Significance
Danish and Greenland Inuit epidemiologic studies in the 1970s reported that eicosapentaenoic acid (EPA) reduces the risk of death after myocardial infarction, and further clinical studies have indicated its analgesic, anti-(neuro)inflammatory, platelet aggregation inhibition, blood triglyceride and glucose decrease, and insulin resistance improvement properties. However, the molecular target of EPA remains unclear. In this study, we demonstrated that EPA is a potent physiological blocker of vesicular nucleotide transporter (VNUT) and potently alleviates neuropathic and inflammatory pain and insulin resistance with few side effects via VNUT inhibition. Thus, we identified VNUT as a molecular target of EPA. Our results can help develop unique nutrient-based treatment and prevention strategies by targeting purinergic chemical transmission for inflammatory, neurological, and metabolic diseases.
Keywords: eicosapentaenoic acid, omega-3 polyunsaturated fatty acid, vesicular nucleotide transporter, purinergic chemical transmission, neuropathic pain
Abstract
Eicosapentaenoic acid (EPA), an omega-3 (ω-3) polyunsaturated fatty acid, is an essential nutrient that exhibits antiinflammatory, neuroprotective, and cardiovascular-protective activities. Although EPA is used as a nutrient-based pharmaceutical agent or dietary supplement, its molecular target(s) is debatable. Here, we showed that EPA and its metabolites strongly and reversibly inhibit vesicular nucleotide transporter (VNUT), a key molecule for vesicular storage and release of adenosine triphosphate (ATP) in purinergic chemical transmission. In vitro analysis showed that EPA inhibits human VNUT-mediated ATP uptake at a half-maximal inhibitory concentration (IC50) of 67 nM, acting as an allosteric modulator through competition with Cl−. EPA impaired vesicular ATP release from neurons without affecting the vesicular release of other neurotransmitters. In vivo, VNUT−/− mice showed a delay in the onset of neuropathic pain and resistance to both neuropathic and inflammatory pain. EPA potently attenuated neuropathic and inflammatory pain in wild-type mice but not in VNUT−/− mice without affecting the basal nociception. The analgesic effect of EPA was canceled by the intrathecal injection of purinoceptor agonists and was stronger than that of existing drugs used for neuropathic pain treatment, with few side effects. Neuropathic pain impaired insulin sensitivity in previous studies, which was improved by EPA in the wild-type mice but not in the VNUT−/− mice. Our results showed that VNUT is a molecular target of EPA that attenuates neuropathic and inflammatory pain and insulin resistance. EPA may represent a unique nutrient-based treatment and prevention strategy for neurological, immunological, and metabolic diseases by targeting purinergic chemical transmission.
Omega-3 (ω-3) polyunsaturated fatty acids (PUFAs) are essential nutrients that contain multiple double bonds. PUFAs can be classified into ω-3 and ω-6 depending on the position of the bonds. As humans cannot produce PUFAs, they must be acquired from the diet to maintain homeostasis. Omega-3 PUFAs, such as eicosapentaenoic acid (EPA), are abundantly present in fish and linseed oil and exhibit antiinflammatory, neuroprotective, and cardiovascular-protective activities via the competitive inhibition of cyclooxygenase (COX)-2 in eicosanoid production (1–3). Danish and Greenland Inuit epidemiological studies have reported that EPA reduces the risk of death after myocardial infarction (4, 5), and other studies have reported its influence on analgesia, neuroinflammatory disease (Parkinson’s disease, Alzheimer’s disease, and depression) improvement, platelet aggregation inhibition, decrease in blood triglyceride and glucose levels, and improved insulin resistance (1, 6–11). Omega-3 fatty acid supplementation in COVID-19 patients showed a beneficial effect in managing the cytokine storm (12). Conversely, omega-6 fatty acids, such as arachidonic acid, produce inflammatory eicosanoids and play central roles in the initial stage of inflammatory responses (13). Although arachidonic acid has also been reported to produce antiinflammatory metabolites, omega-6 PUFA-derived linoleate diols have a harmful effect and are biomarkers for severe COVID-19 infection (14). An omega-6 PUFA-enriched Western-style diet, which abundantly contains linoleate, causes neuropathy and chronic pain, but an omega-3 PUFA-enriched diet attenuates these pathological conditions (15).
All therapeutic effects of EPA cannot be explained by COX-2 inhibition alone (16). Typically, COX-2 inhibitors (nonsteroidal antiinflammatory druga [NSAIDs]) are effective for inflammatory pain but ineffective for neuropathic pain (16). However, EPA significantly attenuates both inflammatory and neuropathic pain, which strongly suggests another important molecular target of EPA related to neuropathy (7, 8). Although chronic pain is coincidentally caused by inflammation and neuropathy, there is no therapeutic drug with few side effects to attenuate both inflammatory and neuropathic pain (17–20). In this situation, EPA may affect the key signaling molecule(s) in neurological, metabolic, and immunological functions.
Purinergic chemical transmission is involved in neurological, metabolic, and immunological disruptions and functions, including neuropathic and inflammatory pain, depression, inflammation, increase in blood triglyceride and glucose levels, insulin resistance, and blood coagulation (21, 22). The released adenosine triphosphate (ATP) and degraded adenosine diphosphate (ADP) or adenosine binds to many types of purinoceptors that are intricately involved in biological and pathological processes. In pain perception, ATP and ADP bind to P2X and P2Y receptors and thereby exacerbate neuropathic and inflammatory pain (23). Adenosine binds to P1 receptors and thereby attenuates neuropathic and inflammatory pain (24). However, a vesicular nucleotide transporter (VNUT/SLC17A9) is localized in the secretory vesicles of neuronal, endocrine, and immune cells. It plays an essential role in vesicular ATP storage in a Δψ- and Cl–-dependent manner in the purinergic chemical transmission, which leads to vesicular ATP release (25, 26). Thus, VNUT is a key molecule in the initiation of purinergic signaling for neurological, metabolic, and immunological disruptions and functions. Interestingly, the observed effects of the VNUT inhibitor and phenotypes of VNUT−/− mice were consistent with the above-mentioned therapeutic effects of EPA (27–31). Therefore, we hypothesized that VNUT serves as a molecular target of EPA to attenuate neuropathic and inflammatory pain.
Here, we demonstrated that a low concentration of EPA and its metabolites, but not docosahexaenoic acid (DHA), are potent and selective physiological inhibitors of vesicular ATP release via the blockade of purinergic chemical transmission, which improved neuropathic and inflammatory pain and insulin resistance. Furthermore, EPA is more effective for neuropathic and inflammatory pain and has fewer side effects than existing drugs.
Results
EPA and Its Metabolites Are Potent VNUT Inhibitors.
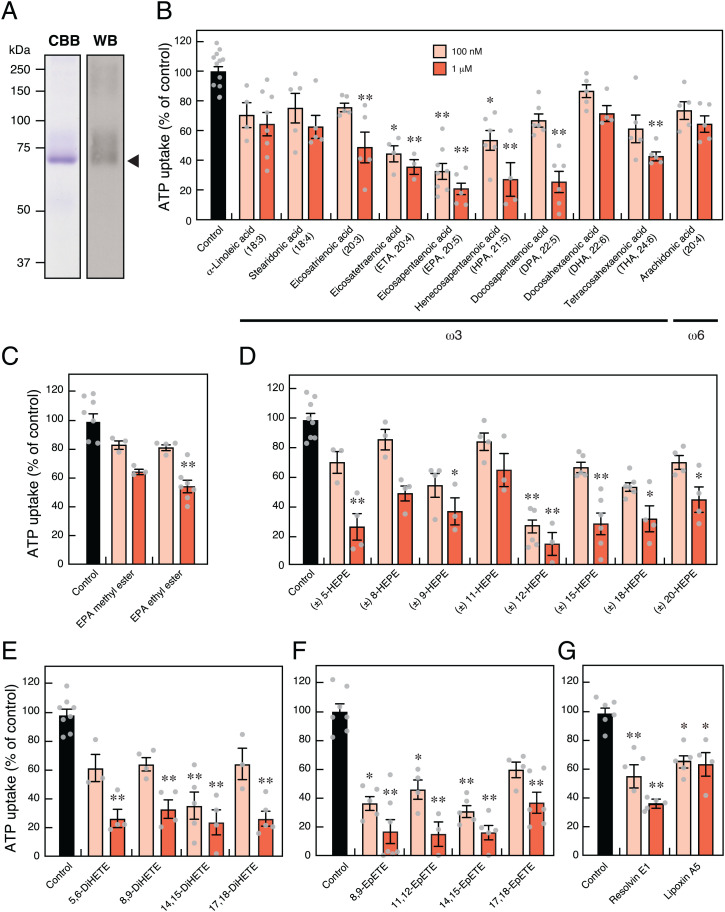
We evaluated VNUT as a molecular target of ω-3 PUFAs using a quantitative transport assay system with proteoliposomes containing only a purified human VNUT protein (Fig. 1A and SI Appendix, Fig. S1). Low-concentration EPA exhibited a potent inhibitory effect on VNUT-mediated ATP uptake. Structure–activity relationship analysis showed that VNUT-mediated ATP uptake was inhibited by EPA (20:5, ω3), eicosatetraenoic acid (ETA: 20:4, ω3), heneicosapentaenoic acid (HPA: 21:5, ω3), and docosapentaenoic acid (DPA: 22:5, ω3) (Fig. 1B). Interestingly, docosahexaenoic acid (DHA: 22:6, ω3), another typical ω-3 PUFA, and arachidonic acid (20:4, ω6), a typical ω-6 PUFA, α-linoleic acid (18:3, ω3), stearidonic acid (18:4, ω3), and eicosatrienoic acid (20:3, ω3) (Fig. 1B) did not effectively inhibit VNUT-mediated ATP uptake, suggesting that chain length and the number and position of double bonds are important for exerting inhibitory effects. EPA ethyl ester (Epadel) and EPA + DHA ethyl ester (Lotriga) are used as prodrugs of EPA in clinical practice or dietary supplements for hyperlipidemia. EPA methyl ester and EPA ethyl ester significantly reduced the inhibitory effect of VNUT-mediated ATP uptake when compared with EPA (Fig. 1C), suggesting that the carboxyl group is important for VNUT inhibition. Furthermore, VNUT-mediated ATP transport was inhibited by EPA metabolites, such as hydroxyeicosapentaenoic acid (HEPE), dihydroxyeicosatetraenoic acid, epoxyeicosatetraenoic acid (EpETE), resolvin E1, and lipoxin A5, suggesting that the structure of ω-3 PUFA with side chains, as shown in SI Appendix, Fig. S1, is necessary for VNUT inhibition (Fig. 1D–G).
Fig. 1.
EPA and its metabolites are potent inhibitors of VNUT-mediated ATP uptake. (A) Coomassie Brilliant Blue (CBB) staining and immunoblotting (WB) using 6×His-tag antibodies against purified human VNUT protein (3 μg) subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Arrowhead shows the position of recombinant VNUT protein. (B–G) Inhibitory effect of 100 nM or 1 μM polyunsaturated fatty acid on VNUT (B), EPA-ester (C), HEPE (D), DiHETE (E), EpETE (F), and resolvin E1 and lipoxin A5 (G) assayed under physiological intracellular concentration of 10 mM Cl– for 2 min; n = 3 to 14. Data represent mean ± SEM. *P < 0.05, **P < 0.01 (one-way ANOVA followed by Dunnett’s test).
EPA Works as an Allosteric Modulator through Competition with Cl−.
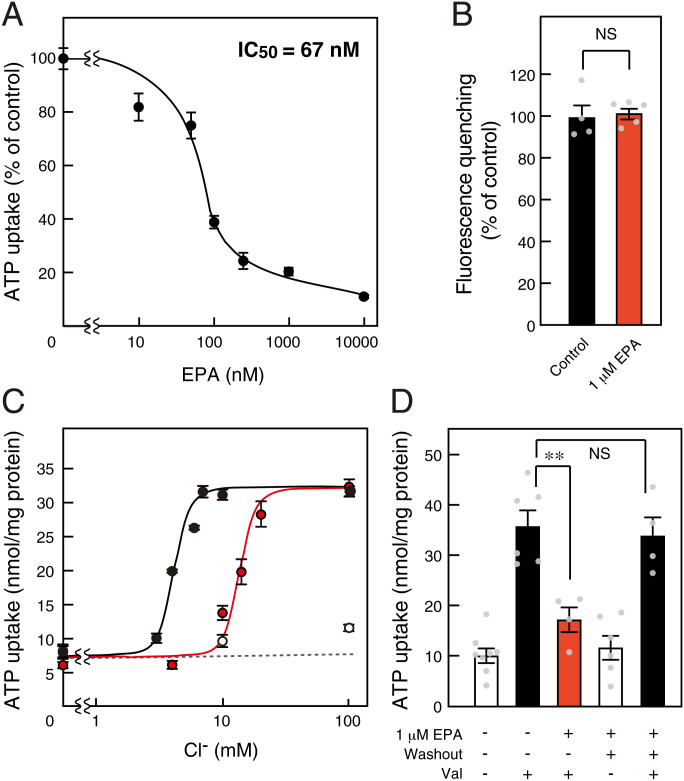
We further elucidated the inhibitory mechanism of EPA on VNUT-mediated ATP uptake. EPA inhibited VNUT-mediated ATP uptake at a half-maximal inhibitory concentration (IC50) of 67 nM (Fig. 2A). A high concentration of EPA had no effect on the Δψ driving force, as observed following the oxonol V fluorescence quenching (Fig. 2B). VNUT shows unique transport properties; its Cl–-dependent activation exhibited strong positive cooperativity for ATP transport, with a Hill coefficient of ∼3 for Cl– (30). EPA-mediated VNUT inhibition decreased as the Cl– concentration increased, suggesting a competitive interaction between Cl– and EPA (Fig. 2C). Furthermore, the inhibitory effect of EPA on VNUT was completely recovered by washout, which strongly suggests that a reversible reaction occurred (Fig. 2D).
Fig. 2.
EPA reversibly inhibits VNUT via Cl– competition. (A) ATP uptake assayed under 10 mM Cl– for 2 min. IC50: inhibitory concentration of EPA; n = 5 to 14. (B) Valinomycin (Val)-evoked Δψ formation measured for 2 min for quenching of oxonol V fluorescence in the presence (red bar) or absence (closed bar) of 1 μM EPA; n = 4 to 5. (C) ATP uptake for 1 min at various Cl– concentrations in the presence (red circles) or absence (closed circles) of 1 μM EPA, or in the absence of valinomycin (open circles); n = 3 to 11. (D) Inhibition of ATP uptake by 1 μM EPA fully reversed after washing proteoliposomes; n = 4 to 8. Data represent mean ± SEM. **P < 0.01; NS, not significant (two-tailed paired Student’s t test).
EPA Modulates Vesicular ATP Release.
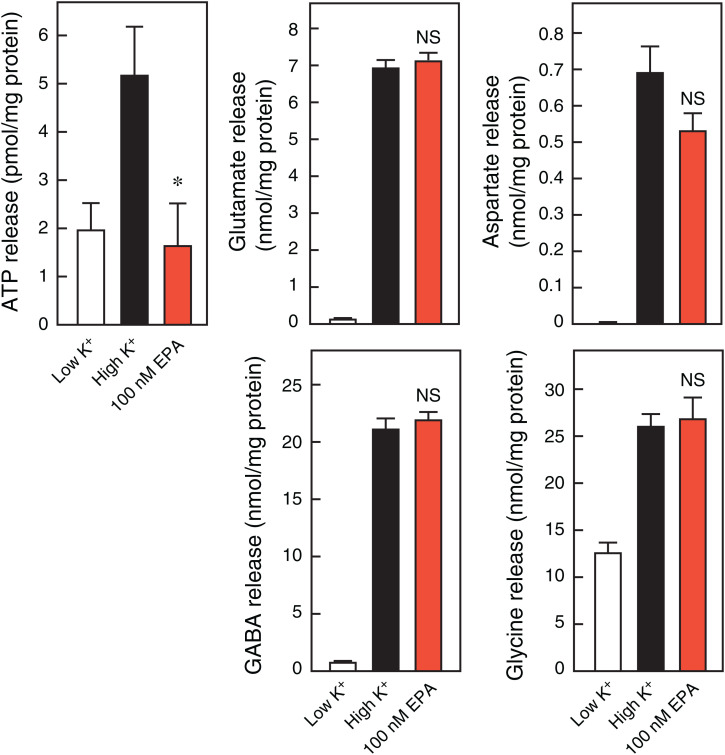
ATP is released from neurons through VNUT-mediated exocytosis (27, 30), which is important for the onset of neuropathic pain (28). Low-concentration EPA (100 nM) completely inhibited vesicular ATP release upon depolarization-dependent stimulation via increasing K+ concentration in the rat neurons (Fig. 3). In a parallel experiment of vesicular ATP release, EPA did not inhibit the release of other excitatory or inhibitory neurotransmitters such as glutamate, aspartate, gamma-aminobutyric acid (GABA), and glycine (Fig. 3). These observations strongly suggest that EPA selectively and effectively inhibits vesicular ATP release.
Fig. 3.
EPA is a selective inhibitor of vesicular ATP release. KCl-dependent release of neurotransmitters such as ATP, glutamate, aspartate, GABA, and glycine from rat cultured neurons after 20 min, assayed in the presence (red bars) or absence (closed bars) of 100 nM EPA; n = 5 to 7. Data represent mean ± SEM. *P < 0.05; NS, not significant (two-tailed paired Student’s t test).
EPA Attenuates Chemotherapy-Induced Neuropathic Pain via VNUT Inhibition.
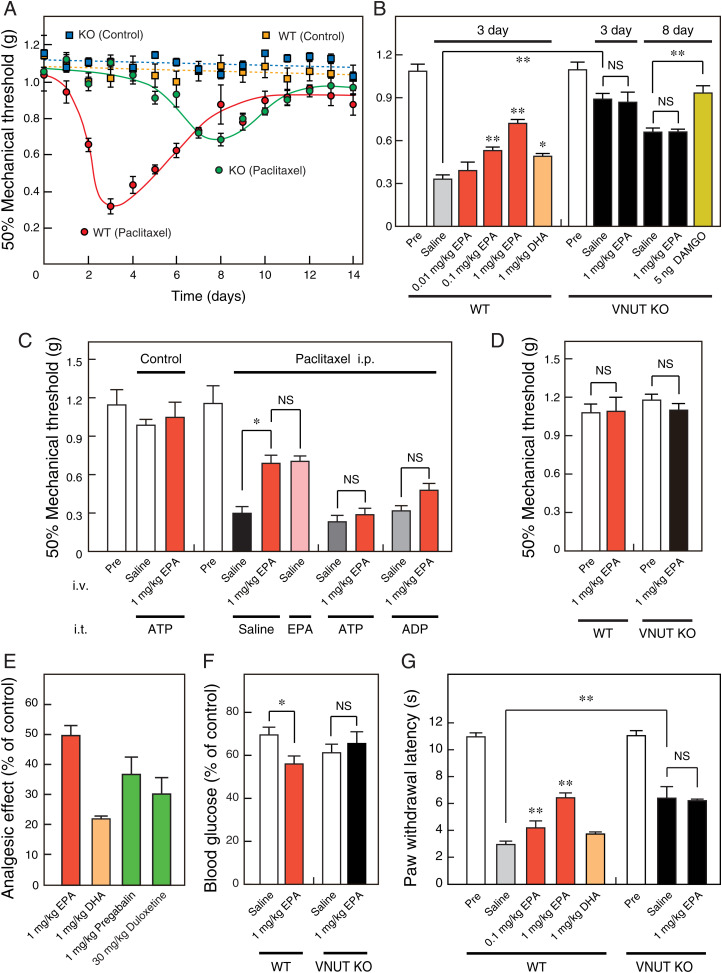
We evaluated the analgesic effects of EPA on chemotherapy-induced neuropathic pain in wild-type (WT) and VNUT−/− mice because chemotherapy causes a high incidence of peripheral neuropathy, and omega-3 PUFA is effective for chemotherapy-induced neuropathy (8, 32). The onset of anticancer agent (paclitaxel)-evoked neuropathy and the accompanying pain were significantly delayed from 3 to 8 d in the VNUT−/− mice compared with the wild-type mice (Fig. 4A). In addition, the VNUT−/− mice exhibited reduced maximal hyperalgesia (by 60%) compared with the wild-type mice (Fig. 4A), suggesting that VNUT inhibition contributes to the prevention and analgesia of neuropathy and the accompanying pain.
Fig. 4.
EPA attenuates neuropathic and inflammatory pain via VNUT inhibition. In wild-type and VNUT−/− mice, (A) von Frey tests were performed each day after an intraperitoneal injection of paclitaxel or solvent (0 d) without EPA injection (n = 7 to 9), and (B and C) 45 min (time of maximal EPA effect) after intravenous injection of saline or compound at 3 d (WT) or 3 and 8 d (knockout [KO]) after paclitaxel injection (n = 5 to 10). DAMGO (5 ng) was injected intrathecally 15 min before the von Frey test in VNUT−/− mice (B). ATP and ADP (0.6 pg) were injected intrathecally 30 min before the von Frey test in wild-type mice (C). EPA (10 ng) was injected intrathecally 45 min before the von Frey test in wild-type mice (C). (D) Von Frey test was performed 45 min after the intravenous injection of saline or 1 mg/kg EPA in a normal condition of wild-type and VNUT−/− mice without intraperitoneal injection of paclitaxel (n = 5 to 7). (E) Omega-3 polyunsaturated fatty acids and analgesic agents for neuropathic pain at the indicated dose were assayed by intravenous injection at 3 d after paclitaxel injection in wild-type mice (n = 5 to 7). Pregabalin and duloxetine were injected intravenously at 3 h and 1 h (time of maximal effect) before the von Frey test, respectively. (F) Insulin tolerance tests were performed 45 min after intravenous injection of saline or 1 mg/kg EPA at 3 d (WT) or 8 d (KO) after paclitaxel injection (n = 5 to 12). Insulin sensitivity (% of control) was estimated by the ratio of blood glucose before and after insulin injection. (G) Plantar test performed 45 min after intravenous injection of saline or compound at 4 h after carrageenan injection in wild-type and VNUT−/− mice (n = 5 to 6). Control experiments used saline + 0.04% ethanol corresponding to the solvent of 1 mg/kg EPA and DHA. Data represent mean ± SEM. *P < 0.05, **P < 0.01; NS, not significant (one-way ANOVA followed by Dunnett’s test or two-tailed paired Student’s t test).
Intravenous injection of EPA in the wild-type mice significantly reduced the paclitaxel-induced neuropathic pain in a time-dependent manner. The time of maximal effect was 45 min after EPA injection (SI Appendix, Fig. S2A and Fig. 4B). DHA, which did not exhibit a potent inhibitory effect on VNUT, had a weaker analgesic effect than EPA (Fig. 4B). As expected, the analgesic effect of EPA, but not mu-opioid receptor agonist (DAMGO) as another analgesic target, was not observed in the VNUT−/− mice (Fig. 4B). In addition, the EPA analgesic effect was completely abolished by the intrathecal injection of ATP and ADP, which are P2X and P2Y receptor agonists, respectively (Fig. 4C). This result is consistent with the previous reports that ATP released from neurons bind to P2X4, P2X7, and P2Y12 receptors in microglia, leading to neuropathic pain (23, 28). To decrease the possibilities of peripheral effects and EPA metabolism, we showed that the intrathecal injection of EPA attenuates neuropathic pain at a level comparable to the intravenous injection of EPA (Fig. 4C). EPA did not change the baseline sensory threshold values (Fig. 4D). These observations indicate that EPA attenuates neuropathic pain, without affecting basal nociception via the specific inhibition of vesicular ATP release.
The analgesic effect of EPA was stronger than that of analgesic agents, such as pregabalin and duloxetine, which are clinically used for neuropathic pain treatment (Fig. 4E). Pregabalin and duloxetine at their effective dose induce drowsiness and reduced exploratory behavior. However, these severe side effects were not observed in the case of EPA, even at the dose of 1 mg/kg (Movie S1). EPA ethyl ester, a prodrug that facilitates absorption and distribution, was metabolized to EPA and ethanol by intracellular carboxylesterase. Thus, it was effective for treating neuropathic pain, without causing any severe side effects (SI Appendix, Figs. S2B and S3 and Movie S1).
A recent study reported that the omega-3 fatty acids–evoked analgesic effect is canceled by intrathecal injection at a high dose (1 μg) of CTOP, a potent mu-opioid receptor antagonist in the diabetic neuropathic pain rat model (33). We reconfirmed that the intrathecal injection of DAMGO, a potent mu-opioid receptor agonist, attenuated the paclitaxel-induced neuropathic pain, and the analgesic effect was largely blocked by the intrathecal injection of CTOP at a low dose (1 ng). As expected, the intrathecal injection of 1 ng CTOP did not significantly cancel the EPA-evoked analgesic effect (SI Appendix, Fig. S4), supporting the finding that DAMGO was effective in VNUT−/− mice (Fig. 4B). Although the molecular target of EPA is debatable, these results strongly suggest that EPA attenuates neuropathic pain in a VNUT-dependent manner.
EPA Improves Neuropathy-Induced Insulin Resistance via VNUT Inhibition.
Neuropathic pain is involved in insulin resistance via the insulin receptor–PI3K–Akt signaling pathway (34). Therefore, we examined insulin resistance in the paclitaxel-induced neuropathic pain model. The intravenous injection of EPA improved insulin resistance in the wild-type mice at the same time of the maximal analgesic effect (Fig. 4F). However, EPA was ineffective in improving insulin resistance in the VNUT−/− mice (Fig. 4F), suggesting that EPA improves neuropathy-induced insulin resistance in a VNUT-dependent manner.
EPA Attenuates Neuropathic and Inflammatory Pain via VNUT Inhibition.
We further examined the analgesic effects of EPA on the partial sciatic nerve ligation–induced neuropathic pain and the carrageenan-induced inflammatory pain in wild-type and VNUT−/− mice. EPA significantly attenuated both neuropathic and inflammatory pain in the wild-type mice but not the VNUT−/− mice (SI Appendix, Fig. S5 and Fig. 4G). This analgesic effect on the inflammatory pain was comparable with that of the nonnarcotic opioid tramadol in the therapeutic range, as shown in our previous report (30). These results strongly suggest that the EPA-evoked VNUT inhibition is effective for a wide range of chronic pain.
Discussion
Previous attempts at identifying the molecular targets of EPA, including the epidemiologic study of Danish and Greenland Inuits, have been unsuccessful (4, 5). In our study, we showed that low-concentration EPA, a typical omega-3 PUFA, but not DHA, is a potent and selective physiological VNUT inhibitor. In addition, our findings indicate that the EPA-evoked inhibition of vesicular ATP release is important for treating and preventing neuropathy and the accompanying pain. To the best of our knowledge, this study identifies one of the major molecular targets of EPA and the mechanisms underlying its neuropathic and inflammatory pain treatment.
VNUT−/− mice had improved pathological conditions, such as neuropathy and accompanying pain, without any significant change in their phenotype via the blockade of vesicular storage and release of ATP (Fig. 4). Our findings and previous reports suggest that the vesicular ATP release is a risk factor for various conditions and diseases associated with metabolic syndromes and EPA deficiency (21, 22, 27–31). In addition, chemotherapy-induced side effects involve not only neuropathy and insulin resistance but also cardiomyopathy and nephropathy (35, 36). Because these side effects are caused by the abnormality of purinergic chemical transmission (36), EPA may attenuate these side effects in a VNUT-dependent manner. We revealed that the first generation bisphosphonate clodronate is also a VNUT inhibitor (IC50 = 15.6 nM), and clodronate and EPA attenuated neuropathic and inflammatory pain with the same maximal efficacy. Approximately 60% and 40% attenuation of the partial sciatic nerve ligation–induced neuropathic pain and the carrageenan-induced inflammatory pain were observed following the injection of EPA and clodronate, respectively (30). Interestingly, EPA showed an instantaneous effect, whereas clodronate showed a long-lasting effect, on neuropathic pain, suggesting the difference in their metabolism. In contrast, COX-2 expression increases during inflammation, enhancing the production of inflammatory eicosanoids from omega-6 PUFA arachidonic acid, thereby leading to an inflammatory response (3, 13). The production of inflammatory eicosanoids is competitively inhibited by EPA with an IC50 value of 7.1 μM (3). Therefore, EPA blocks purinergic signaling (via VNUT inhibition: IC50 = 67 nM) and inflammatory eicosanoid production (via COX-2 inhibition). As COX-2−/− mice showed reduced viability due to conditions such as renal alterations, renal dysplasia, and cardiac fibrosis (37), the phenotypes of COX-2−/− mice were not consistent with EPA efficacy. It should be stressed that EPA-evoked VNUT inhibition was more effective than EPA-evoked COX-2 inhibition. Further studies evaluating other therapeutic effects induced by EPA-evoked VNUT inhibition are currently underway in our laboratory.
We found that VNUT-mediated ATP transport was inhibited by various EPA metabolites at the nanomolar level (Fig. 1). EPA and its metabolites bind to the specific G protein–coupled receptors (GPCRs), such as GPR120 and GPR40, but only at the micromolar levels, which have an independent mechanism to VNUT (38, 39). GPR120 and GPR40 are also involved in glucose homeostasis and the inflammatory process. These results indicate that EPA and its metabolites are effective for VNUT at lower concentrations than for the GPCRs. Among EPA metabolites, EpETE was slightly more effective in the VNUT-mediated ATP transport than EPA. Cytochrome P450 produces 17,18-EpETE, which modulates allergy and inflammation via mast cell activation and neutrophil mobility (39, 40). Cytochrome P450 produces 12-HEPE, which effectively inhibits VNUT and is involved in glucose homeostasis, insulin resistance, and atherosclerosis (41, 42). Vascular hyperpermeability is attenuated by 5,6-DiHETE during inflammation (43). However, these other physiological functions still remain unclear. Interestingly, resolvin E1, a proresolving mediator, is produced from 18-HEPE and binds to the specific GPCRs, such as ChemR23 and leukotriene B4 receptor at the nanomolar level, leading to the attenuation of inflammatory pain rather than neuropathic pain (44–48). Pharmacologically, resolvin E1 exerts various physiological effects, such as antineuropathic and antiinflammatory effects, via agonistic GPCR and antagonistic VNUT binding. As the physiological functions of other EPA metabolites are also unknown, further studies evaluating their physiological functions in the purinergic chemical transmission are currently underway in our laboratory.
Nerve injury, inflammation, and other pathological processes cause chronic pain, with a worldwide prevalence of 20 to 25% and an annual treatment cost of more than US$600 billion (49, 50). The increase in treatment cost is due to the increase in the prevalence of chronic pain. As optimal drug treatment regimens with fewer side effects are unavailable (17–20), patients suffer from considerable chronic pain and side effects, leading to decreased quality of life and increased economic burden. Moreover, addiction, dependency, respiratory depression, sedation, nausea, vomiting, constipation, and tolerance are some of the other problems associated with opioid treatment in the United States (18, 19). Therefore, it is clinically and nutritionally important to confirm that EPA is not involved in side effects such as addiction and dependency. However, the EPA-evoked analgesic effect was not canceled by the mu-opioid receptor antagonist. Hence, EPA-induced VNUT inhibition may be a unique treatment and prevention for neuropathic and inflammatory pain without affecting basal nociception and with fewer side effects and less insulin resistance. Although EPA was more effective for VNUT than DHA (Fig. 1B), EPA and DHA are commonly used together as pharmaceutical agents or dietary supplements. Recent clinical trials also showed the clinical importance of EPA-induced VNUT inhibition as it reported that EPA-mediated attenuation of neurological diseases and hyperlipidemia was more effective than that mediated by EPA + DHA agent (9, 10, 51, 52). Our finding emphasizes that enrichment of EPA but not DHA is important in neurological, immunological, and metabolic diseases.
EPA improved neuropathic and inflammatory pain and insulin resistance in a VNUT-dependent manner, but the other therapeutic effects of EPA are also consistent with the phenotypes of VNUT−/− mice and the efficacy of the VNUT inhibitor. Therefore, VNUT is a hopeful molecular target for other therapeutic effects of EPA. In future studies, a wide range of in vivo and in vitro techniques should be employed to reveal these therapeutic mechanisms, and further investigation into whether this molecular target of EPA and its metabolites is VNUT and how vesicular ATP release inhibition leads to signaling cascades is required.
To summarize, low EPA concentration selectively and potently inhibited VNUT and safely regulated purinergic chemical transmission in vivo. The reduction in purinergic chemical transmission attenuated and prevented neuropathic and inflammatory pain and insulin resistance. Thus, using EPA may be a unique nutrient-based treatment and prevention strategy for neurological, immunological, and metabolic diseases, in addition to hyperlipidemia. Notably, EPA has been approved for clinical and dietary use in many countries, and its clinical safety in humans is well established. Oral supplementation with omega-3 PUFA shows the transfer of EPA across the blood–brain barrier (53), and oral administration of EPA at 1,800 mg/d (dose per day of Epadel) results in mild side effects such as nausea, diarrhea, discomfort in the upper abdomen, and belching rarely. Considering the potency of EPA and the side effects of existing analgesics, EPA and its metabolites are potential neurological, immunological, and metabolic therapeutic drugs that function via the blockade of vesicular ATP release.
Materials and Methods
Reconstitution.
Human VNUT was expressed in Escherichia coli and purified as previously described (30). Aliquots of 20 μg of purified protein were mixed with 550 μg of liposomes and frozen at −80 °C for at least 15 min. The mixture was thawed quickly by holding the sample tube in hand and diluted 60-fold with reconstitution buffer containing 20 mM 3-(N-morpholino)propanesulfonic acid (MOPS)-Tris (pH 7.0), 150 mM sodium acetate, and 5 mM magnesium acetate. Reconstituted proteoliposomes were obtained by centrifugation at 200,000 × g for 1 h at 4 °C and then suspended in 0.2 mL of reconstitution buffer. Asolectin liposomes were prepared using a tip sonifier, as previously described (30).
Transport Assay.
A reaction mixture (130 μL) consisting of 0.3 μg of VNUT protein incorporated into proteoliposomes, 20 mM MOPS-Tris (pH 7.0), 140 mM potassium acetate, 5 mM magnesium acetate, 10 mM KCl, 2 μM valinomycin, and 100 μM [3H]-ATP (0.5 MBq/μmol; PerkinElmer) was incubated at 27 °C. At the indicated time points, the proteoliposomes were separated from the external medium using centrifuge columns containing Sephadex G-50 (fine) to terminate the transport. The radioactivity in the eluate was measured by liquid scintillation counting (PerkinElmer).
Animal Experiments.
All animal experiments were performed in accordance with the guidelines set by the Animal Ethics Committee of Okayama University and approved institutional guidelines. C57BL/6 mice and Wistar rats were purchased from Japan SLC, Inc. We used VNUT−/− mice and the wild-type littermates, which were also used in the previous studies (27, 30, 31). These animals were housed individually or in groups of no more than four animals per cage at a temperature of 23 ± 1 °C under a 12-h light/dark cycle and allowed ad libitum access to food and water.
For the chemotherapy-induced neuropathic pain model, 6 mg/kg paclitaxel (Adipogen) in solvent (cremophor EL:ethanol = 1:1) was administered intraperitoneally into the C57BL/6 mice (male, 7 to 9 wk old, 22 to 30 g at the time of the test) using a 1,000-μL Terumo syringe with a 26-gauge needle. For the inflammatory pain model, 20 μL of 1% λ-carrageenan solution (Sigma-Aldrich) was administered into the plantar surface of the left hind paw of the C57BL/6 mice (male, 7 to 9 wk old, and weighing 22 to 30 g at the time of the test) using a 100-μL Hamilton microsyringe with a 27-gauge needle. The von Frey and plantar tests were performed to evaluate the neuropathic and inflammatory pain, respectively (SI Appendix, Supplementary Information). Drug administration was performed via intravenous (a volume of 100 μL per 10 g of body weight) or intrathecal injection (a volume of 5 μL). All the animal experiments were randomized and double blind.
Data Analysis.
The sample size was selected to allow statistical analysis of the results according to the previous animal experiments (30). All numerical values are presented as the mean ± SEM. Two-tailed paired Student’s t test or one-way ANOVA followed by Dunnett’s test for multiple comparisons was performed to determine the statistical significance of results using GraphPad Prism 6 software (GraphPad Software). *P < 0.05, **P < 0.01 were considered statistically significant; NS, not significant.
Supplementary Material
Acknowledgments
We thank Prof. Yoshinori Moriyama (current affiliation: Kurume University), Dr. Hiroshi Omote, Dr. Narinobu Juge, and Dr. Miki Hiasa at Okayama University, Dr. Jin Endo and Dr. Motoaki Sano at Keio University, and Prof. Masatoshi Nomura (current affiliation: Kurume University) and Dr. Shohei Sakamoto at Kyushu University for their suggestions. We thank Ms. Miku Komatsu and Ms. Manami Nishimura at Okayama University for their help with this study. We thank Prof. Nathan Nelson at Tel Aviv University for providing the bacterial expression vector. This work was supported in part by the Japan Agency for Medical Research and Development (Nos. 19gm5910019h0004 and JP19lm023008) to T.M., a Grant-in-Aid for Scientific Research (B) (Nos. 18H03179 and 22H03534), a Grant-in-Aid for Challenging Research (Exploratory) (Nos. 17K19489 and 21K19338) to T.M., the Astellas Foundation for Research on Metabolic Disorders, the Salt Science Research Foundation (Nos. 2038 and 2140), the Uehara Memorial Foundation, the Lotte Foundation, the Naito Foundation, and the Takeda Science Foundation to T.M.; a Grant-in-Aid for Young Scientists (No. 18K14903) to Y.K.; and the Takeda Science Foundation to Y.K.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2122158119/-/DCSupplemental.
Data Availability
All study data are included in the article and/or supporting information.
References
- 1.Simopoulos A. P., Omega-3 fatty acids in inflammation and autoimmune diseases. J. Am. Coll. Nutr. 21, 495–505 (2002). [DOI] [PubMed] [Google Scholar]
- 2.Rodriguez-Leyva D., Dupasquier C. M., McCullough R., Pierce G. N., The cardiovascular effects of flaxseed and its omega-3 fatty acid, alpha-linolenic acid. Can. J. Cardiol. 26, 489–496 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ringbom T., et al. , Cox-2 inhibitory effects of naturally occurring and modified fatty acids. J. Nat. Prod. 64, 745–749 (2001). [DOI] [PubMed] [Google Scholar]
- 4.Dyerberg J., Bang H. O., Stoffersen E., Moncada S., Vane J. R., Eicosapentaenoic acid and prevention of thrombosis and atherosclerosis? Lancet 2, 117–119 (1978). [DOI] [PubMed] [Google Scholar]
- 5.Bang H. O., Dyerberg J., Sinclair H. M., The composition of the Eskimo food in north western Greenland. Am. J. Clin. Nutr. 33, 2657–2661 (1980). [DOI] [PubMed] [Google Scholar]
- 6.Yokoyama M., et al. ; Japan EPA lipid intervention study (JELIS) Investigators, Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): A randomised open-label, blinded endpoint analysis. Lancet 369, 1090–1098 (2007). [DOI] [PubMed] [Google Scholar]
- 7.Ko G. D., Nowacki N. B., Arseneau L., Eitel M., Hum A., Omega-3 fatty acids for neuropathic pain: Case series. Clin. J. Pain 26, 168–172 (2010). [DOI] [PubMed] [Google Scholar]
- 8.Ghoreishi Z., et al. , Omega-3 fatty acids are protective against paclitaxel-induced peripheral neuropathy: A randomized double-blind placebo controlled trial. BMC Cancer 12, 355 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liao Y., et al. , Efficacy of omega-3 PUFAs in depression: A meta-analysis. Transl. Psychiatry 9, 190 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dyall S. C., Long-chain omega-3 fatty acids and the brain: A review of the independent and shared effects of EPA, DPA and DHA. Front. Aging Neurosci. 7, 52 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poudyal H., Panchal S. K., Diwan V., Brown L., Omega-3 fatty acids and metabolic syndrome: Effects and emerging mechanisms of action. Prog. Lipid Res. 50, 372–387 (2011). [DOI] [PubMed] [Google Scholar]
- 12.Szabó Z., et al. , The potential beneficial effect of EPA and DHA supplementation managing cytokine storm in coronavirus disease. Front. Physiol. 11, 752 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harizi H., Corcuff J. B., Gualde N., Arachidonic-acid-derived eicosanoids: Roles in biology and immunopathology. Trends Mol. Med. 14, 461–469 (2008). [DOI] [PubMed] [Google Scholar]
- 14.McReynolds C. B., et al. , Plasma linoleate diols are potential biomarker for sever COVID-19 infections. Front. Physiol. 12, 663869 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boyd J. T., et al. , Elevated dietary ω-6 polyunsaturated fatty acids induce reversible peripheral nerve dysfunction that exacerbates comorbid pain conditions. Nat. Metab. 3, 762–773 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moore R. A., Chi C. C., Wiffen P. J., Derry S., Rice A. S. C., Oral nonsteroidal anti-inflammatory drugs for neuropathic pain. Cochrane Database Syst. Rev. 10, CD010902 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harirforoosh S., Asghar W., Jamali F., Adverse effects of nonsteroidal antiinflammatory drugs: An update of gastrointestinal, cardiovascular and renal complications. J. Pharm. Pharm. Sci. 16, 821–847 (2013). [DOI] [PubMed] [Google Scholar]
- 18.Woolf C. J., Mu and delta opioid receptors diverge. Cell 137, 987–988 (2009). [DOI] [PubMed] [Google Scholar]
- 19.Porreca F., Ossipov M. H., Nausea and vomiting side effects with opioid analgesics during treatment of chronic pain: Mechanisms, implications, and management options. Pain Med. 10, 654–662 (2009). [DOI] [PubMed] [Google Scholar]
- 20.Jensen T. S., Madsen C. S., Finnerup N. B., Pharmacology and treatment of neuropathic pains. Curr. Opin. Neurol. 22, 467–474 (2009). [DOI] [PubMed] [Google Scholar]
- 21.Burnstock G., Physiology and pathophysiology of purinergic neurotransmission. Physiol. Rev. 87, 659–797 (2007). [DOI] [PubMed] [Google Scholar]
- 22.Hiasa M., et al. , Essential role of vesicular nucleotide transporter in vesicular storage and release of nucleotides in platelets. Physiol. Rep. 2, e12034 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsuda M., Inoue K., Salter M. W., Neuropathic pain and spinal microglia: A big problem from molecules in “small” glia. Trends Neurosci. 28, 101–107 (2005). [DOI] [PubMed] [Google Scholar]
- 24.Sawynok J., Adenosine receptor targets for pain. Neuroscience 338, 1–18 (2016). [DOI] [PubMed] [Google Scholar]
- 25.Sawada K., et al. , Identification of a vesicular nucleotide transporter. Proc. Natl. Acad. Sci. U.S.A. 105, 5683–5686 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miyaji T., Sawada K., Omote H., Moriyama Y., Divalent cation transport by vesicular nucleotide transporter. J. Biol. Chem. 286, 42881–42887 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sakamoto S., et al. , Impairment of vesicular ATP release affects glucose metabolism and increases insulin sensitivity. Sci. Rep. 4, 6689 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Masuda T., et al. , Dorsal horn neurons release extracellular ATP in a VNUT-dependent manner that underlies neuropathic pain. Nat. Commun. 7, 12529 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tatsushima K., et al. , Vesicular ATP release from hepatocytes plays a role in the progression of nonalcoholic steatohepatitis. Biochim. Biophys. Acta Mol. Basis Dis. 1867, 166013 (2021). [DOI] [PubMed] [Google Scholar]
- 30.Kato Y., et al. , Identification of a vesicular ATP release inhibitor for the treatment of neuropathic and inflammatory pain. Proc. Natl. Acad. Sci. U.S.A. 114, E6297–E6305 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harada Y., et al. , Vesicular nucleotide transporter mediates ATP release and migration in neutrophils. J. Biol. Chem. 293, 3770–3779 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seretny M., et al. , Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: A systematic review and meta-analysis. Pain 155, 2461–2470 (2014). [DOI] [PubMed] [Google Scholar]
- 33.Redivo D. D. B., Jesus C. H. A., Sotomaior B. B., Gasparin A. T., Cunha J. M., Acute antinociceptive effect of fish oil or its major compounds, eicosapentaenoic and docosahexaenoic acids on diabetic neuropathic pain depends on opioid system activation. Behav. Brain Res. 372, 111992 (2019). [DOI] [PubMed] [Google Scholar]
- 34.Grote C. W., Wright D. E., A role for insulin in diabetic neuropathy. Front. Neurosci. 10, 581 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yeh E. T. H., et al. , Cardiovascular complications of cancer therapy: Diagnosis, pathogenesis, and management. Circulation 109, 3122–3131 (2004). [DOI] [PubMed] [Google Scholar]
- 36.Dupre T. V., et al. , Suramin protects from cisplatin-induced acute kidney injury. Am. J. Physiol. Renal Physiol. 310, F248–F258 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dinchuk J. E., et al. , Renal abnormalities and an altered inflammatory response in mice lacking cyclooxygenase II. Nature 378, 406–409 (1995). [DOI] [PubMed] [Google Scholar]
- 38.Oh D. Y., et al. , GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 142, 687–698 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nagatake T., et al. , The 17,18-epoxyeicosatetraenoic acid-G protein-coupled receptor 40 axis ameliorates contact hypersensitivity by inhibiting neutrophil mobility in mice and cynomolgus macaques. J. Allergy Clin. Immunol. 142, 470–484.e12 (2018). [DOI] [PubMed] [Google Scholar]
- 40.Shimanaka Y., et al. , Omega-3 fatty acid epoxides are autocrine mediators that control the magnitude of IgE-mediated mast cell activation. Nat. Med. 23, 1287–1297 (2017). [DOI] [PubMed] [Google Scholar]
- 41.Leiria L. O., et al. , 12-lipoxygenase regulates cold adaptation and glucose metabolism by producing the omega-3 lipid 12-HEPE from brown fat. Cell Metab. 30, 768–783.e7 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nagatake T., et al. , 12-Hydroxyeicosapentaenoic acid inhibits foam cell formation and ameliorates high-fat diet-induced pathology of atherosclerosis in mice. Sci. Rep. 11, 10426 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hamabata T., Nakamura T., Tachibana Y., Horikami D., Murata T., 5,6-DiHETE attenuates vascular hyperpermeability by inhibiting Ca2+ elevation in endothelial cells. J. Lipid Res. 59, 1864–1870 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schwab J. M., Chiang N., Arita M., Serhan C. N., Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature 447, 869–874 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arita M., et al. , Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation. J. Immunol. 178, 3912–3917 (2007). [DOI] [PubMed] [Google Scholar]
- 46.Duan J., Song Y., Zhang X., Wang C., Effect of ω-3 polyunsaturated fatty acids-derived bioactive lipids on metabolic disorders. Front. Physiol. 12, 646491 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu Z. Z., et al. , Resolvins RvE1 and RvD1 attenuate inflammatory pain via central and peripheral actions. Nat. Med. 16, 592–597, 1p following 597 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu Z. Z., Berta T., Ji R. R., Resolvin E1 inhibits neuropathic pain and spinal cord microglial activation following peripheral nerve injury. J. Neuroimmune Pharmacol. 8, 37–41 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gold M. S., Gebhart G. F., Nociceptor sensitization in pain pathogenesis. Nat. Med. 16, 1248–1257 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Holmes D., The pain drain. Nature 535, S2–S3 (2016). [DOI] [PubMed] [Google Scholar]
- 51.Skulas-Ray A. C., et al. ; American Heart Association Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; and Council on Clinical Cardiology, Omega-3 fatty acids for the management of hypertriglyceridemia: A science advisory from the American Heart Association. Circulation 140, e673–e691 (2019). [DOI] [PubMed] [Google Scholar]
- 52.Khan S. U., et al. , Effect of omega-3 fatty acids on cardiovascular outcomes: A systematic review and meta-analysis. EClinicalMedicine 38, 100997 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Freund Levi Y., et al. , Transfer of omega-3 fatty acids across the blood-brain barrier after dietary supplementation with a docosahexaenoic acid-rich omega-3 fatty acid preparation in patients with Alzheimer’s disease: The OmegAD study. J. Intern. Med. 275, 428–436 (2014). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or supporting information.