—could decrease the deamidation rate up to ∼100-fold (22). Thus, as previously employed for long-acting MS∼peptide conjugates (12), we substituted Gln for the labile Asn residues to form [Gln6,14]CNP-38. [Gln6,14]CNP-38 was incubated for 4 wk at pH 7.4 and 37 °C, and samples were analyzed weekly by HPLC. The data indicated that [Gln6,14]CNP-38 degraded 2.7%/wk, indicating a t1/2 of ∼5 mo.
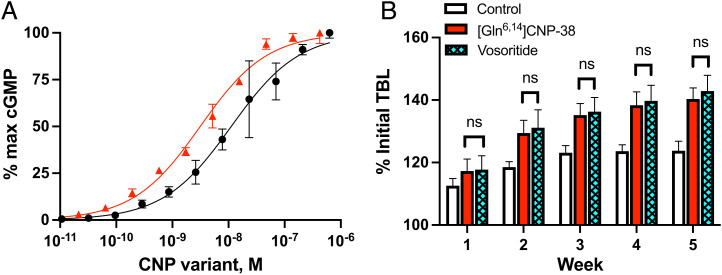
When assayed in the 3T3 cell NPR-B guanylate cyclase assay (24), [Gln6,14]CNP-38 showed a half maximal effective concentration (EC50) of 3 nM compared with 11 nM for vosoritide (Fig. 2A). These values are similar to those reported for vosoritide (4.9 nM) and CNP-38 (6.8 nM) (7), indicating that the Asn to Gln substitution has little or no effect on the EC50. Using a model that measures growth of normal juvenile FVB/nJ mice (7), we found no statistical difference between QD 70-nmol/kg doses of [Gln6,14]CNP-38 and vosoritide in the total body length (TBL; nasoanal + tail length) (SI Appendix) change over a 5-wk study period (Fig. 2B); at study end, growth of the treated animals exceeded that of the vehicle control group by 100%. Interestingly, these CNP variants as well as others tested (below) caused significant growth beyond the 3-wk period of growth of untreated animals. Hence, [Gln6,14]CNP-38 is as potent as vosoritide in cell-based and murine growth assays but—unlike vosoritide and CNP-38—is quite stable toward deamidation.
Fig. 2.
Potency of [Gln6,14]CNP-38 in cell-based and growth assays. (A) Stimulation of 3T3 cell guanylate cyclase by [Gln6,14]CNP-38 ( ) and vosoritide (•). (B) Effects of [Gln6,14]CNP-38 and vosoritide on TBL (nasoanal + tail length) growth of 3-wk-old FVB/nJ male mice. Groups of 3-wk-old wild-type mice (n = 6) were given daily subcutaneous administrations of 70 nmol/kg CNP variants or vehicle for 5 wk. cGMP, cyclic guanosine monophosphate. ns, not significant.
) and vosoritide (•). (B) Effects of [Gln6,14]CNP-38 and vosoritide on TBL (nasoanal + tail length) growth of 3-wk-old FVB/nJ male mice. Groups of 3-wk-old wild-type mice (n = 6) were given daily subcutaneous administrations of 70 nmol/kg CNP variants or vehicle for 5 wk. cGMP, cyclic guanosine monophosphate. ns, not significant.
Synthesis and Characterization of MS∼[Gln6,14]CNP-38.
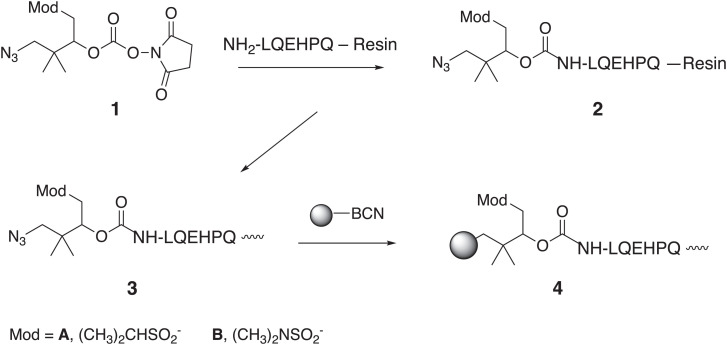
As in Scheme 2, [Gln6,14]CNP-38 was prepared by solid-phase peptide synthesis (SPPS), and after removal of the N-terminal fluorenylmethyloxycarbonyl (Fmoc) group, N3-linker N-hydroxysuccinymidocarbonates containing isopropyl sulfone (1A) and N,N-dimethyl-sulfonamide (1B) modulators were added to the N terminus. N3-linker peptides were cleaved from the resins and purified by HPLC. The N3-linker peptides, 3A and 3B, were coupled to bicyclononyne-modified MSs by strain-promoted azide-alkyne cycloaddition (SPAAC) to give the MS∼[Gln6,14]CNP-38 conjugates 4A and 4B at ∼3.5 μmol/mL (25). In the present work, the amounts of 4A or 4B refer to the [Gln6,14]CNP-38 contained in the MSs.
Scheme 2.
Synthesis of MS∼[Gln6,14]CNP-38.
When treated at pH 9.4 and 37 °C, MS∼[Gln6,14]CNP-38 conjugates showed release t1/2 values of 6.0 h for 4A and 15.8 h for 4B at pH 9.4 and 37 °C, corresponding to t1/2 values of 600 and 1,580 h, respectively, at pH 7.4 and 37 °C. The purity of [Gln6,14]CNP-38 in MS∼[Gln6,14]CNP-38 conjugates was assessed by HPLC of proteins released from the MSs versus time at pH 9.4; when extrapolated to t = 0, the fraction of released protein that is [Gln6,14]CNP-38 represents its purity on the MSs. In the above preparations, ≥95% of the total peptide on 4A and 4B analyzed as [Gln6,14]CNP-38.
Pharmacokinetics of MS∼[Gln6,14]CNP-38 Conjugates 4A and 4B.
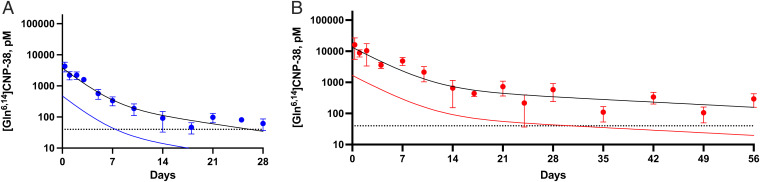
Pharmacokinetic studies of 4A and 4B were not assessed in juvenile mice because of anticipated difficulties in analysis of long-acting prodrugs in rapidly growing animals. Fig. 3 shows concentration versus time profiles of single injections of 4A and 4B in adult CD1 mice fitted to biexponential equations. The [Gln6,14]CNP-38 released from the MS conjugates shows initial elimination t1/2,α of ∼40 and 60 h for 4A and 4B, respectively, followed by a slower terminal phase with t1/2,β of ∼212 h for 4A and ∼610 h for 4B. The data for released [Gln6,14]CNP-38 could also be fit to single exponential equations over limited time periods; here, the best-fit t1/2 values over 2 wk for 4A and 1 mo from 4B were 60 and 100 h, respectively, corresponding to the sums of the rates in the biexponential fits.
Fig. 3.
C versus t plots of [Gln6,14]CNP-38 released from MS∼[Gln6,14]CNP-38 in CD1 mice. (A) Single dose of MS∼[Gln6,14]CNP-38 4A containing 220 nmol [Gln6,14]CNP-38/mouse ( ), and assuming dose linearity the simulated dose adjustment to 20 nmol to keep the peptide ∼40 pM (
), and assuming dose linearity the simulated dose adjustment to 20 nmol to keep the peptide ∼40 pM ( ) for 1 wk. (B) Single dose of 4B containing 700 nmol [Gln6,14]CNP-38/mouse (
) for 1 wk. (B) Single dose of 4B containing 700 nmol [Gln6,14]CNP-38/mouse ( ) and simulated dose-adjustment to 85 nmol to maintain the released peptide ≥40 pM (
) and simulated dose-adjustment to 85 nmol to maintain the released peptide ≥40 pM ( ) for 1 mo. Data points are mean ± SD.
) for 1 mo. Data points are mean ± SD.
Using the pharmacokinetic data in Fig. 3 and aiming for a minimal plasma concentration of ∼40 pM—an effective bone growth–promoting level of CNP-38 in the monkey (9)—we estimated dosing schedules of 4A and 4B to guide anticipated pharmacodynamic experiments in mice. Assuming dose linearity, simulations for conjugate 4A indicated that a dose of 20 nmol/mouse would give ∼35 pM [Gln6,14]CNP-38 1 wk after the first dose (Fig. 3A); by superposition of sequential QWk doses, steady state would be reached at the fourth dose with Cmin of ∼55 pM and Cmax of 300 pM [Gln6,14]CNP-38, >600-fold lower than the 200 nM Cmax of the effective QD 70-nmol/kg dose of vosoritide (7). A single dose of 85 nmol 4B is predicted to give a Cmax of ∼1 nM and Cmin of ≥40 pM for [Gln6,14]CNP-38 over 1 mo (Fig. 3B).
Growth-Promoting Effects of MS∼[Gln6,14]CNP-38.
Vosoritide and related CNP variants correct growth deficits in the Fgfr3ACH/+ mouse model of ACH and promote significant growth in normal juvenile mice (7, 9). Using 3-wk-old ∼15-g FVB/nJ male mice, we studied the growth stimulatory effects of various regimens of 4A and 4B MS∼[Gln6,14]CNP-38 conjugates as well as QD 70 nmol/kg [Gln6,14]CNP-38 and vosoritide as positive controls. As reported for normal mice (26) and rats (27) treated with CNP-22 or CNP-53, we observed no difference in the body weights of treated versus untreated mice (SI Appendix, Fig. S2). The nasoanal and tail length growth were measured (SI Appendix, Figs. S3 and S4) and combined to give the TBL measurements used in figures. Radiographic measurements of the tibia, humerus, femur, ulna, and spine obtained after the study period are provided in SI Appendix, Table S1.
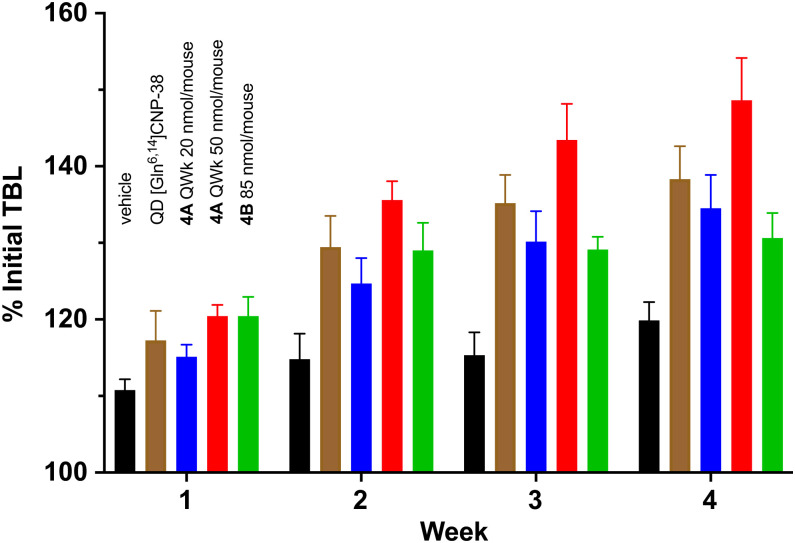
In initial exploratory studies, we examined the growth-stimulatory effects of 4A and 4B MS∼[Gln6,14]CNP-38 conjugates administered at constant amounts per animal each dosing interval. Fig. 4 shows plots of the percentages of the initial TBL versus time for vehicle control and CNP variant–treated mice over 4 wk of the 5-wk study period; replicate and additional studies are presented in SI Appendix. Untreated normal mice grew rapidly for ∼3 wk, after which growth plateaued; as shown, most treatments with CNP variants that caused an increase in growth also showed continued growth beyond the growth period of untreated mice. As projected from the pharmacokinetics, QWk treatment with 20 nmol 4A elicited similar growth as QD [Gln6,14]CNP-38, and QWk 50 nmol 4A caused significantly increased growth over the QD-administered peptide; over the study period, the QWk 50 nmol dose of 4A gave mice that were ∼25% longer than the vehicle control. Likewise, the growth of juvenile mice treated with a single dose of 85 nmol 4B was similar to QD [Gln6,14]CNP-38 over 3 wk and then, plateaued. Thus, both 4A and 4B dosed at appropriate constant amounts over appropriate intervals can achieve or surpass growth stimulated by QD administration of [Gln6,14]CNP-38 or vosoritide.
Fig. 4.
Growth of mice treated with [Gln6,14]CNP-38 and MS∼[Gln6,14]CNP-38 conjugates 4A and 4B at constant amounts per mouse each dosing interval. From left to right are vehicle control (black), QD [Gln6,14]CNP-38 (brown), QWk 4A 20 nmol/mouse (blue), QWk 4A 50 nmol/mouse (red), and single-dose 4B 85 nmol/mouse (green). They are plotted as the percentage of initial TBL versus time; values are displayed as the mean ± SD.
In the above experiments, animals were treated with the same amount of MS∼CNP each dosing interval, but since mice were rapidly growing over the initial part of the study, the dose per kilogram of body weight (BW) decreased with time. Guided by these results, we designed and performed a study in which the amount of CNP variant was dosed per kilogram of BW. In addition to selected weekly and monthly regimens, we also tested biweekly (Q2Wk) dosing of 4A.
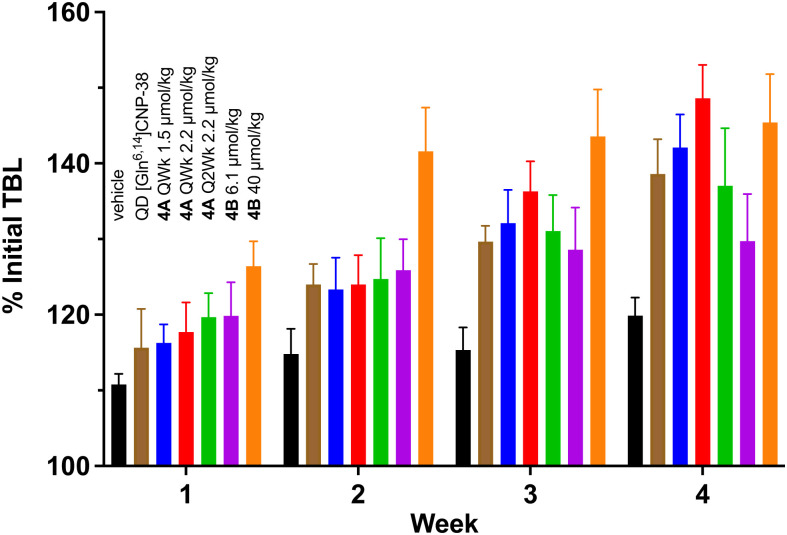
QWk treatment with 1.5 and 2.2 μmol/kg 4A elicited similar and superior growth stimulation, respectively, as the QD 70-nmol/kg [Gln6,14]CNP-38 control (Fig. 5). Reduction of the dose frequency of 2.2 μmol/kg 4A to Q2Wk also attained the growth stimulation of QD [Gln6,14]CNP-38. The growth of mice treated with a single dose of 85 nmol 4B was similar to QD [Gln6,14]CNP-38 over 3 wk but plateaued during week 4, suggesting reduction of the CNP below its most effective concentration. A single very high dose of 40 μmol/kg 4B resulted in very rapid growth over the initial 2 wk before saturating and exceeded that of other regimens, except the QWk 1.5 and 2.2 μmol/kg 4A; although the dose is impractical for translation to humans, the Cmin over 1 mo is estimated to be ≥300 pM (Fig. 3B), and the growth rate it achieves may represent the highest feasible with a CNP variant. Taken together, the data suggest that the most promising dosing regimens tested in mice that may be translated to humans are QWk 1.5 μmol/kg and Q2Wk 2.2 μmol/kg for 4A and monthly (QMo) 6.1 μmol/kg for 4B.
Fig. 5.
Growth of mice treated with [Gln6,14]CNP-38 and MS∼[Gln6,14]CNP-38 conjugates 4A and 4B at body weight–determined dosing (micromoles per kilogram). From left to right are vehicle control (black), QD [Gln6,14]CNP-38 (brown), QWk 4A 1.5 μmol/kg (blue), QWk 4A 2.2 μmol/kg (red), Q2Wk 2.2 μmol/kg (green), single-dose 4B 6.1 μmol/kg (purple), and single-dose 4B 40 μmol/kg (orange). They are plotted as the percentage of initial TBL versus time; values are displayed as the mean ± SD.
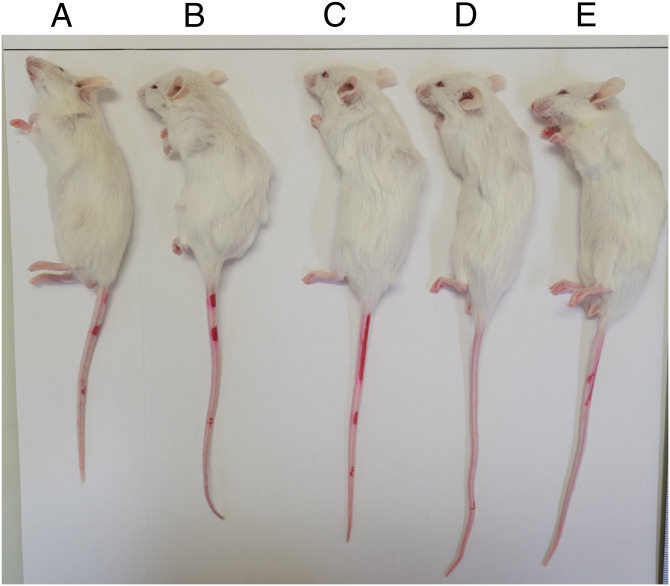
Fig. 6.
Representative mice treated with [Gln6,14]CNP-38 or 4A for 5 wk. (A) Vehicle control. (B) QD [Gln6,14]CNP-38 70 nmol/kg. (C) Q2Wk 2.2 μmol/kg. (D) QWk 2.2 μmol/kg. (E) QWk 1.5 μmol/kg. Anesthetized mice were initially positioned for measurements with their heads extended, noses aligned to the horizontal guideline, and tails straight; here, the distance between the top guideline and the end of the tail best represents TBL.
As a measure of the efficiencies of 4A and 4B, we estimated the cumulative dose (CD) of [Gln6,14]CNP-38 delivered per mouse by the most promising regimens of 4A and 4B over a 4-wk period (CD4Wk) (Table 1). Whereas QD 70 nmol/kg [Gln6,14]CNP-38 has a CD4Wk of 44 nmol, 4A administered as QWk 20 nmol/mouse or QWk 1.5 μmol/kg has CD4Wk values of 80 and 132 nmol, respectively, and Q2Wk 2.2 μmol/kg 4A has a CD4Wk of 86 nmol. For 4B, the CD4Wk for a single 6.1-μmol/kg dose was 95 nmol/mouse. Hence, selected regimens of 4A and 4B can achieve equieffective growth as QD [Gln6,14]CNP-38 with only approximately two- to threefold higher CD4Wk.
Table 1.
[Gln6,14]CNP-38 delivered per mouse with different dosing regimens
| Dosing regimen | [Gln6,14]CNP-38 administered per week | ||||
|---|---|---|---|---|---|
| QD [Gln6,14]CNP-38, 70 nmol/kg | QWk 4A, 20 nmol/mouse | QWk 4A, 1.5 μmol/kg | Q2Wk 4A, 2.2 μmol/kg | QMo 4B, 6.1 μmol/kg | |
| Week 1 | 9 | 20 | 23 | 34 | 95 |
| Week 2 | 11 | 20 | 33 | 0 | 0 |
| Week 3 | 11 | 20 | 36 | 52 | 0 |
| Week 4 | 13 | 20 | 40 | 0 | 0 |
| CD4Wk | 44 | 80 | 132 | 86 | 95 |
The data for QWk [Gln6,14]CNP-38 at 20 nmol/mouse versus 1.5 μmol/kg 4A (Fig. 4 and Fig. 5 and Table 1) also suggest that optimal dosing may vary according to the growth period during which the CNP is administered. For example, near-equal growth stimulation is achieved by QWk dosing of 4A as 20 nmol/mouse of [Gln6,14]CNP-38 or by a BW-determined dose of 1.5 μmol/kg. Although the dose of either regimen is initially similar at ∼20 nmol per animal, the [Gln6,14]CNP-38 delivered to animals receiving the BW-determined dose rapidly increases over time; by week 4, it is twice that of mice receiving a constant dose per animal and has a CD4Wk that is 1.7-fold higher. The high early growth is recapitulated by 40 μmol/kg 4B, where all growth occurs during the first 2 wk, although levels of [Gln6,14]CNP-38 are calculated to be ≥300 pM through the study period. The data suggest that a high dose intensity is most effective during the early period of rapid growth and less important during later stages. This could be due to different CNP-dose responses or changing pharmacokinetic parameters at different growth phases. If correct, improved harmonization of CNP delivery with growth phase may allow for dose reductions with concomitant improved safety and without affecting growth enhancement. Clearly, such customized dosing of a long-acting CNP would be simpler to achieve in species that do not grow as rapidly as mice.
Estimations of Human Equivalent Doses of 4A and 4B.
The efficacious dose of vosoritide is 70 nmol (290 μg)/kg per day in mice (7) and as in the phase 3 trial (8), 3.7 nmol (15 μg)/kg per day in achondroplastic children. These doses represent a 19-fold higher dose per kilogram in the mouse compared with humans, consistent with the ∼12-fold difference predicted by allometric scaling (28). We used this 19-fold scaling factor to estimate the human equivalent dose of the most promising regimens of 4A and 4B. We calculate that the average 8.4-y-old patient in the human trial weighing ∼20 kg (29) received 74 nmol (300 μg)/d of vosoritide. The individual doses of QWk 1.5 μmol/kg and Q2Wk 2.2 μmol/kg 4A in the mouse—targeting the 40 pM effective level in the monkey—scale to 1.6 and 2.3 μmol per 20-kg ACH patient, respectively, and the QMo 6.1 μmol/kg 4B dose in the mouse is equivalent to 6.4 μmol per 20-kg ACH child. The CDs over 4 wk for these regimens in a 20-kg human are 2.2 μmol vosoritide, 6.3 μmol QWk 4A, 4.6 μmol Q2Wk 4A, and 6.4 μmol QMo 4B. However, if the target trough level in humans is ≤20 pM, as indicated by early clinical trials (30), the scaling factor would be ∼40-fold, and human dosing would be ≤50% of what is given above. Pharmacokinetics in the cyno monkey and simulations in humans (SI Appendix Fig. S5 and S6) suggest that the [Gln6,14]CNP-38 released from 4A should remain in the therapeutic window for up to one month.
Discussion
A phase 3 clinical trial has demonstrated efficacy and safety of vosoritide in the treatment of ACH in children (8), and the peptide has recently been approved for this use by the European Commission and the US Food and Drug Administration in 2021. Nevertheless, the 39–amino acid peptide has a short t1/2 of only ∼20 to 45 min, requires daily SC injections over multiple years, and it shows a high Cmax and daily peak-to-trough excursions. In response to these shortcomings, Transcon CNP, a PEGylated prodrug of CNP-38, was developed that has a t1/2 in humans of 120 h and allows for once weekly SC administration, and it shows a low Cmax and peak-to-trough excursions (9, 30). However, there are potential disadvantages to the PEGylated CNP-38 prodrug. First, vacuolation of PEG that is 40-kDa molecular weight occurs in many tissues (31) and is of particular concern in the pediatric population that would be targeted with PEG40kDa-CNP (32); although our MSs also contain PEG, the monomer degradation products are only 10 kDa, and the amount of PEG per CNP is 10-fold less than that in Transcon CNP. Second, since the renal elimination half-life of the PEG carrier in Transcon CNP is ∼6 d, it is unlikely that the lifetime of the prodrug could be further extended. Finally, both vosoritide and CNP-38 have two Asn residues that may undergo degradative deamidation that limits long-term in vivo residence and complicates formulations for long-term storage.
The primary objective of the present work was to develop a long-acting, stable prodrug of CNP for treatment of ACH. First, we describe a 38–amino acid CNP analog—[Gln6,14]CNP-38—that has equal potency and efficacy as vosoritide and CNP-38, but it does not undergo deamidation and should survive long-term in vivo residence. Next, we report the synthesis and characterization of MS∼[Gln6,14]CNP-38 conjugates designed for once weekly and once monthly administration of [Gln6,14]CNP-38. Then, we describe the pharmacokinetics of the conjugates in mice, which show long terminal half-lives of released [Gln6,14]CNP-38. Finally, we demonstrate remarkable growth-promoting effects of these long-acting CNPs in juvenile normal mice.
Since a long-acting prodrug of a CNP variant must reside in vivo for long periods, stability of the CNP component for the duration of in vivo exposure and for long-term storage is a critical feature of the drug product. We found that vosoritide and CNP-38 were unstable at physiological pH and temperature, with a t1/2 for degradation of ≤1 wk. Using PIMT, we observed isoAsp formation that demonstrated that the degradation of the peptides was due to deamidation of Asn. For QD-administered vosoritide, the in vivo deamidation rate is insignificant compared with the rapid clearance of the peptide. However, with the longer-acting Transcon CNP prodrug, a significant amount of the CNP-38 could be degraded by Asn deamidation during its in vivo residence. Also, for both vosoritide and CNP-38, deamidation can present challenging issues in developing formulations suitable for long-term storage. One solution to the problem of Asn deamidation is to simply substitute a stable amino acid (e.g., Gln) for hydrolytically labile Asn residues (12). Indeed, when the Asn6,14 residues of CNP-38 were substituted by Gln to give [Gln6,14]CNP-38, the analog was stable for several months under physiological conditions. When assayed in the 3T3 cell NPR-B guanylate cyclase assay, [Gln6,14]CNP-38 showed an approximately fourfold lower EC50 than that of vosoritide, and QD injections stimulated growth of normal young mice at the same rate as QD vosoritide. Hence, [Gln6,14]CNP-38 is as potent and effective as vosoritide or CNP-38 but is stable toward deamidation.
We next attached [Gln6,14]CNP-38 to hydrogel MSs by β-eliminative releasable linkers. Azido linkers containing modulators suitable for QWk and QMo dosing intervals were added to the N terminus of [Gln6,14]CNP-38 during SPPS. After cleavage from the resin and HPLC purification, the azido-linker peptides were coupled to cyclooctyne-modified MSs by SPAAC to give the two MS∼[Gln6,14]CNP-38 conjugates 4A and 4B, respectively. Over 95% of the material on the MS conjugates analyzed as [Gln6,14]CNP-38, and 4A and 4B released [Gln6,14]CNP-38 with in vitro t1/2 values of 185 and 1,200 h, respectively, at pH 7.4 and 37 °C.
In addition to deamidation, CNP is rapidly cleared from the circulation by cell-associated NPR-C and NEP. Both vosoritide and Transcon CNP-38 were structurally optimized to resist clearance by NEP (7, 9) but despite optimization, are still susceptible. In the present case, [Gln6,14]CNP-38 is dispersed throughout the interior of the MSs. Since the porosity of the particles is ≤10 nm, the MSs provide a protective shelter for [Gln6,14]CNP-38 that is impenetrable by large cell-associated NPR-C and NEP. However, once released from the MSs, free [Gln6,14]CNP-38 would be susceptible to degradation by these pathways, which could affect its pharmacokinetic disposition.
After single injections of the MS∼[Gln6,14]CNP-38 conjugates in adult mice, the plasma [Gln6,14]CNP-38 from the MSs showed biphasic elimination with initial t1/2,α of ∼40 and 60 h for QWk 4A and QMo 4B, respectively, followed by a slower phase with t1/2,β values of ∼212 and 610 h, respectively. Reasons for the biphasic pharmacokinetic behavior are not clear, but the very different in vitro release rates yet similar in vivo t1/2,α values of 4A versus 4B suggest that the observed plasma half-lives are not completely controlled by peptide release from the MSs. A potential component of the initial elimination phase may involve target-mediated drug disposition (33, 34), such as might result from the aforementioned clearance of released [Gln6,14]CNP-38 from the circulation by NPR-C (6). From the pharmacokinetic data, we estimated to continuously maintain the presumed effective concentration of 40 pM [Gln6,14]CNP-38 at steady state (9), that 4A would be dosed at 20 nmol [Gln6,14]CNP-38 per mouse per week and have a Cmax of only 0.3 nM and that 4B would be dosed at 85 nmol [Gln6,14]CNP-38 per mouse per month and have a Cmax of ∼1 nM. In contrast, the effective QD 70-nmol/kg dose of vosoritide gives a very high Cmax of 200 nM (7). Hence, in mice, both the QWk and QMo MS conjugates provide [Gln6,14]CNP-38 at therapeutic levels but have much lower Cmax values and peak-to-trough excursions than vosoritide.
Potential hemodynamic side effects of high doses of CNP involve lowered blood pressure (BP) and increased heart rate (HR). In mice, 70 nmol/kg vosoritide—with Cmax of 200 nM—produced acute decreases in blood pressure and increases in heart rate within acceptable limits (7). The timing of such effects correlated with peak drug levels, and there was a clear pharmacokinetic–pharmacodynamic relationship with Cmax. In monkeys, 12 nmol/kg vosoritide giving a Cmax of ∼10 nM resulted in no changes in systemic BP or HR (9). With a slow-releasing, low-Cmax CNP, such as Transcon CNP, there were no changes in systemic BP or HR in mice or monkeys over long periods. Since the Cmax of the [Gln6,14]CNP-38 slowly released from 4A and 4B is far below those of vosoritide, which causes hemodynamic effects in mice and monkeys, there seems little reason for concern about such side effects with these prodrugs.
Exogenous administration of CNP corrects growth defects of rodent models of ACH and causes acceleration of growth of normal mice, rats, and monkeys (7, 9, 27). To measure the pharmacodynamics of CNP variants, we monitored growth of normal juvenile FVB/nJ mice (7). We established that daily vosoritide elicited the previously reported increase in growth of normal mice and that equivalent dosing of [Gln6,14]CNP-38 had the same effects. We then investigated varying dosing regimens of QWk 4A and QMo 4B MS∼[Gln6,14]CNP-38 in this model. Our results showed that a weekly dose of 4A containing 1.5 μmol/kg [Gln6,14]CNP-38 or biweekly dose containing 2.2 μmol/kg [Gln6,14]CNP-38 increased growth equal to or exceeding that of QD [Gln6,14]CNP-38 or vosoritide. A single dose of 6.1 μmol/kg 4B also supported increased growth comparable to QD [Gln6,14]CNP-38 for at least 3 wk before stabilizing. Thus, appropriate regimens of long-acting 4A and 4B delivered weekly, biweekly, or monthly can achieve similar or greater growth enhancement of juvenile mice as daily dosing of vosoritide or other CNP variants.
Based on the effective doses of vosoritide in the mouse and humans (7, 8), the human equivalent per body weight dose was calculated to be ∼20-fold lower than the dose in mice; thus, we estimated the most promising regimens to be 1.6 and 2.3 μmol per 20-kg child for QWk and Q2Wk 4A, respectively, and 6.4 μmol per 20-kg child for QMo 4B. Here, we targeted blood levels of the 40-pM effective dose in the monkey. However, if the effective trough level of CNP-38 in humans is ≤20 pM, as indicated by early clinical trials (30), the human dosing would be ≤50% of what is given above. Since the MSs used here have a capacity of ∼5 μmol [Gln6,14]CNP-38/mL, these regimens should require ≤1-mL SC injections. Further, the CDs of 4A or 4B over a 1-mo period are only slightly higher that of QD vosoritide, which seems a small price to pay for the decreased frequency of injections. Hence, the long-acting MS conjugates 4A and 4B should be feasible and practical for use in achondroplastic children.
In summary, the MS∼[Gln6,14]CNP-38 prodrug provides a long-acting source of [Gln6,14]CNP-38, an NPR-B agonist having similar activity as vosoritide. The [Gln6,14]CNP-38 released from the MSs has a low Cmax and causes significant increased growth of juvenile normal mice that is comparable to daily dosing with vosoritide. Estimations of human equivalent regimens from these data, as well as pharmacokinetics in the cyno monkey and simulations in humans, suggest the single MS conjugate 4A should be feasible, practical, and acceptable for QWk, Q2Wk, and likely, QMo dosing in achondroplastic children. From our perspective, important future work entails additional studies of MS∼[Gln6,14]CNP-38 in nonhuman primates and then, in human clinical trials. Since the pharmacokinetics of [Gln6,14]CNP-38 are governed largely by the β-eliminative linker connecting the peptide to the carrier and the rate of release is generally species independent (11, 12), we are optimistic that the results obtained here will translate from mouse to monkey to man.
Materials and Methods
The sources of specialized materials are provided along with their use in SI Appendix. Detailed synthetic, conjugation, and analytical procedures are described. In vitro kinetic procedures are provided as are in vivo pharmacokinetic and pharmacodynamic methods and analyses. All animal handling and care was performed by MuriGenics (Vallejo, California) and conformed to IACUC recommendations.
Supplementary Material
Footnotes
Competing interest statement: All authors are employees and hold options or stock in ProLynx.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2201067119/-/DCSupplemental.
Data Availability
All study data are included in the article and/or SI Appendix.
References
- 1.Pauli R. M., Achondroplasia: A comprehensive clinical review. Orphanet J. Rare Dis. 14, 1 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Legeai-Mallet L., Savarirayan R., Novel therapeutic approaches for the treatment of achondroplasia. Bone 141, 115579 (2020). [DOI] [PubMed] [Google Scholar]
- 3.Foreman P. K., et al. , Birth prevalence of achondroplasia: A systematic literature review and meta-analysis. Am. J. Med. Genet. A. 182, 2297–2316 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sabir A. H., Cole T., The evolving therapeutic landscape of genetic skeletal disorders. Orphanet J. Rare Dis. 14, 300 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prickett T. C., A Espiner E., Circulating products of C-type natriuretic peptide and links with organ function in health and disease. Peptides 132, 170363 (2020). [DOI] [PubMed] [Google Scholar]
- 6.Potter L. R., Natriuretic peptide metabolism, clearance and degradation. FEBS J. 278, 1808–1817 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wendt D. J., et al. , Neutral endopeptidase-resistant C-type natriuretic peptide variant represents a new therapeutic approach for treatment of fibroblast growth factor receptor 3-related dwarfism. J. Pharmacol. Exp. Ther. 353, 132–149 (2015). [DOI] [PubMed] [Google Scholar]
- 8.Savarirayan R., et al. , Once-daily, subcutaneous vosoritide therapy in children with achondroplasia: A randomised, double-blind, phase 3, placebo-controlled, multicentre trial. Lancet 396, 684–692 (2020). [DOI] [PubMed] [Google Scholar]
- 9.Breinholt V. M., et al. , TransCon CNP, a sustained-release C-type natriuretic peptide prodrug, a potentially safe and efficacious new therapeutic modality for the treatment of comorbidities associated with fibroblast growth factor receptor 3-related skeletal dysplasias. J. Pharmacol. Exp. Ther. 370, 459–471 (2019). [DOI] [PubMed] [Google Scholar]
- 10.Morozumi N., et al. , ASB20123: A novel C-type natriuretic peptide derivative for treatment of growth failure and dwarfism. PLoS One 14, e0212680 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Santi D. V., Schneider E. L., Reid R., Robinson L., Ashley G. W., Predictable and tunable half-life extension of therapeutic agents by controlled chemical release from macromolecular conjugates. Proc. Natl. Acad. Sci. U.S.A. 109, 6211–6216 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schneider E. L., et al. , A hydrogel-microsphere drug delivery system that supports once-monthly administration of a GLP-1 receptor agonist. ACS Chem. Biol. 12, 2107–2116 (2017). [DOI] [PubMed] [Google Scholar]
- 13.Henise J., Yao B., Ashley G. W., Santi D. V., Autoclave sterilization of tetra-polyethylene glycol hydrogel biomaterials with β-eliminative crosslinks. Eng. Rep. 2, e12091 (2020). [Google Scholar]
- 14.Ashley G. W., Henise J., Reid R., Santi D. V., Hydrogel drug delivery system with predictable and tunable drug release and degradation rates. Proc. Natl. Acad. Sci. U.S.A. 110, 2318–2323 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henise J., Hearn B. R., Ashley G. W., Santi D. V., Biodegradable tetra-PEG hydrogels as carriers for a releasable drug delivery system. Bioconjug. Chem. 26, 270–278 (2015). [DOI] [PubMed] [Google Scholar]
- 16.Henise J., et al. , High-throughput, aseptic production of injectable Tetra-PEG hydrogel microspheres for delivery of releasable covalently bound drugs. Eng. Rep. 2, e12213 (2020). [Google Scholar]
- 17.Henise J., Yao B., Ashley G. W., Santi D. V., Facile preparation of tetra-polyethylene glycol hydrogel microspheres for drug delivery by cross-flow membrane emulsification. Eng. Rep. 3, e12412 (2021). [Google Scholar]
- 18.Henise J., et al. , In vitro-in vivo correlation for the degradation of tetra-PEG hydrogel microspheres with tunable β-eliminative crosslink cleavage rates. Int. J. Polym. Sci. 2019, 9483127 (2019). [Google Scholar]
- 19.Wakankar A. A., Borchardt R. T., Formulation considerations for proteins susceptible to asparagine deamidation and aspartate isomerization. J. Pharm. Sci. 95, 2321–2336 (2006). [DOI] [PubMed] [Google Scholar]
- 20.Li B., Borchardt R. T., Topp E. M., VanderVelde D., Schowen R. L., Racemization of an asparagine residue during peptide deamidation. J. Am. Chem. Soc. 125, 11486–11487 (2003). [DOI] [PubMed] [Google Scholar]
- 21.Riggs D. L., Gomez S. V., Julian R. R., Sequence and solution effects on the prevalence of d-isomers produced by deamidation. ACS Chem. Biol. 12, 2875–2882 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robinson N. E., et al. , Structure-dependent nonenzymatic deamidation of glutaminyl and asparaginyl pentapeptides. J. Pept. Res. 63, 426–436 (2004). [DOI] [PubMed] [Google Scholar]
- 23.Riggs D. L., Silzel J. W., Lyon Y. A., Kang A. S., Julian R. R., Analysis of glutamine deamidation: Products, pathways, and kinetics. Anal. Chem. 91, 13032–13038 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abbey S. E., Potter L. R., Lysophosphatidic acid inhibits C-type natriuretic peptide activation of guanylyl cyclase-B. Endocrinology 144, 240–246 (2003). [DOI] [PubMed] [Google Scholar]
- 25.Schneider E. L., Henise J., Reid R., Ashley G. W., Santi D. V., Hydrogel drug delivery system using self-cleaving covalent linkers for once-a-week administration of exenatide. Bioconjug. Chem. 27, 1210–1215 (2016). [DOI] [PubMed] [Google Scholar]
- 26.Yasoda A., et al. , Systemic administration of C-type natriuretic peptide as a novel therapeutic strategy for skeletal dysplasias. Endocrinology 150, 3138–3144 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hirota K., et al. , Exogenous C-type natriuretic peptide restores normal growth and prevents early growth plate closure in its deficient rats. PLoS One 13, e0204172 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nair A. B., Jacob S., A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 7, 27–31 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoover-Fong J. E., McGready J., Schulze K. J., Barnes H., Scott C. I., Weight for age charts for children with achondroplasia. Am. J. Med. Genet. A. 143A, 2227–2235 (2007). [DOI] [PubMed] [Google Scholar]
- 30.Breinholt V. M., et al. , Phase 1 safety, tolerability, pharmacokinetics and pharmacodynamics results of a long-acting C-type natriuretic peptide prodrug, TransCon CNP. Br. J. Clin. Pharmacol., 10.1111/bcp.15369 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rudmann D. G., Alston J. T., Hanson J. C., Heidel S., High molecular weight polyethylene glycol cellular distribution and PEG-associated cytoplasmic vacuolation is molecular weight dependent and does not require conjugation to proteins. Toxicol. Pathol. 41, 970–983 (2013). [DOI] [PubMed] [Google Scholar]
- 32.CHMP Safety Working Party, CHMP Safety Working Party’s response to the PDCO regarding the use of PEGylated drug products in the paediatric population (2012). https://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2012/2011/WC500135123.pdf. Accessed 29 June 2022.
- 33.Mager D. E., Target-mediated drug disposition and dynamics. Biochem. Pharmacol. 72, 1–10 (2006). [DOI] [PubMed] [Google Scholar]
- 34.An G., Concept of pharmacologic target-mediated drug disposition in large-molecule and small-molecule compounds. J. Clin. Pharmacol. 60, 149–163 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or SI Appendix.