Abstract
Background
Pericarditis caused by Methicillin-resistant Staphylococcus aureus (MRSA) is a rare infection, often seen in patients with chronic kidney disease, immunosuppression, or previous pericardial disease. The presentation can be dramatic with acute illness leading to septic and/or obstructive shock due to pericardial tamponade. Occasionally disease can have a more protracted, indolent, subacute clinical course.
Case report
We report a case of a 57-year-old male patient with a previous history of smoking and moderate alcohol use who presented with progressive dyspnea and cough. He was found to have a disseminated MRSA infection with pericarditis complicated by pericardial tamponade. Urgent pericardiocentesis yielded 1.1 liters of purulent fluid that grew MRSA. MRSA was also isolated from the blood and pleural fluid. The patient underwent left thoracotomy, decortication, and pericardial window and completed 3 weeks of intravenous vancomycin therapy, concluding in an excellent outcome.
Conclusion
Bacterial pericarditis is an exceptionally rare form of pericarditis which been traditionally associated with chronic medical conditions requiring a prolonged healthcare stay. However, it has lately been observed in healthy individuals with social habits such as smoking and alcohol consumption. Bacterial pericarditis must be recognized in a timely fashion and managed aggressively to prevent a devastating outcome. A multidisciplinary approach is advised, which includes a combination of pericardial drainage and aggressive antibiotic therapy. Such treatment often yields a positive outcome and good long-term prognosis.
Keywords: Methicillin-resistant Staphylococcus aureus, MRSA, Purulent pericarditis, Bacterial pericarditis
Introduction
Methicillin-resistant Staphylococcus aureus (MRSA) is a Gram- and coagulase-positive spherical bacterium, genetically distinct from other Staphylococcus aureus strains [1]. Knowledge of S. aureus infections dates from the 19th century, but the clinical significance of antibiotic resistance emerged following the introduction of penicillin and the subsequent development of penicillin resistance which was first detected in 1942 [1]. Following the development of semi-synthetic penicillins (i.e. methicillin) a decade later, the first MRSA strain was identified in 1961 [1]. MRSA is characterized by multiple drug resistance to beta-lactam antibiotics [1]. Infections due to MRSA are associated with higher mortality rates compared to methicillin-sensitive strains [1]. MRSA is a common pathogen in healthcare facilities, including hospitals, transitional care units, nursing homes, and dialysis units. While traditionally recognized as a nosocomial pathogen, MRSA has recently become common in the community and in livestock. Hence, current terminology differentiates between healthcare-associated MRSA (HA-MRSA), community-associated MRSA (CA-MRSA), and livestock-associated MRSA (LA-MRSA) [2].
Human MRSA infections present in a variety of ways leading to significant morbidity and mortality. Individuals with open wounds, intravascular devices, and a compromised immune system are at a higher risk of developing severe MRSA infections [3]. In addition to skin, soft tissue infection, and postoperative wounds, MRSA commonly affects the bones, lungs, or heart causing endocarditis [1]. MRSA pericarditis and pericardial abscesses are uncommon and traditionally associated with chronic medical conditions requiring a prolonged healthcare stay [1], [3]. Resistance against multiple antibiotic classes significantly limits treatment options [1]. Bacterial pericarditis is therefore fatal if not quickly recognized and appropriately treated. However, it can have an excellent outcome with appropriate antibiotic therapy and pericardial drainage [4].
To our knowledge, this is the first case of MRSA pericarditis reported in an otherwise healthy individual without an identified source of infection, whose only risk factors for the infectious disease were the social habits of smoking tobacco and alcoholism.
Case presentation
A 57-year-old man presented with a 3-week history of progressively worsening shortness of breath, and cough. Dyspnea was present at rest and on exertion, and his cough was nonproductive. He denied chest pain, fever, chills, and nocturnal diaphoresis. The patient did not have any major medical problems, was not taking any prescription medications, and had not seen a physician for many years. His social history was significant for daily beer drinking (about 70 standard drinks per week) and smoking (20 pack-years). He was unemployed and denied illicit drug use.
Initial physical examination was remarkable for a low body mass index of 18.3 kg/m2, tachycardia of 110 beats/minute, tachypnea of 22 breaths/minute, and he was afebrile with normal blood pressure. Estimated jugular venous pressure was 10 cm of water with a positive hepatojugular reflex and a two-component pericardial friction rub. No track marks or signs of intravenous (IV) drug use were detected on the skin exam. A cardiac point-of-care ultrasound (POCUS) showed a moderate-sized pericardial effusion with mobile adhesions attached to the epicardium, without signs of tamponade physiology. Electrocardiogram (ECG) was remarkable for sinus tachycardia with low QRS complexes voltage, without electrical alternans. Initial laboratory workup demonstrated elevated total leukocytes of 19,300 per microliter, with neutrophilic predominance (83%), lactic acid of 2.6 microgram/deciliter, and elevated inflammatory markers: C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR): 61.4 milligrams/liter and 120 millimeters/hour, respectively. D-dimer was 2117 micrograms/milliliter (ref value: 570 micrograms/milliliter, adjusted for age). High sensitivity troponin was elevated at 20 nanograms/liter, and N-terminal pro-brain Natriuretic Peptide (NT-proBNP) was 5654 picograms/milliliter. Serial troponin measurements remained unchanged. After 13 h, blood cultures grew MRSA and the patient was started on IV vancomycin. The blood cultures remained repeatedly positive for the following 2 days. Computerized tomography (CT) scan of the chest showed a large pericardial effusion, and bilateral pleural effusions with loculation seen on the left (Fig. 1).
Fig. 1.
Coronal (A) and axial (B) planes of the chest CT scan with intravenous contrast showing significant pericardial and pleural effusions with the subtle enhancement of the parietal and visceral pericardium and bilateral pleural loculations.
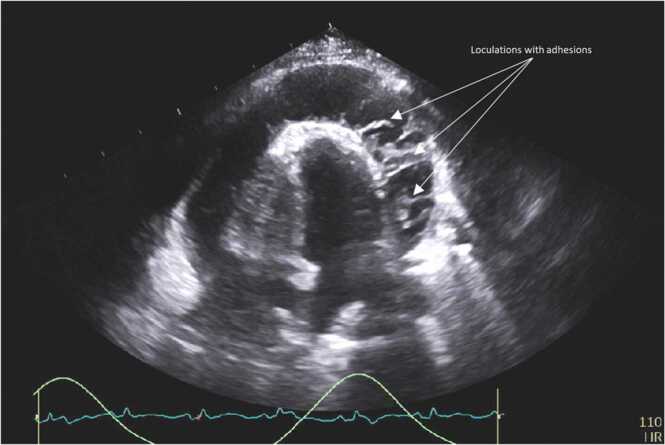
Transthoracic echocardiogram (TTE) confirmed a large circumferential pericardial effusion with fibrinous exudate in the pericardial space (Fig. 2), with right ventricle collapse in diastole, suggestive of cardiac tamponade (Fig. 3). Urgent pericardiocentesis was performed, with the removal of 1.1 liter of purulent pericardial fluid. Immediately after, the patient improved symptomatically with reduced cough and dyspnea. Pericardial fluid analysis showed: total nucleated cells of 174,951 cells/microliter with 91 % of neutrophils, lactate dehydrogenase > 2500 units/liter, total protein 4.7 g/deciliter (serum protein: 6.8 g/deciliter), and glucose of less than 2 milligrams/deciliter (serum glucose: 137 milligrams/deciliter). MRSA was also isolated from the pericardial fluid. Fungal and mycobacterial cultures remained sterile. Purulent fluid continued in the pericardial drain for 48 h, after which it became serosanguinous. Transesophageal echocardiogram (TEE) was negative for valvular vegetations.
Fig. 2.
Transthoracic echocardiogram (four-chamber view) showing large circumferential exudative pericardial effusion with substantial mobile adhesions.
Fig. 3.
A - Diastolic Right ventricle collapse by 2D indicative of cardiac tamponade; B - Focused diastolic Right ventricle collapse by M-mode indicative of cardiac tamponade; C - Combined septal bounce and diastolic Right ventricle collapse by M-mode indicative of cardiac tamponade; D – Pulse-Doppler of Hepatic Vein showing reversal of diastolic flow with expiration and systolic venous flow predominance).
Two days after the pericardiocentesis, the patient underwent ultrasound-guided bilateral thoracentesis with pleural drain placement. MRSA was isolated from the pleural fluid as well. Fibrinolytic treatment was performed daily for the following 3 days. Despite these interventions, pleural effusions remained partially loculated and unchanged in appearance on repeated CT scan of the chest. The patient then underwent left muscle-sparing thoracotomy, decortication, and pericardial window. During the surgery, the patient was found to have a left pleural abscess, dense pleural adhesions, and markedly thickened pericardium without significant fluid in the pericardial space. Chest tubes and surgical drains were removed gradually over the following 6 days. IV vancomycin was continued 1 g every 8 h for the following 3 weeks in a transitional care unit. The patient subsequently recovered well, with the resolution of leukocytosis after 2.5 weeks, and normalization of CRP after 3 weeks. The patient remained asymptomatic and a follow-up TTE one month after discharge showed no recurrence of pericardial effusion and no evidence of constriction.
Discussion
Pericarditis is the most common form of pericardial disease, and the etiology can be classified as infectious or noninfectious [5]. The etiology remains uncertain (i.e. idiopathic) in 80–90 % of the cases after routine workup, with most of these cases presumed to be of viral etiology – coxsackieviruses, echoviruses, herpesviruses, but also adenoviruses and parvovirus B19 in children [5]. Besides viruses, other common causes are connective tissue and systemic autoimmune diseases, metabolic diseases, malignancy, post-traumatic, and post-cardiac surgery (iatrogenic). Bacterial pericarditis (excluding Mycobacterium tuberculosis in developing countries) is rare and represents less than 1 % of all pericarditis cases [4], [5]. In developed parts of the world, streptococci and staphylococci are the most prevalent pathogens, with Coxiella burnetii and Borrelia burgdorferi commonly encountered [4], [5]. In recent years, unusual organisms have been documented in an increasing number of immunocompromised patients [6], [7].
Although MRSA has been traditionally associated with the healthcare setting (HA-MRSA), the epidemiology has been shifting to CA-MRSA with the emerging community and nosocomial outbreaks [1]. The other clinically important distinction is multidrug resistance associated with HA-MRSA compared to limited β-lactam resistance in CA-MRSA [1]. This antibiotic resistance mainly occurs due to the production of altered penicillin-binding protein (PBP) that exhibits decreased affinity to most of the semi synthetic penicillins [1], [8]. The genetic component responsible for this resistance is mecA which is carried on staphylococcal cassette chromosome mec (SCCmec) [1], [8]. To date, 13 SCCmec types have been identified [1], [8]. CA-MRSA is genetically distinct from HA-MRSA, carrying a smaller version of SCCmec, and often producing a cytotoxin: Panton-Valentine leukocidin (PVL) [1]. MRSA lineages and strains can be identified using various typing methods, including SCCmec genotyping. This information can be epidemiologically useful for identifying the likely source of colonization, distinguishing between community and hospital strains, and tracing outbreaks [1]. Clinical, epidemiological, and genetic distinctions between CA-MRSA and HA-MRSA are not well defined, as CA-MRSA infections have been rising in a hospital setting as well, causing numerous nosocomial outbreaks [1]. We did not perform genotyping on the isolate for SCCmec or PVL as our patient did not have the risk for HA-MRSA and culture sensitivity was compatible with CA-MRSA isolates.
Bacterial pericarditis occurs through one of the two mechanisms, hematogenous dissemination or direct spread [4]. Expression of the cell wall-anchored surface (CWS) protein in MRSA is the reason for multi-focal infection development [1] and hematogenous spread to the pericardium. Direct spread commonly occurs from infected surrounding structures (e.g. pneumonia or pleural empyema), but iatrogenic inoculation, such as with intrathoracic surgeries or chest trauma is also possible [5]. Rarely, tumor infiltration into the pericardial sac can lead to infection as documented in a case of esophago-pericardial fistula secondary to esophageal cancer [9]. Endocarditis is unique in that it can cause septic emboli or hematogenous spread to the pericardium, but also direct spread through the myocardium, with periannular abscess and fistula formation [10]. Predisposing risk factors for bacterial pericarditis are chronic kidney disease, immunosuppression, previous and/or pre-existing pericardial disease, cardiothoracic surgery, prolonged hospitalizations, indwelling vascular catheters/devices, trauma, and alcohol use [11]. Interestingly, our patient didn’t have traditionally recognized risk factors, apart from alcohol use disorder [12]. Cigarette smoking has been recently recognized as a risk factor for MRSA infections due to various mechanisms. These include modulation of genes involved in surface adhesion and biofilm formation and changes of the surface charge and hydrophobicity of the staphylococcal cell wall [13]. These mechanisms allow the pathogen to escape antimicrobial peptides of innate immunity [14].
Bacterial pericarditis generally manifests as an acute febrile illness that can rapidly progress to sepsis and septic shock. Patients often present with refractory shock due to sepsis frequently complicated by cardiac tamponade (i.e. obstructive shock) [5]. Elevated jugular venous pressure and pulsus paradoxus are common physical findings. Dullness to percussion over the left subscapular area (known as Ewart’s sign) can also be present. It is not uncommon for the fever to be absent when the infection is localized (e.g. pericardial abscess), particularly in immunocompromised patients who have difficulty mounting a systemic inflammatory response [15], [16]. Despite the presence of a circumferential pericardial effusion and pericarditis with significant purulent collection, our patient did not have a fever on presentation or throughout the hospital course. Similarly, several previous MRSA pericarditis cases [9], [15], [17], [18], [19] were afebrile, but all had chronic co-morbidities that could affect their immune response, except for a case reported by Karuyanna et al. - a middle-aged woman with longstanding smoking and alcohol abuse history who was later found to have esophageal cancer [9]. Other common symptoms and signs of patients with bacterial pericarditis are: weakness, dyspnea, tachycardia, and syncope. Syncopal episodes occur because of decreased cardiac output and cerebral hypoperfusion in the setting of tamponade [4]. Our patient had tachycardia and dyspnea, but no syncope occurred in the course of the disease.
The diagnosis can be challenging as other conditions may resemble purulent pericarditis and there is a broad differential for sepsis with hemodynamic instability. Initial X-ray or CT scan at the emergency department may reveal cardiomegaly and pericardial effusion, respectively [4], [5]. In refractory shock, POCUS is essential for diagnosing cardiac tamponade [20]. In correlation with sepsis presentation, suspicion of purulent pericarditis should be high in the differential and urgent pericardiocentesis is indicated if there is evidence of cardiac tamponade [4], [5]. Besides providing relief of symptoms, pericardiocentesis provides essential laboratory information about the nature of pericarditis. In MRSA pericarditis, pericardial fluid is often frankly purulent, but additional laboratory data is necessary to further evaluate the effusion. Elevated white cell count with neutrophilia, high LDH and protein levels, and low glucose are characteristic [4]. Bacterial cultures provide a definitive microbiological diagnosis. Studies should also include fungal and Tuberculosis testing [4]. European guidelines for diagnosis and management of pericardial diseases particularly recommend testing for Tuberculosis, Coxiella burnetii, and Borrelia spp [4]. In our case, pericardial fluid laboratory perfectly fit the picture of bacterial pericarditis, with cultures yielding MRSA. Chest imaging provides additional information about the etiology of pericarditis, such as in cases of malignancy, continuous spread (e.g. esophago-pericardial fistula) or trauma or pneumonia. It can also detect other complications such as pleural effusion or empyema [4], which were reported in many cases – including our patient. This further complicates the clinical course and often requires invasive interventions, such as thoracentesis, thoracotomy, or decortication.
Purulent pericarditis should be managed aggressively since death is inevitable if left untreated. The survival rate depends on etiology and ranges from 60 % to 85 % with comprehensive therapy [4], [5]. A multi-disciplinary approach along with a combination of aggressive antibiotic coverage and pericardial drainage is essential for a positive outcome [4], [5]. Intravenous antimicrobial therapy should be started empirically until microbiological results are available [4]. Pericardiocentesis is indicated in symptomatic moderate to large pericardial effusion, especially in cases of bacterial or neoplastic tamponade [4], [5]. Re-accumulation is frequent and often requires the placement of a pericardial drain for recurrent washouts [4], [5]. Intrapericardial thrombolysis is sometimes used for loculated effusions in order to achieve adequate drainage before resorting to surgery [4]. In cases of ineffective drainage, pericardial window, pericardiotomy or even pericardiectomy is required to achieve appropriate drainage [4], [5]. In our case, decortication was necessary due to severe pleural and pericardial adhesions with loculations, as well as left pleural abscess.
The most common complication of purulent pericarditis is constrictive pericarditis with incidence of 20–30 % depending on etiology [4]. Our patient had no re-accumulation of pericardial effusion on a 1-month follow-up TTE, and no evidence of constrictive pericarditis on subsequent follow up.
Prognosis of acute pericarditis is essentially related to the etiology, with the size of the pericardial effusion correlating with the prognosis [4]. Moderate to large pericardial effusions are more often seen in bacterial infections and neoplastic conditions, hence prognosis is often poor [5].
Conclusion
MRSA pericarditis is potentially a fatal infection if not timely recognized and aggressively treated. It is becoming more prevalent, especially in patients with multiple comorbidities, but it can also be seen in healthy individuals with alcohol and tobacco use. It should be listed highly in the differential when patients present acutely ill with a shock-like syndrome. It must be promptly recognized and aggressively managed in order to facilitate positive outcome.
Consent
Written, informed consent for publication was obtained from the patient for the case report and imaging.
Disclosure/conflict of interest
None to be reported.
Sources of financial support
None to be reported.
Acknowledgements
Dr. Joseph C. Wildenberg, M.D., Ph.D. for providing diagnostic imaging and Ms. Jennifer N. Goodrich, Mayo Clinic Health System Library Specialist.
References
- 1.Lakhundi S., Zhang K. Methicillin-resistant staphylococcus aureus: molecular characterization, evolution, and epidemiology. Clin Microbiol Rev. 2018;31:4. doi: 10.1128/CMR.00020-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stone M.J., et al. An outbreak of livestock-associated meticillin-resistant Staphylococcus aureus (LA-MRSA) clonal complex 398 in a regional burns centre. J Hosp Infect. 2021 doi: 10.1016/j.jhin.2021.12.005. [DOI] [PubMed] [Google Scholar]
- 3.Rodvold K.A., McConeghy K.W. Methicillin-resistant Staphylococcus aureus therapy: past, present, and future. Clin Infect Dis. 2014;58(Suppl 1):S20–S27. doi: 10.1093/cid/cit614. [DOI] [PubMed] [Google Scholar]
- 4.Adler Y., et al. ESC Guidelines for the diagnosis and management of pericardial diseases: the task force for the diagnosis and management of pericardial diseases of the European Society of Cardiology (ESC)endorsed by: the european association for cardio-thoracic surgery (EACTS) Eur Heart J. 2015;36(42):2921–2964. doi: 10.1093/eurheartj/ehv318. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Imazio M., Gaita F., LeWinter M. Evaluation and treatment of pericarditis: a systematic review. Jama. 2015;314(14):1498–1506. doi: 10.1001/jama.2015.12763. [DOI] [PubMed] [Google Scholar]
- 6.Ortiz D., et al. Nontyphoidal cardiac salmonellosis: two case reports and a review of the literature. Tex Heart Inst J. 2014;41(4):401–406. doi: 10.14503/THIJ-13-3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cho Y.K., et al. Morganella morganii pericarditis in a child with X-linked agammaglobulinemia. Pedia Int. 2010;52(3):489–491. doi: 10.1111/j.1442-200X.2010.03036.x. [DOI] [PubMed] [Google Scholar]
- 8.Katayama Y., Ito T., Hiramatsu K. A new class of genetic element, staphylococcus cassette chromosome mec, encodes methicillin resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 2000;44(6):1549–1555. doi: 10.1128/aac.44.6.1549-1555.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kariyanna P.T., et al. Esophago-pericardial fistula induced community acquired methicillin resistant staphylococcus aureus (CA-MRSA) Cardiac Tamponade - a rare case report and literature review. Am J Med Case Rep. 2018;6(6):109–113. [Google Scholar]
- 10.Regueiro A., et al. Risk factors of pericardial effusion in native valve infective endocarditis and its influence on outcome: a multicenter prospective cohort study. Int J Cardiol. 2018;273:193–198. doi: 10.1016/j.ijcard.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 11.Sagristà-Sauleda J., et al. Purulent pericarditis: review of a 20-year experience in a general hospital. J Am Coll Cardiol. 1993;22(6):1661–1665. doi: 10.1016/0735-1097(93)90592-o. [DOI] [PubMed] [Google Scholar]
- 12.Moore C., et al. Risk factors for methicillin-resistant Staphylococcus aureus (MRSA) acquisition in roommate contacts of patients colonized or infected with MRSA in an acute-care hospital. Infect Control Hosp Epidemiol. 2008;29(7):600–606. doi: 10.1086/588567. [DOI] [PubMed] [Google Scholar]
- 13.Kulkarni R., et al. Cigarette smoke increases Staphylococcus aureus biofilm formation via oxidative stress. Infect Immun. 2012;80(11):3804–3811. doi: 10.1128/IAI.00689-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McEachern E.K., et al. Analysis of the effects of cigarette smoke on staphylococcal virulence phenotypes. Infect Immun. 2015;83(6):2443–2452. doi: 10.1128/IAI.00303-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tan T.L., et al. Pericardial abscess: the corollary of disseminated Methicillin-resistant Staphylococcus aureus following diabetic foot ulcer infection. Med J Malays. 2020;75(6):742–744. [PubMed] [Google Scholar]
- 16.Tsai J., Shands J.W., Jr. Staphylococcal pericarditis. An atypical presentation. Arch Intern Med. 1989;149(4):953–954. [PubMed] [Google Scholar]
- 17.Oizumi H., et al. Pericardial window for methicillin-resistant staphylococcus aureus pericarditis. Ann Thorac Surg. 2019;107(1):e27–e29. doi: 10.1016/j.athoracsur.2018.05.053. [DOI] [PubMed] [Google Scholar]
- 18.Mada P.K., et al. Disseminated MRSA infection with purulent pericarditis. BMJ Case Rep. 2017:2017. doi: 10.1136/bcr-2016-218463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dherange P.A., et al. From bone to heart: a case of MRSA osteomyelitis with haematogenous spread to the pericardium. BMJ Case Rep. 2015:2015. doi: 10.1136/bcr-2015-211410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alerhand S., Carter J.M. What echocardiographic findings suggest a pericardial effusion is causing tamponade? Am J Emerg Med. 2019;37(2):321–326. doi: 10.1016/j.ajem.2018.11.004. [DOI] [PubMed] [Google Scholar]