Abstract
Cancer therapy has evolved from being broadly directed towards tumor types, to highly specific treatment protocols that target individual molecular subtypes of tumors. With the ever-increasing data on imaging characteristics of tumor subtypes and advancements in imaging techniques, it is now often possible for radiologists to differentiate tumor subtypes on imaging. Armed with this knowledge, radiologists may be able to provide specific information that can obviate the need for invasive methods to identify tumor subtypes. Different tumor subtypes also differ in their patterns of metastatic spread. Awareness of these differences can direct radiologists to relevant anatomical sites to screen for early metastases that may otherwise be difficult to detect during cursory inspection. Likewise, this knowledge will help radiologists to interpret indeterminate findings in a more specific manner.
Keywords: Cancer, Tumor subtype, Precision medicine, Radiomics, Artificial intelligence
Highlights
-
•
Tumor subtypes can be identified based on their different imaging characteristics.
-
•
Awareness of tumor subtype can help radiologists chose the appropriate modality for additional imaging workup.
-
•
Awareness of differences in metastatic pattern between tumor subtypes can be helpful to identify early metastases.
1. Background
Precision medicine is a broad concept that refers to individualized care for each patient, as opposed to the more common historically evolved one-size-fits-all method, based on broad disease categories. Perhaps the most conspicuous branch of precision medicine is precision cancer therapy [1]. Personalizing cancer therapy has been made possible by the tremendous advancements in the field of molecular biology and genomics over the last few decades. These advancements have resulted in an increase in the understanding of how tumor cells develop, survive and progress. This further paved the way for the development of new anticancer agents that target specific pathways. Thus, present day cancer therapy requires characterization not only of the histological type of the tumor, but also its genomic or molecular subtype [2].
Advancements in cancer therapy have been paralleled by advancements in imaging technologies. In addition, over the last few decades, there has been a significant increase in the understanding of how tumors differ in their imaging characteristics based on their subtypes [3], [4]. In a growing number of instances, this knowledge helps radiologists non-invasively predict the subtype of the tumor, understand how to screen for and identify early metastatic disease and chose the appropriate imaging modality or technique that best suits further determination of the tumor subtype.
The first part of this review describes imaging features that can differentiate the subtypes of select tumors and explains how some tumor subtypes are best evaluated with certain imaging modalities. The second part of this review describes how select tumor subtypes differ in their metastatic patterns. We have not included advanced techniques such as texture analysis that are currently mostly utilized for research. Instead, our review discusses imaging techniques that are already in wide clinical use.
2. Radiological differentiation of tumor subtypes
2.1. Lung cancer
Lung cancer remains the leading cause of cancer mortality worldwide with non-small cell lung cancer (NSCLC) representing approximately 85 % of cases [5]. While clinical outcome for NSCLC is directly related to the pathologic stage at time of diagnosis [6], [7], the development of therapies targeting specific genomic subtypes has significantly improved survival (not mortality) [8]. A combination of clinical and imaging features can be helpful in identifying certain subtypes of NSCLC.
Mutations in both epidermal growth factor receptor (EGFR) and anaplastic lymphoma kinase (ALK) subtypes of NSCLC occur in younger individuals with light or no smoking history, with EGFR mutated lung cancer preferentially occurring in females [9], [10].
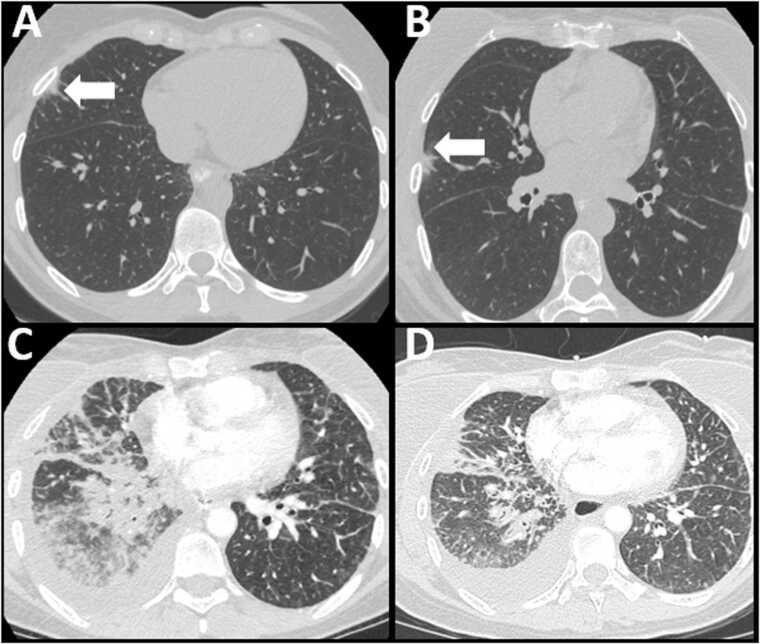
Imaging features that favor EGFR subtype are peripheral lesions with a ground glass opacity (GGO) ratio of greater than 50 %, size less than 3 cm, spiculated margins, pleural retraction and air bronchograms [11], [12]. An important point for the radiologist to consider is that EGFR mutant NSCLC can be mistaken for pneumonia or an inflammatory process (Fig. 1), both due to overlapping imaging features, and a common clinical presentation consisting of nonspecific respiratory symptoms such as cough and shortness of breath [13].
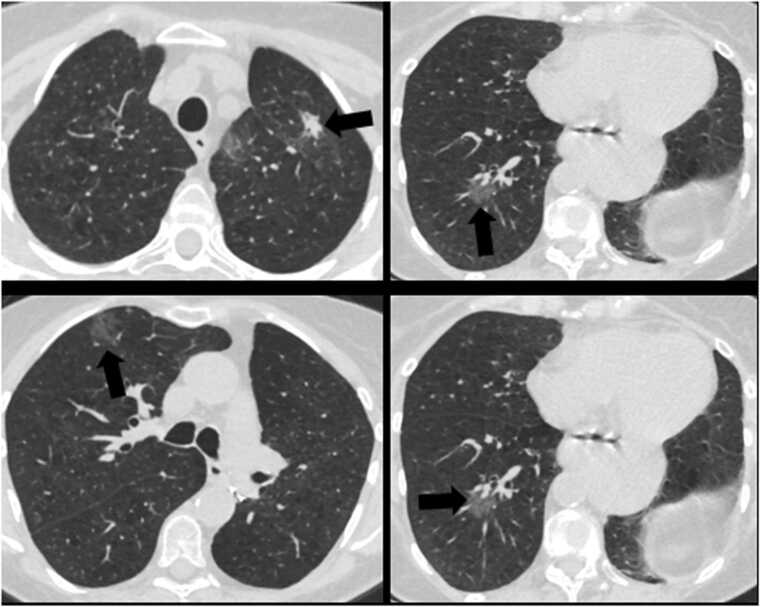
Fig. 1.
72 year old female with shortness of breath. Axial CT images of the chest in lung window show multifocal ground glass and part solid opacities (arrows). This was initially considered to be an infectious or inflammatory process. However, as the patient had a history of treated EGFR mutated lung cancer, possibility of metastatic disease was raised, which was subsequently confirmed with biopsy.
Imaging features that favor ALK rearranged NSCLC compared to ALK negative NSCLC, are solid hypoattenuating lesions that do not demonstrate cavitation. Advanced disease is more likely to be associated with lymphadenopathy, lymphangitic carcinomatosis, and pleural and pericardial metastasis most commonly in the form of effusions [14]. The solid nature of ALK positive NSCLC is thought to be secondary to its histologic appearance of abundant intracellular mucin with small marinated nuclei [15].
2.2. Renal cell carcinoma
Renal cell carcinoma (RCC) occurs twice as commonly in males compared to females. North America has one of the highest rate of cases worldwide, with approximately 76,000 new cases and 14,000 yearly deaths [16], [17], [18]. CT and MRI with and without contrast demonstrate similar accuracy in the diagnosis of small renal masses (SRM; renal mass less than 4 cm in maximal diameter), with MRI being more sensitive to detect contrast enhancement [19], [20]. Contrast-enhanced dual-energy CT with material attenuation analysis is found to improve specificity when assessing for a possible SRM [21].
Approximately 75–85 % of RCC are of the clear cell carcinoma subtype, which arise from the proximal tubule and tend to have a 3p chromosome deletion including von-Hippel Lindau gene (vHL) mutation [22]. Papillary RCC comprises approximately 10–15 % of all RCC, and is further divided into type 1 and type 2 papillary RCC. The former is associated with MET mutations and tends to be more indolent while the latter has a more aggressive course and often presents with advanced disease [23], [24]. Chromophobe RCC is less common than both papillary and clear cell subtypes, tends to present at a lower stage and has a much lower risk of disease progression in comparison to clear cell carcinoma [25], [26].
Clear cell carcinoma is typically iso to hyperintense on T2 weighted images with respect to renal cortex [27], whereas almost all papillary RCC will present as a homogenously hypointense lesion [28] (Fig. 2). Clear cell RCC tends to enhance rapidly and avidly and reaches maximal enhancement by the corticomedullary phase (Fig. 3), whereas papillary demonstrates gradual enhancement, peaking during the excretory phase of renal enhancement [28], [29]. Clear cell RCCs contain microscopic fat and lose signal intensity on out-of-phase images while papillary RCCs can contain macroscopic fat [30]. Chromophobe RCC enhances to a degree between clear cell RCC and papillary RCC [31], [32]. Chromophobe and papillary RCC can be further differentiated from clear cell RCC by their significantly lower apparent diffusion coefficient values [33]. Chromophobe RCCs can demonstrate a ‘spoke-wheel’ pattern of enhancement.
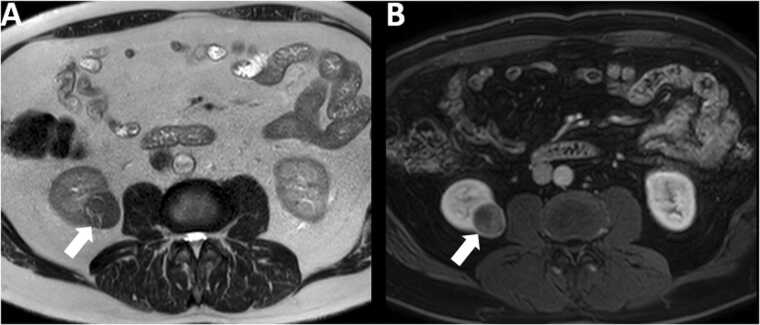
Fig. 2.
61 year old male with papillary renal cell carcinoma. Note the hypointense signal on T2 weighted image (arrow in A) and relative hypoenhancement on post contrast T1 weighted image (arrow in B) of the mass.
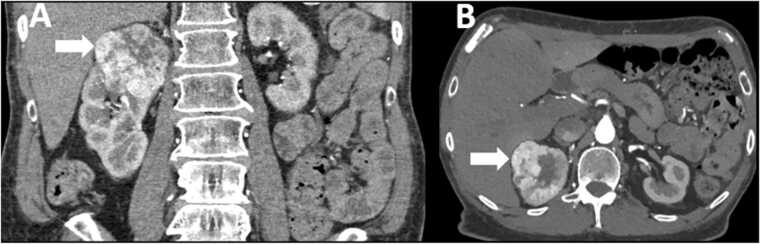
Fig. 3.
48 year old male with clear cell renal cell carcinoma. Note the hypervascular appearance of the mass (arrows) on the corticomedullary phase post contrast CT images in the coronal (A) and axial (B) planes.
A multiphase CT renal protocol of a papillary RCC may show indeterminate levels of enhancement (10–20 HU) and sometimes even absent attenuation (less than 10 HU) secondary to its tendency to be hypovascular and homogenous [34]. Papillary RCC lacks the Von Hippel-Lindau (VHL)- hypoxia inducible factor (HIF)- vascular endothelial growth factor (VEGF) pathway that often features prominently in clear cell RCC, which explains its relative lack of vascularity. A subtype of RCC known as “clear cell papillary RCC” can demonstrate imaging and histopathologic features common to both papillary and clear cell RCC. Fortunately, this type of RCC has low malignant potential [35], [36].
2.3. Pancreatic tumors
Pancreatic cancer is the fourth leading cause of cancer related mortality in the United States [16]. Exocrine pancreatic tumors, or adenocarcinomas, make up approximately 85 % of all pancreatic cancers [37]. Pancreatic neuroendocrine tumors (NETs) are rare and usually sporadic, but they are also associated with several hereditary endocrinopathies [38].
Exocrine pancreatic tumors appear as ill-defined hypoattenuating masses in the pancreas [39] (Fig. 4). These are best seen on the “pancreatic phase”, which is a period after peak enhancement of the aorta in arterial phase but before peak hepatic enhancement in the portal venous phase [40], [41].
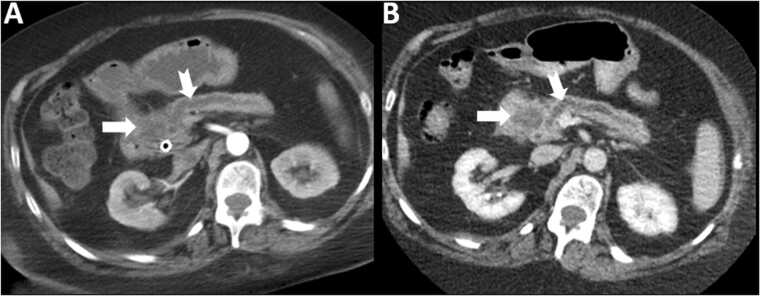
Fig. 4.
Pancreatic adenocarcinoma. 61 year old male with vague epigastric pain and jaundice. The ill defined mass in the pancreatic head (arrows) enhances lesser than the pancreatic parenchyma on both arterial phase (A) and portal venous phase (B) images. Note upstream duct dilatation (notched arrows) and parenchymal atrophy.
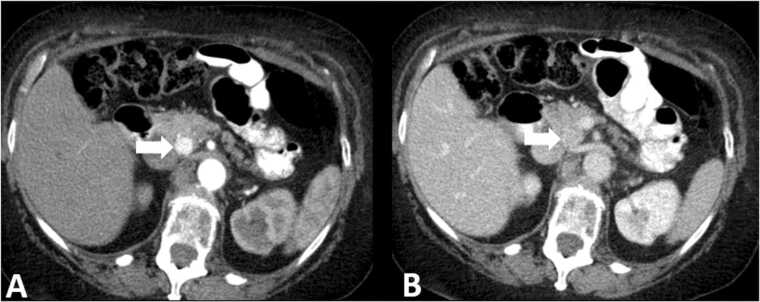
Pancreatic NETs are best seen on arterial phase images as hypervascular lesions [42], [43], [44], [45] (Fig. 5). Somatostatin receptor-based imaging is an important tool that takes advantage of the overexpression of somatostatin receptors in most well differentiated NETs. Multiple positron emission tomography (PET) tracers currently in use, including 68-Ga DOTATATE, 68-Ga DOTATOC, and copper Cu-64 DOTATATE are often preferred over traditional somatostatin imaging such as the Octreoscan [46], [47], [48], [49] due to increased sensitivity for detecting lesions (Fig. 6). One important consideration for the radiologist is that insulinomas, the most common functioning type of pancreatic NET, tends to have fewer levels of the somatostatin receptor and can be difficult to detect or occult on somatostatin receptor-based imaging [50], [51].
Fig. 5.
Pancreatic neuroendocrine tumor. 53 year old female with flushing. Mass in the uncinate process of the pancreas (arrows) shows increased enhancement compared to the pancreatic parenchyma on the arterial phase (A) and is iso-attenuating on the portal venous phase (B).
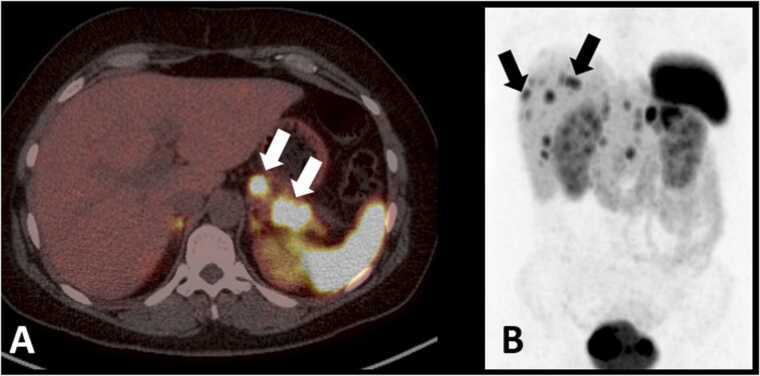
Fig. 6.
68Ga DOTATATE PET/CT for pancreatic neuroendocrine tumor. 42 year old female with months of epigastric pain and flushing. Fused axial PET-CT (A) shows multiple foci of uptake in the body and tail of pancreas (white arrows). Coronal PET maximum intensity projection image (B) shows multiple foci of uptake in the liver, consistent with metastatic disease (black arrows show representative lesions).
2.4. Colorectal cancer
Colorectal cancer (CRC) is a common disease with approximately 150,000 new cases diagnosed annually in the United States alone and nearly 53,000 Americans expected to die of colorectal cancer yearly [16], [52]. Colorectal adenocarcinoma can be differentiated histologically into several subtypes [53]. However, this paper will discuss how certain imaging features of mucinous adenocarcinoma and signet ring adenocarcinoma subtypes can help differentiate them from the classic adenocarcinoma subtype.
2.4.1. Mucinous adenocarcinoma
Mucinous CRCs comprise nearly 11–17 % of all CRCs [54], [55]. In comparison to classic adenocarcinoma, the mucinous subtype has a predilection for arising from the right colon [56]. With nearly 25% of cases misclassified secondary to sampling errors on initial biopsy [57], MRI becomes a useful tool to identify the mucinous subtype. Due to the presence of extensive extracellular mucin, mucinous colorectal cancers have significantly higher T2 signal (Fig. 7) when compared to nonmucinous cancers (Fig. 8) with a sensitivity and specificity of 94–100 % and 95–98 % respectively on T2 sequences. Thus MRI can be a superior tool for diagnosing the mucinous subtype compared to a biopsy [58]. Additionally, due to relatively low cellularity, mucinous CRCs demonstrate less enhancement and higher ADC values when compared to classic adenocarcinoma [59], [60]. This is an important point for the radiologist to consider as a relative lack of enhancement and diffusion restriction in a colonic mass does not exclude neoplastic etiology, and may in fact point towards a mucinous subtype.
Fig. 7.
62 year old male with mucinous rectal carcinoma. Note the T2 hyperintense signal of the primary rectal mass (arrows in A) and a metastatic lymph node (notched arrow in B). Note a low density perihepatic metastatic deposit (arrow in C).
Fig. 8.
57 year old male with non-mucinous rectal carcinoma. The primary rectal mass (arrows) does not have the T2 hyperintense signal associated with mucinous rectal carcinoma.
FDG-PET has variable utility in the setting of mucinous CRC with a negative correlation found between amount of mucin and inherent FDG avidity [61]. Interestingly, when compared to its colonic counterpart, mucinous cancer of the rectum demonstrates FDG avidity similar to non-mucinous subtypes [62].
Characteristic CT features of mucinous tumors when compared to classic adenocarcinoma include lower attenuation, heterogenous enhancement, eccentric wall thickening, and more common intra-tumoral calcifications, with increasing specificity on CT if several of these features are present [58].
2.4.2. Signet ring cell adenocarcinoma
Accounting for 1–2 % of all cases, a CRC is labeled as a signet ring cell subtype when greater than 50 % of its cells contain intracellular mucin that displaces the cell nuclei to the periphery [63], [64]. This subtype tends to have an aggressive course with frequent transmural spread and can have extensive peritoneal carcinomatosis at diagnosis [64], [65]. Similar to mucinous adenocarcinoma, signet ring cell carcinoma (SRCC) tends to arise in the right colon, and in addition to its propensity for peritoneal spread, SRCC is prone to vascular and nerve infiltration, resulting in poorer prognosis [66]. Like mucinous CRC, the abundant intracellular mucin of SRCC results in T2 hyperintense signal and high ADC values [65]. There are limited reports of the imaging characteristics of SRCC, likely due to its rarity and late stage of presentation.
2.5. Cervical cancer
Cervical cancer accounts for an estimated 342,000 deaths annually worldwide [16], [67]. Squamous cell carcinoma (SCC) comprises the majority of cervical cancer subtypes, accounting for 70–75 %, with adenocarcinoma accounting for nearly 25 %, and all other subtypes, including neuroendocrine, being extremely rare [68].
Neuroendocrine cervical cancer (NECC) variants comprise approximately 2 % of all cervical cancers [69], [70]. Small cell neuroendocrine cervical cancer is the most common variant of neuroendocrine cervical cancer overall [71]. In MRI, NECC tend to demonstrate higher signal intensity on T2 and homogenous enhancement when compared to non NECCs [71], [72]. Additionally, due to the high cellularity nature of NECCs, ADC maps and DWI sequence can be useful to differentiate NECCs from other cervical cancers with a sensitivity of approximately 63 % and a specificity of 95 % [71], [72].
3. Tumor subtypes and patterns of metastases
3.1. Cervical cancer
3.1.1. Adenocarcinoma versus SCC
SCC of the cervix typically portends a better prognosis compared to adenocarcinoma [73], [74]. Adenocarcinoma and SCC of the cervix share many of the same prognostic factors, including stage, nodal status, tumor volume, grade, depth of stromal invasion and lymphovascular invasion [75], [76]. However, adenocarcinomas more commonly exhibit ovarian metastasis (5 % compared to 0.8 %) [77], distant metastasis (36.8 % compared to 21.2 %) [78], hydrothorax and ascites [79]. Adenocarcinoma of the cervix also has a higher predilection for spreading to the paraaortic lymph nodes and uterine body [79].
3.1.2. Neuroendocrine cervical cancer
The dissemination pattern of NECC, in particular the small cell subset, is more similar to small cell cancer of the lung than other cervical cancer subtypes, with higher likelihood of spread to bone, liver, lungs, lymphatic system, and soft tissues [80]. There is also a higher incidence of brain metastasis in NECCs; - another similarity with small cell cancer of the lung [81], [82]. 18F-FDG PET/CT and 68Ga-DOTATATE PET/CT, in somatostatin receptor positive NECCs, are both excellent for early staging and are superior to both MRI and CT in detecting distant metastasis [71], [78].
3.2. Gastric cancer
Despite the decline in incidence and mortality of gastric cancer and the current advancements in understanding epidemiology and molecular pathology, gastric cancer remains one of the leading causes of cancer deaths worldwide [83], [84]. Cancers of the gastric cardia demonstrate a twofold higher rate of lung metastasis when compared to non-cardia cancers [85] and also have a higher rate of liver metastasis [86]. The WHO categorizes gastric cancer into its five histological subtypes: tubular, papillary, mucinous, poorly cohesive, and rare variants [84].
Signet ring gastric carcinoma (SRGC) is categorized under the poorly cohesive histological subtype of gastric cancers. When compared to other gastric cancers, SRCC more frequently metastasizes to the peritoneum and bones and less to the liver and lungs [86], [87]. Pulmonary metastases of SRCC can manifest as lymphangitic carcinomatosis (Fig. 9). Such patients often present with acutely progressive dyspnea, thus mimicking an infectious process on imaging [88]. Indeed, there are several case reports of young patients presenting with acute dyspnea as a first manifestation of their undiagnosed SRCC with thoracic metastases [89], [90].
Fig. 9.
Lymphangitis carcinomatosis pattern with metastatic signet ring gastric cancer (SRGC). 49 year old female with history of SRGC presenting with shortness of breath. Chest CT at the time of presentation (A, B) shows small ground glass opacities (arrows in A and B), initially thought to be infectious or inflammatory in etiology. However in view of history of SRCC, possibility of metastatic disease was considered. Chest CT three weeks later (C,D) shows extensive consolidative opacities and septal thickening throughout the right lung with new small right pleural effusion, confirmed to be lymphangitic carcinomatosis from SRGC on cytology.
3.3. Germ cell tumors of the testes
Germ cell tumors are the most common solid neoplasms in men between the ages of 15 and 34 [91]. By subtype, germ cell tumors are subdivided into seminomatous germ cell tumors (SGCT) and non-seminomatous germ cell tumors (NSGCT), with the latter further subdivided into embryonal carcinoma, teratomas, yolk sack tumors and choriocarcinoma [92]. Potential metastatic sites include lungs, liver, bones and CNS with different prognostic implications for each site [93].
Less than 5 % of cases of pure seminomas present with stage 3 disease and spread beyond the retroperitoneal lymph nodes [94]. There is a twenty fold greater chance of intrathoracic metastasis with NSGCT when compared to SGCT [95], [96]. Pulmonary metastases of seminomas tend to be larger in size, measuring around 1–2 cm, and are more homogenous [95], [97]. Pulmonary metastases of NSGCT classically manifest as small, bilateral peripheral pulmonary nodules [97]. Seminomas are more likely to spread to the posterior mediastinum, contiguous with the thoracic duct, with mediastinal disease being present in nearly 70 % of all cases with intrathoracic manifestations [98], [99]. Intrathoracic metastases from NSGCT involve the anterior and middle mediastinum (visceral mediastinum) and usually spare the posterior mediastinum [98], [100]. Brain metastases are far more likely with NSGCT [101].
NSGCT metastases containing choriocarcinoma histology can be hemorrhagic [94]. Osseous metastases are more likely with tumors that have embryonal and yolk sack histology [102], [103], [104].
3.4. Breast cancer
Breast cancer is one of the most common cancers among women with new case numbers rising yearly [105], [106]. Invasive ductal carcinoma (IDC), which comprises approximately 85% of invasive breast cancers [107], follows a predictable pattern of spread based on its expression of hormone markers. For example, triple negative breast cancer has increased propensity for the lungs and brain, and is less likely to spread to bone and liver when compared to ER or PR positive breast cancers [108], [109]. Patients with high grade myoepithelial cancers, a closely related subgroup, have a higher tendency to metastasize to the lungs and brain compared to other subsets of IDC [110], [111].
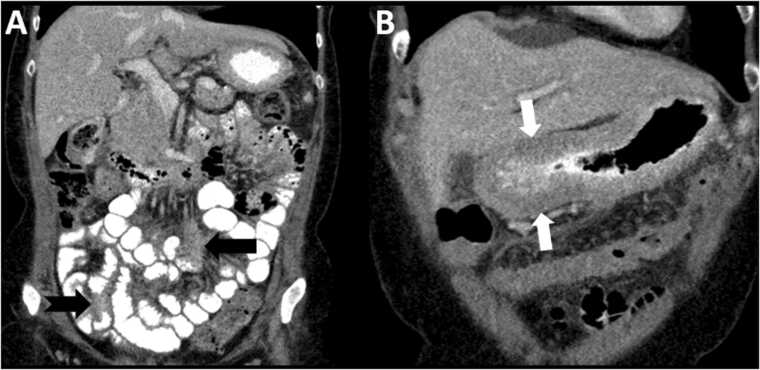
Invasive lobular carcinoma (ILC) is the second most common subtype of breast cancer [112]. It has a much greater propensity for gastrointestinal/peritoneal, pleural and ovarian metastasis [113], [114], [115] (Fig. 10). It is postulated that E-cadherin downregulation/dysfunction, which is noted in ILC, ovarian and gastric cancers, may contribute to ILC’s proclivity for these unusual site of metastases [116], [117].
Fig. 10.
Peritoneal metastatic disease from lobular breast cancer. 68 year old female presenting with abdominal discomfort, diarrhea and fecal incontinence. She has a past history of ER+ lobular breast cancer. CT of the abdomen and pelvis with oral contrast shows ill-defined mesenteric soft tissue (black arrow) and serosal thickening of the small bowel (notched black arrow) and stomach (white arrows), indicating peritoneal metastatic disease.
Patients with prior history of breast cancer presenting with signs of intestinal obstruction need careful work up and understanding of the subtype of the patient’s breast cancer, as intraabdominal metastatic spread of ILC can occur remote to initial diagnosis and can be the first sign of recurrence [118], [119]. The typical sites of intraabdominal metastasis of lobular carcinoma include the peritoneum/retroperitoneum and liver, with the rest of the GI tract including colon and stomach being less likely [115]. Interestingly, when ILC metastasizes to the stomach, it poses a dilemma for the pathologist as it is identical in appearance to signet ring cell carcinoma of the stomach [120].
3.5. Prostate cancer
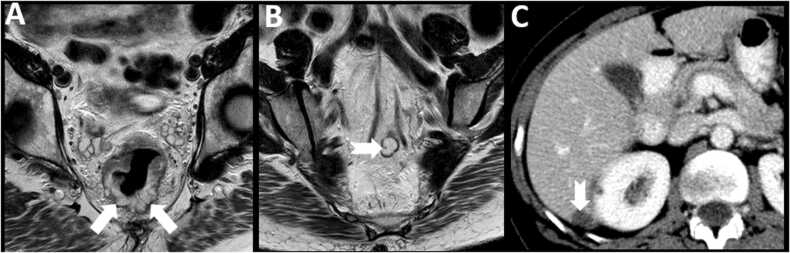
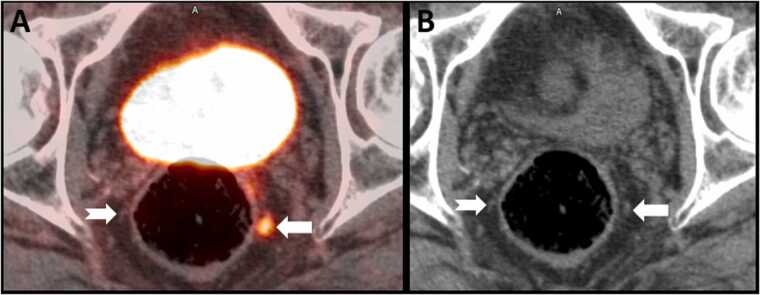
Prostate cancer has an annual estimated incidence of 1,600,000 cases and results in approximately 360,000 deaths worldwide, and has become one of the most common cancers in men [121]. Many prostate cancers are indolent, and in general patients diagnosed with prostate cancer are far more likely to die of other causes [122]. Lymph nodes, bones, liver and lung are the most frequent sites of metastatic spread in prostate adenocarcinoma [123]. PSMA PET/CT has narrowed the interval between biochemical and imaging recurrence and can detect prostate adenocarcinoma’s spread at an earlier stage than in the pre-PMSA era [124], [125] (Fig. 11).
Fig. 11.
18F PSMA PET/CT for prostate carcinoma. 66 year old male with new diagnosis of Gleason 4 + 5 prostate adenocarcinoma. Axial fused PET-CT image (A) shows intense tracer uptake in a 3 mm left perirectal node (arrow), indicating metastatic disease. A contralateral node (notched arrow) does now show increased tracer uptake. On the CT image (B) the left perirectal node is not enlarged as per size criteria (arrow) and is similar to the uninvolved right perirectal node (notched arrow).
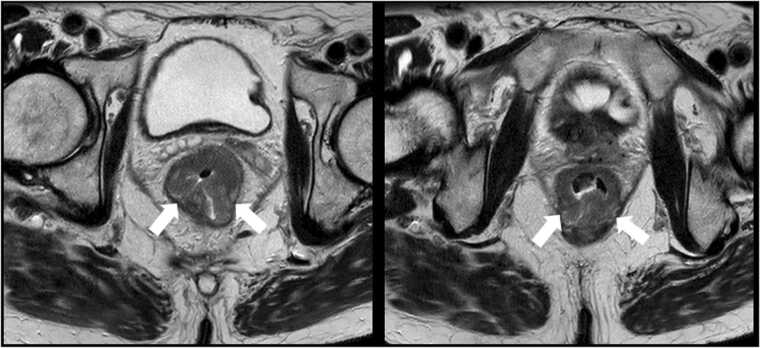
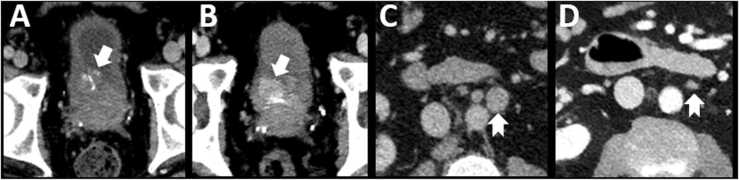
Prostate cancers are classified into two broad histologic subtypes - prostate adenocarcinoma and neuroendocrine prostate cancer (NEPC) [126]. NEPC, also referred to as castration resistant prostate cancers, comprises a group of neuroendocrine subsets including small cell carcinoma, carcinoid, large cell neuroendocrine, Paneth cell, and mixed neuroendocrine/adenocarcinoma [126], [127]. De novo primary NEPC represents about 1 % of all prostate cancer cases whereas focal neuroendocrine differentiation of an existing prostatic adenocarcinoma occurs in about 5–10 % of all prostate cancer cases [127] (Fig. 12).
Fig. 12.
Neuroendocrine differentiation in prostate cancer. 65 year old male with metastatic prostate adenocarcinoma on hormonal therapy. Serial CT scans at one month (A, C) and four months (B, D) after initiation of treatment. There is progressive increase in size of a urinary bladder mass at the right ureterovesical junction (arrows in A and B), an unusual site of metastasis for prostate cancer. This was noted despite response at typical sites of metastatic disease including retroperitoneal lymph nodes (notched arrows in C and D) and bones. Biopsy of the bladder mass showed focal neuroendocrine differentiation of prostate cancer.
Prostate cancer has a preponderance for metastasis to the vertebral bodies with 90% of autopsied metastatic prostate cancer patients having histologically proven vertebral metastasis [128].
Instead of the expected sclerotic lesion seen with prostatic adenocarcinoma, non-epithelial tumors of the prostate can cause lytic bone metastasis, particularly the more aggressive small cell carcinoma subset [129], [130]. Osteolytic prostate metastatic lesions typically demonstrate intense uptake on 18F-FDG PET as opposed to predominantly osteoblastic lesions which are not as avid [131], [132].
Current guidelines recommend a bone scan with CT and MRI for osseous lesion characterization and cord involvement respectively, in patients with known vertebral metastases from prostate cancer. However as small osteolytic metastatic foci can often be missed on these modalities, in patients with known small cell neuroendocrine variant of prostate cancer, radiologists should consider recommending 18F-FDG PET/CT to optimally localize sub-clinical lesions, particularly in patients with known biochemical relapse [133], [134].
3.6. Renal cell carcinoma
About 18 % of patients with RCC have metastases at diagnosis, and about 25–50 % of those without metastatic disease at diagnosis will develop metastasis during the course of therapy [135], [136]. While higher stage and higher nuclear grade of the tumor are associated with increased risk of metastatic disease [136], [137], the histologic subtype is also an important factor. More than 90 % of patients with metastatic RCC have clear cell RCC [138]. However, it is important to note the clear cell RCC is also the most common subtype of RCC. Collecting duct (Bellini duct) carcinoma, medullary carcinoma, and sarcomatoid variants of RCC are almost always associated with metastatic disease at presentation [135].
The histologic subtype of RCC also determines the pattern of metastatic spread. Lung, adrenal, brain, pancreatic and renal metastases are more common with clear cell RCC, lymph nodal and peritoneal metastases are more common with papillary RCC (Fig. 13) and liver metastases are more common with chromophobe RCC [4], [139].
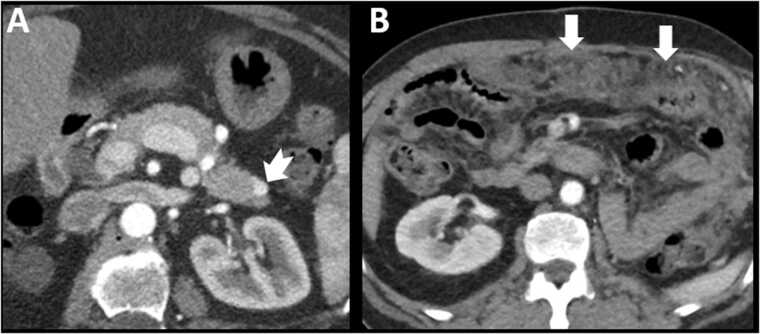
Fig. 13.
Subtypes of renal cell carcinoma and patterns of metastatic disease. Fig. 13A – 55 year old female with metastatic clear cell renal cell carcinoma on antiangiogenic therapy. Note the hypervascular nodule in the pancreatic tail (notched arrow). Fig. 13B – 62 year old male, status post left nephrectomy for papillary cell renal cell carcinoma. Note diffuse nodularity and stranding of the omentum indicating peritoneal metastatic disease (arrows).
3.7. Future directions – radiomics
The exponential increase in medical image analysis has led to the development of a field called radiomics. Broadly, radiomics refers to the process of segmenting and extracting a multitude of features from a region of interest contained within medical images. These features are then studied for their association with a variety of medically relevant dimensions of information including diagnosis, prognosis and treatment response [140], [141]. Although multiple publications have demonstrated the utility of radiomics, its real-world applicability is limited by the magnitude of the data that needs to be processed and the large number of features that can be extracted. For example, a study by Stefania et al. utilized 20 imaging features, in combination with age, gender and history of smoking, to differentiate molecular subtypes of NSCLC [3]. Such an extensive and complex analysis is not feasible in a clinical setting without routine computational support. Artificial intelligence-based solutions hold promise in this regard, but their application to a broad array of tumors, and suitability for routine clinical use requires extensive further exploration [142], [143]. Conceivably, neural networks could be trained to correlate clinical downstream data such as genomic or histologic tumor typing with upstream radiomic analyses, early proof of concept, interdisciplinary studies are needed in this regard. Future efforts must be directed to develop systems that are well integrated into the production workflow of the radiologist to provide insights in real time at the time of reporting. This holds promise to vastly advance the quality of their work product, the radiology report. One can envision a future in which radiomics may obviate the need for biopsy and histological diagnosis, or accelerate therapeutic intervention, at least in suitable tumors.
4. Conclusion
Subtypes of tumors differ in their imaging characteristics and patterns of metastatic spread. Awareness of these differences will help radiologists to predict tumor subtype at the time of diagnosis and recommend appropriate imaging modalities for additional imaging workup. Likewise, knowledge about patterns of spread will enable radiologists to create additional specific search patterns to identify early and typical metastases. This added skillset will enhance the ability of radiologists to participate in comprehensive cancer care.
Funding statement
This submission has not received any funding.
Ethics Statement
All authors declare that the contents of this submission are original. These contents have not been submitted to another journal. This review article does not involve human or animal subjects. We have taken all efforts to remove patient identifiers from the images. We have ensured that the integrity of the images have been maintained.
CRediT authorship contribution statement
All authors have made important contributions to this review article and are thoroughly familiar with the contents of the manuscript. All authors are responsible for the contents and have read and approved the manuscript. Ali Khader: Writing – review & editing. Marta Braschi Amirfarzan: Writing – review & editing, figures. Lacey J. McIntosh: Writing – review & editing, figures. Babina Gosangi: Writing – review & editing, figures. Jeremy R. Wortman: Writing – review & editing, figures. Christoph Wald: Writing – review & editing. Richard Thomas: Writing – review & editing, figures.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
None.
Disclosures
All authors have no competing interests for this submission. The following authors have non-competing disclosures: Lacey J. McIntosh – Bioclinica, Consultant. Jeremy R. Wortman – United Imaging Intelligence – Consultant, Philips - Speaker Agreement. Christoph Wald - Chair of the Informatics Commission of the ACR and Member of Board of Chancellors. Other authors do not have any disclosures.
Contributor Information
Ali Khader, Email: ali.khader@lahey.org.
Marta Braschi-Amirfarzan, Email: marta.braschiamirfarzan@lahey.org.
Lacey J. McIntosh, Email: lacey.mcintosh@umassmemorial.org.
Babina Gosangi, Email: babina.gosangi@yale.edu.
Jeremy R. Wortman, Email: jeremy.wortman@lahey.org.
Christoph Wald, Email: christoph.wald@lahey.org.
Richard Thomas, Email: richard.thomas1@lahey.org.
References
- 1.Vineis P., Wild C.P. The science of precision prevention of cancer. Lancet Oncol. 2017;18:997–998. doi: 10.1016/S1470-2045(17)30331-5. [DOI] [PubMed] [Google Scholar]
- 2.Kou T., Kanai M., Matsumoto S., Okuno Y., Muto M. The possibility of clinical sequencing in the management of cancer. Jpn. J. Clin. Oncol. 2016;46:399–406. doi: 10.1093/jjco/hyw018. [DOI] [PubMed] [Google Scholar]
- 3.Rizzo S., Petrella F., Buscarino V., De Maria F., Raimondi S., Barberis M., et al. CT radiogenomic characterization of EGFR, K-RAS, and ALK mutations in non-small cell lung cancer. Eur. Radiol. 2016;26:32–42. doi: 10.1007/s00330-015-3814-0. [DOI] [PubMed] [Google Scholar]
- 4.Shinagare A.B., Krajewski K.M., Braschi-Amirfarzan M., Ramaiya N.H. Advanced renal cell carcinoma: role of the radiologist in the era of precision medicine. Radiology. 2017;284:333–351. doi: 10.1148/radiol.2017160343. [DOI] [PubMed] [Google Scholar]
- 5.Torre L.A., Bray F., Siegel R.L., Ferlay J., Lortet-Tieulent J., Jemal A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 6.Goldwasser D.L. Estimation of the tumor size at cure threshold among aggressive non-small cell lung cancers (NSCLCs): evidence from the surveillance, epidemiology, and end results (SEER) program and the national lung screening trial (NLST) Int. J. Cancer. 2017;140:1280–1292. doi: 10.1002/ijc.30548. [DOI] [PubMed] [Google Scholar]
- 7.Flieder D.B., Port J.L., Korst R.J., Christos P.J., Levin M.A., Becker D.E., et al. Tumor size is a determinant of stage distribution in t1 non-small cell lung cancer. Chest. 2005;128:2304–2308. doi: 10.1378/chest.128.4.2304. [DOI] [PubMed] [Google Scholar]
- 8.Howlader N., Forjaz G., Mooradian M.J., Meza R., Kong C.Y., Cronin K.A., et al. The effect of advances in lung-cancer treatment on population mortality. N. Engl. J. Med. 2020;383:640–649. doi: 10.1056/NEJMoa1916623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Y., Kim J., Qu F., Liu S., Wang H., Balagurunathan Y., et al. CT features associated with epidermal growth factor receptor mutation status in patients with lung adenocarcinoma. Radiology. 2016;280:271–280. doi: 10.1148/radiol.2016151455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shaw A.T., Yeap B.Y., Mino-Kenudson M., Digumarthy S.R., Costa D.B., Heist R.S., et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J. Clin. Oncol. 2009;27:4247–4253. doi: 10.1200/JCO.2009.22.6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yano M., Sasaki H., Kobayashi Y., Yukiue H., Haneda H., Suzuki E., et al. Epidermal growth factor receptor gene mutation and computed tomographic findings in peripheral pulmonary adenocarcinoma. J. Thorac. Oncol. 2006;1:413–416. [PubMed] [Google Scholar]
- 12.Zhou J.Y., Zheng J., Yu Z.F., Xiao W.B., Zhao J., Sun K., et al. Comparative analysis of clinicoradiologic characteristics of lung adenocarcinomas with ALK rearrangements or EGFR mutations. Eur. Radiol. 2015;25:1257–1266. doi: 10.1007/s00330-014-3516-z. [DOI] [PubMed] [Google Scholar]
- 13.Garrana S.H., Dagogo-Jack I., Cobb R., Kuo A.H., Mendoza D.P., Zhang E.W., et al. Clinical and imaging features of non–small-cell lung cancer in young patients. Clin. Lung Cancer. 2021;22:23–31. doi: 10.1016/j.cllc.2020.10.012. [DOI] [PubMed] [Google Scholar]
- 14.Mendoza D.P., Stowell J., Muzikansky A., Shepard J.-A.O., Shaw A.T., Digumarthy S.R. Computed tomography imaging characteristics of non–small-cell lung cancer with anaplastic lymphoma kinase rearrangements: a systematic review and meta-analysis. Clin. Lung Cancer. 2019;20:339–349. doi: 10.1016/j.cllc.2019.05.006. [DOI] [PubMed] [Google Scholar]
- 15.Kim T.J., Lee C.-T., Jheon S.H., Park J.-S., Chung J.-H. Radiologic characteristics of surgically resected non-small cell lung cancer with ALK rearrangement or EGFR mutations. Ann. Thorac. Surg. 2016;101:473–480. doi: 10.1016/j.athoracsur.2015.07.062. [DOI] [PubMed] [Google Scholar]
- 16.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics, 2021. CA Cancer J. Clin. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 17.Chow W.-H., Dong L.M., Devesa S.S. Epidemiology and risk factors for kidney cancer. Nat. Rev. Urol. 2010;7:245–257. doi: 10.1038/nrurol.2010.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Znaor A., Lortet-Tieulent J., Laversanne M., Jemal A., Bray F. International variations and trends in renal cell carcinoma incidence and mortality. Eur. Urol. 2015;67:519–530. doi: 10.1016/j.eururo.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 19.Beer A.J., Dobritz M., Zantl N., Weirich G., Stollfuss J., Rummeny E.J. Comparison of 16-MDCT and MRI for characterization of kidney lesions. AJR Am. J. Roentgenol. 2006;186:1639–1650. doi: 10.2214/AJR.04.1545. [DOI] [PubMed] [Google Scholar]
- 20.Wang Z.J., Westphalen A.C., Zagoria R.J. CT and MRI of small renal masses. Br. J. Radiol. 2018;91 doi: 10.1259/bjr.20180131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marin D., Davis D., Roy Choudhury K., Patel B., Gupta R.T., Mileto A., et al. Characterization of small focal renal lesions: diagnostic accuracy with single-phase contrast-enhanced dual-energy CT with material attenuation analysis compared with conventional attenuation measurements. Radiology. 2017;284:737–747. doi: 10.1148/radiol.2017161872. [DOI] [PubMed] [Google Scholar]
- 22.Presti J.C., Rao P.H., Chen Q., Reuter V.E., Li F.P., Fair W.R., et al. Histopathological, cytogenetic, and molecular characterization of renal cortical tumors. Cancer Res. 1991;51:1544–1552. [PubMed] [Google Scholar]
- 23.Choueiri T.K., Plimack E., Arkenau H.-T., Jonasch E., Heng D.Y.C., Powles T., et al. Biomarker-based phase II trial of savolitinib in patients with advanced papillary renal cell cancer. J. Clin. Oncol. 2017;35:2993–3001. doi: 10.1200/JCO.2017.72.2967. [DOI] [PubMed] [Google Scholar]
- 24.Linehan W.M., Spellman P.T., Ricketts C.J., Creighton C.J., Fei S.S., et al. Cancer Genome Atlas Research Network Comprehensive molecular characterization of papillary renal-cell carcinoma. N. Engl. J. Med. 2016;374:135–145. doi: 10.1056/NEJMoa1505917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Volpe A., Novara G., Antonelli A., Bertini R., Billia M., Carmignani G., et al. Chromophobe renal cell carcinoma (RCC): oncological outcomes and prognostic factors in a large multicentre series. BJU Int. 2012;110:76–83. doi: 10.1111/j.1464-410X.2011.10690.x. [DOI] [PubMed] [Google Scholar]
- 26.Klatte T., Han K., Said J.W., Böhm M., Allhoff E.P., Kabbinavar F.F., et al. Pathobiology and prognosis of chromophobe renal cell carcinoma. Urol. Oncol. 2008;26:604–609. doi: 10.1016/j.urolonc.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 27.Vikram R., Ng C.S., Tamboli P., Tannir N.M., Jonasch E., Matin S.F., et al. Papillary renal cell carcinoma: radiologic-pathologic correlation and spectrum of disease. RadioGraphics. 2009;29:741–754. doi: 10.1148/rg.293085190. [DOI] [PubMed] [Google Scholar]
- 28.Tsuda K., Kinouchi T., Tanikawa G., Yasuhara Y., Yanagawa M., Kakimoto K., et al. Imaging characteristics of papillary renal cell carcinoma by computed tomography scan and magnetic resonance imaging. Int. J. Urol. 2005;12:795–800. doi: 10.1111/j.1442-2042.2005.01126.x. [DOI] [PubMed] [Google Scholar]
- 29.Walker J.B., Loloi J., Birk A., Raman J.D. Computed tomography imaging characteristics of histologically confirmed papillary renal cell carcinoma—implications for ancillary imaging. J. Kidney Cancer VHL. 2019;6:10–14. doi: 10.15586/jkcvhl.2019.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoshimitsu K., Irie H., Tajima T., Nishie A., Asayama Y., Hirakawa M., et al. MR imaging of renal cell carcinoma: its role in determining cell type. Radiat. Med. 2004;22:371–376. [PubMed] [Google Scholar]
- 31.Vargas H.A., Chaim J., Lefkowitz R.A., Lakhman Y., Zheng J., Moskowitz C.S., et al. Renal cortical tumors: use of multiphasic contrast-enhanced MR imaging to differentiate benign and malignant histologic subtypes. Radiology. 2012;264:779–788. doi: 10.1148/radiol.12110746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun M.R.M., Ngo L., Genega E.M., Atkins M.B., Finn M.E., Rofsky N.M., et al. Renal cell carcinoma: dynamic contrast-enhanced MR imaging for differentiation of tumor subtypes–correlation with pathologic findings. Radiology. 2009;250:793–802. doi: 10.1148/radiol.2503080995. [DOI] [PubMed] [Google Scholar]
- 33.Wang H., Cheng L., Zhang X., Wang D., Guo A., Gao Y., et al. Renal cell carcinoma: diffusion-weighted MR imaging for subtype differentiation at 3.0 T. Radiology. 2010;257:135–143. doi: 10.1148/radiol.10092396. [DOI] [PubMed] [Google Scholar]
- 34.Herts B.R., Coll D.M., Novick A.C., Obuchowski N., Linnell G., Wirth S.L., et al. Enhancement characteristics of papillary renal neoplasms revealed on triphasic helical CT of the kidneys. AJR Am. J. Roentgenol. 2002;178:367–372. doi: 10.2214/ajr.178.2.1780367. [DOI] [PubMed] [Google Scholar]
- 35.Wang K., Zarzour J., Rais-Bahrami S., Gordetsky J. Clear cell papillary renal cell carcinoma: new clinical and imaging characteristics. Urology. 2017;103:136–141. doi: 10.1016/j.urology.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 36.Williamson S.R. Clear cell papillary renal cell carcinoma: an update after 15 years. Pathology. 2021;53:109–119. doi: 10.1016/j.pathol.2020.10.002. [DOI] [PubMed] [Google Scholar]
- 37.Ilic M., Ilic I. Epidemiology of pancreatic cancer. World J. Gastroenterol. 2016;22:9694–9705. doi: 10.3748/wjg.v22.i44.9694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Metz D.C., Jensen R.T. Gastrointestinal neuroendocrine tumors: pancreatic endocrine tumors. Gastroenterology. 2008;135:1469–1492. doi: 10.1053/j.gastro.2008.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoon S.H., Lee J.M., Cho J.Y., Lee K.B., Kim J.E., Moon S.K., et al. Small (≤ 20 mm) pancreatic adenocarcinomas: analysis of enhancement patterns and secondary signs with multiphasic multidetector CT. Radiology. 2011;259:442–452. doi: 10.1148/radiol.11101133. [DOI] [PubMed] [Google Scholar]
- 40.Fletcher J.G., Wiersema M.J., Farrell M.A., Fidler J.L., Burgart L.J., Koyama T., et al. Pancreatic malignancy: value of arterial, pancreatic, and hepatic phase imaging with multi-detector row CT. Radiology. 2003;229:81–90. doi: 10.1148/radiol.2291020582. [DOI] [PubMed] [Google Scholar]
- 41.Lu D.S., Vedantham S., Krasny R.M., Kadell B., Berger W.L., Reber H.A. Two-phase helical CT for pancreatic tumors: pancreatic versus hepatic phase enhancement of tumor, pancreas, and vascular structures. Radiology. 1996;199:697–701. doi: 10.1148/radiology.199.3.8637990. [DOI] [PubMed] [Google Scholar]
- 42.Khashab M.A., Yong E., Lennon A.M., Shin E.J., Amateau S., Hruban R.H., et al. EUS is still superior to multidetector computerized tomography for detection of pancreatic neuroendocrine tumors. Gastrointest. Endosc. 2011;73:691–696. doi: 10.1016/j.gie.2010.08.030. [DOI] [PubMed] [Google Scholar]
- 43.Legmann P., Vignaux O., Dousset B., Baraza A.J., Palazzo L., Dumontier I., et al. Pancreatic tumors: comparison of dual-phase helical CT and endoscopic sonography. AJR Am. J. Roentgenol. 1998;170:1315–1322. doi: 10.2214/ajr.170.5.9574609. [DOI] [PubMed] [Google Scholar]
- 44.King C.M., Reznek R.H., Dacie J.E., Wass J.A. Imaging islet cell tumours. Clin. Radiol. 1994;49:295–303. doi: 10.1016/s0009-9260(05)81790-8. [DOI] [PubMed] [Google Scholar]
- 45.Wang S.C., Parekh J.R., Zuraek M.B., Venook A.P., Bergsland E.K., Warren R.S., et al. Identification of unknown primary tumors in patients with neuroendocrine liver metastases. Arch. Surg. 2010;145:276–280. doi: 10.1001/archsurg.2010.10. [DOI] [PubMed] [Google Scholar]
- 46.Crown A., Rocha F.G., Raghu P., Lin B., Funk G., Alseidi A., et al. Impact of initial imaging with gallium-68 dotatate PET/CT on diagnosis and management of patients with neuroendocrine tumors. J. Surg. Oncol. 2020;121:480–485. doi: 10.1002/jso.25812. [DOI] [PubMed] [Google Scholar]
- 47.Johnbeck C.B., Knigge U., Loft A., Berthelsen A.K., Mortensen J., Oturai P., et al. Head-to-head comparison of 64Cu-DOTATATE and 68Ga-DOTATOC PET/CT: a prospective study of 59 patients with neuroendocrine tumors. J. Nucl. Med. 2017;58:451–457. doi: 10.2967/jnumed.116.180430. [DOI] [PubMed] [Google Scholar]
- 48.Pfeifer A., Knigge U., Binderup T., Mortensen J., Oturai P., Loft A., et al. 64Cu-DOTATATE PET for neuroendocrine tumors: a prospective head-to-head comparison with 111In-DTPA-octreotide in 112 patients. J. Nucl. Med. 2015;56:847–854. doi: 10.2967/jnumed.115.156539. [DOI] [PubMed] [Google Scholar]
- 49.Sadowski S.M., Neychev V., Millo C., Shih J., Nilubol N., Herscovitch P., et al. Prospective study of 68Ga-DOTATATE positron emission tomography/computed tomography for detecting gastro-entero-pancreatic neuroendocrine tumors and unknown primary sites. J. Clin. Oncol. 2016;34:588–596. doi: 10.1200/JCO.2015.64.0987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kimura N., Pilichowska M., Date F., Kimura I., Schindler M. Immunohistochemical expression of somatostatin type 2A receptor in neuroendocrine tumors. Clin. Cancer Res. 1999;5:3483–3487. [PubMed] [Google Scholar]
- 51.Zimmer T., Stölzel U., Bäder M., Koppenhagen K., Hamm B., Buhr H., et al. Endoscopic ultrasonography and somatostatin receptor scintigraphy in the preoperative localisation of insulinomas and gastrinomas. Gut. 1996;39:562–568. doi: 10.1136/gut.39.4.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Islami F., Ward E.M., Sung H., Cronin K.A., Tangka F.K.L., Sherman R.L., et al. Annual report to the nation on the status of cancer, Part 1: national cancer statistics. J. Natl. Cancer Inst. 2021:djab131. doi: 10.1093/jnci/djab131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fleming M., Ravula S., Tatishchev S.F., Wang H.L. Colorectal carcinoma: pathologic aspects. J. Gastrointest. Oncol. 2012;3:153–173. doi: 10.3978/j.issn.2078-6891.2012.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Green J.B., Timmcke A.E., Mitchell W.T., Hicks T.C., Gathright J.B., Ray J.E. Mucinous carcinoma–just another colon cancer? Dis. Colon Rectum. 1993;36:49–54. doi: 10.1007/BF02050301. [DOI] [PubMed] [Google Scholar]
- 55.Secco G.B., Fardelli R., Campora E., Lapertosa G., Gentile R., Zoli S., et al. Primary mucinous adenocarcinomas and signet-ring cell carcinomas of colon and rectum. Oncology. 1994;51:30–34. doi: 10.1159/000227306. [DOI] [PubMed] [Google Scholar]
- 56.Nitsche U., Zimmermann A., Späth C., Müller T., Maak M., Schuster T., et al. Mucinous and signet-ring cell colorectal cancers differ from classical adenocarcinomas in tumor biology and prognosis. Ann. Surg. 2013;258:775–782. doi: 10.1097/SLA.0b013e3182a69f7e. (discussion 782-783) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Younes M., Katikaneni P.R., Lechago J. The value of the preoperative mucosal biopsy in the diagnosis of colorectal mucinous adenocarcinoma. Cancer. 1993;72:3588–3592. doi: 10.1002/1097-0142(19931215)72:12<3588::aid-cncr2820721207>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 58.Wnorowski A.M., Menias C.O., Pickhardt P.J., Kim D.H., Hara A.K., Lubner M.G. Mucin-containing rectal carcinomas: overview of unique clinical and imaging features. Am. J. Roentgenol. 2019;213:26–34. doi: 10.2214/AJR.18.20864. [DOI] [PubMed] [Google Scholar]
- 59.Barbaro B., Leccisotti L., Vecchio F.M., Di Matteo M., Serra T., Salsano M., et al. The potential predictive value of MRI and PET-CT in mucinous and nonmucinous rectal cancer to identify patients at high risk of metastatic disease. Br. J. Radiol. 2017;90 doi: 10.1259/bjr.20150836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nasu K., Kuroki Y., Minami M. Diffusion-weighted imaging findings of mucinous carcinoma arising in the ano-rectal region: comparison of apparent diffusion coefficient with that of tubular adenocarcinoma. Jpn. J. Radiol. 2012;30:120–127. doi: 10.1007/s11604-011-0023-x. [DOI] [PubMed] [Google Scholar]
- 61.Berger K.L., Nicholson S.A., Dehdashti F., Siegel B.A.F.D.G. PET evaluation of mucinous neoplasms. Am. J. Roentgenol. 2000;174:1005–1008. doi: 10.2214/ajr.174.4.1741005. [DOI] [PubMed] [Google Scholar]
- 62.Dos Anjos D.A., Habr-Gama A., Vailati B.B., Rossi C.B., Coturel A.E., Perez R.O., et al. 18)F-FDG uptake by rectal cancer is similar in mucinous and nonmucinous histological subtypes. Ann. Nucl. Med. 2016;30:513–517. doi: 10.1007/s12149-016-1089-4. [DOI] [PubMed] [Google Scholar]
- 63.Nissan A., Guillem J.G., Paty P.B., Wong W.D., Cohen A.M. Signet-ring cell carcinoma of the colon and rectum: a matched control study. Dis. Colon Rectum. 1999;42:1176–1180. doi: 10.1007/BF02238570. [DOI] [PubMed] [Google Scholar]
- 64.Psathakis D., Schiedeck T.H., Krug F., Oevermann E., Kujath P., Bruch H.P. Ordinary colorectal adenocarcinoma vs. primary colorectal signet-ring cell carcinoma: study matched for age, gender, grade, and stage. Dis. Colon Rectum. 1999;42:1618–1625. doi: 10.1007/BF02236218. [DOI] [PubMed] [Google Scholar]
- 65.Suthar M., Baheti A.D., Ankathi S.K., Choudhari A., Haria P.D., Engineer R., et al. MRI features of signet ring rectal cancer. Abdom. Radiol. 2021 doi: 10.1007/s00261-021-03250-1. [DOI] [PubMed] [Google Scholar]
- 66.Wang L., Hirano Y., Heng G., Ishii T., Kondo H., Hara K., et al. Does signet ring cell carcinoma component signify worse outcomes for patients with colorectal cancer. Asian J. Surg. 2021;44:105–110. doi: 10.1016/j.asjsur.2020.03.017. [DOI] [PubMed] [Google Scholar]
- 67.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 68.Adegoke O., Kulasingam S., Virnig B. Cervical cancer trends in the United States: a 35-year population-based analysis. J. Women’s Health. 2012;21:1031–1037. doi: 10.1089/jwh.2011.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Albores-Saavedra J., Larraza O., Poucell S., Rodríguez Martínez H.A. Carcinoid of the uterine cervix: additional observations on a new tumor entity. Cancer. 1976;38:2328–2342. doi: 10.1002/1097-0142(197612)38:6<2328::aid-cncr2820380620>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 70.Miller B., Dockter M., el Torky M., Photopulos G. Small cell carcinoma of the cervix: a clinical and flow-cytometric study. Gynecol. Oncol. 1991;42:27–33. doi: 10.1016/0090-8258(91)90225-t. [DOI] [PubMed] [Google Scholar]
- 71.Elsherif S., Odisio E.G.L.C., Faria S., Javadi S., Yedururi S., Frumovitz M., et al. Imaging and staging of neuroendocrine cervical cancer. Abdom. Radiol. 2018;43:3468–3478. doi: 10.1007/s00261-018-1667-0. [DOI] [PubMed] [Google Scholar]
- 72.Duan X., Ban X., Zhang X., Hu H., Li G., Wang D., et al. MR imaging features and staging of neuroendocrine carcinomas of the uterine cervix with pathological correlations. Eur. Radiol. 2016;26:4293–4302. doi: 10.1007/s00330-016-4327-1. [DOI] [PubMed] [Google Scholar]
- 73.Eifel P.J., Burke T.W., Morris M., Smith T.L. Adenocarcinoma as an independent risk factor for disease recurrence in patients with stage IB cervical carcinoma. Gynecol. Oncol. 1995;59:38–44. doi: 10.1006/gyno.1995.1265. [DOI] [PubMed] [Google Scholar]
- 74.Intaraphet S., Kasatpibal N., Søgaard M., Khunamornpong S., Patumanond J., Chandacham A., et al. Histological type-specific prognostic factors of cervical small cell neuroendocrine carcinoma, adenocarcinoma, and squamous cell carcinoma. Onco Targets Ther. 2014;7:1205–1214. doi: 10.2147/OTT.S64714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kilgore L.C., Soong S.J., Gore H., Shingleton H.M., Hatch K.D., Partridge E.E. Analysis of prognostic features in adenocarcinoma of the cervix. Gynecol. Oncol. 1988;31:137–153. doi: 10.1016/0090-8258(88)90281-8. [DOI] [PubMed] [Google Scholar]
- 76.Eifel P.J., Morris M., Oswald M.J., Wharton J.T., Delclos L. Adenocarcinoma of the uterine cervix. Prognosis and patterns of failure in 367 cases. Cancer. 1990;65:2507–2514. doi: 10.1002/1097-0142(19900601)65:11<2507::aid-cncr2820651120>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 77.Shimada M., Kigawa J., Nishimura R., Yamaguchi S., Kuzuya K., Nakanishi T., et al. Ovarian metastasis in carcinoma of the uterine cervix. Gynecol. Oncol. 2006;101:234–237. doi: 10.1016/j.ygyno.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 78.Park K.J., Braschi-Amirfarzan M., DiPiro P.J., Giardino A.A., Jagannathan J.P., Howard S.A., et al. Multimodality imaging of locally recurrent and metastatic cervical cancer: emphasis on histology, prognosis, and management. Abdom. Radiol. 2016;41:2496–2508. doi: 10.1007/s00261-016-0825-5. [DOI] [PubMed] [Google Scholar]
- 79.Drescher C.W., Hopkins M.P., Roberts J.A. Comparison of the pattern of metastatic spread of squamous cell cancer and adenocarcinoma of the uterine cervix. Gynecol. Oncol. 1989;33:340–343. doi: 10.1016/0090-8258(89)90524-6. [DOI] [PubMed] [Google Scholar]
- 80.Viswanathan A.N., Deavers M.T., Jhingran A., Ramirez P.T., Levenback C., Eifel P.J. Small cell neuroendocrine carcinoma of the cervix: outcome and patterns of recurrence. Gynecol. Oncol. 2004;93:27–33. doi: 10.1016/j.ygyno.2003.12.027. [DOI] [PubMed] [Google Scholar]
- 81.Sheets E.E., Berman M.L., Hrountas C.K., Liao S.Y., DiSaia P.J. Surgically treated, early-stage neuroendocrine small-cell cervical carcinoma. Obstet. Gynecol. 1988;71:10–14. [PubMed] [Google Scholar]
- 82.Perrin L., Ward B. Small cell carcinoma of the cervix. Int. J. Gynecol. Cancer. 1995:5. doi: 10.1046/j.1525-1438.1995.05030200.x. [DOI] [PubMed] [Google Scholar]
- 83.Van Cutsem E., Sagaert X., Topal B., Haustermans K., Prenen H. Gastric cancer. Lancet. 2016;388:2654–2664. doi: 10.1016/S0140-6736(16)30354-3. [DOI] [PubMed] [Google Scholar]
- 84.Nagtegaal I.D., Odze R.D., Klimstra D., Paradis V., Rugge M., Schirmacher P., et al. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182–188. doi: 10.1111/his.13975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Viadana E., Bross I.D., Pickren J.W. The metastatic spread of cancers of the digestive system in man. Oncology. 1978;35:114–126. doi: 10.1159/000225269. [DOI] [PubMed] [Google Scholar]
- 86.Riihimäki M., Hemminki A., Sundquist K., Sundquist J., Hemminki K. Metastatic spread in patients with gastric cancer. Oncotarget. 2016;7:52307–52316. doi: 10.18632/oncotarget.10740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Qiu M.-Z., Shi S.-M., Chen Z.-H., Yu H.-E., Sheng H., Jin Y., et al. Frequency and clinicopathological features of metastasis to liver, lung, bone, and brain from gastric cancer: a SEER-based study. Cancer Med. 2018;7:3662–3672. doi: 10.1002/cam4.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dennstedt F.E., Greenberg S.D., Kim H.-S., Weilbaecher D.G., Bloom K. Pulmonary lymphangitic carcinomatosis from occult stomach carcinoma in young adults an unusual cause of dyspnea. Chest. 1983;84:787–788. doi: 10.1378/chest.84.6.787. [DOI] [PubMed] [Google Scholar]
- 89.Sakai Y., Minouchi K., Ohta H., Annen Y., Sugimoto T. Cardiac tamponade originating from primary gastric signet ring cell carcinoma. J. Gastroenterol. 1999;34:250–252. doi: 10.1007/s005350050252. [DOI] [PubMed] [Google Scholar]
- 90.Moubax K., Wuyts W., Vandecaveye V., Prenen H. Pulmonary lymphangitic carcinomatosis as a primary manifestation of gastric carcinoma in a young adult: a case report and review of the literature. BMC Res. Notes. 2012;5:638. doi: 10.1186/1756-0500-5-638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Motzer R.J., Agarwal N., Beard C., Bhayani S., Bolger G.B., Buyyounouski M.K., et al. Testicular cancer. J. Natl. Compr. Cancer Netw. 2012;10:502–535. doi: 10.6004/jnccn.2012.0050. [DOI] [PubMed] [Google Scholar]
- 92.Bosl G.J., Motzer R.J. Testicular germ-cell cancer. N. Engl. J. Med. 1997;337:242–253. doi: 10.1056/NEJM199707243370406. [DOI] [PubMed] [Google Scholar]
- 93.Mead G., Stenning S., Cook P., Fossa S., Horwich A., Kaye S., et al. International germ cell consensus classification: a prognostic factor-erased staging system for metastatic germ cell cancers. J. Clin. Oncol. 1997;15:594–603. doi: 10.1200/JCO.1997.15.2.594. [DOI] [PubMed] [Google Scholar]
- 94.Wood M.J., Thomas R., Howard S.A., Braschi-Amirfarzan M. Imaging of metastatic germ cell tumors in male patients from initial diagnosis to treatment-related toxicities: a primer for radiologists. Am. J. Roentgenol. 2020;214:24–33. doi: 10.2214/AJR.19.21623. [DOI] [PubMed] [Google Scholar]
- 95.White P.M., Adamson D.J., Howard G.C., Wright A.R. Imaging of the thorax in the management of germ cell testicular tumours. Clin. Radiol. 1999;54:207–211. doi: 10.1016/s0009-9260(99)91152-2. [DOI] [PubMed] [Google Scholar]
- 96.Horwich A., Nicol D., Huddart R. Testicular germ cell tumours. BMJ. 2013;347:f5526. doi: 10.1136/bmj.f5526. [DOI] [PubMed] [Google Scholar]
- 97.Sohaib S.A., Cook G., Koh D.-M. Imaging studies for germ cell tumors. Hematol. Oncol. Clin. N. Am. 2011;25(vii):487–502. doi: 10.1016/j.hoc.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 98.Wood A., Robson N., Tung K., Mead G. Patterns of supradiaphragmatic metastases in testicular germ cell tumours. Clin. Radiol. 1996;51:273–276. doi: 10.1016/s0009-9260(96)80345-x. [DOI] [PubMed] [Google Scholar]
- 99.Williams M., Husband J., Heron C. Intrathoracic manifestations of metastatic testicular seminoma: a comparison of chest radiographic and CT findings. Am. J. Roentgenol. 1987;149:473–475. doi: 10.2214/ajr.149.3.473. [DOI] [PubMed] [Google Scholar]
- 100.Kesler K.A., Brooks J.A., Rieger K.M., Fineberg N.S., Einhorn L.H., Brown J.W. Mediastinal metastases from testicular nonseminomatous germ cell tumors: patterns of dissemination and predictors of long-term survival with surgery. J. Thorac. Cardiovasc. Surg. 2003;125:913–923. doi: 10.1067/mtc.2003.407. [DOI] [PubMed] [Google Scholar]
- 101.Oechsle K., Bokemeyer C. Treatment of brain metastases from germ cell tumors. Hematol. Oncol. Clin. N. Am. 2011;25(ix):605–613. doi: 10.1016/j.hoc.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 102.Hitchins R.N., Philip P.A., Wignall B., Newlands E.S., Begent R.H., Rustin G.J., et al. Bone disease in testicular and extragonadal germ cell tumours. Br. J. Cancer. 1988;58:793–796. doi: 10.1038/bjc.1988.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Oechsle K., Bokemeyer C., Kollmannsberger C., Mayer F., Berger L.A., Oing C., et al. Bone metastases in germ cell tumor patients. J. Cancer Res. Clin. Oncol. 2012;138:947–952. doi: 10.1007/s00432-012-1169-3. [DOI] [PubMed] [Google Scholar]
- 104.Jamal-Hanjani M., Karpathakis A., Kwan A., Mazhar D., Ansell W., Shamash J., et al. Bone metastases in germ cell tumours: lessons learnt from a large retrospective study. BJU Int. 2013;112:176–181. doi: 10.1111/bju.12218. [DOI] [PubMed] [Google Scholar]
- 105.CDCBreastCancer, Breast Cancer Statistics, Centers for Disease Control and Prevention, 2020. 〈https://www.cdc.gov/cancer/breast/statistics/index.htm〉, (Accessed 26 January 2021).
- 106.Jemal A., Siegel R., Ward E., Hao Y., Xu J., Thun M.J. Cancer statistics, 2009. CA: Cancer J. Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 107.Li C.I., Anderson B.O., Daling J.R., Moe R.E. Trends in incidence rates of invasive lobular and ductal breast carcinoma. JAMA. 2003;289:1421–1424. doi: 10.1001/jama.289.11.1421. [DOI] [PubMed] [Google Scholar]
- 108.Dent R., Hanna W.M., Trudeau M., Rawlinson E., Sun P., Narod S.A. Pattern of metastatic spread in triple-negative breast cancer. Breast Cancer Res. Treat. 2009;115:423–428. doi: 10.1007/s10549-008-0086-2. [DOI] [PubMed] [Google Scholar]
- 109.Fulford L.G., Reis-Filho J.S., Ryder K., Jones C., Gillett C.E., Hanby A., et al. Basal-like grade III invasive ductal carcinoma of the breast: patterns of metastasis and long-term survival. Breast Cancer Res. 2007;9:R4. doi: 10.1186/bcr1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Albiges L., André F., Balleyguier C., Gomez-Abuin G., Chompret A., Delaloge S. Spectrum of breast cancer metastasis in BRCA1 mutation carriers: highly increased incidence of brain metastases. Ann. Oncol. 2005;16:1846–1847. doi: 10.1093/annonc/mdi351. [DOI] [PubMed] [Google Scholar]
- 111.Hicks D.G., Short S.M., Prescott N.L., Tarr S.M., Coleman K.A., Yoder B.J., et al. Breast cancers with brain metastases are more likely to be estrogen receptor negative, express the basal cytokeratin CK5/6, and overexpress HER2 or EGFR. Am. J. Surg. Pathol. 2006;30:1097–1104. doi: 10.1097/01.pas.0000213306.05811.b9. [DOI] [PubMed] [Google Scholar]
- 112.Biglia N., Mariani L., Sgro L., Mininanni P., Moggio G., Sismondi P. Increased incidence of lobular breast cancer in women treated with hormone replacement therapy: implications for diagnosis, surgical and medical treatment. Endocr. Relat. Cancer. 2007;14:549–567. doi: 10.1677/ERC-06-0060. [DOI] [PubMed] [Google Scholar]
- 113.Ilgen J.S., Marr A.L. Cancer emergencies: the acute abdomen. Emerg. Med. Clin. N. Am. 2009;27:381–399. doi: 10.1016/j.emc.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 114.Beniey M. Peritoneal metastases from breast cancer: a scoping review. Cureus. 2019:11. doi: 10.7759/cureus.5367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.DiPiro P.J., Tirumani S.H., Cruz G.P., Ramaiya N.H., Lester S.C., Shinagare A.B. Lobular breast cancer: patterns of intraabdominal metastatic spread on imaging and prognostic significance. Abdom. Radiol. 2019;44:362–369. doi: 10.1007/s00261-018-1722-x. http://dx.doi.org.ezproxy.library.tufts.edu/10.1007/s00261-018-1722-x. [DOI] [PubMed] [Google Scholar]
- 116.Mathew A., Rajagopal P.S., Villgran V., Sandhu G.S., Jankowitz R.C., Jacob M., et al. Distinct pattern of metastases in patients with invasive lobular carcinoma of the breast. Geburtshilfe Frau. 2017;77:660–666. doi: 10.1055/s-0043-109374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.El-Hage A., Ruel C., Afif W., Wissanji H., Hogue J.-C., Desbiens C., et al. Metastatic pattern of invasive lobular carcinoma of the breast—emphasis on gastric metastases. J. Surg. Oncol. 2016;114:543–547. doi: 10.1002/jso.24362. [DOI] [PubMed] [Google Scholar]
- 118.Inoue M., Nakagomi H., Nakada H., Furuya K., Ikegame K., Watanabe H., et al. Specific sites of metastases in invasive lobular carcinoma: a retrospective cohort study of metastatic breast cancer. Breast Cancer. 2017;24:667–672. doi: 10.1007/s12282-017-0753-4. [DOI] [PubMed] [Google Scholar]
- 119.Winn J.S., Baker M.G., Fanous I.S., Slack-Davis J.K., Atkins K.A., Dillon P.M. Lobular breast cancer and abdominal metastases: a retrospective review and impact on survival. Oncology. 2016;91:135–143. doi: 10.1159/000447264. [DOI] [PubMed] [Google Scholar]
- 120.O’Connell F.P., Wang H.H., Odze R.D. Utility of immunohistochemistry in distinguishing primary adenocarcinomas from metastatic breast carcinomas in the gastrointestinal tract. Arch. Pathol. Lab. Med. 2005;129:338–347. doi: 10.1043/1543-2165(2005)129<338:UOIIDP>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 121.Fitzmaurice C., Allen C., Barber R.M., Barregard L., Bhutta Z.A., et al. Global Burden of Disease Cancer Collaboration Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA Oncol. 2017;3:524–548. doi: 10.1001/jamaoncol.2016.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cooperberg M.R., Broering J.M., Carroll P.R. Risk assessment for prostate cancer metastasis and mortality at the time of diagnosis. JNCI: J. Natl. Cancer Inst. 2009;101:878–887. doi: 10.1093/jnci/djp122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bubendorf L., Schöpfer A., Wagner U., Sauter G., Moch H., Willi N., et al. Metastatic patterns of prostate cancer: an autopsy study of 1589 patients. Hum. Pathol. 2000;31:578–583. doi: 10.1053/hp.2000.6698. [DOI] [PubMed] [Google Scholar]
- 124.Barbosa F.G., Queiroz M.A., Nunes R.F., Viana P.C.C., Marin J.F.G., Cerri G.G., et al. Revisiting prostate cancer recurrence with PSMA PET: atlas of typical and atypical patterns of spread. RadioGraphics. 2019;39:186–212. doi: 10.1148/rg.2019180079. [DOI] [PubMed] [Google Scholar]
- 125.Hofman M.S., Hicks R.J., Maurer T., Eiber M. Prostate-specific membrane antigen PET: clinical utility in prostate cancer, normal patterns, pearls, and pitfalls. RadioGraphics. 2018;38:200–217. doi: 10.1148/rg.2018170108. [DOI] [PubMed] [Google Scholar]
- 126.Parimi V., Goyal R., Poropatich K., Yang X.J. Neuroendocrine differentiation of prostate cancer: a review. Am. J. Clin. Exp. Urol. 2014;2:273–285. [PMC free article] [PubMed] [Google Scholar]
- 127.Aggarwal R., Zhang T., Small E.J., Armstrong A.J. Neuroendocrine prostate cancer: subtypes, biology, and clinical outcomes. J. Natl. Compr. Cancer Netw. 2014;12:719–726. doi: 10.6004/jnccn.2014.0073. [DOI] [PubMed] [Google Scholar]
- 128.Prasad D., Schiff D. Malignant spinal-cord compression. Lancet Oncol. 2005;6:15–24. doi: 10.1016/S1470-2045(04)01709-7. [DOI] [PubMed] [Google Scholar]
- 129.Nepal P., Nagar A., Tirumani S.H., Ojili V. Imaging of non-epithelial neoplasms of the prostate. Abdom. Radiol. 2020;45:4117–4132. doi: 10.1007/s00261-020-02774-2. [DOI] [PubMed] [Google Scholar]
- 130.Palmgren J.S., Karavadia S.S., Wakefield M.R. Unusual and underappreciated: small cell carcinoma of the prostate. Semin. Oncol. 2007;34:22–29. doi: 10.1053/j.seminoncol.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 131.Parent E.E., Schuster D.M. Update on 18F-fluciclovine PET for prostate cancer imaging. J. Nucl. Med. 2018;59:733–739. doi: 10.2967/jnumed.117.204032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nanni C., Zanoni L., Bach-Gansmo T., Minn H., Willoch F., Bogsrud T.V., et al. [18F]Fluciclovine PET/CT: joint EANM and SNMMI procedure guideline for prostate cancer imaging—version 1.0. Eur. J. Nucl. Med. Mol. Imaging. 2020;47:579–591. doi: 10.1007/s00259-019-04614-y. [DOI] [PubMed] [Google Scholar]
- 133.Jadvar H. Is there use for FDG-PET in prostate cancer? Semin. Nucl. Med. 2016;46:502–506. doi: 10.1053/j.semnuclmed.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wallitt K.L., Khan S.R., Dubash S., Tam H.H., Khan S., Barwick T.D. Clinical PET imaging in prostate cancer. RadioGraphics. 2017;37:1512–1536. doi: 10.1148/rg.2017170035. [DOI] [PubMed] [Google Scholar]
- 135.Brufau B.P., Cerqueda C.S., Villalba L.B., Izquierdo R.S., González B.M., Molina C.N. Metastatic renal cell carcinoma: radiologic findings and assessment of response to targeted antiangiogenic therapy by using multidetector CT. RadioGraphics. 2013;33:1691–1716. doi: 10.1148/rg.336125110. [DOI] [PubMed] [Google Scholar]
- 136.Janzen N.K., Kim H.L., Figlin R.A., Belldegrun A.S. Surveillance after radical or partial nephrectomy for localized renal cell carcinoma and management of recurrent disease. Urol. Clin. N. Am. 2003;30:843–852. doi: 10.1016/S0094-0143(03)00056-9. [DOI] [PubMed] [Google Scholar]
- 137.Fuhrman S.A., Lasky L.C., Limas C. Prognostic significance of morphologic parameters in renal cell carcinoma. Am. J. Surg. Pathol. 1982;6:655–664. doi: 10.1097/00000478-198210000-00007. [DOI] [PubMed] [Google Scholar]
- 138.Motzer R.J., Bacik J., Mariani T., Russo P., Mazumdar M., Reuter V. Treatment outcome and survival associated with metastatic renal cell carcinoma of non–clear-cell histology. JCO. 2002;20:2376–2381. doi: 10.1200/JCO.2002.11.123. [DOI] [PubMed] [Google Scholar]
- 139.Dudani S., de Velasco G., Wells J.C., Gan C.L., Donskov F., Porta C., et al. Evaluation of clear cell, papillary, and chromophobe renal cell carcinoma metastasis sites and association with survival. JAMA Netw. Open. 2021;4 doi: 10.1001/jamanetworkopen.2020.21869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gillies R.J., Kinahan P.E., Hricak H. Radiomics: images are more than pictures, they are data. Radiology. 2016;278:563–577. doi: 10.1148/radiol.2015151169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lambin P., Leijenaar R.T.H., Deist T.M., Peerlings J., de Jong E.E.C., van Timmeren J., et al. Radiomics: the bridge between medical imaging and personalized medicine. Nat. Rev. Clin. Oncol. 2017;14:749–762. doi: 10.1038/nrclinonc.2017.141. [DOI] [PubMed] [Google Scholar]
- 142.Koçak B., Durmaz E.Ş., Ateş E., Kılıçkesmez Ö. Radiomics with artificial intelligence: a practical guide for beginners. Diagn. Interv. Radiol. 2019;25:485–495. doi: 10.5152/dir.2019.19321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Bera K., Braman N., Gupta A., Velcheti V., Madabhushi A. Predicting cancer outcomes with radiomics and artificial intelligence in radiology. Nat. Rev. Clin. Oncol. 2022;19:132–146. doi: 10.1038/s41571-021-00560-7. [DOI] [PMC free article] [PubMed] [Google Scholar]