Abstract
Socioeconomic disadvantage is associated with volumetric differences in stress-sensitive neural structures, including the hippocampus, and deficits in episodic memory. Rodent studies provide evidence that memory deficits arise via stress-related structural differences in hippocampal subdivisions; however, human studies have only provided limited evidence to support this notion. We used a sample of 10,695 9–13-year-old participants from two timepoints of the Adolescent Brain and Cognitive Development (ABCD) Study to assess whether socioeconomic disadvantage relates to episodic memory performance through hippocampal volumes. We explored associations among socioeconomic disadvantage, measured via the Area Deprivation Index (ADI), concurrent subregion (anterior, posterior) and subfield volumes (CA1, CA3, CA4/DG, subiculum), and episodic memory, assessed via the NIH Toolbox Picture Sequence Memory Test at baseline and 2-year follow-up (Time 2). Results showed that higher baseline ADI related to smaller concurrent anterior, CA1, CA4/DG, and subiculum volumes and poorer Time 2 memory performance controlling for baseline memory. Moreover, anterior, CA1, and subiculum volumes mediated the longitudinal association between the ADI and memory. Results suggest that greater socioeconomic disadvantage relates to smaller hippocampal subregion and subfield volumes and less age-related improvement in memory. These findings shed light on the neural mechanisms linking socioeconomic disadvantage and cognitive ability in childhood.
Keywords: Socioeconomic disadvantage, Hippocampus, Episodic memory, Development
Highlights
-
•
Socioeconomic disadvantage relates to smaller hippocampal subregion and subfield volumes.
-
•
Socioeconomic disadvantage relates to less growth in memory over a 2-year period.
-
•
Hippocampal volumes mediate the relation between socioeconomic disadvantage and memory.
-
•
Results offer evidence of a neural mechanism linking disadvantage and cognition.
1. Introduction
Socioeconomic disadvantage is a leading risk factor for an array of negative outcomes in children (Calem et al., 2017, Engle and Black, 2008, Jensen et al., 2017). The stress associated with living in a low socioeconomic status (SES) environment can be chronic and far reaching in nature. In addition, children of low SES often learn and are raised in less cognitively enriching environments than children of higher SES. Seminal work has identified neural differences in children and adults who grew up in such environments (Brito and Noble, 2014, Farah, 2017, Vargas et al., 2020), including both widespread impacts on the brain and more specific impacts on stress-sensitive neural regions. One of these regions, the hippocampus, is highly susceptible to stress and other variations in the environment relative to other brain regions due to its high density of glucocorticoid receptors (i.e., stress hormone receptors; Sapolsky et al., 1990; Virgin et al., 1991).
The hippocampus is integral to children’s ability to bind features to form memories, referred to as episodic memory (Eichenbaum, 1999, Tulving and Markowitsch, 1998). Volume of the hippocampus has been shown to relate to memory performance in children and adolescents (e.g., Botdorf et al., 2022). In addition to neural effects of low SES, research shows poorer memory ability in children from low SES backgrounds and superior memory performance in those from high SES or cognitively enriching backgrounds (Botdorf et al., 2019; Decker et al., 2020; Farah et al., 2008; Taylor et al., 2020). Given that successful academic performance is dependent on memory, it is important to understand how socioeconomic disadvantage may affect the hippocampus and this cognitive ability (Eichenbaum, 1999, Hassevoort et al., 2018).
Research focused on the neural impacts of socioeconomic disadvantage has mainly assessed the hippocampus as a homogeneous structure and shown that variations in SES relate to smaller total hippocampal volume in both child and adult samples (e.g., Ellwood-Lowe et al., 2018; Hanson et al., 2011; Luby et al., 2013; Noble et al., 2012; Raffington et al., 2019; Taylor et al., 2020; Yu et al., 2018). This work further shows that both low SES and total hippocampal volume relate to memory performance; however, evidence of mediation of effects has not been established. For example, one study assessed associations among income, total hippocampal volume, and memory performance on an associative memory task but did not provide evidence of mediating effects of hippocampal volume on the association between income and memory (Raffington et al., 2019). These null effects may have been due to the limited variability in the sample with regards to SES, the limited sample size (~80 participants provided MRI data), or the assessment of the whole hippocampus. Another recent assessment included a wide array of neural and behavioral metrics from the Adolescent Brain and Cognitive Development (ABCD) study and explored effects of neighborhood disadvantage (assessed using the Area Deprivation Index [ADI]). Findings from this investigation suggested that the ADI was related to smaller right hippocampal volume at baseline and poorer performance on the NIH Toolbox Picture Sequence Memory Task at baseline (Taylor et al., 2020). Finally, another smaller study of 31 children and adolescents showed a positive relation between SES and total hippocampal volume but failed to find a link between SES and memory (Yu et al., 2018). Again, these null effects may have been due to the limited sample size and assessment of the whole hippocampus.
Effects of socioeconomic status on the hippocampus may not be distributed equally throughout the hippocampus, given this structure’s heterogenous nature. The hippocampus can be divided along its longitudinal axis into anterior and posterior subregions (Poppenk et al., 2013). It can also be divided into functionally specific subfields (cornu ammonis (CA) regions 1–4, dentate gyrus (DG), subiculum), which have distinct cell types and are distributed differentially along the longitudinal axis (Duvernoy, 1998, Insausti and Amaral, 2003, Lavenex and Banta Lavenex, 2013). For example, anterior hippocampus has the largest proportion of the CA1–3 subfields compared to posterior hippocampus, and the most posterior portion of the hippocampus has the smallest amount of subiculum compared to the more anterior portions (e.g., Malykhin et al., 2010). These subregions and subfields also exhibit differential developmental trajectories in children (Canada et al., 2020, Canada et al., 2021a, Canada et al., 2021b, Lee et al., 2020, Tamnes et al., 2018) and relate to children’s memory performance in a region-dependent manner (Daugherty et al., 2017, Demaster et al., 2013; Lee et a, 2014; Riggins et al., 2018;). Given their distinct properties, focusing on subregions and subfields of the hippocampus allows for assessing this structure in a more fine-grained manner and ensuring that region-specific effects are not obscured.
Rodent studies provide evidence that memory deficits associated with low SES arise via structural differences in hippocampal subfields and subregions. This work shows that rearing animals in paradigms designed to mimic the scarcity of resources associated with low SES (i.e., limited bedding and nesting) results in smaller hippocampal subfield volumes while rearing an animal in a cognitively enriching environment tends to result in the opposite effect (i.e., larger volumes; Derks et al., 2016; Naninck et al., 2015; Rocha et al., 2021; Youssef et al., 2019). Volumetric differences arise from deficits in neurogenesis, dendritic branching, and synaptogenesis of the hippocampus among other neurodevelopmental processes due to the prolonged activation of the HPA axis and lack of cognitive stimulation. Importantly, these structural deficits in the hippocampus relate to performance on memory tasks, including object recognition tasks (Naninck et al., 2015, Rocha et al., 2021, Youssef et al., 2019).
Volumetric differences are more apparent in certain subfields that are known to have a higher density of glucocorticoid receptors, including CA1, CA3, and DG (Bath et al., 2016, Champagne et al., 2008, Naninck et al., 2015, Youssef et al., 2019). In addition, postnatal neurogenesis, which occurs in DG, makes this subfield even more vulnerable to variations in the environment given its unique plasticity. Impacts have also been reported on other subfields, including subiculum, but to a lesser degree (Bath et al., 2016). Regarding subregions, compared to posterior hippocampus (dorsal hippocampus in rodents), work has highlighted the anterior portion of the hippocampus (ventral hippocampus in rodents) as being particularly impacted by stress in both rodent and human samples (Fanselow and Dong, 2010; Vogel et al., 2020). One reason for this may be the direct structural connections between anterior hippocampus and other stress sensitive regions, including vmPFC, amygdala, and nucleus accumbens (Poppenk et al., 2013). Additionally, gene expression in anterior hippocampus may relate to neural regions implicated in stress, including the amygdala (Fanselow and Dong, 2010). Taken together, this work suggests certain subregions and subfields are more susceptible to SES-related effects than others.
Few studies have assessed hippocampal subregion or subfield volumes when investigating SES in human children. This limited work shows that smaller DG and CA3 volumes are associated with socioeconomic disparity in children and young adults (Brody et al., 2017, Merz et al., 2019) and that this association is mediated by variations in hair cortisol levels, an index of chronic physiological stress (Merz et al., 2019). However, these studies are small in sample size and limited in their representation. A larger, more representative study using subregion volumes showed that higher income relates to larger anterior, but not posterior, hippocampal volume and superior memory performance on the NIH Toolbox Picture Sequence Memory Task (Decker et al., 2020). This study further showed that this association is stronger in children of lower income families and that anterior hippocampal volumes mediated the association between income and memory performance. However, these data were fully cross-sectional in nature, which limits the interpretation of causal relations. Taken together, this research suggests associations between socioeconomic disadvantage and smaller hippocampal subregion and subfield volumes in humans. However, more research is needed to provide evidence that structural differences in subfields serve as a mechanism linking SES and memory in human children.
The current study aims to extend prior work and provide further specificity by assessing longitudinal associations between socioeconomic disadvantage and episodic memory ability via hippocampal structure in a large, diverse sample of adolescents from the ABCD study (Volkow et al., 2018). Assessing subregions and subfields as opposed to total hippocampal volume offers greater specificity and allows for making connections with rodent work, which offers a translational understanding of how stressful experiences impact the hippocampus and memory ability. The use of a large sample of adolescents provides a wide range of variability in socioeconomic disadvantage.
Given that CA1, CA3, and DG have a high density of glucocorticoid receptors and have consistently shown to be impacted by stress in rodent samples (Champagne et al., 2008), we hypothesized an association between higher levels of disadvantage and smaller volumes of CA1, CA3, and DG. In addition, given research showing that there are direct structural connections between anterior hippocampus and other stress sensitive regions, such as the amygdala and vmPFC, we hypothesized an association between disadvantage and anterior hippocampal volume. We also hypothesized a negative relation between baseline socioeconomic adversity and memory at a two-year follow-up (Farah et al., 2008, Taylor et al., 2020). Finally, we hypothesized that subfield volumes will mediate longitudinal associations between disadvantage and poorer memory performance at the two-year follow-up. These findings will allow for further understanding the neural mechanisms through which low SES relates to cognitive outcomes.
2. Methods
2.1. Participants
This project utilized data from the ABCD Study release 2.0 (Volkow et al., 2018). This is a large, diverse sample of 11,878 participants initially designed to assess substance abuse in adolescents. Participants were 9–10 years old at study entry. Parental consent and child assent were obtained for participants in the study. Children and their parents completed a series of questionnaires, and children completed a structural MRI scan. Questionnaire data and MRI data at baseline (Time 1, 9–10 years old) were used in this study. Data from the NIH Toolbox Picture Sequence Memory Task at baseline (Time 1) and the 2-year follow up (Time 2; 11–13 years old) were also used in the current study.
Of the initial 11,878 subjects, 1183 were not included because they were missing either a T1 or T2-weighted scan, they did not pass the quality control screening, or it was deemed the MRI scan was not protocol compliant as defined by ABCD researchers (Hagler et al., 2019). Thus, our sample assessing ADI and hippocampal subfields consisted of 10,695 participants. Descriptive statistics specific to the subsample used in this study are reported in Table 1. A smaller sample of 5776 participants who had memory data at Time 2 as of November 2021 were used in the analyses assessing longitudinal relations with memory.
Table 1.
Demographic characteristics of the ABCD subsample included in this study (N = 10,695).
| Demographic Variable | |
|---|---|
| Age (yrs), Time 1 [M (SD)] | 9.92 (0.62) |
| Age (yrs), Time 2 [M (SD)] | 11.96 (0.64) |
| Child sex, female [n (%)] | 5531 (51.72%) |
| Child race/ethnicity, [n (%)] | |
| Asian | 228 (2%) |
| Black | 1537 (14%) |
| Hispanic | 2181 (20%) |
| Multi-Racial/Other | 1107 (10%) |
| White | 5640 (53%) |
| Area Deprivation Index [M (SD)] | 38.79 (27.01) |
| Family income [n (%)] | |
| < $50,000 | 2833 (26%) |
| $50,001 to $100,000 | 2773 (26%) |
| > $100,001 | 4186 (39%) |
| Did not disclose | 943 (9%) |
| Parental education [n (%)] | |
| At least one parent with a 4-year college degree | 6446 (60%) |
The data were split into discovery and replication samples to probe the replicability of findings. Data were stratified by the ADI, study site, and sex to ensure there was an equal distribution of these variables across the two samples. There were 5284 subjects in the discovery sample and 5411 subjects in the replication sample. When results were similar between the discovery and replication samples, only results from the discovery sample were reported. When results did not replicate across samples, deviations are indicated. All statistics from models using the replication sample are reported in the Supplementary Material (Supplemental Tables S3-S7; Fig. S1).
2.2. Tasks and questionnaires
2.2.1. Sociodemographic variables
Several variables from the demographics survey were used, including the participant’s parent-reported age and sex. Sex was coded as a dichotomous variable where males received a value of 0 and females a value of 1.
2.2.2. Area Deprivation Index (ADI)
The ADI was used to provide an index of socioeconomic disadvantage (Kind and Buckingham, 2018). This metric is derived using the home address for the participant, which is provided as part of the Residential History Questionnaire and was created using the American Community Survey (U.S. Census Bureau, 2012). The ADI assesses disadvantage at the neighborhood or community level by accounting for numerous socioeconomic variables making it a more robust measure of SES than income or parent education, which are two variables often used to provide an index of SES. Specifically, it includes 17 factors, including income, education, employment, and housing quality. Supplemental Table S1 provides a list of these factors. The ADI is a validated metric (Kind et al., 2014) and ranges in value from 0% to 100% with a higher number indicating greater neighborhood deprivation. The ADI was similar in the discovery (M = 38.65, SD=26.95) and replication (M = 38.93, SD = 27.08) samples.
2.2.3. Pubertal status
Parent-reported pubertal status was assessed using the Pubertal Development Scale and Menstrual Cycle Survey (Petersen et al., 1988), which includes 5 questions specific to males or females. Scores were summed across the 5 questions. Pubertal status was included as a covariate given research suggesting that puberty is influenced by stressful experiences and is related to maturation of the hippocampus (Herting and Sowell, 2017, Selmeczy et al., 2018).
2.2.4. Memory assessment
The NIH Toolbox Picture Sequence Memory Task was used to provide an index of episodic memory at Time 1 and Time 2 (Bauer et al., 2013, Dikmen et al., 2014). Participants were shown a series of 15 pictures presented on the computer and were asked to remember the order of the images. After two learning trials, they were asked to reproduce the sequence that was presented to them. The number of adjacent pairs summed across trials served as the variable of interest as this is an indicator of the ability to retain details (i.e., temporal order) in addition to item information. This variable was age-corrected for use in analyses. Time 1 and Time 2 memory scores were moderately correlated (r = .44, p < .001).
2.3. MRI assessment
Data were collected at 22 sites across the United States using one of three different scanners (i.e., Siemens Prisma, Philips Achieva, and GE MR750). Participants first completed a “prescan” during which they were trained to ensure motion would not impact results and they were screened for MR contraindications. Then, they completed a series of structural and functional scans. The T1-weighted and T2-weighted structural scans were the focus of this study. Motion was tracked in real time for the structural scans. Scan parameters differed by scanner type for the T1-weighted and T2-weighted scan and are reported in Supplemental Table S2 (for further information, see Casey et al., 2018; Hagler et al., 2019).
Raw T1-weighted and T2-weighted data were acquired from the ABCD database (https://nda.nih.gov/abcd), and data were processed using Freesurfer v7.1 (Fischl et al., 2002; Fischl, 2012). Specifically, a series of preprocessing steps, including skull stripping, motion correction, normalization, and cortical and subcortical segmentation, were applied to the data among other steps. T2-weighted data were included with T1-weighted data to improve processing of structural data, including cortical and subcortical segmentations. Data were processed using both a local server and University supercomputing resources, which took several months to complete given the magnitude of the processing and the large number of participants.
2.3.1. Hippocampal subregions and subfields
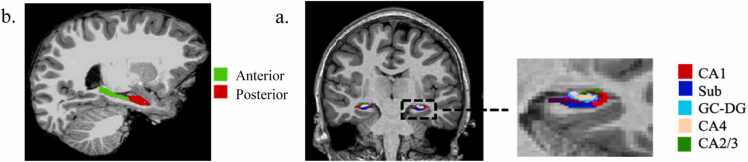
Freesurfer v7.1 (http://surfer.nmr.mgh.harvard.edu; Fischl et al., 2002; Iglesias et al., 2015) was then used to segment hippocampal subregions and subfields and to generate volumes. Hippocampal head volume was used to provide a measure of anterior hippocampal volume, and hippocampal body and tail volumes were summed to provide a measure of posterior hippocampal volume. Freesurfer has the capability of segmenting several subfields in the hippocampus using different parcellations, but the current report will focus on the parcellation that includes CA1, CA3 (which also includes CA2), CA4, DG, and subiculum (FS360 parcellation; Iglesias et al., 2015). Subfields in the head and body subregions of the hippocampus were summed to create single volumes for each subfield. To limit the number of dependent variables assessed, CA4 and DG were combined, as is often done in manual tracing due to their proximity to each other. Fig. 1 shows an example subregion and subfield segmentation for one subject.
Fig. 1.
Example segmentation of a. hippocampal subregions and b. subfields from Freesurfer v7.1.
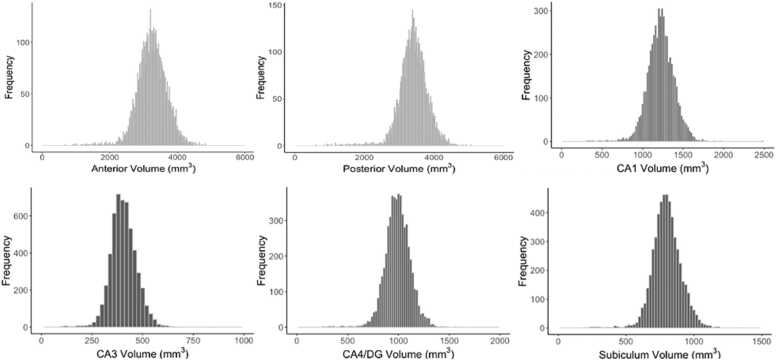
In addition to hippocampal volumes, estimated total intracranial volume (eTICV) was extracted using Freesurfer. eTICV was included as a covariate in analyses assessing hippocampal volumes, which ensured that differences in brain size were not driving effects. Given that findings were similar with and without this covariate, analyses including eTICV are reported. Descriptive statistics for hippocampal volumes and histograms of the distribution of each subregion and subfield are presented in Table 2 and Fig. 2.
Table 2.
Descriptive statistics for hippocampal subregion and subfield volumes from the discovery sample.
| M | SD | Minimum Value | Maximum Value | |
|---|---|---|---|---|
| Anterior | 3238.12 | 422.72 | 896.75 | 5058.64 |
| Posterior | 3401.39 | 397.11 | 924.41 | 5085.83 |
| CA1 | 1223.11 | 164.31 | 322.26 | 2488.89 |
| CA3 | 405.39 | 62.99 | 103.92 | 830.03 |
| CA4/DG | 991.12 | 125.96 | 260.08 | 2090.14 |
| Subiculum | 799.82 | 104.36 | 203.39 | 1640.91 |
Notes. M = mean. SD = standard deviation. Volumes are in mm3 units.
Fig. 2.
Histograms representing frequency distributions of each hippocampal subregion and subfield assessed from the discovery sample.
2.4. Data analysis plan
2.4.1. Covariates
Fixed effect covariates included age at Time 1, sex, eTICV, and pubertal status. Random effect covariates include site and family ID given differences that may arise from geographic location and the fact that siblings from the same family often participated in the study. Family ID was nested within site given the hierarchical relation between these two variables. Time 1 memory was also included as a covariate in analyses assessing Time 2 memory to ensure that effects were specific to Time 2 memory and not due to associations with concurrent memory. Given that Time 1 memory was included as a covariate, the effects of the predictors (i.e., ADI, subregion volumes, subfield volumes) on the dependent variable (i.e., memory) reflect change in memory from Time 1 to Time 2.
Study developers attempted to match the ABCD sample with that of the United States population on key demographic variables (e.g., sex, race; Dick et al., 2021; Garavan et al., 2018). However, differences still exist that are important to account for using post-stratification weighting (Heeringa and Berglund, 2020). This weighting was done using the American Community Survey as a fixed population reference (Heeringa and Berglund, 2020, U.S. Census Bureau, 2012). All analyses included this weighting as a covariate to ensure the sample was representative and results were generalizable to the population.
2.4.2. Analytic methods
Multilevel mixed-effects models were used to analyze the data using the lme4 package in R version 1.3 (R Core Team, 2020, Bates et al., 2015). Mixed effects models allow for assessing more complex models, such as those with multiple levels to the data. These models account for the hierarchical structure of the data and allows for missing data across variables.
To test associations between socioeconomic disadvantage and subregion (i.e., anterior, posterior) and subfield volumes (i.e., CA1, CA3, CA4/DG, and subiculum), the ADI was entered as the independent variable and hippocampal volumes were entered as the dependent variable along with the covariates listed above. All subregion and subfield volumes were run in separate models. To test associations between socioeconomic disadvantage and memory, the ADI was entered as the independent variable and the Time 1 or Time 2 memory score was entered as the dependent variable along with the necessary covariates. To test associations between subregion/subfield volumes and memory, each volume was entered as the independent variable and the Time 1 or Time 2 memory score was entered as the dependent variable. To make connections with the literature assessing total hippocampal volume, we also explored relations between the ADI, total hippocampal volume, and memory performance at the 2-year follow-up.
Finally, to assess whether performance on the memory task was due to ADI-related differences in hippocampal volumes, a multilevel mediation model was run using Mplus (Muthén and Muthén, 1998–2017). The ADI was entered as the predictor along with the covariates listed above, each hippocampal volume was entered as the mediator, and Time 2 memory score was entered as the dependent variable. The dependent variable therefore represents residuals; that is, the effect of the predictors on the dependent variable (memory) reflects change in memory from one time point to the next. Separate models were run for each subregion and subfield that showed significant relations with the ADI and memory performance in the previous analyses. Maximum likelihood was used to calculate confidence intervals for the indirect effect.
Multiple comparisons were accounted for via a Bonferroni correction. Alpha levels were adjusted to .0125 for analyses assessing subfield volumes given that 4 subfields were assessed and .025 for analyses assessing subregions given that two subregions were assessed. Thus, Pcorrected< 0.05 indicates significance values that satisfy the threshold imposed by this correction. In the mediation analyses, the alpha level was adjusted based on the number of subregions or subfields that showed significant relations with the ADI and memory performance.
3. Results
3.1. Associations with covariates
Bivariate correlations are presented in Table 3. Sex was related to all subregion and subfield volumes, such that males had significantly larger volumes compared to females. There was a positive association between Time 1 age and memory performance at Time 2, but not at Time 1. There was also a positive correlation between Time 1 age and each of the hippocampal volumes and a negative correlation between pubertal status and each of the hippocampal volumes.
Table 3.
Bivariate correlations in the discovery sample between variables included in the mixed-effects models.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Age (T1) | ||||||||||||||
| 2. Age (T2) | .97** | |||||||||||||
| 3. Sex | -0.03 | -0.01 | ||||||||||||
| 4. Pubertal Status | .18** | .18** | .46** | |||||||||||
| 5. ADI | -0.04* | -0.07** | .03 | .18** | ||||||||||
| 6. Memory (T1) | .02 | .04 | .09** | -.03 | -0.15** | |||||||||
| 7. Memory (T2) | .12** | .13** | .06** | -.04 | -0.16** | .43** | ||||||||
| 8. Anterior volume | .09** | .04 | -0.28** | -.22** | -.21** | .05** | .08** | |||||||
| 9. Posterior volume | .06** | .05* | -0.24** | -.17** | -.13** | .04* | .07** | .81** | ||||||
| 10. CA1 volume | .10** | .10** | -.26** | -.19** | -.21** | .07** | .11** | .96** | .83** | |||||
| 11. CA3 volume | .08** | .05* | -0.20** | -.13** | -.16** | .06** | .07** | .79** | .75** | .79** | ||||
| 12. CA4/DG volume | .08** | .08** | -.27** | -.18** | -.16** | .06** | .09** | .90** | .89** | .90** | .83** | |||
| 13. Sub. volume | .09** | .10** | -.23** | -.17** | -.19** | .06** | .09** | .85** | .86** | .82** | .60** | .83** | ||
| 14. eTICV | .07** | .10** | -.39** | -.23** | -.16** | .03 | .06* | .51** | .49** | .50** | .40** | .55** | .52** | |
| 15. Total hipp. volume | .07** | .05* | -0.27** | -.21** | -.18** | .05** | .08** | .96** | 95** | .94** | .81** | .94** | .90** | .53** |
Note. *p < .01; **p < .001. Sex, parental education, income, and race are coded 0, 1. 10 = Male, 1 = Female. ADI = Area Deprivation Index. eTICV = estimated total intracranial volume. Hipp. = hippocampal. Sub. = subiculum volume. T1 = Time 1. T2 = Time 2. Memory was assessed using the Picture Sequence task from the NIH Toolbox.
3.2. Associations between the Area Deprivation Index and hippocampal volumes
Results from the mixed-effects models assessing the ADI and hippocampal volumes showed a replicable effect between a higher ADI and smaller anterior, but not posterior, hippocampal subregion volumes. In addition, a higher ADI was related to smaller CA1 and subiculum subfield volumes across samples. A higher ADI was also related to smaller CA4/DG volume in both samples; however, the association did not survive the stringent correction for multiple comparisons in the replication sample (p < .021). Given that the Bonferroni correction is a very conservative threshold, and the association was significant in the discovery sample and just over the threshold of p < .0125 in the replication sample, the association between the ADI and CA4/DG volume was deemed worthy of further study. No associations emerged between the ADI and CA3 volume. Statistics for each model are presented in Table 4 and statistics from the replication sample are reported in Supplemental Table S3. Consistent with Taylor et al. (2020), there was also a replicable negative association between the ADI and total hippocampal volume (b = −1.614, SE =0.444, p = .0003).
Table 4.
Results from multilevel mixed-effects models assessing the relation between the Area Deprivation Index (ADI) and hippocampal volumes in the discovery sample.
| Hippocampal volume | b | SE | t | p | |
|---|---|---|---|---|---|
| Anterior volume | Intercept | 854.204* | 101.593 | 8.408 | < 0.001 |
| ADI | -1.739* | 0.239 | -7.285 | < 0.001 | |
| Age | 3.160* | 0.639 | 4.946 | < 0.001 | |
| Sex | -44.273* | 11.504 | -3.849 | < 0.001 | |
| Pubertal status | -17.067* | 6.469 | -2.638 | 0.009 | |
| eTICV | 0.002* | < 0.001 | 40.390 | < 0.001 | |
| Weighting | 0.028 | 0.017 | 1.681 | 0.094 | |
| b | SE | t | p | ||
| Posterior volumea | Intercept | 1294.425* | 99.274 | 13.039 | < 0.001 |
| ADI | -1.217* | 0.237 | -5.142 | < 0.001 | |
| Age | 2.411* | 0.646 | 3.733 | < 0.001 | |
| Sex | -39.458* | 11.562 | -3.413 | < 0.001 | |
| Pubertal status | -0.530 | 6.503 | -0.082 | 0.935 | |
| eTICV | 0.001* | < 0.001 | 35.785 | < 0.001 | |
| Weighting | 0.022 | 0.017 | 1.301 | 0.194 | |
| b | SE | t | p | ||
| CA1 volume | Intercept | 337.508* | 39.973 | 8.443 | < 0.001 |
| ADI | -0.611* | 0.095 | -6.435 | < 0.001 | |
| Age | 1.257* | 0.258 | 4.872 | < 0.001 | |
| Sex | -15.576* | 4.621 | -3.371 | < 0.001 | |
| Pubertal status | -4.547 | 2.599 | -1.749 | 0.081 | |
| eTICV | 0.001* | < 0.001 | 36.605 | < 0.001 | |
| Weighting | 0.009 | 0.007 | 1.378 | 0.169 | |
| b | SE | t | p | ||
| CA3 volume | Intercept | 98.332* | 16.171 | 6.081 | < 0.001 |
| ADI | -0.067 | 0.037 | -1.802 | 0.072 | |
| Age | 0.368* | 0.102 | 3.596 | < 0.001 | |
| Sex | -2.309 | 1.817 | -1.271 | 0.205 | |
| Pubertal status | -0.015 | 1.022 | -0.015 | 0.988 | |
| eTICV | < 0.001* | < 0.001 | 32.087 | < 0.001 | |
| Weighting | 0.002 | 0.003 | 0.590 | 0.556 | |
| b | SE | t | p | ||
| CA4/DG volume | Intercept | 311.040* | 30.120 | 10.327 | < 0.001 |
| ADI | -0.354* | 0.072 | -4.936 | < 0.001 | |
| Age | 0.663* | 0.197 | 3.360 | < 0.001 | |
| Sex | -13.044* | 3.526 | -3.699 | < 0.001 | |
| Pubertal status | -1.849 | 1.984 | -0.932 | 0.352 | |
| eTICV | < 0.001* | < 0.001 | 38.634 | < 0.001 | |
| Weighting | 0.010 | 0.005 | 1.968 | 0.050 | |
| b | SE | t | p | ||
| Subiculum volume | Intercept | 256.270* | 25.859 | 9.910 | < 0.001 |
| ADI | -0.419* | 0.061 | -6.820 | < 0.001 | |
| Age | 0.632* | 0.171 | 3.698 | < 0.001 | |
| Sex | -4.829 | 3.043 | -1.587 | 0.114 | |
| Pubertal status | -2.554 | 1.712 | -1.492 | 0.137 | |
| eTICV | < 0.001* | < 0.001 | 34.989 | < 0.001 | |
| Weighting | 0.005 | 0.004 | 1.247 | 0.213 |
Note. Significant effects are denoted by *Pcorrected< 0.05. †Puncorrected< 0.05. Site and family ID are included in the model as nested random effect covariates. a Findings did not replicate across samples. ADI = Area Deprivation Index. eTICV = estimated total intracranial volume. Weighting = post-stratification weighting.
3.3. Associations between the Area Deprivation Index and memory
Results from the mixed-effects models assessing the ADI and memory ability showed that there was a significant negative association between the ADI and the Time 2 memory score (Table 5; see Supplemental Table S4 for statistics from replication sample). Consistent with Taylor and colleagues (2020), a higher ADI was also associated with poorer memory scores at Time 1 (b= −0.09, SE=0.010, p < .001). Importantly, the association between the ADI and Time 2 memory was present even when controlling for memory scores at Time 1, suggesting that a higher ADI was related to less age-related improvement in memory ability from Time 1 to Time 2.
Table 5.
Results from multilevel mixed-effects models assessing the relation between the Area Deprivation Index (ADI) and Time 2 memory performance in the discovery sample.
| Memory (Time 2) | b | SE | t | p | |
|---|---|---|---|---|---|
| Intercept | 36.379* | 4.972 | 7.317 | < 0.001 | |
| ADI | -0.068* | 0.013 | -5.433 | < 0.001 | |
| Age | 0.250* | 0.039 | 6.502 | < 0.001 | |
| Sex | 2.250* | 0.630 | 3.572 | < 0.001 | |
| Pubertal status | -1.433* | 0.382 | -3.751 | < 0.001 | |
| Weighting | 0.001 | 0.001 | 1.110 | 0.269 | |
| Memory (Time 1) | 0.405* | 0.017 | 23.511 | < 0.001 |
Note. Significant effects are denoted by *Pcorrected< 0.05. †Puncorrected< 0.05. Site and family ID are included in the model as nested random effect covariates. Memory was assessed via the NIH Picture Sequence Memory Task. ADI = Area Deprivation Index. Weighting = post-stratification weighting.
3.4. Associations between hippocampal volume and memory
Results from the mixed-effects models assessing hippocampal volumes and Time 2 memory ability showed that a smaller anterior, but not posterior, hippocampus volume related to poorer memory performance at Time 2. In addition, there was a significant positive association between both CA1 volume and Time 2 memory scores and subiculum and Time 2 memory scores (Table 6). All significant relations were consistent when controlling for Time 1 memory. Associations between both CA3 volume and Time 2 memory and CA4/DG volume and Time 2 memory were non-significant as they did not replicate across samples (Table 6).
Table 6.
Results from multilevel mixed-effects models assessing the relation between hippocampal volumes and Time 2 memory performance in the discovery sample.
| Memory (Time 2) | b | SE | t | p | |
|---|---|---|---|---|---|
| Intercept | 19.181* | 5.802 | 3.306 | 0.001 | |
| Anterior volume | 0.003* | 0.001 | 2.931 | 0.004 | |
| Age | 0.251* | 0.038 | 6.562 | 0.000 | |
| Sex | 3.294* | 0.673 | 4.892 | 0.000 | |
| Pubertal status | -1.582* | 0.376 | -4.202 | 0.000 | |
| eTICV | 0.000 | 0.000 | 1.492 | 0.137 | |
| Weighting | 0.000 | 0.001 | -0.358 | 0.721 | |
| Memory (Time 1) | 0.410* | 0.017 | 24.216 | 0.000 | |
| b | SE | t | p | ||
| Intercept | 18.177* | 5.869 | 3.097 | 0.002 | |
| Posterior volumea | 0.002* | 0.001 | 2.603 | 0.010 | |
| Age | 0.254* | 0.038 | 6.641 | 0.000 | |
| Sex | 3.282* | 0.674 | 4.872 | 0.000 | |
| Pubertal status | -1.657* | 0.375 | -4.421 | 0.000 | |
| eTICV | 0.000 | 0.000 | 1.736 | 0.084 | |
| Weighting | 0.000 | 0.001 | -0.384 | 0.701 | |
| Memory (Time 1) | 0.410* | 0.017 | 24.249 | 0.000 | |
| b | SE | t | p | ||
| Intercept | 19.149* | 5.794 | 3.305 | 0.001 | |
| CA1 volume | 0.007* | 0.002 | 3.424 | 0.001 | |
| Age | 0.249* | 0.038 | 6.511 | 0.000 | |
| Sex | 3.285* | 0.673 | 4.881 | 0.000 | |
| Pubertal status | -1.587* | 0.376 | -4.225 | 0.000 | |
| eTICV | 0.000 | 0.000 | 1.330 | 0.185 | |
| Weighting | 0.000 | 0.001 | -0.357 | 0.721 | |
| Memory (Time 1) | 0.409* | 0.017 | 24.198 | 0.000 | |
| b | SE | t | p | ||
| Intercept | 19.817* | 5.813 | 3.409 | 0.001 | |
| CA3 volume | 0.009 | 0.005 | 1.741 | 0.083 | |
| Age | 0.256* | 0.038 | 6.710 | 0.000 | |
| Sex | 3.255* | 0.674 | 4.828 | 0.000 | |
| Pubertal status | -1.676* | 0.375 | -4.470 | 0.000 | |
| eTICV | 0.000* | 0.000 | 2.513 | 0.013 | |
| Weighting | 0.000 | 0.001 | -0.350 | 0.727 | |
| Memory (Time 1) | 0.410* | 0.017 | 24.226 | 0.000 | |
| b | SE | t | p | ||
| Intercept | 19.074* | 5.824 | 3.275 | 0.001 | |
| CA4/DG volumea | 0.007† | 0.003 | 2.491 | 0.014 | |
| Age | 0.255* | 0.038 | 6.685 | 0.000 | |
| Sex | 3.276* | 0.674 | 4.863 | 0.000 | |
| Pubertal status | -1.635* | 0.375 | -4.356 | 0.000 | |
| eTICV | 0.000 | 0.000 | 1.597 | 0.112 | |
| Weighting | 0.000 | 0.001 | -0.414 | 0.680 | |
| Memory (Time 1) | 0.410* | 0.017 | 24.227 | 0.000 | |
| b | SE | t | p | ||
| Intercept | 19.194* | 5.816 | 3.300 | 0.001 | |
| Subiculum volume | 0.009* | 0.003 | 2.629 | 0.009 | |
| Age | 0.253* | 0.038 | 6.621 | 0.000 | |
| Sex | 3.217* | 0.673 | 4.777 | 0.000 | |
| Pubertal status | -1.625* | 0.375 | -4.328 | 0.000 | |
| eTICV | 0.000 | 0.000 | 1.812 | 0.072 | |
| Weighting | 0.000 | 0.001 | -0.367 | 0.714 | |
| Memory (Time 1) | 0.411* | 0.017 | 24.276 | 0.000 |
Note. Significant effects are denoted by *Pcorrected< 0.05. †Puncorrected< 0.05. Site and family ID are included in the model as nested random effect covariates. Memory was assessed via the NIH Picture Sequence Memory Task. a Findings did not replicate across samples. ADI = Area Deprivation Index. Weighting = post-stratification weighting.
There was a positive association between Time 1 memory scores and volume of anterior hippocampus, CA1, CA4/DG, and subiculum (Supplemental Tables S6 and S7). There was no association between Time 1 memory scores and CA3 volume. Finally, results showed a positive association between total hippocampal volume and memory performance both at Time 1 (b =0.001, SE = <0.001, p = .005) and at Time 2, which was robust when controlling for Time 1 memory (b =0.001, SE = <0.001, p = .029).
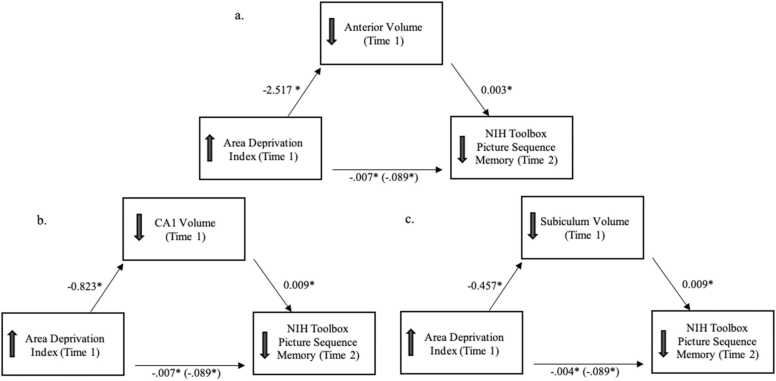
3.5. Mediating effects among the area deprivation index, subfield volume, and memory
Given significant associations between both the ADI and hippocampal volumes and hippocampal volumes and memory, multilevel mediation was assessed with anterior hippocampus, CA1 and subiculum volumes. Results of mediation analyses showed significant mediation such that there was a significant indirect effect of higher ADI on poorer Time 2 memory scores via smaller subregion and subfield volumes. This mediating effect was evident for anterior hippocampus, CA1, and subiculum volumes (Fig. 3). Finally, total hippocampal volume also mediated the longitudinal relation between the ADI and memory performance (indirect effect: b = −0.007, SE =0.003, p = .018); however, this relation did not replicate across samples (indirect effect: b = −0.004, SE =0.002, p = .059).
Fig. 3.
Mediation model depicting the indirect effect of the Area Deprivation Index (ADI) at Time 1 on episodic memory performance at Time 2 via subregion (a. anterior hippocampus) and subfield volumes (b. CA1, c. subiculum) in the discovery sample. Note. *Pcorrected< 0.05. Unstandardized coefficients are reported. Coefficients in parentheses represent the total effect of the ADI on memory performance.
4. Discussion
This study investigated associations among socioeconomic disadvantage, measured via the Area Deprivation Index (ADI), hippocampal subregion and subfield volumes, and episodic memory in the large sample of adolescents from the ABCD study. Findings showed that a higher ADI was related to smaller anterior hippocampus, CA1, CA4/DG, and subiculum volumes along with poorer memory performance both at Time 1 and Time 2. In addition, smaller volumes of anterior hippocampus, CA1, and subiculum were related to poorer Time 2 memory performance. Longitudinal associations between the ADI, hippocampal volumes, and Time 2 memory were robust when controlling for Time 1 memory ability suggesting that both a higher baseline ADI and smaller subfield volumes were related to less age-related improvements in memory ability from Time 1 to Time 2. Importantly, results showed that variations in both subregion (i.e., anterior) and subfield volumes (i.e., CA1, subiculum) mediated the association between socioeconomic disadvantage and poorer memory ability at Time 2. These findings shed light on the neural mechanism linking socioeconomic disadvantage to episodic memory ability via hippocampal structure during adolescence.
4.1. Socioeconomic disadvantage and hippocampal volumes
Greater levels of socioeconomic disadvantage were related to smaller anterior hippocampus, CA1, CA4/DG, and subiculum volumes. These results align with those from rodent studies, which often report that exposure to scarcity of resources is associated with structural deficits in hippocampal subfields (Champagne et al., 2008; Naninck et al., 2015). Findings of an association with anterior hippocampal volume are consistent with previous research which has shown a similar positive relation between income and volume of anterior, but not posterior, hippocampus (Decker et al., 2020) and broader research suggesting differentiation of stress-effects along the anterior/posterior axis of the hippocampus (Fanselow and Dong, 2010, Satpute et al., 2012).
These findings are also consistent with studies which have assessed the hippocampus as a homogeneous structure and shown that lower SES relates to smaller total hippocampal volume in children (e.g., Noble et al., 2012; Raffington et al., 2019; Taylor et al., 2020; Yu et al., 2018). Importantly, results provide greater specificity by identifying specific subregions and subfields of the hippocampus that are impacted by low SES adding to the limited studies that have assessed low SES and these specific regions of the hippocampus in human samples (Brody et al., 2017; Decker et al., 2020; Merz et al., 2019). Our findings showed relations between the ADI and total hippocampal volume, but assessing subregions suggested that effects were driven by anterior hippocampus. Subfields provided the most specificity by suggesting that the CA1 and subiculum subfields (which anterior hippocampus has a large proportion of) were related to the ADI and mediated the association between disadvantage and memory.
Prior studies that assessed subfields indicated that CA3 and DG volumes were related to low SES (Brody et al., 2017, Merz et al., 2019). This study found evidence that DG (assessed as a combined subfield with CA4) was related to socioeconomic disadvantage but did not find evidence of an association with CA3 volume. Null findings with CA3 are also in contrast to rodent work which highlights this subfield as being impacted by stress (Champagne et al., 2008). The current findings with CA3 volume may be due to the larger and more diverse sample included in this study. These results may also be due to the automated parcellation was used to delineate subfields. In this parcellation, CA3 includes the CA2 subfield as well so it is possible that the segmentation method obscured findings. However, both studies that assessed low SES and subfields also used Freesurfer’s subfield parcellation (though these studies used Freesurfer v6.0).
Interestingly, subiculum volume showed an association with socioeconomic disadvantage. Although this finding was contrary to hypotheses, research suggests that subiculum can be impacted by stress, just less so due to its lower density of glucocorticoid receptors (Bath et al., 2016). In addition, studies which have assessed the effect of other forms of stress, such as childhood maltreatment, on subfield volumes have shown that this subfield may be impacted by early life stress (Lee et al., 2018, Teicher et al., 2012). This suggests that subiculum may be important to examine in future studies assessing impacts of low SES on hippocampal structure.
4.2. Hippocampal volumes and memory
Hippocampal subregion and subfield volumes were related to episodic memory ability in this study. Specifically, larger anterior, but not posterior, hippocampal volume related to memory at both Time 1 and Time 2. In addition, larger volumes of CA1 and subiculum, but not CA3 or CA4/DG, were related to superior performance and smaller volumes were related to poorer performance on the NIH Picture Sequence Memory Task at Time 2. The positive relation observed between hippocampal volumes and memory performance aligns with work using rodent samples which has shown associations between subfield structure and performance on memory tasks (Rocha et al., 2021) and work using human samples which has also shown that structural differences in subregions and subfields relate to memory performance in this age range (Daugherty et al., 2017; Decker et al., 2020; Lee et al., 2014, Lee et al., 2020). Importantly, some studies have reported negative associations between subregion/subfield volumes and memory in children and adolescents (Schlichting et al., 2017; Tamnes et al., 2014). The discrepancy in findings across studies may be due to several factors, including the subfield parcellation method used, the scan resolution, the age range of participants, and the memory task assessed. Results also showed a positive association between total hippocampal volume and memory at Time 1 and Time 2. The direction of effects at both time points is consistent with recent work suggesting a positive association between hippocampal volume and memory ability in children and adolescents (Botdorf et al., 2022).
4.3. Socioeconomic disadvantage and memory
Results showed that higher levels of socioeconomic disadvantage were associated with poorer memory at Time 2. Given that these associations remained when controlling for baseline memory performance, these findings can be interpreted such that higher levels of socioeconomic disadvantage relate to less growth in children’s memory ability. These results replicate those observed in other studies assessing socioeconomic disadvantage and memory, which have found poorer memory in those of low SES (Farah et al., 2008, Taylor et al., 2020). Findings also extend previous research by showing that socioeconomic disadvantage relates to age-related improvements of memory ability during adolescence when this cognitive process is still undergoing development. This study underscores socioeconomic adversity as being a key factor that may impact the development of memory in children. Results also align with those assessing other aspects of the child’s environment, such as stressful life events, which show that these experiences relate to poorer memory performance (van der Heijden et al., 2011). Some studies have not found an association between low SES and poorer memory ability (Yu et al., 2018); however, these studies used small samples, which may account for findings. In addition, differences in the memory task used in each study may also account for discrepancies in results.
4.4. Mediating effects among socioeconomic disadvantage, hippocampal volumes, and memory
Findings provide evidence of significant mediation, such that greater socioeconomic disadvantage was related to poorer memory ability via variations in anterior, CA1, and subiculum volumes. Studies have assessed these variables previously, but few have included them all in the same model. By assessing subregion and subfield volumes, we provide greater specificity and also provide evidence of mediating effects, which mirror those that are often observed in rodent studies. Interestingly, there was not a replicable indirect effect of total hippocampal volume on the association between the ADI and memory performance, which further underscores the importance of assessing subregions and subfields of the hippocampus.
These findings of a significant mediation with anterior hippocampus support those obtained in a previous study which assessed concurrent relations among income, hippocampal subregion volume and memory, and showed that anterior hippocampal volume mediated the association between income and memory performance (Decker et al., 2020). Our results replicate and extend this finding by assessing subfields in addition to subregions and by assessing longitudinal, rather than concurrent, relations with memory.
Although not included in the current study, stress physiology likely plays an important role in this model. In particular, low SES likely relates to differences in stress hormone (i.e., cortisol) levels, which then impact subfield structure. One prior study provided support for this notion by showing that hair cortisol levels mediate the association between low SES and subfield volumes (Merz et al., 2019). Other mechanisms are likely at play as well. Cognitive stimulation is an additional pathway through which socioeconomic disparities may impact children’s memory abilities. Research shows that children of low SES families often receive less cognitive stimulation, which is likely related to the lack of resources and time that the parents can spend with the child. These children may also go to less optimal schools with fewer resources and higher student to teacher ratios. Future work should include measures of stress physiology, such as cortisol data, and assess variables related to the caregiving environment as this will help to further our mechanistic understanding of how socioeconomic disadvantage impacts cognitive outcomes.
4.5. Strengths
A strength of this study is the use of a large, diverse dataset, which allowed for the detection of small associations with more precision, which may not have been possible with a smaller dataset (Dick et al., 2021). Some observed effects in the current study were small in size, but given the large sample size, they are well-powered. Another strength is the use of hippocampal subregions and subfields, which allowed for moving beyond investigating the hippocampus as a whole and starting to understand what specific subfields are driving associations often observed between socioeconomic disadvantage and the hippocampus as a whole. The use of longitudinal memory task data is also a strength of the current study as it allowed for assessing change in children’s memory ability. Finally, this study’s use of the ADI is a strength as it accounts for the complex nature of SES by using a variable that accounts for 17 aspects of SES. This provides more information than simply assessing income or parent education.
4.6. Limitations and future directions
Although this study broke new ground in the ABCD sample by examining hippocampal subfields, this was done using an automated software package, Freesurfer 7.1, to segment hippocampal subfields. It is possible that using Freesurfer introduced some form of bias when compared to manual tracing (Schmidt et al., 2018, Wisse et al., 2014, Wisse et al., 2021). However, previous research has shown that the potential bias introduced in segmenting the hippocampus, at least as a homogenous structure, using Freesurfer is consistent across subjects. Therefore, any potential bias may not be of much concern (Schoemaker et al., 2016). In a sample this large, using an automated toolbox, like Freesurfer, not only saves resources (Schmidt et al., 2018), but makes it possible to use big datasets in a way that may not be possible otherwise.
These findings are also limited by the concurrent assessment of the ADI and subfields at Time 1. Thus, the mediation model did not fully meet the assumption of temporal precedence of each of the three indicators. However, the memory data are from a later time point. Therefore, there is a lag between the predictor and dependent variable and mediator and dependent variable. Moreover, it is theoretically unlikely that a child’s hippocampal volume impacts the ADI given that the literature suggests that neighborhood level environmental variables exert impacts on an individual child’s brain and not vice versa. In addition, because the ADI is a metric that assesses disadvantage at the neighborhood level, it is unlikely to change much over the span of several years. Nevertheless, future research should aim to assess how change in socioeconomic disadvantage, hippocampal volume, and memory all relate to one another in the ABCD sample or in another large, diverse sample with all assessment timepoints spaced out over time.
Finally, the effects observed in this study are not deterministic. Instead, additional factors in the child’s environment may buffer the impact of socioeconomic disadvantage on the brain, such as parental support. Some research has shown that positive parenting moderates the impacts of socioeconomic disadvantage on other stress-sensitive neural regions (Brody et al., 2017). These moderating factors will be important to assess in a larger, more diverse sample, such as the one used in the current study. The current findings also provide motivation for future work to assess interventions to limit the impact of socioeconomic disadvantage on brain and cognitive development in children. One study found that a supportive parenting intervention reduces the impact of low SES on subfield volumes (Whittle et al., 2017). It will be important to assess whether this also reduces impacts on memory ability. Future research should also investigate how findings may vary by race or ethnicity. In particular, environmental stressors related to race (e.g., chronic discrimination) are important to assess and should be included in future research.
5. Conclusions
Results from this study show that socioeconomic disadvantage, as measured by the Area Deprivation Index, negatively relates to hippocampal subregion volumes, subfield volumes, and memory performance in adolescents. In addition, results showed that the association between socioeconomic disadvantage and memory ability assessed two years later is mediated by anterior hippocampus, CA1, and subiculum volumes. These findings provide evidence of a neural mechanism through which socioeconomic disadvantage impacts episodic memory ability via alterations in hippocampal structure. They also provide avenues for future research to assess the implications of findings especially with regards to interventions aimed at reducing the impact of socioeconomic disadvantage on cognition in children.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the University of Maryland’s Graduate School Dean’s Fellowship (to M.B.), the National Institute of Mental Health grant R01MH122487 (to L.R.D.), and the National Institute of Mental Health grant R25MH125545. The authors would thank the families who participated in the ABCD study and the researchers who collected the data.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.dcn.2022.101138.
Appendix A. Supplementary material
Supplementary material
.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Bates D., Mächler M., Bolker B., Walker S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015;67(1):1–48. doi: 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- Bath K.G., Manzano-Nieves G., Goodwill H. Early life stress accelerates behavioral and neural maturation of the hippocampus in male mice. Horm. Behav. 2016;82:64–71. doi: 10.1016/j.yhbeh.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer P.J., Dikmen S.S., Heaton R.K., Mungas D., Slotkin J., Beaumont J.L. III. NIH Toolbox Cognition Battery (CB): measuring episodic memory. Monogr. Soc. Res. Child Dev. 2013;78(4):34–48. doi: 10.1111/mono.12033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botdorf M., Riggins T., Dougherty L.R. Early positive parenting and maternal depression history predict children’s relational binding ability at school-age. Dev. Psychol. 2019;55(11) doi: 10.1037/dev0000803. [DOI] [PubMed] [Google Scholar]
- Botdorf M., Canada K., Riggins T. A meta-analysis of the relation between hippocampal volume and memory ability in typically developing children and adolescents. Hippocampus. 2022;32(5):386–400. doi: 10.1002/hipo.23414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito N.H., Noble K.G. Socioeconomic status and structural brain development. Front. Neurosci. 2014;8(SEP):1–12. doi: 10.3389/fnins.2014.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody G.H., Gray J.C., Yu T., Barton A.W., Beach S.R.H., Galván A., MacKillop J., Windle M., Chen E., Miller G.E., Sweet L.H. Protective prevention effects on the association of poverty with brain development. JAMA Pediatr. 2017;171(1):46. doi: 10.1001/jamapediatrics.2016.2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calem M., Bromis K., McGuire P., Morgan C., Kempton M.J. Meta-analysis of associations between childhood adversity and hippocampus and amygdala volume in non-clinical and general population samples. NeuroImage Clin. 2017;14:471–479. doi: 10.1016/j.nicl.2017.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canada K.L., Botdorf M., Riggins T. Longitudinal development of hippocampal subregions from early- to mid-childhood. Hippocampus. 2020;30:1098–1111. doi: 10.1002/hipo.23218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canada K.L., Hancock G.R., Riggins T. Modeling longitudinal changes in hippocampal subfields and relations with memory from early- to mid-childhood. Dev. Cogn. Neurosci. 2021;48 doi: 10.1016/j.dcn.2021.100947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canada K.L., Hancock G.R., Riggins T. Modeling longitudinal changes in hippocampal subfields and relations to memory from early- to mid-childhood. Dev. Cogn. Neurosci. 2021 doi: 10.1016/j.dcn.2021.100947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B.J., Cannonier T., Conley M.I., Cohen A.O., Barch D.M., Heitzeg M.M., Dale A.M. The Adolescent Brain Cognitive Development (ABCD) study: imaging acquisition across 21 sites. Dev. Cogn. Neurosci. 2018;32:43–54. doi: 10.1016/j.dcn.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne D.L., Bagot R.C., van Hasselt F., Ramakers G., Meaney M.J., de Kloet E.R., Krugers H. Maternal care and hippocampal plasticity: evidence for experience-dependent structural plasticity, altered synaptic functioning, and differential responsiveness to glucocorticoids and stress. J. Neurosci. 2008;28(23):6037–6045. doi: 10.1523/jneurosci.0526-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugherty A.M., Flinn R., Ofen N. Hippocampal CA3-dentate gyrus volume uniquely linked to improvement in associative memory from childhood to adulthood. NeuroImage. 2017;153:75–85. doi: 10.1016/j.neuroimage.2017.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaster, D., Pathman, T., Lee, J.K., Ghetti, S., & Dem, D. (2013). Structural Development of the Hippocampus and Episodic Memory: Developmental Differences Along the Anterior/Posterior Axis. https://doi.org/10.1093/cercor/bht160. [DOI] [PubMed]
- Decker A.L., Duncan K., Finn A.S., Mabbott D.J. Children’s family income is associated with cognitive function and volume of anterior not posterior hippocampus. Nature Communications. 2020;11:4040. doi: 10.1038/s41467-020-17854-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derks N.A.V., Krugers H.J., Hoogenraad C.C., Joëls M., Sarabdjitsingh R.A. Effects of early life stress on synaptic plasticity in the developing hippocampus of male and female rats. PLoS One. 2016;11(10) doi: 10.1371/journal.pone.0164551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick A.S., Lopez D.A., Watts A.L., Heeringa S., Reuter C., Bartsch H., Fan C.C., Kennedy D.N., Palmer C., Marshall A., Haist F., Hawes S., Nichols T.E., Barch D.M., Jernigan T.L., Garavan H., Grant S., Pariyadath V., Hoffman E., Thompson W.K. Meaningful associations in the adolescent brain cognitive development study. NeuroImage. 2021;239 doi: 10.1016/j.neuroimage.2021.118262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikmen S.S., Bauer P.J., Weintraub S., Mungas D., Slotkin J., Beaumont J.L., Heaton R.K. Measuring episodic memory across the lifespan: NIH toolbox picture sequence memory test. J. Int. Neuropsychol. Soc. 2014;20(6):611–619. doi: 10.1017/S1355617714000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvernoy H.M. The Human Hippocampus, Functional Anatomy, Vascularization, and Serial Sections with MRI. 2nd ed. Spinger; 1998. [Google Scholar]
- Eichenbaum H. The hippocampus and mechanisms of declarative memory. Behav. Brain Res. 1999;103(2):123–133. doi: 10.1016/S0166-4328(99)00044-3. [DOI] [PubMed] [Google Scholar]
- Ellwood-Lowe M.E., Humphreys K.L., Ordaz S.J., Camacho M.C., Sacchet M.D., Gotlib I.H. Time-varying effects of income on hippocampal volume trajectories in adolescent girls. Dev. Cogn. Neurosci. 2018;30:41–50. doi: 10.1016/j.dcn.2017.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engle P.L., Black M.M. The effect of poverty on child development and educational outcomes. Ann. N. Y. Acad. Sci. 2008;1136(1):243–256. doi: 10.1196/annals.1425.023. [DOI] [PubMed] [Google Scholar]
- Fanselow M.S., Dong H.-W. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65(1):7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farah M.J. The neuroscience of socioeconomic status: correlates, causes, and consequences. Neuron. 2017;96(1):56–71. doi: 10.1016/j.neuron.2017.08.034. [DOI] [PubMed] [Google Scholar]
- Farah M.J., Betancourt L., Shera D.M., Savage J.H., Giannetta J.M., Brodsky N.L., Malmud E.K., Hurt H. Environmental stimulation, parental nurturance and cognitive development in humans. Dev. Sci. 2008;11(5):793–801. doi: 10.1111/j.1467-7687.2008.00688.x. [DOI] [PubMed] [Google Scholar]
- Fischl B. FreeSurfer. NeuroImage. 2012;62(2):774–781. doi: 10.1016/j.neuroimage.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B., Salat D.H., Busa E., Albert M., Dieterich M., Haselgrove C., van der Kouwe A., Killiany R., Kennedy D., Klaveness S., Montillo A., Makris N., Rosen B., Dale A.M. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Garavan H., Bartsch H., Conway K., Decastro A., Goldstein R.Z., Heeringa S., Zahs D. Recruiting the ABCD sample: Design considerations and procedures. Dev. Cogn. Neurosci. 2018;32:16–22. doi: 10.1016/j.dcn.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagler D.J., Hatton S.N., Cornejo M.D., Makowski C., Fair D.A., Dick A.S., Dale A.M. Image processing and analysis methods for the Adolescent Brain Cognitive Development Study. NeuroImage. 2019 doi: 10.1016/j.neuroimage.2019.116091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson J.L., Chandra A., Wolfe B.L., Pollak S.D. Association between income and the hippocampus. PLoS One. 2011;6(5) doi: 10.1371/journal.pone.0018712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassevoort K.M., Khan N.A., Hillman C.H., Kramer A.F., Cohen N.J. Relational memory is associated with academic achievement in preadolescent children. Trends Neurosci. Educ. 2018;13:8–16. doi: 10.1016/j.tine.2018.09.001. [DOI] [Google Scholar]
- Heeringa, S., Berglund, P. (2020). A Guide forPopulation-based Analysis of the Adolescent Brain Cognitive Development (ABCD) Study Baseline Data, (2018), 1–36. 10.1101/2020.02.10.942011. [DOI]
- Herting M.M., Sowell E.R. Puberty and structural brain development in humans. Front. Neuroendocrinol. 2017;44:122–137. doi: 10.1016/j.yfrne.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias J.E., Augustinack J.C., Nguyen K., Player C.M., Player A., Wright M., Van Leemput K. A computational atlas of the hippocampal formation using ex vivo, ultra-high resolution MRI: application to adaptive segmentation of in vivo MRI. NeuroImage. 2015;115:117–137. doi: 10.1016/j.neuroimage.2015.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insausti R., Amaral D.G. The Human Nervous System: Second Edition. Elsevier Inc; 2003. Hippocampal formation; pp. 871–914. [DOI] [Google Scholar]
- Jensen S.K.G., Berens A.E., Nelson C.A. Effects of poverty on interacting biological systems underlying child development. Lancet Child Adolesc. Health. 2017;1(3):225–239. doi: 10.1016/S2352-4642(17)30024-X. [DOI] [PubMed] [Google Scholar]
- Kind A., Buckingham W.R. Making neighborhood-disadvantage metrics accessible - the neighborhood atlas. N. Engl. J. Med. 2018;378(26):2456–2458. doi: 10.1056/NEJMp1802313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kind A.J., Jencks S., Brock J., Yu M., Bartels C., Ehlenbach W., Greenberg C., Smith M. Neighborhood socioeconomic disadvantage and 30-day rehospitalization: a retrospective cohort study. Ann. Intern. Med. 2014;161(11):765–774. doi: 10.7326/M13-2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavenex P., Banta Lavenex P. Building hippocampal circuits to learn and remember: Insights into the development of human memory. Behav. Brain Res. 2013;254:8–21. doi: 10.1016/j.bbr.2013.02.007. [DOI] [PubMed] [Google Scholar]
- Lee J.K., Ekstrom A.D., Ghetti S. Volume of hippocampal subfields and episodic memory in childhood and adolescence. NeuroImage. 2014;94:162–171. doi: 10.1016/j.neuroimage.2014.03.019. [DOI] [PubMed] [Google Scholar]
- Lee J.K., Fandakova Y., Johnson E.G., Cohen N.J., Bunge S.A., Ghetti S. Changes in anterior and posterior hippocampus differentially predict item-space, item-time, and item-item memory improvement. Dev. Cogn. Neurosci. 2020:41. doi: 10.1016/j.dcn.2019.100741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.W., Yoo J.H., Kim K.W., Kim D., Park H.W., Choi J., Jeong B. Hippocampal subfields volume reduction in high schoolers with previous verbal abuse experiences. Clin. Psychopharmacol. Neurosci. 2018;16(1):46–56. doi: 10.9758/cpn.2018.16.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby J., Belden A., Botteron K., Marrus N., Harms M.P., Babb C., Barch D. The effects of poverty on childhood brain development: the mediating effect of caregiving and stressful life events. JAMA Pediatr. 2013;167(12):1135–1142. doi: 10.1001/jamapediatrics.2013.3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malykhin N.V., Lebel R.M., Coupland N.J., Wilman A.H., Carter R. In vivo quantification of hippocampal subfields using 4.7 T fast spin echo imaging. NeuroImage. 2010;49(2):1224–1230. doi: 10.1016/j.neuroimage.2009.09.042. [DOI] [PubMed] [Google Scholar]
- Merz E.C., Desai P.M., Maskus E.A., Melvin S.A., Rehman R., Torres S.D., Noble K.G. Socioeconomic disparities in chronic physiologic stress are associated with brain structure in children. Biol. Psychiatry. 2019;86(12):921–929. doi: 10.1016/j.biopsych.2019.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén, L.K., Muthén, B.O. (1998–2017). Mplus User's Guide. Eight Edition. Los Angeles, CA: Muthén & Muthén.
- Naninck E.F.G., Hoeijmakers L., Kakava-Georgiadou N., Meesters A., Lazic S.E., Lucassen P.J., Korosi A. Chronic early life stress alters developmental and adult neurogenesis and impairs cognitive function in mice. Hippocampus. 2015;25(3):309–328. doi: 10.1002/hipo.22374. [DOI] [PubMed] [Google Scholar]
- Noble K.G., Grieve S.M., Korgaonkar M.S., Engelhardt L.E., Griffith E.Y., Williams L.M., Brickman A.M. Hippocampal volume varies with educational attainment across the life-span. Front. Hum. Neurosci. 2012;6 doi: 10.3389/fnhum.2012.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppenk J., Evensmoen H.R., Moscovitch M., Nadel L. Long-axis specialization of the human hippocampus. Trends Cogn. Sci. 2013 doi: 10.1016/j.tics.2013.03.005. [DOI] [PubMed] [Google Scholar]
- R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2020. R: A Language and Environment for Statistical Computing.https://www.R-project.org/ [Google Scholar]
- Raffington L., Czamara D., Mohn J.J., Falck J., Schmoll V., Heim C., Binder, Elisabeth B., Shing Y.L. Stable longitudinal associations of family income with childrens hippocampal volume and memory persist after controlling for polygenic scores of educational attainment. Dev. Cogn. Neurosci. 2019 doi: 10.1016/j.dcn.2019.100720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggins T., Geng F., Botdorf M., Canada K., Cox L., Hancock G.R. Protracted hippocampal development is associated with age-related improvements in memory during early childhood. NeuroImage. 2018;174:127–137. doi: 10.1016/j.neuroimage.2018.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha M., Wang D., Avila-Quintero V., Bloch M.H., Kaffman A. Deficits in hippocampal-dependent memory across different rodent models of early life stress: systematic review and meta-analysis. Transl. Psychiatry. 2021;11(1):231. doi: 10.1038/s41398-021-01352-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky R.M., Uno H., Rebert C.S., Finch C.E. Hippocampal damage associated with prolonged glucocorticoid exposure in primates. J. Neurosci. 1990;10(9):2897–2902. doi: 10.1523/jneurosci.10-09-02897.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satpute A.B., Mumford J.A., Naliboff B.D., Poldrack R.A. Human anterior and posterior hippocampus respond distinctly to state and trait anxiety. Emotion. 2012;12(1):58–68. doi: 10.1037/a0026517. [DOI] [PubMed] [Google Scholar]
- Schlichting M.L., Guarino K.F., Schapiro A.C., Turk-Browne N.B., Preston A.R. Hippocampal structure predicts statistical learning and associative inference abilities during development. J. Cogn. Neurosci. 2017;29(1):37–51. doi: 10.1162/jocn_a_01028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M.F., Storrs J.M., Freeman K.B., Jack C.R., Turner S.T., Griswold M.E., Mosley T.H. A comparison of manual tracing and FreeSurfer for estimating hippocampal volume over the adult lifespan. Hum. Brain Mapp. 2018;39(6):2500–2513. doi: 10.1002/hbm.24017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoemaker D., Buss C., Head K., Sandman C.A., Davis E.P., Chakravarty M.M., Pruessner J.C. Hippocampus and amygdala volumes from magnetic resonance images in children: assessing accuracy of FreeSurfer and FSL against manual segmentation. NeuroImage. 2016;129:1–14. doi: 10.1016/j.neuroimage.2016.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selmeczy D., Fandakova Y., Grimm K.J., Bunge S.A., Ghetti S. Longitudinal trajectories of hippocampal and prefrontal contributions to episodic retrieval: effects of age and puberty. Dev. Cogn. Neurosci. 2018 doi: 10.1016/j.dcn.2018.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamnes C., Bos M., van de Kamp F., Peters S., Crone E. Longitudinal development of hippocampal subregions from childhood to adulthood. Dev. Cogn. Neurosci. 2018;30:212–222. doi: 10.1016/j.dcn.2018.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamnes C.K., Walhovd K.B., Engvig A., Grydeland H., Krogsrud S.K., Østby Y., Holland D., Dale A.M., Fjell A.M. Regional hippocampal volumes and development predict learning and memory. Developmental Neuroscience. 2014;34:161–174. doi: 10.1159/000362445. [DOI] [PubMed] [Google Scholar]
- Taylor R.L., Cooper S.R., Jackson J.J., Barch D.M. Assessment of neighborhood poverty, cognitive function, and prefrontal and hippocampal volumes in children. JAMA Netw. Open. 2020;3(11) doi: 10.1001/jamanetworkopen.2020.23774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher M.H., Anderson C.M., Polcari A. Childhood maltreatment is associated with reduced volume in the hippocampal subfields CA3, dentate gyrus, and subiculum. Proc. Natl. Acad. Sci. USA. 2012;109(9):E563–E572. doi: 10.1073/pnas.1115396109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E., Markowitsch H.J. Episodic and declarative memory: role of the hippocampus. Hippocampus. 1998;8(3):198–204. doi: 10.1002/(sici)1098-1063(1998)8:3%3C198::aid-hipo2%3E3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- U.S. Census Bureau. (2012). 2009–2011 American Community Survey 3-year Public Use Microdata Samples [SAS Data file]. Retrieved from 〈https://factfinder.census.gov/faces/nav/jsf/pages/searchresults.xhtml?refresh=t〉.
- van der Heijden, Kristiaan B., Suurland J., Swaab H., de Sonneville LeoM.J. Relationship between the number of life events and memory capacity in children. Child Neuropsychol. 2011;17(6):580–598. doi: 10.1080/09297049.2011.554391. [DOI] [PubMed] [Google Scholar]
- Vargas T., Damme K.S.F., Mittal V.A. Neighborhood deprivation, prefrontal morphology and neurocognition in late childhood to early adolescence. NeuroImage. 2020;220 doi: 10.1016/j.neuroimage.2020.117086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virgin C.E., Ha T.P., Packan D.R., Tombaugh G.C., Yang S.H., Homer H.C., Sapolsky R.M. Glucocorticoids inhibit glucose transport and glutamate uptake in hippocampal astrocytes: implications for glucocorticoid neurotoxicity. J. Neurochem. 1991;57(4):1422–1428. doi: 10.1111/j.1471-4159.1991.tb08309.x. [DOI] [PubMed] [Google Scholar]
- Volkow N.D., Koob G.F., Croyle R.T., Bianchi D.W., Gordon J.A., Koroshetz W.J., Weiss S.R.B. The conception of the ABCD study: from substance use to a broad NIH collaboration. Dev. Cogn. Neurosci. 2018 doi: 10.1016/j.dcn.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle S., Simmons J.G., Hendriksma S., Vijayakumar N., Byrne M.L., Dennison M., Allen N.B. Childhood maltreatment, psychopathology, and the development of hippocampal subregions during adolescence. Brain Behav. 2017;7(2):1–9. doi: 10.1002/brb3.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisse L.E.M., Biessels G.J., Geerlings M.I., Malykhin N. A critical appraisal of the hippocampal subfield segmentation package in FreeSurfer. Front. Aging Neurosci. 2014:6. doi: 10.3389/fnagi.2014.00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisse L.E.M., Chételat G., Daugherty A.M., de Flores R., la Joie R., Mueller S.G., Carr V.A. Hippocampal subfield volumetry from structural isotropic 1 mm3 MRI scans: a note of caution. Hum. Brain Mapp. 2021;42(2):539–550. doi: 10.1002/hbm.25234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youssef M., Atsak P., Cardenas J., Kosmidis S., Leonardo E.D., Dranovsky A. Early life stress delays hippocampal development and diminishes the adult stem cell pool in mice. Sci. Rep. 2019;9(1) doi: 10.1038/s41598-019-40868-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q., Daugherty A.M., Anderson D.M., Nishimura M., Brush D., Hardwick A., Lacey W., Raz S., Ofen N. Socioeconomic status and hippocampal volume in children and young adults. Developmental Science. 2018;21(3) doi: 10.1111/desc.12561. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.