The amounts of methane and δ13CCH4 evolving from pyrolysis of the Sample Analysis of Mars evolved gas analysis (SAM-EGA) instrument suite as reported in ref. 1 are inconsistent with fundamental observations of thermogenic formation of methane from organic matter precursors. In pyrolysis, samples with organic carbon are heated, whereby the carbon isotopic composition of the evolving methane (δ13CCH4) is depleted in 13C compared to the organic carbon precursor and increases systematically with increasing temperature (e.g., figure 11 in ref. 2 and figure 5 in ref. 3). The pyrolysis setup of the SAM-EGA instrument suite is that of the so-called open-system pyrolysis for which quantitative data are available (3).
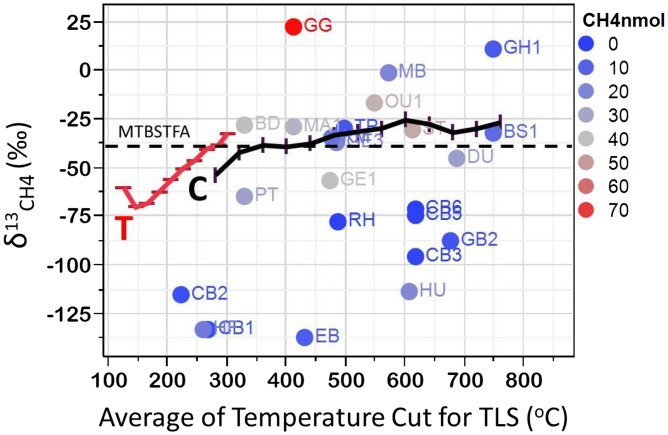
In comparison to these laboratory calibrations (“T” and “C” in Fig. 1), the SAM-EGA δ13CCH4 values are erratic, and do not exhibit any relationship with temperature and/or the amount of evolved CH4 (Fig. 1). Specifically, δ13CCH4 values in 10 out of 16 samples are either more negative than −100‰ or more positive than −25‰, values never measured in pyrolysis; moreover, δ13CCH4 values of eight samples >20 nmol are similar to that of N-tert-butyldimethylsilyl-N-methyl-trifluoroacetamide (MTBSTFA), a potential contaminant (Fig. 1).
Fig. 1.
SAM-EGA pyrolysis carbon isotopic composition of the evolved methane related to the pyrolysis temperature in comparison with laboratory data. The red line “T” is the trend for cumulative methane evolution in sealed gold tube pyrolysis from Tang et al.’s (2) figure 11. The black line “C” is from open-system pyrolysis with instantaneous methane formation (3). The SAM pyrolysis δ13Cmethane exhibit no correlation with temperature or methane concentration but rather plot randomly with an unrealistic range from −137‰ (EB) to +11‰ (GH1). Sample ID is from ref. 1. MTBSTFA is a potential contaminant from earlier wet chemistry experiments on the SAM instrument (1).
Despite these serious analytical issues, the authors (1) conclude, from the 10 most-depleted δ13CCH4 values, that “biologically produced methane from the Martian interior could result in the deposition of 13C-depleted organic material after photolysis,” thus inferring that 13C-depleted methane from a high-temperature process is of biological origin! Finally, the authors offer “Martian methanotrophy” and infer the widespread existence of “methane [that] was already 13C depleted because of its biological production during methanogenesis.” Again, biogenesis of methane based on 9 out of 24 depleted δ13CCH4 values from a high-temperature process to infer methane as a proven biosignature on Mars.
Footnotes
The author declares no competing interest.
References
- 1.House C. H., Wong G. M., Webster C. R., Mahaffy P. R., Depleted carbon isotope compositions observed at Gale crater, Mars. Proc. Natl. Acad. Sci. U.S.A. 119, 10.1073/pnas.2115651119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang Y., Perry J. K., Jenden P., Schoell M., Mathematical modeling of stable carbon isotope ratios in natural gases. Geochim. Cosmochim. Acta 64, 2673–2687 (2000). [Google Scholar]
- 3.Cramer B., Krooss B. M., Littke R., Modeling isotope fractionation during primary cracking of natural gas: A reaction kinetic approach. Chem. Geol. 149, 235–250 (1998). [Google Scholar]