Abstract
Cardiovascular involvement is a common complication of COVID-19 infection and is associated to increased risk of unfavorable outcome. Advanced imaging modalities (coronary CT angiography and Cardiac Magnetic Resonance) play a crucial role in the diagnosis, follow-up and risk stratification of patients affected by COVID-19 pneumonia with suspected cardiovascular involvement. In the present manuscript we firstly review current knowledge on the mechanisms by which SARS-CoV-2 can trigger endothelial and myocardial damage. Secondly, the implications of the cardiovascular damage on patient's prognosis are presented. Finally, we provide an overview of the main findings at advanced cardiac imaging characterizing COVID-19 in the acute setting, in the post-acute syndrome, and after vaccination, emphasizing the potentiality of CT and CMR, the indication and their clinical implications.
Keywords: COVID-19, Coronary CT angiography, Cardiac Magnetic Resonance, Pulmonary embolism, Myocarditis, Vaccine
1. Introduction
Coronavirus disease 2019 (COVID-19) has become a worldwide pandemic. COVID-19 is caused by the novel severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2), that primarily manifests as an interstitial pneumonia and can rapidly progress towards severe acute respiratory distress syndrome [1]. Despite the respiratory system is the primary target, multiorgan involvement frequently occurs. Cardiovascular involvement in COVID-19 is common: an increase in levels of biomarkers of cardiac injury or dysfunction (troponin I and T, creatine kinase-MB, myoglobin, NT-proBNP) is described in up to 40% of cases, especially in patients with cardiovascular risk factors [2] and severe disease 3., 4..
Pre-existing cardiovascular risk factors and the occurrence of acute cardiac injury are both predictors of adverse events 5., 6., being associated to more severe disease and higher mortality rate 1., 3., 7., 8., 9., 10., 11., 12., 13., 14., 15., 16..
The spectrum of SARS-CoV-2 cardiovascular manifestations is wide and encompasses multiple clinical presentations, including acute coronary syndromes (ACS), heart failure, myocarditis, and arrhythmias [17] and the underlying mechanism is complex, multifaceted, and still not completely understood [18].
Cardiac symptoms might persist months after recovery from COVID-19 [19]. The introduction of COVID-19 vaccination has significantly reduced the incidence of severe COVID-19. Nevertheless, several reports have raised concerns about myopericarditis occurrence after different types of COVID-19 vaccines 20., 21., 22..
Advanced cardiac imaging plays a role in diagnosis and risk stratification of COVID-19 patients with cardiovascular complications. CT has a pivotal rule in ruling-out coronary artery disease and pulmonary embolism. Vascular evaluation may be coupled with the assessment of pneumonia severity and to myocardial tissue characterization, excluding myocardial scars 23., 24.. Additionally, CT may provide quantitative information about subclinical comorbidities or COVID-19 related complications capable of improving risk stratification of COVID-19 patients 7., 9., 10., 25., 26., 27. (Fig. 1 ). Cardiac Magnetic Resonance (CMR) is the imaging of choice for the non-invasive characterization of myocardium, allowing to accurately assess ventricular function, myocardial edema, and myocardial injury. CMR imaging is useful for the non-invasive detection of cardiac alteration related to acute COVID-19, post-acute sequelae, and vaccination [28].
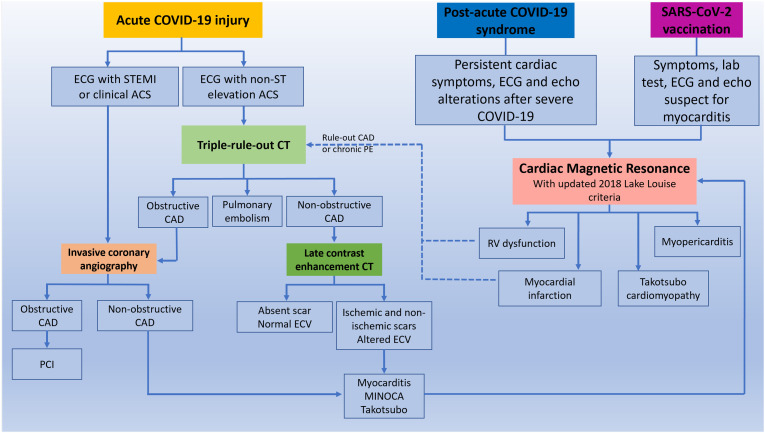
Fig. 1.
Role of CT and CMR in the diagnostic algorithm of COVID-19 related cardiac complication. After clinical evaluation, patients with chest pain, ST elevation ACS, high pretest probability of CAD and high risk of mortality should be referred to emergent invasive coronary angiography (ICA). Patients with NSTEMI, atypical symptoms and ECG abnormality should be referred to CT. A triple rule-out protocol should be preferred for the simultaneous exclusion of pulmonary embolism (PE) and coronary artery disease (CAD). Patients with obstructive CAD should be referred to ICA for percutaneous intervention (PCI), while patients with non-obstructive CAD to tissue characterization. This could be obtained directly from CT, and in presence of scar and ECV alteration according to multidisciplinary evaluation a diagnostic confirmation with CMR can be performed.
In patients with suspected long COVID-19 syndrome and post-vaccination symptoms, CMR is the first level examination. CT can have a role subsequently in order to exclude chronic PE or obstructive CAD in patients with long COVID-19 syndrome.
In the present manuscript, we aim to provide an overview about indication, potentialities, and main findings of cardiothoracic CT and CMR in the most frequent scenarios of COVID-19 related manifestation.
2. COVID-19 related cardiovascular damage physiopathology
Most frequently, COVID-19 related acute myocardial damage manifestation include [18]: i) right ventricular dysfunction because of COVID-19 associated pulmonary embolism or pulmonary hypertension; ii) acute coronary ischemia because of focal epicardial coronary artery thrombosis (type 1 myocardial infarction) or diffuse myocardial ischemia sustained by extensive microvascular dysfunction, hypoxemia and vasoconstriction due to oxygen demand/supply mismatch (type 2 myocardial infarction) 17., 29., 30., resulting from direct vascular infection, endotheliitis, microvascular remodeling, and thrombosis secondary to a hypercoagulability status; iii) myopericarditis following myocardial cell binding, direct/indirect cell damage due to ACE-2 receptor interaction or cytotoxic damage and microcirculation dysfunction as a result of the so-called cytokine storm; iv) vasoplegic shock due to sepsis and dysregulation of the renin-angiotensin system;
Takotsubo syndrome [31] is also reported and considered to be due to unbalanced sympathetic stimulation.
Additionally, COVID-19 patients may suffer from arrhythmias typically ranging from supraventricular arrhythmias in clinically stable patients [32], to major bradyarrhythmias, such as complete heart block and ventricular tachyarrhythmia in complicated infections 33., 34., triggered by hypoxia, electrolyte derangements, myocardial strain, inflammatory microenvironment and drug side effects [35].
Differently from other cardiotropic viruses, SARS-CoV-2 viral particles have been found inside endothelial cells and cardiac macrophages, but never inside cardiomyocytes 33., 36., 37., 38., while macrophage infiltration, inflammation, and microthrombi were the most common finding at autopsy (48% of cases) [39].
Immune-mediated mechanisms such as molecular mimicry are thought to contribute to persistent cardiac dysfunction due to a chronic and uncontrolled cytokine response also in post-acute and chronic phases.
Cardiac complication according to the stage of the disease is reported in Table 1 .
Table 1.
Cardiac complication and physiopathology according to the stage of disease.
| Cardiac complication | Pathophysiology | Time of onset | |
|---|---|---|---|
| Acute COVID-19 | - Right ventricular dysfunction | Pulmonary embolism or pulmonary hypertension for hypercoagulability status, endothelial dysfunction, Hypoxemia and vasoconstriction | From acute symptom onset to symptoms resolution |
| - Type I myocardial infarction | - Endothelial dysfunction - Hypercoagulability status |
||
| - Type II myocardial infarction | - Endothelial dysfunction - Hypoxemia and vasoconstriction |
||
| - Myocarditis, pericarditis | - Direct viral injury - Cytotoxic damage due to cytokine storm |
||
| - Takotsubo cardiomyopathy | - Unbalanced sympathetic stimulation | ||
| - Arrhythmias | - Hypoxia, electrolyte derangements, myocardial inflammation | ||
| Cardiac post-acute COVID-19 syndrome | - Myocarditis - Pericarditis |
- Chronic inflammatory response for persistent viral reservoirs - Chronic autoimmune inflammation due to molecular mimicry |
3–4 weeks after COVID-19 onset |
| - Microvascular ischemia and myocardial infarction | - Endothelial dysfunction | ||
| SARS-CoV-2 vaccination | - Myocarditis, pericarditis | - Delayed hypersensitivity reaction - Molecular mimicry - Systemic inflammatory response |
Within 14 days after second shot |
3. Cardiac imaging in COVID-19
Transthoracic echocardiography is the first imaging technique used in the diagnostic work-up [40] of COVID-19 patients with suspected cardiovascular involvement providing information directly at bedside.
Bonnemain et al. [41] reviewed 151 articles about echocardiogram in COVID-19 patients and found right ventricle (RV) alteration as the most common finding, with RV dilation in up to 49% of patients and RV systolic dysfunction in up to 40%. RV alterations resulted correlated to the severity of lung involvement and to pulmonary hypertension [41] and were associated to increased level of biomarkers of cardiac injury (troponin and NT-pro-BNP), inflammation (C-reactive protein), and pro-thrombotic status (D-dimer). In a prospective multicenter study including 1216 hospitalized acute COVID-19 patients, 55% of them had abnormal echocardiograms, however the underlying cause of alteration was not identified in most cases [42]. Therefore, echocardiography plays a crucial role in selecting patients for advanced cardiac imaging, reducing unnecessary exams and diagnostic delays. The diagnostic flow-chart varied according to national and international guidelines and institution according to local expertise and resource availability [40]. The flow-chart we used is reported in Fig. 1.
However, CT is the imaging modality of choice for the evaluation of cardiothoracic complications related to COVID-19 [43], providing useful information in a short time also in unstable patients, while reducing exposure time of patients and personnel 44., 45..
In a recent position paper, Cosyns et al. [46] stated that the pre-test probability of coronary artery disease may represents the primary guidance in the diagnostic work-up of COVID-19 patients with myocardial injury and that coronary computed tomography angiography (CCTA) should be preferred for patients with low-to-intermediate risk of acute coronary syndrome (ACS) because of its high negative predictive value. However, Stefanini et al. found out that 39% of COVID-19 patients with STEMI had negative invasive coronary angiography (ICA) probably for the higher prevalence of type-2 MI in patients during the acute phase of COVID-19. These data further supported the use of CCTA in COVID-19 patients [47], also having the advantage to simultaneously rule-out coronary artery disease (CAD) and pulmonary embolism (PE) using a triple rule-out scanning protocol 23., 24. and to refine risk stratification of COVID-19 patients with the quantification of coronary artery calcium score (CAC) 7., 9., 10., 16., 25., 48..
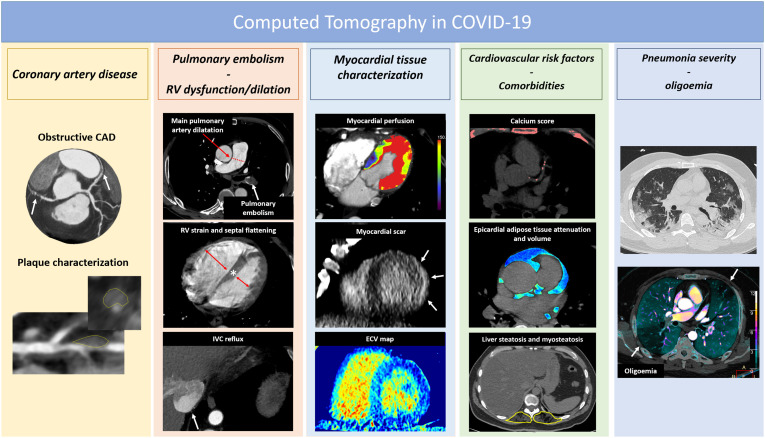
CT assessment offers the additional advantage of COVID-19 pneumonia severity assessment [1] and to reveal subclinical CV risk factors such as liver steatosis [49], myosteatosis [50], and epicardial fat volume and attenuation 11., 51.. Finally, CT may offer the opportunity to characterize myocardial scar and extracellular volume fraction (ECV) with the addition of a late contrast enhancement scan 23., 24., 52., 53.. This may be particularly useful and effective especially in the emergency setting [23], providing a full range of diagnosis with a single examination requiring few minutes, without the need of CMR [24] (Fig. 2 ).
Fig. 2.
The spectrum of CT potentialities in the setting of COVID-19.
Therefore, a comprehensive CT acquisition protocol in suspected acute myocardial damage should include: i) precontrast CT scan for the assessment of pneumonia severity and calcium score; ii) an angiographic triple rule out scan for exclusion of CAD, PE and acute aortic injury, acquired with retrospective gating in order to obtain multiphase reconstruction (0–90% of R-R interval) for the assessment of wall motion alteration; iii) a late contrast enhancement scan acquired 5 to 10 min after contrast administration for the assessment of myocardial scars and for ECV quantification 54., 55., 56., 57., 58. Table 2 .
Table 2.
CT protocol in suspected COVID-19 related cardiovascular injury.
| CT scan | Indication | Parameters | Information | Severity cut-off value |
|---|---|---|---|---|
| Non-contrast scan | -Pneumonia assessment -Coronary artery calcium |
Standard Large FOV for pneumonia evaluation Cardiac FOV prospective ECG-gated scan at 75% of the R-R interval |
- Pneumonia severity - Coronary artery calcium - Total thoracic calcium - Main pulmonary artery diameter/Hypertension - Epicardial adipose tissue attenuation - Liver steatosis - Myosteatosis |
>50% lung volumea1., 27. >400 AUa[25] ≥1068 cca[10] ≥31 mma[27] ≥−96.3 HUa[11] ≤−40 HUb[49] <34.3 (F) and <38.5 (M) HUb[50] |
| Angiographic scan | -Pulmonary embolism - CAD -Triple-rule out |
Chest FOV, Single energy or DECT, standard parameters Cardiac FOV. Retrospective gating with automatic tube current modulation (100% in 60–80% or 40–80% for HR <65 bpm or >65 bpm with 4% current in other phases). 80 kVp for BMI < 20; 100 kVp for BMI ≥ 20 and <30; 120 kVp for BMI ≥ 30 Same for CAD protocol but chest FOV |
- Pulmonary artery embolism - Oligoemia - RV dysfunction - Obstructive CAD - Wall motion abnormalities - Obstructive CAD - Pulmonary artery embolism - Acute aortic injury |
Presence Presence RV/LV diameter ratio > 0.9a[26] IVC reflux [26] ≥50% [69] presence ≥50%b[69] Presence Presence |
| Late contrast enhancement | Myocardial tissue characterization | Cardiac FOV. Single energy: 80KV, p prospective ECG-gating at 75% of the R-R interval 5–10 minute post contrast |
Myocardial scar Extracellular volume fraction |
Presence ≥27%b53., 54. |
Abbreviation: CAD: coronary artery disease, FOV: field-of-view; LV: left ventricle, RV: right ventricle, IVC: inferior vena cava.
Cut-off values associated with increased mortality in COVID-19 setting.
Cut-off values derived from population studies or other clinical settings.
Late contrast enhancement scan needs higher contrast volume compared to standard CCTA, with a total iodine dose of 600 mg per kilogram of body weight 55., 59..
However, tissue characterization in CT has still limited application worldwide mainly for limited contrast-to-noise ratio, requiring experience for scar detection [55], hence CMR remains the gold standard for non-invasive characterization of myocardial tissue. CMR should be considered in COVID-19 patients with high pretest probability for acute myocardial injury, in particular in those with chest pain and unobstructed coronary arteries to differentiate between acute myocarditis, Takotsubo cardiomyopathy, and MINOCA 40., 60., 61., avoiding diagnostic and treatment delay.
Several authors 40., 61., 62. proposed short CMR protocols to improve resource allocation and to reduce the infection risk (Table 3 ). A tailored CMR protocol should include cine-sequences for functional assessment, T2-based imaging to evaluate myocardial edema (i.e., T2w-STIR and T2 mapping), and T1-based imaging (i.e., T1 mapping and late gadolinium enhancement evaluation) to evaluate myocardial edema, hyperemia/capillary leak, necrosis, and pre-existing fibrosis. To exclude pulmonary embolism, a 3D pulmonary angiography could be acquired during gadolinium injection [63], while, to assess lung pathology, a breath hold T2-w sequence can be used [40] Table 3.
Table 3.
Short CMR protocol in suspected COVID-19 cardiac damage.
| Timeline | Sequence | Planes and coverage | Finding |
|---|---|---|---|
| Precontrast | Black Blood STIR T2w sequence | Entire ventricle coverage | Edema |
| T2 mapping | 3 short axis (base, mid, apex) |
Edema | |
| Native T1 mapping | 3 short axis (base, mid, apex) |
Edema, fibrosis | |
| Gadolinium injection | FLASH 3D pulmonary angiography | 3D entire chest | Pulmonary embolism |
| 2–5 min post contrast | SSFP cine | Entire ventricle coverage | Volume and function |
| 10 min post contrast | Inversion recovery or 3D-PSIR | Entire ventricle coverage | Myocardial scar |
| 15 min post contrast | Post-contrast T1 mapping |
3 short axis (base, mid, apex) |
Extracellular volume fraction |
| Optional | T2w | Chest | Pneumonia |
Based on the high rate of myocarditis in patients with COVID-19 myocardial injury, mapping techniques are crucial for the identification of subtle myocardial inflammation according to the updated Lake Louise criteria [64], while the evaluation of standard CMR criteria are enough for the identification of Takotsubo cardiomyopathy [65] and MINOCA [66] also in COVID-19 setting.
Main CT and CMR findings according to the stage of disease are reported in Table 4 .
Table 4.
Main cardiac complication at CT and CMR according to the stage of disease.
| Reference | Patients (n) | Age (y); male (%) | Follow-up time | Cardiovascular symptom or signs (%) | Main CT findings | Main CMR findings | |
|---|---|---|---|---|---|---|---|
| Acute cardiovascular complications | |||||||
| LV function alterations | Kato et al. [28] | 1414 | NR; NR | NR | NR | Mean difference in LVEF between COVID-19 patients and controls = −2.84 (CI, −5.11 to −0.56) | |
| Takotsubo cardiomyopathy | Ojha et al. [76] | 199 | NR; 57 | NR | NR | Takotsubo cardiomyopathy in 1.5% | |
| Esposito et al. [75] | 10 | 52 ± 6; 20 | 3 (IQR, 2–4) days after symptoms onset | Chest pain (80%) Dyspnea (20%) |
Takotsubo cardiomyopathy in 20% | ||
| Myocardial edema | Ojha et al. [76] | 199 | NR; 57 | NR | NR | Myocardial edema in 63% of patients by increased T2 mapping values | |
| Kato et al. [28] | 1414 | NR; NR | NR | NR | Myocardial edema in 39.5% of patients by increased T2 mapping/T2w images | ||
| Myocarditis or pericarditis | Pontone et al. [24] | 1 | 59; 1 | 1 day after COVID-19 disease diagnosis | Dyspnea and chest pain | Subepicardial (non-ischemic) late iodine enhancement (LIE) in the basal-mid inferolateral wall of the left ventricle | |
| Peretto et al. [33] | 7 | 51 ± 9; 57 | 0–12 days after COVID-19 disease diagnosis | Heart-failure presentation (57%); ACS-like presentation (43%) |
Mid-basal septal or infero-lateral active myocarditis. In only one patient (PCR) analysis revealed an intra-myocardial SARS-CoV-2 genome | ||
| Esposito et al. [75] | 10 | 52 ± 6; 20 | 3 (IQR, 2–4) days after symptoms onset | Chest pain (80%) Dyspnea (20%) |
Acute myocarditis in 80%. LGE was positive in only 3 patients with thin and shadowed sub-epicardial striae |
||
| Ojha et al. (66) | 199 | NR; 57 | NR | NR | Myocarditis in 40.2% of population in inferior/infero-lateral basal segments of the LV | ||
| Kato et al. [28] | 1414 | NR; NR | NR | NR | Prevalence of myocarditis in 17.6% Pericardial LGE enhancement in 11.9% |
||
| Myocardial ischemic alterations | Ojha et al. [76] | 199 | NR; 57 | NR | NR | Ischemic pattern of LGE (subendocardial in coronary distribution) in 10% | |
| Pulmonary embolism | Loffi et al. [67] | 333 | 67 (IQR, 57–67); 67 | Examinations performed at admission in ED | Inadequate clinical response to high oxygen flow therapy; high D-dimer levels; signs of right ventricle dysfunction at echocardiography | PE in 33% of patients with bilateral distribution 49% of patients. 71% of the patients showed PE mainly located in lung consolidation areas | |
| Grillet et al. [68] | 100 | 66 ± 13; 70 | 9 ± 5 days after symptoms onset | 39% recovered in ICU | PE in 23% of patients; PE more frequent in ICU patients (74% vs 29%) |
||
| Pulmonary artery hypertension | Esposito et al. [27] | 761 | 69.25 (IQR, 58.01–76.87); 71 | Examinations performed at admission in ED | NR | Enlarged main pulmonary artery diameter (≥ 31 mm) is a predictor of mortality | |
| RV alterations | Planek et al. [26] | 189 | 58 (IQR, 46.75–73.25); 56 | NR | NR | Septal flattening and IVC reflux are associated with higher risk of 60-day mortality and MACE | |
| Vasculitis and epicardial adipose tissue inflammation | Feuchtner et al. [30] | 1 | 48; 0 | 1 day after COVID-19 disease diagnosis | Chest pain | Irregular coronary walls thickening and perivascular edema, defined as a perivascular fat attenuation index of >−70HU | |
| Conte et al. [11] | 192 | 60 (IQR 53–70); 54 | 3 (1.0; 6.5) days after hospital admission | 59% presented ARDS | Median epicardial adipose tissue was 95.8 (99.1; 93.0) HU and correlated with systemic inflammation | ||
| Post-acute cardiovascular complications | |||||||
| RV dysfunction | Cassar et al. [87] | 58 | 55 ± 13; 58.6 | 2–3 months and 6 months after COVID-19 infection |
Shortness of breath (43.5%) Palpitations (28.3%) Chest pain (17.4%) |
Reduction of RV function compared to controls at 2–3 months follow-up | |
| Clark et al. [81] | 19 | 26.5 (23−31); 98 | 139 days after COVID-19 infection | Abnormal ECG or transthoracic echocardiogram (48%) Chest pain (42%) Palpitations (10%) |
RVEF reduction compared to controls | ||
| Tanacli et al. [77] | 32 | 48 ± 14; 59 | 95 ± 59 days after COVID-19 infection | Fatigue (28%) Arrhythmia (28%) |
RV dysfunction in 28% with RV stroke volume significantly lower compared to controls | ||
| LV dysfunction | Kotecha et al. [85] | 148 | 64 ± 12; 70 | Median 68 days after COVID-19 infection | NR | LV dysfunction in 11% | |
| Myocardial edema | Breitbart et al. [80] | 56 | 45.7 ± 12.2; 46.4 | 70.7 ± 66 days after COVID-19 infection | Fatigue (75.0%) Chest pain (71.4%) Shortness of breath (66.1%) |
Diffuse myocardial edema in 5.3% of patients by increased T2 mapping values | |
| Huang et al. [82] | 26 | 38 (32–45); 38 | 47 (36–58) days after COVID-19 infection | Chest distress (23%) Palpitations (88%) Chest pain (12%) |
Myocardial edema in 54% of patients, involving 33% of LV segments by increased T2 signal | ||
| Tanacli et al. [77] | 32 | 48 ± 14; 59 | 95 ± 59 days after COVID-19 infection | Fatigue (28%) Arrhythmia (28%) |
Diffuse myocardial edema in 13% of patients by increased T2 mapping values | ||
| Myocarditis or pericarditis | Breitbart et al. [80] | 56 | 45.7 ± 12.2; 46.4 | 70.7 ± 66 days after COVID-19 infection | Fatigue (75.0%) Chest pain (71.4%) Shortness of breath (66.1%) |
Active myocarditis in 1.8% Non-ischemic subepicardial LGE in 10.7% |
|
| Cassar et al. [87] | 58 | 55 ± 13; 58.6 | 2–3 months and 6 months after COVID-19 disease | Shortness of breath (43.5%) Palpitations (28.3%) Chest pain (17.4%) |
Non-ischemic subepicardial LGE in 10.7% | ||
| Clark et al. [81] | 19 | 26.5 (23–31); 98 | Median 139 days after COVID-19 infection | Abnormal ECG or transthoracic echocardiogram (48%) Chest pain (42%) Palpitations (10%) |
Active myocarditis in 1 patient (2%) Non-ischemic subepicardial LGE in 8% |
||
| Huang et al. [82] | 26 | 38 (32–45); 38 | 47 (36–58) days after COVID-19 infection | Chest distress (23%) Palpitations (88%) Chest pain (12%) |
Non-ischemic subepicardial LGE in 31% | ||
| Kotecha et al. [85] | 148 | 64 ± 12; 70 | Median 68 days after COVID-19 infection | NR | Active myocarditis in 8% Non-ischemic subepicardial LGE in 26% |
||
| Tanacli et al. [77] | 32 | 48 ± 14; 59 | 95 ± 59 days after COVID-19 infection | Fatigue (28%) Arrhythmia (28%) |
Active myocarditis in 9% Pericarditis in 25% Non-ischemic subepicardial LGE in 25% |
||
| Myocardial ischemic alterations | Kotecha et al. [85] | 148 | 64 ± 12; 70 | Median 68 days after COVID-19 infection | NR | Ischemic LGE in 23% | |
| Post-vaccine complications | |||||||
| Myocarditis or pericarditis | Fronza et al. [96] | 21 | 31 ± 14; 81 | 33 (25–41) days after COVID-19 vaccination | Chest pain (100%) 3 (IQR, 1–7) days after 2nd dose (81%) or first dose (19%) of COVID-19 mRNA vaccines | Non-ischemic sub-epicardial LGE in 81% of patients; hyperintense signal on T2-weighted imaging in 79% | |
| Ammirati et al. [20] | 1 | 56; 1 | 3 days after 2nd dose of COVID-19 BNT162b2 mRNA vaccine | Chest pain | Non-ischemic subepicardial LGE involving the basal and apical segments of the infero-lateral wall, colocalized with signs suggestive for edema on T2 weighted images | ||
| D'Angelo et al. [21] | 1 | 30; 1 | 3 days after 2nd dose of COVID-19 BNT162b2 mRNA vaccine | Dyspnea and chest pain | Non-ischemic subepicardial LGE and increased myocardial and pericardial signal intensity on T2-weighted images | ||
| Abu Mouch et al. [22] | 6 | 22; 1 | 24–72 h (83%) or 16 days (17%) after 2nd dose of COVID-19 mRNA vaccines | Chest pain | Non-ischemic subepicardial LGE and increased myocardial signal intensity on T2-weighted images | ||
RV: right ventricle; LV: left ventricle; IQR: interquartile range; CI: confidence interval EF: ejection fraction; LGE: late gadolinium enhancement; MACE: major adverse cardiovascular events; IVC: inferior vena cava; ED: emergency department; NR: not reported.
4. Acute cardiovascular damage: the role of CT
Right ventricle dysfunction due to pulmonary embolism or hypertension is the most frequent alteration occurring in the acute setting [41] and CT is indicated to rule-out PE when D-dimer levels are significantly elevated. In COVID-19 patients, PE was found to affect up to 30% of hospitalized patients and to involve vessels mainly located in areas of parenchymal consolidation [67]. Additionally, vessel enlargement within or outside pulmonary opacities, dilated distal subsegmental vessels touching pleura or fissures, and the mosaic attenuation pattern were reported to be probably related to vascular inflammation, endothelial damage, micro-thrombosis, and dysfunctional vasoregulation [68].
Inflammatory thrombogenic vasculopathy leads to increased pulmonary peripheral resistance and pulmonary hypertension. Enlarged pulmonary artery on CT scan is a biomarker of pulmonary hypertension, and a main pulmonary artery diameter ≥ 31 mm was found to be an independent predictor of COVID-19 outcome [27]. This measurement would be highly reliable compared to the ratio between main pulmonary artery diameter (PA) to aorta calliper (Ao) for the risk of false negative results due to enlarged ascending aorta. The extraction of both these measurements does not require administration of contrast agent and can be obtained from standard non-contrast chest CT performed for lung assessment. On the other hand, CCTA and triple rule-out CT may show ancillary findings suggestive for right ventricle (RV) dysfunction. RV dysfunction is a common occurrence in COVID-19 pneumonia associated to severity of lung involvement and pulmonary hypertension [41]. CT findings suggestive of RV dilation/dysfunction are RV strain (RV to LV diameter ratio > 0.9), septal flattening due to increased RV pressure, and contrast agent reflux in the inferior vena cava (IVC) [26] (Fig. 2). Planek et al. [26] investigated the predictive values of these parameters in a cohort of 245 COVID-19 patients and found that septal flattening and IVC reflux were independently associated with higher risk of 60-day mortality and MACE. All these data are easy to be extracted and should be routinely reported to improve the identification of high-risk patients.
CCTA is indicated in patients with COVID-19 pneumonia with elevated troponin serum levels and non-ST elevation for the exclusion of an ACS [45], reducing useless ICAs. Moreover, because NSTEMI management depends on patients' cardiovascular risk, CCTA can improve risk stratification.
CCTA can quickly rule out or confirm the presence of clinically significant CAD and identify the features of vulnerable plaques, such as low attenuation (<30 HU), positive remodeling, spotty calcifications [69], and napkin-ring sign, becoming an essential tool for selecting patients eligible for invasive imaging [70] or for identifying coronary wall alteration due to COVID-19 related vasculitis [30].
Coronary artery calcium score (CAC) is an established biomarker for risk stratification in patients with suspected CAD and its value has been also documented in COVID-19 setting [25]. Several studies 9., 10., 16., 25. showed that elevated CAC score, specially >400 AU, is associated to poor prognosis. Female patients showed lower mortality compared to men. However, this gender mortality gap disappears in the subgroup of patients with CAC >100 AU [16], suggesting that the differences in outcomes can be at least partially explained by the gender difference in cardiovascular risk profiles and that CAC is a risk modifier. This could be explained by the biological meaning of CAC, being a biomarker of vascular senescence and atherosclerosis, therefore suggestive for higher susceptibility to endothelial damage. Additionally, CAC resulted associated to hypertension [7], a comorbidity commonly associated to COVID-19 infection and severity. Moreover, CAC revealed subclinical CAD in COVID-19 patients [9], improving risk stratification. Furthermore, calcium score of the aortic valve, known marker of aortic stenosis, and of the thoracic aorta, marker of atherosclerosis, resulted prognosticators together with CAC 10., 26..
Additionally, from the same CT examination, information about epicardial adipose tissue attenuation (EAT) (Fig. 2, Table 2), a marker of inflammation associated to plaque vulnerability [71] and COVID-19 severity 11., 72., could be extracted.
EAT attenuation ranges between −45 HU and −195 HU while it is increased in case of inflammation [73]. However, different cut-off values were found in previous studies on COVID-19 patients, probably due to methodological issues (e.g., segmentation method, analysis) and to the limited sample size 11., 44., 72..
Vascular evaluation can be combined with the assessment of lung parenchyma, providing information about COVID-19 pneumonia severity and disease stage 1., 74. and identifying other diagnosis responsible of symptoms and laboratory markers alteration.
5. Acute cardiovascular damage: the role of CMR
In May 2020, Sala et al. [31] firstly described cardiovascular involvement studied with CMR and endomyocardial biopsy in a 43-year-old woman affected by COVID-19 with severe myocardial edema and reverse Takotsubo motion pattern, diagnosed as acute myocarditis at histology. Initial reports on COVID-19 patients with acute myocardial injury that underwent CMR 33., 75. showed myocarditis as the most frequent finding, with diffuse myocardial edema and minimal or negligible LGE, followed by Takotsubo cardiomyopathy.
These initial data were confirmed by Ojha et al. [76] that firstly conducted a meta-analysis on COVID-19 patients who underwent CMR. The most common diagnosis (40.2%) in a total of 34 studies and 199 patients was myocarditis, while CMR was negative in 21% of cases, a finding partially due to the time gap between symptoms onset and CMR, which reached up to 71 days. The most common findings were increased T1 (73%) and T2 (63%) myocardial mapping values, with LGE being less common (43%). When present, LGE had non-ischemic pattern involving a few segments with subepicardial distribution (81%) in the inferior/infero-lateral basal segments of the left ventricle.
Kato et al. [28] published an updated meta-analysis in 2022 that included 10.462 COVID-19 patients who underwent CMR and found a minimal reduction in left (−2.84%) and right (−2.69%) ventricular ejection fraction in COVID-19 patients compared to controls, with LV LGE abnormalities in 27.5%, pericardial involvement in 11.9%, T1 mapping alteration in 39.5%, T2 mapping or T2-weighted sequences alterations in 38.1%, with a prevalence of myocarditis of 17.6%.
These confirm that LV involvement is common in COVID-19 patients, CMR is useful in detecting cardiac abnormalities, and myocarditis is the most common finding. It was reported an 18-fold increased risk to develop myocarditis in COVID-19 [13], independently of patients' age, related to multisystem inflammatory syndrome [13].
Advanced CMR imaging techniques (i.e. mapping techniques) outperform “traditional” techniques such as LGE in COVID-19 patients (Fig. 3 ) due to the absent or limited necrosis 77., 78..
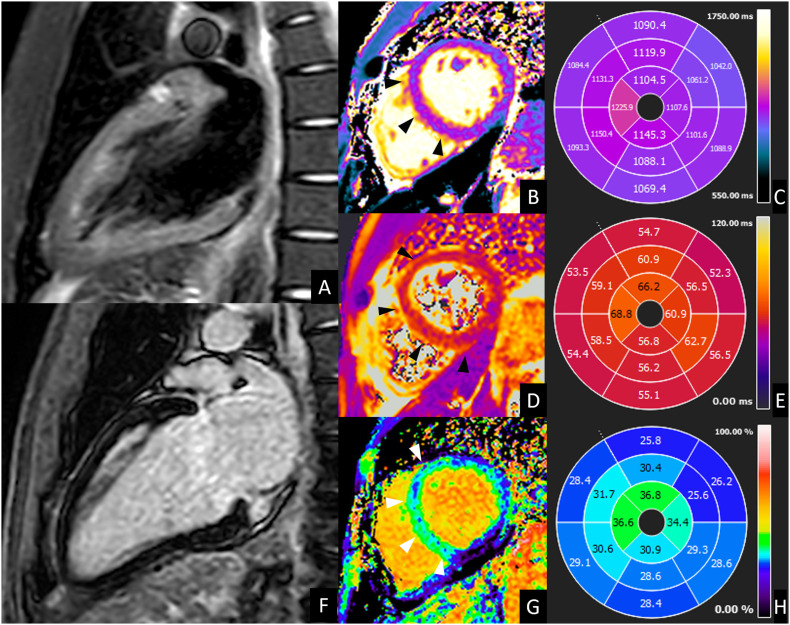
Fig. 3.
CMR of acute left ventricle dysfunction during COVID-19. A 39-year-old male presented to the emergency department for fever, caught and dyspnea. Nasopharyngeal swab was positive for SARS-CoV 2 infection. Laboratory tests showed increased troponin T level (42,6 ng/mL, normal value <14 ng/mL) and a moderate depression of left ventricle systolic function (ejection fraction <40%) was documented at echocardiography. CMR was performed 8 days later and showed a slight diffuse hypokinesia of left ventricle (LV ejection fraction 51%) with absent focal edema on short-tau inversion recovery images (A) and absent LGE (B), but diffuse alteration of T2 values (B) (56 ms, normal value ≤ 50 ms; C), of native T1 (D) (1084 ms, normal value ≤ 1045 ms E) and of extracellular volume fraction (G) (28%, normal value ≤ 27%; H) with higher values in mid-apical septum and mid-apical anterior wall (arrows in B, D and G). These findings were suggestive for acute myocarditis according to 2018 Lake Louise criteria. Endomyocardial biopsy confirmed these findings, showing diffuse edema and macrophage infiltrate. After 1 month, the patient was discharged with complete resolution of cardiac alteration.
6. Cardiac post-acute COVID-19 syndrome
Cardiac post-acute COVID-19 syndrome (cPACS) is generally defined as the persistence of COVID-19 cardiovascular symptoms or signs for >3–4 weeks after recovery, mainly including lasting chest pain, shortness of breath, palpitations, or troponin levels elevation. Mechanisms responsible for persistence of post-acute cardiac damage are still poorly understood. Possible explanations are chronic inflammatory response for persistent viral reservoirs, autoimmune inflammation due to molecular mimicry, and chronic thromboembolic pulmonary hypertension [19]. In cPACS patients with suspected myocardial involvement, CMR is highly recommended [79] to exclude ischemia, preexisting cardiomyopathies and to assess COVID-19 associated myocardial alteration, including myocardial inflammation, scar, and pericardial effusion. Persistent troponin rise was mainly associated to active myocardial inflammation and reported in 14% to 54% of screened cPACS patients 77., 80., 81., 82. with higher prevalence in patients with severe disease [82]. Edema was in fewer cases associated to LGE (8–31%) 80., 81., 82., 83. mainly with non-ischemic pattern involving the infero-lateral wall. Ischemic LGE has been reported less frequently. In a case control study on 90 hospitalized patients with troponin-positive COVID-19 infection [84], CMR performed 2 months after recovery showed post-myocarditis scar in 34% of cases and post-ischemic scar in 17% of cases. Notably, 36% of patients showed adenosine-induced regional perfusion defects. Similar findings were reported by Kotecha et al. [85] on a large series (148) of cPACS patients, with myocarditis-like scar involving three or less myocardial segments as the most frequent finding (26%) (Fig. 4 ), followed by post ischemic scar (19%); 26% of patients had inducible ischemia. Most patients with inducible ischemia or ischemic scar (66%) had no previous history of coronary artery disease. Despite in cPACS patients LV function seem to be preserved, subclinical alterations were reported in term of strain reduction within 2 and 4 months after moderate to severe COVID-19 infection, respectively [86], mainly associated to edema at early stage and LGE in late stage 86., 87.. Notably, right ventricle dysfunction has been reported as a possible indirect effect of COVID-19 related lung disease and improves over time returning to normality 6 months after recovery 81., 82., 87..
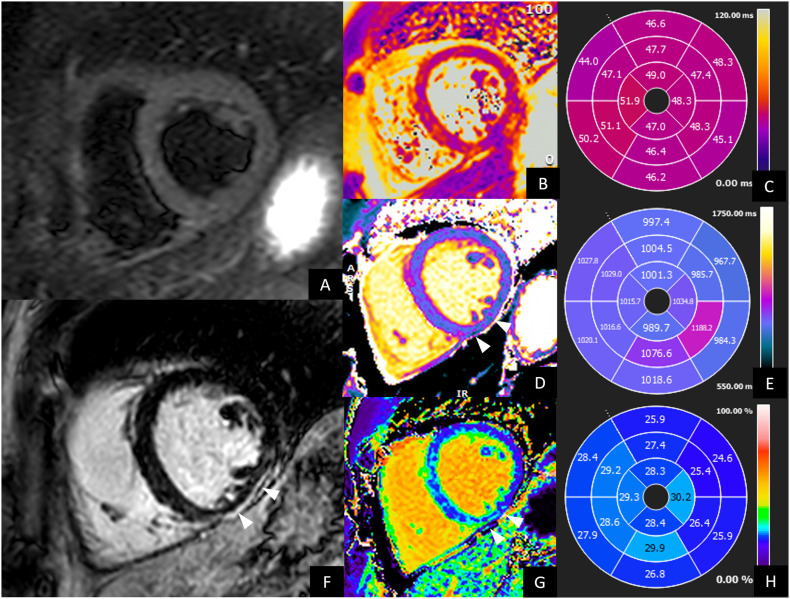
Fig. 4.
CMR of a 33-year-old male with persistent palpitation and tachycardia especially during physical activity at 1 year after COVID-19 recovery. Holter ECG documented frequent ectopic ventricular beats. Hence, CMR was performed. CMR showed preserved left and right ventricle ejection fraction, without wall motion alteration. No edema was evident on short-tau inversion recovery images (A) neither on T2 maps (B and C). LGE images (F) showed a thin subepicardial scar on the inferior mid-ventricular wall, associated to increased native T1 (arrows in D, values in E) and ECV values (arrows in H, values in G). These findings were suggestive for post-myocarditis scar.
In asymptomatic patients recovered from mild-to-moderate COVID-19 infection, there is no increased risk in long-term cardiac sequelae. In a prospective study, no differences were identified at CMR performed 6 months post-infection between 74 asymptomatic healthcare workers and age, sex, and ethnicity matched controls [88]. Similarly, Petersen et al. [89] found non-significant CMR alterations in a population of 443 asymptomatic post-COVID patients compared to 1380 matched controls.
7. Cardiac Imaging findings after SARS-CoV-2 vaccine
The COVID-19 vaccines have determined a substantial worldwide decline in morbidity and mortality, with reduction of hospitalization related to severe disease. All approved vaccines have shown to provide benefits that obscure their potential risks across different age groups [90].
Since the beginning of the vaccination program, more reports have been raising concerns for the association of myopericarditis to different types of COVID-19 vaccines 20., 21., 22..
In pre-COVID-19 era, vaccine-related myocarditis or pericarditis had a reported incidence of 0.1%, according to Vaccine Adverse Event Reporting System (VAERS) files collected between 1990 and 2018. Of these, 79% of cases were observed in males [91]. Since COVID-19 vaccines rollout, a rate of 12.6 cases of myocarditis per million doses has been related to the second vaccine shot, in individuals aged between 12 and 39 years.
However, VAERS data collection system cannot be used to determine the real incidence of vaccine adverse events, since it is primarily a safety signal detection and hypothesis-generating system [92]. Certainly, myocarditis has been described as the most frequent vaccine-related adverse event occurring mainly in patients having smallpox vaccination rather than in patients receiving vaccines for single-stranded RNA viruses [93].
However, an association between mRNA COVID-19 vaccines (mRNA-1273 [Moderna] and BNT162b2 [Pfizer-BioNTech]) myocarditis and pericarditis cases has been found, particularly after the second shot of vaccination [94].
Most of the reported cases presented abnormal ECG with ST elevation and elevated cardiac troponin peaking three days after vaccination, usually within 14 days of COVID-19 vaccination [95].
Most subjects had rapid recovery and high antibody levels for SARS-CoV-2 spike protein suggesting effective immunization. Echocardiogram was abnormal in only 40% of cases, with a minimal percentage of patients presenting reduced left ventricular ejection fraction [94]. Conversely, CMR showed abnormalities in all tested patients, depicting findings such as myocardial edema and subepicardial late gadolinium enhancement suggestive of myocarditis.
Recently, Fronza et al. showed that COVID-19 vaccine-related myocarditis has different imaging patterns compared to other causes of myocarditis such as COVID-19-related myocarditis, independently from patients' age or sex and from interval between symptoms onset and imaging [96].
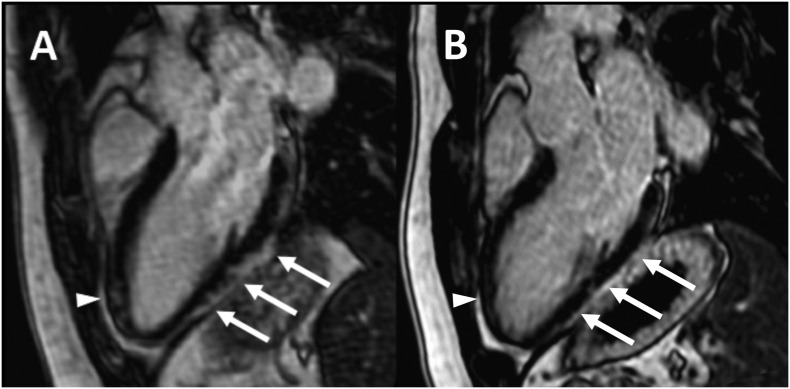
In particular, the authors found that, in vaccine-related myocarditis, right and left ventricular ejection fraction, strain values, and myocardial native T1-value are less altered, while LGE is less extensive and mainly involves the infero-lateral segments compared to other causes of myocarditis (Fig. 5A).
Fig. 5.
CMR of a 30-year-old male with COVID-19 vaccine-related myocarditis. LGE imaging performed along three-chambers view 5 days after the onset of patient's symptoms (A) shows subepicardial enhancement along the infero-lateral myocardial segments (arrows) with minimal involvement of the anterior wall in the apical region (arrowhead). Cardiac MRI performed 3-months later (B) shows almost complete resolution of myocardial LGE in the same segments.
The etiology of myocardial inflammation following COVID-19 vaccination is still unknown. Different mechanisms have been proposed: i) a delayed hypersensitivity reaction, with sensitization occurring after the first COVID-19 vaccine shot; ii) a mechanism of molecular mimicry between the SARS-CoV-2 spike proteins, encoded by the mRNA vaccines, and cardiomyocyte antigens, which may provoke an immune response in predisposed subjects; iii) a systemic inflammatory response triggered by the antigenic mRNA, leading to myocardial inflammation.
However, almost all reports confirm that symptoms resolution, as well as diagnostic markers and imaging findings normalization, is rapid either with or without treatment (Fig. 5B).
Clinicians should be aware of the existing risk of myocarditis and pericarditis related to COVID-19 vaccination, especially in young male individuals presenting with chest pain shortly after vaccination.
8. Conclusions
Advanced cardiac imaging in COVID-19 provides effective and non-invasive characterization of COVID-19 related cardiovascular manifestations and improves risk stratification, minimizing the use of unnecessary and invasive procedures and speeding-up the diagnostic pathways.
The choice of the most appropriate imaging modality and acquisition protocol needs to be tailored to patient's clinical features and suspicion. CT angiography allows accurately characterizing vessels involvement. Moreover, independently by the selected protocol, CT can provide a multiplicity of ancillary information useful for a more comprehensive patients' characterization and risk stratification. CMR has the advantage of enabling accurate myocardial tissue characterization, being able to exclude preexisting cardiomyopathies and to identify subclinical cardiac injury, myocardial inflammation, and abnormalities potentially affecting quality of life or increasing risk of future events.
References
- 1.Esposito A., Palmisano A., Cao R., Rancoita P., Landoni G., Grippaldi D., et al. Quantitative assessment of lung involvement on chest CT at admission: impact on hypoxia and outcome in COVID-19 patients. Clin Imaging. 2021;77:194–201. doi: 10.1016/J.CLINIMAG.2021.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giustino G., Croft L.B., Stefanini G.G., Bragato R., Silbiger J.J., Vicenzi M., et al. Characterization of myocardial injury in patients with COVID-19. J Am Coll Cardiol. 2020;76:2043–2055. doi: 10.1016/J.JACC.2020.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chung M.K., Zidar D.A., Bristow M.R., Cameron S.J., Chan T., Harding C.V., et al. COVID-19 and cardiovascular disease: from bench to bedside. Circ Res. 2021;128:1214–1236. doi: 10.1161/CIRCRESAHA.121.317997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bansal M. Cardiovascular disease and COVID-19. Diabetes Metab Syndr Clin Res Rev. 2020;14:247–250. doi: 10.1016/J.DSX.2020.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lala A., Johnson K.W., Januzzi J.L., Russak A.J., Paranjpe I., Richter F., et al. Prevalence and impact of myocardial injury in patients hospitalized with COVID-19 infection. J Am Coll Cardiol. 2020;76:533–546. doi: 10.1016/J.JACC.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.An W., Kang J.S., Wang Q., Kim T.E. Cardiac biomarkers and COVID-19: a systematic review and meta-analysis. J Infect Public Health. 2021;14:1191–1197. doi: 10.1016/j.jiph.2021.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cereda A., Toselli M., Palmisano A., Vignale D., Khokhar A., Campo G., et al. Coronary calcium score as a predictor of outcomes in the hypertensive Covid-19 population: results from the italian (S) Core-Covid-19 registry. Hypertens Res. 2022;45:333–343. doi: 10.1038/S41440-021-00798-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sticchi A., Cereda A., Toselli M., Esposito A., Palmisano A., Vignale D., et al. Diabetes and mortality in patients with COVID-19: are we missing the link? Anatol J Cardiol. 2021;25:376–379. doi: 10.5152/ANATOLJCARDIOL.2021.29132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scoccia A., Gallone G., Cereda A., Palmisano A., Vignale D., Leone R., et al. Impact of clinical and subclinical coronary artery disease as assessed by coronary artery calcium in COVID-19. Atherosclerosis. 2021;328:136–143. doi: 10.1016/J.ATHEROSCLEROSIS.2021.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giannini F., Toselli M., Palmisano A., Cereda A., Vignale D., Leone R., et al. Coronary and total thoracic calcium scores predict mortality and provides pathophysiologic insights in COVID-19 patients. J Cardiovasc Comput Tomogr. 2021;15:421–430. doi: 10.1016/J.JCCT.2021.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conte C., Esposito A., de Lorenzo R., di Filippo L., Palmisano A., Vignale D., et al. Epicardial adipose tissue characteristics, obesity and clinical outcomes in COVID-19: a post-hoc analysis of a prospective cohort study. Nutr Metab Cardiovasc Dis. 2021;31:2156–2164. doi: 10.1016/J.NUMECD.2021.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo T., Fan Y., Chen M., Wu X., Zhang L., He T., et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:811–818. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boehmer T.K., Kompaniyets L., Lavery A.M., Hsu J., Ko J.Y., Yusuf H., et al. Association between COVID-19 and myocarditis using hospital-based administrative data — United States, March 2020-January 2021. MMWR Recomm Rep. 2021;70:1228–1232. doi: 10.15585/mmwr.mm7035e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jaiswal V., Sarfraz Z., Sarfraz A., Mukherjee D., Batra N., Hitawala G. COVID-19 infection and myocarditis: a state-of-the-art systematic review. J Prim Care Community Health. 2021:12. doi: 10.1177/21501327211056800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Godeau D., Petit A., Richard I., Roquelaure Y., Descatha A. Return-to-work, disabilities and occupational health in the age of covid-19. Scand J Work Environ Health. 2021;47:408–409. doi: 10.5271/sjweh.3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cereda A., Toselli M., Palmisano A., Vignale D., Leone R., Nicoletti V., et al. The hidden interplay between sex and COVID-19 mortality: the role of cardiovascular calcification. Geroscience. 2021;43:2215–2229. doi: 10.1007/s11357-021-00409-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hendren N.S., Drazner M.H., Bozkurt B., Cooper L.T. Description and proposed management of the acute COVID-19 cardiovascular syndrome. Circulation. 2020;141:1903–1914. doi: 10.1161/CIRCULATIONAHA.120.047349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Augoustides J.G. Cardiovascular consequences and considerations of coronavirus infection – perspectives for the cardiothoracic anesthesiologist and intensivist during the coronavirus crisis. J Cardiothorac Vasc Anesth. 2020;34:1713–1716. doi: 10.1053/J.JVCA.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raman B., Bluemke D.A., Lüscher T.F., Neubauer S. Long COVID: post-acute sequelae of COVID-19 with a cardiovascular focus. Eur Heart J. 2022;43:1157–1172. doi: 10.1093/EURHEARTJ/EHAC031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ammirati E., Cavalotti C., Milazzo A., Pedrotti P., Soriano F., Schroeder J.W., et al. Temporal relation between second dose BNT162b2 mRNA Covid-19 vaccine and cardiac involvement in a patient with previous SARS-COV-2 infection. Int J Cardiol Heart Vasc. 2021;34 doi: 10.1016/J.IJCHA.2021.100774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.D’Angelo T., Cattafi A., Carerj M.L., Booz C., Ascenti G., Cicero G., et al. Myocarditis after SARS-CoV-2 vaccination: a vaccine-induced Reaction? Can J Cardiol. 2021;37:1665–1667. doi: 10.1016/J.CJCA.2021.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abu Mouch S., Roguin A., Hellou E., Ishai A., Shoshan U., Mahamid L., et al. Myocarditis following COVID-19 mRNA vaccination. Vaccine. 2021;39:3790–3793. doi: 10.1016/J.VACCINE.2021.05.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palmisano A., Vignale D., Tadic M., Moroni F., de Stefano D., Gatti M., et al. Myocardial late contrast enhancement CT in troponin-positive acute chest pain syndrome. Radiology. 2022;302:545–553. doi: 10.1148/RADIOL.211288. [DOI] [PubMed] [Google Scholar]
- 24.Pontone G., Baggiano A., Conte E., Teruzzi G., Cosentino N., Campodonico J., et al. “Quadruple rule-out” with computed tomography in a COVID-19 patient with equivocal acute coronary syndrome presentation. JACC Cardiovasc Imaging. 2020;13:1854–1856. doi: 10.1016/J.JCMG.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dillinger J.G., Benmessaoud F.A., Pezel T., Voicu S., Sideris G., Chergui N., et al. Coronary artery calcification and complications in patients with COVID-19. JACC Cardiovasc Imaging. 2020;13:2468–2470. doi: 10.1016/J.JCMG.2020.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Planek M.I.C., Ruge M., du Fay de Lavallaz J.M., Kyung S.B., Gomez J.M.D., Suboc T.M. Cardiovascular findings on chest computed tomography associated with COVID-19 adverse clinical outcomes. Am Heart J Plus: Cardiol Res Pract. 2021;11 doi: 10.1016/J.AHJO.2021.100052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Esposito A., Palmisano A., Toselli M., Vignale D., Cereda A., Rancoita P.M.V., et al. Chest CT-derived pulmonary artery enlargement at the admission predicts overall survival in COVID-19 patients: insight from 1461 consecutive patients in Italy. Eur Radiol. 2021;31:4031–4041. doi: 10.1007/S00330-020-07622-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kato S., Azuma M., Fukui K., Kodama S., Nakayama N., Kitamura H. Cardiac involvement in coronavirus disease 2019 assessed by cardiac magnetic resonance imaging: a meta-analysis. Heart Vessels. 2022 doi: 10.1007/S00380-022-02055-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bangalore S., Sharma A., Slotwiner A., Yatskar L., Harari R., Shah B., et al. ST-segment elevation in patients with Covid-19 - a case series. N Engl J Med. 2020;382:2478–2480. doi: 10.1056/NEJMC2009020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feuchtner G.M., Barbieri F., Luger A., Skalla E., Kountchev J., Widmann G., et al. Myocardial injury in COVID-19: the role of coronary computed tomography angiography (CTA) J Cardiovasc Comput Tomogr. 2021;15:e3–e6. doi: 10.1016/J.JCCT.2020.07.002/ATTACHMENT/FA1612AA-253C-4BCC-84B4-ED5E4A0B596E/MMC2.MP4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sala S., Peretto G., Gramegna M., Palmisano A., Villatore A., Vignale D., et al. Acute myocarditis presenting as a reverse tako-tsubo syndrome in a patient with SARS-CoV-2 respiratory infection. Eur Heart J. 2020;41:1861–1862. doi: 10.1093/EURHEARTJ/EHAA286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sala S., Peretto G., de Luca G., Farina N., Campochiaro C., Tresoldi M., et al. Low prevalence of arrhythmias in clinically stable COVID-19 patients. Pacing Clin Electrophysiol. 2020;43:891–893. doi: 10.1111/PACE.13987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peretto G., Villatore A., Rizzo S., Esposito A., de Luca G., Palmisano A., et al. The Spectrum of COVID-19-associated myocarditis: a patient-tailored multidisciplinary approach. J Clin Med. 2021;10 doi: 10.3390/JCM10091974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mazzone P., Peretto G., Radinovic A., Limite L.R., Marzi A., Sala S. The COVID-19 challenge to cardiac electrophysiologists: optimizing resources at a referral center. J Interv Card Electrophysiol. 2020:59. doi: 10.1007/S10840-020-00761-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dherange P., Lang J., Qian P., Oberfeld B., Sauer W.H., Koplan B., et al. Arrhythmias and COVID-19: a review. JACC Clin Electrophysiol. 2020;6:1193–1204. doi: 10.1016/J.JACEP.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peretto G., Sala S., Caforio A.L.P. Acute myocardial injury, MINOCA, or myocarditis? Improving characterization of coronavirus-associated myocardial involvement. Eur Heart J. 2020;41:2124–2125. doi: 10.1093/EURHEARTJ/EHAA396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tavazzi G., Pellegrini C., Maurelli M., Belliato M., Sciutti F., Bottazzi A., et al. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur J Heart Fail. 2020;22:911–915. doi: 10.1002/ejhf.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baggiano A., Rizzo S., Basso C., Pontone G. A patient with rapid worsening dyspnoea during Covid-19 pandemic. Eur Heart J. 2021;42:717–718. doi: 10.1093/EURHEARTJ/EHAA988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Halushka M.K., Vander Heide R.S. Myocarditis is rare in COVID-19 autopsies: cardiovascular findings across 277 postmortem examinations. Cardiovasc Pathol. 2021;50 doi: 10.1016/J.CARPATH.2020.107300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beitzke D., Salgado R., Francone M., Kreitner K.F., Natale L., Bremerich J., et al. Cardiac imaging procedures and the COVID-19 pandemic: recommendations of the european Society of Cardiovascular Radiology (ESCR) Int J Cardiovasc Imaging. 2020;36:1801–1810. doi: 10.1007/S10554-020-01892-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bonnemain J., Ltaief Z., Liaudet L. The right ventricle in COVID-19. J Clin Med. 2021;10:2535. doi: 10.3390/JCM10122535. 2021;10:2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dweck M.R., Bularga A., Hahn R.T., Bing R., Lee K.K., Chapman A.R., et al. Global evaluation of echocardiography in patients with COVID-19. Eur Heart J Cardiovasc Imaging. 2020;21:949–958. doi: 10.1093/EHJCI/JEAA178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hothi S.S., Jiang J., Steeds R.P., Moody W.E. Utility of non-invasive cardiac imaging assessment in coronavirus disease 2019. Front Cardiovasc Med. 2021;8:329. doi: 10.3389/fcvm.2021.663864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Onnis C., Muscogiuri G., Paolo Bassareo P., Cau R., Mannelli L., Cadeddu C. Non-invasive coronary imaging in patients with COVID-19: a narrative review. Eur J Radiol. 2022:149. doi: 10.1016/j.ejrad.2022.110188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Skulstad H., Cosyns B., Popescu B.A., Galderisi M., di Salvo G., Donal E., et al. COVID-19 pandemic and cardiac imaging: EACVI recommendations on precautions, indications, prioritization, and protection for patients and healthcare personnel. Eur Heart J Cardiovasc Imaging. 2020;21:592–598. doi: 10.1093/ehjci/jeaa072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cosyns B., Lochy S., Luchian M.L., Gimelli A., Pontone G., Allard S.D., et al. The role of cardiovascular imaging for myocardial injury in hospitalized COVID-19 patients. Eur Heart J Cardiovasc Imaging. 2020;21:709–714. doi: 10.1093/EHJCI/JEAA136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stefanini G.G., Montorfano M., Trabattoni D., Andreini D., Ferrante G., Ancona M., et al. ST-elevation myocardial infarction in patients with COVID-19: clinical and angiographic outcomes. Circulation. 2020;141:2113–2116. doi: 10.1161/CIRCULATIONAHA.120.047525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koch V., Gruenewald L.D., Albrecht M.H., Eichler K., Gruber-Rouh T., Yel I., et al. Lung opacity and coronary artery calcium score: a combined tool for risk stratification and outcome prediction in COVID-19 patients. Acad Radiol. 2022;29:861–870. doi: 10.1016/J.ACRA.2022.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boyce C.J., Pickhardt P.J., Kim D.H., Taylor A.J., Winter T.C., Bruce R.J., et al. Hepatic steatosis (fatty liver disease) in asymptomatic adults identified by unenhanced low-dose CT. AJR Am J Roentgenol. 2010;194:623–628. doi: 10.2214/AJR.09.2590. [DOI] [PubMed] [Google Scholar]
- 50.Derstine B.A., Holcombe S.A., Ross B.E., Wang N.C., Su G.L., Wang S.C. Skeletal muscle cutoff values for sarcopenia diagnosis using T10 to L5 measurements in a healthy US population. Sci Rep. 2018:8. doi: 10.1038/S41598-018-29825-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Palmisano A., Esposito A., Gnasso C., Nicoletti V., Leone R., de Lorenzo R. Myosteatosis significantly predicts persistent dyspnea and mobility problems in COVID-19 survivors. Front Nutr. 2022:9. doi: 10.3389/FNUT.2022.846901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Esposito A., Palmisano A., Barbera M., Vignale D., Benedetti G., Spoladore R., et al. Cardiac computed tomography in troponin-positive chest pain: sometimes the answer lies in the late iodine enhancement or extracellular volume fraction map. JACC Cardiovasc Imaging. 2019;12:745–748. doi: 10.1016/J.JCMG.2018.08.013. [DOI] [PubMed] [Google Scholar]
- 53.Scully P.R., Bastarrika G., Moon J.C., Treibel T.A. Myocardial extracellular volume quantification by cardiovascular magnetic resonance and computed tomography. Curr Cardiol Rep. 2018:20. doi: 10.1007/S11886-018-0961-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Palmisano A., Vignale D., Tadic M., Moroni F., de Stefano D., Gatti M., et al. Myocardial late contrast enhancement CT in TroponinPositive acute chest pain syndrome. Radiology. 2022;302:545–553. doi: 10.1148/radiol.211288. [DOI] [PubMed] [Google Scholar]
- 55.Palmisano A., Vignale D., Benedetti G., del Maschio A., de Cobelli F., Esposito A. Late iodine enhancement cardiac computed tomography for detection of myocardial scars: impact of experience in the clinical practice. Radiologia Medica. 2020;125:128–136. doi: 10.1007/s11547-019-01108-7. [DOI] [PubMed] [Google Scholar]
- 56.Esposito A., Palmisano A., Barbera M., Vignale D., Benedetti G., Spoladore R., et al. Cardiac computed tomography in troponin-positive chest pain: sometimes the answer lies in the late iodine enhancement or extracellular volume fraction map. JACC Cardiovasc Imaging. 2019;12:745–748. doi: 10.1016/j.jcmg.2018.08.013. [DOI] [PubMed] [Google Scholar]
- 57.Esposito A., Palmisano A., Antunes S., Colantoni C., Rancoita P.M.V., Vignale D., et al. Assessment of remote myocardium heterogeneity in patients with ventricular tachycardia using texture analysis of late iodine enhancement (LIE) cardiac computed tomography (cCT) images. Mol Imaging Biol. 2018;20:816–825. doi: 10.1007/s11307-018-1175-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Palmisano A., Vignale D., Peretto G., Busnardo E., Calcagno C., Campochiaro C., et al. Hybrid FDG-PET/MR or FDG-PET/CT to detect disease activity in patients with persisting arrhythmias after myocarditis. JACC Cardiovasc Imaging. 2021;14:288–292. doi: 10.1016/j.jcmg.2020.03.009. [DOI] [PubMed] [Google Scholar]
- 59.Esposito A., Palmisano A., Antunes S., Maccabelli G., Colantoni C., Rancoita P.M.V., et al. Cardiac CT with delayed enhancement in the characterization of ventricular tachycardia structural substrate: relationship between CT-segmented scar and electro-anatomic mapping. JACC Cardiovasc Imaging. 2016;9:822–832. doi: 10.1016/j.jcmg.2015.10.024. [DOI] [PubMed] [Google Scholar]
- 60.Collet J.P., Thiele H., Barbato E., Bauersachs J., Dendale P., Edvardsen T., et al. 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2021;42:1289–1367. doi: 10.1093/EURHEARTJ/EHAA575. [DOI] [PubMed] [Google Scholar]
- 61.Skulstad H., Cosyns B., Popescu B.A., Galderisi M., di Salvo G., Donal E., et al. COVID-19 pandemic and cardiac imaging: EACVI recommendations on precautions, indications, prioritization, and protection for patients and healthcare personnel. Eur Heart J Cardiovasc Imaging. 2020;21:592–598. doi: 10.1093/EHJCI/JEAA072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.D’Angelo T., Grigoratos C., Mazziotti S., Bratis K., Pathan F., Blandino A. High-throughput gadobutrol-enhanced CMR: a time and dose optimization study. J Cardiovasc Magn Reson. 2017:19. doi: 10.1186/S12968-017-0400-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Catapano F., Marchitelli L., Cundari G., Cilia F., Mancuso G., Pambianchi G., et al. Role of advanced imaging in COVID-19 cardiovascular complications. Insights Into Imaging. 2021;12:1–13. doi: 10.1186/S13244-021-00973-Z/FIGURES/8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ferreira V.M., Schulz-Menger J., Holmvang G., Kramer C.M., Carbone I., Sechtem U., et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol. 2018;72:3158–3176. doi: 10.1016/J.JACC.2018.09.072. [DOI] [PubMed] [Google Scholar]
- 65.Eitel I., von Knobelsdorff-Brenkenhoff F., Bernhardt P., Carbone I., Muellerleile K., Aldrovandi A., et al. Clinical characteristics and cardiovascular magnetic resonance findings in stress (takotsubo) cardiomyopathy. JAMA. 2011;306:277–286. doi: 10.1001/JAMA.2011.992. [DOI] [PubMed] [Google Scholar]
- 66.Gatti M., Carisio A., D’Angelo T., Darvizeh F., Dell’Aversana S., Tore D., et al. Cardiovascular magnetic resonance in myocardial infarction with non-obstructive coronary arteries patients: a review. World J Cardiol. 2020;12:248–261. doi: 10.4330/WJC.V12.I6.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Loffi M., Regazzoni V., Toselli M., Cereda A., Palmisano A., Vignale D. Incidence and characterization of acute pulmonary embolism in patients with SARS-CoV-2 pneumonia: a multicenter Italian experience. PLoS One. 2021:16. doi: 10.1371/JOURNAL.PONE.0245565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Grillet F., Behr J., Calame P., Aubry S., Delabrousse E. Acute pulmonary embolism associated with COVID-19 pneumonia detected with pulmonary CT angiography. Radiology. 2020;296:E186–E188. doi: 10.1148/RADIOL.2020201544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cury R.C., Abbara S., Achenbach S., Agatston A., Berman D.S., Budoff M.J. CAD-RADS(TM) coronary artery disease - reporting and data system. An expert consensus document of the Society of Cardiovascular Computed Tomography (SCCT), the American College of Radiology (ACR) and the North American Society for Cardiovascular Imaging (NASCI). Endorsed by the American College of Cardiology. J Cardiovasc Comput Tomogr. 2016;10:269–281. doi: 10.1016/J.JCCT.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 70.Licu R.-A., Blîndu E., Opincariu D., Benedek T. Vulnerable plaques producing an acute coronary syndrome exhibit a different CT phenotype than those that remain silent. J Cardiovasc Emerg. 2020;6:26–34. doi: 10.2478/JCE-2020-0008. [DOI] [Google Scholar]
- 71.Iacobellis G. Epicardial adipose tissue in contemporary cardiology. Nat Rev Cardiol. 2022 doi: 10.1038/S41569-022-00679-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Iacobellis G., Secchi F., Capitanio G., Basilico S., Schiaffino S., Boveri S., et al. Epicardial fat inflammation in severe COVID-19. Obesity. 2020;28:2260–2262. doi: 10.1002/oby.23019. [DOI] [PubMed] [Google Scholar]
- 73.Iacobellis G. Epicardial adipose tissue in contemporary cardiology. Nat Rev Cardiol. 2022 doi: 10.1038/s41569-022-00679-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Palmisano A., Scotti G.M., Ippolito D., Morelli M.J., Vignale D., Gandola D., et al. Chest CT in the emergency department for suspected COVID-19 pneumonia. Radiol Med. 2021;126:498–502. doi: 10.1007/S11547-020-01302-Y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Esposito A., Palmisano A., Natale L., Ligabue G., Peretto G., Lovato L., et al. Cardiac magnetic resonance characterization of myocarditis-like acute cardiac syndrome in COVID-19. JACC Cardiovasc Imaging. 2020;13:2462–2465. doi: 10.1016/J.JCMG.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ojha V., Verma M., Pandey N.N., Mani A., Malhi A.S., Kumar S., et al. Cardiac magnetic resonance imaging in coronavirus disease 2019 (COVID-19): a systematic review of cardiac magnetic resonance imaging findings in 199 patients. J Thorac Imaging. 2021;36:73–83. doi: 10.1097/RTI.0000000000000574. [DOI] [PubMed] [Google Scholar]
- 77.Tanacli R., Doeblin P., Götze C., Zieschang V., Faragli A., Stehning C. COVID-19 vs. classical myocarditis associated myocardial injury evaluated by cardiac magnetic resonance and endomyocardial biopsy. Front Cardiovasc Med. 2021;0:1825. doi: 10.3389/FCVM.2021.737257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Haberka M., Rajewska-Tabor J., Wojtowicz D., Jankowska A., Miszalski-Jamka K., Janus M., et al. Perimyocardial injury specific for SARS-CoV-2-induced myocarditis in comparison with non-COVID-19 myocarditis: a multicenter CMR study. JACC Cardiovasc Imaging. 2022;15:705–707. doi: 10.1016/J.JCMG.2021.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gluckman T.J., Bhave N.M., Allen L.A., Chung E.H., Spatz E.S., Ammirati E., et al. ACC expert consensus decision pathway on cardiovascular sequelae of COVID-19 in adults: myocarditis and other myocardial involvement, post-acute sequelae of SARS-CoV-2 infection, and return to play: a report of the american College of Cardiology Solution set Oversight Committee. J Am Coll Cardiol. 2022;2022 doi: 10.1016/J.JACC.2022.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Breitbart P., Koch A., Schmidt M., Magedanz A., Lindhoff-Last E., Voigtländer T., et al. Clinical and cardiac magnetic resonance findings in post-COVID patients referred for suspected myocarditis. Clin Res Cardiol. 2021;110:1832–1840. doi: 10.1007/S00392-021-01929-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Clark D.E., Dendy J.M., Li D.L., Crum K., Dixon D., George-Durrett K., et al. Cardiovascular magnetic resonance evaluation of soldiers after recovery from symptomatic SARS-CoV-2 infection: a case-control study of cardiovascular post-acute sequelae of SARS-CoV-2 infection (CV PASC) J Cardiovasc Magn Reson. 2021;23 doi: 10.1186/S12968-021-00798-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Huang L., Zhao P., Tang D., Zhu T., Han R., Zhan C., et al. Cardiac involvement in patients recovered from COVID-2019 identified using magnetic resonance imaging. JACC Cardiovasc Imaging. 2020;13:2330–2339. doi: 10.1016/J.JCMG.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang H., Li R., Zhou Z., Jiang H., Yan Z., Tao X. Cardiac involvement in COVID-19 patients: mid-term follow up by cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2021:23. doi: 10.1186/S12968-021-00710-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Thornton G.D., Shetye A., Knight D.S., Knott K., Artico J., Kurdi H. Myocardial perfusion imaging after severe COVID-19 infection demonstrates regional ischemia rather than global blood flow reduction. Front Cardiovasc Med. 2021:8. doi: 10.3389/FCVM.2021.764599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kotecha T., Knight D.S., Razvi Y., Kumar K., Vimalesvaran K., Thornton G., et al. Patterns of myocardial injury in recovered troponin-positive COVID-19 patients assessed by cardiovascular magnetic resonance. Eur Heart J. 2021;42:1866–1878. doi: 10.1093/EURHEARTJ/EHAB075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li X., Wang H., Zhao R., Wang T., Zhu Y., Qian Y., et al. Elevated extracellular volume fraction and reduced global longitudinal strains in participants recovered from COVID-19 without clinical cardiac findings. Radiology. 2021;299:E230–E240. doi: 10.1148/RADIOL.2021203998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cassar M.P., Tunnicliffe E.M., Petousi N., Lewandowski A.J., Xie C., Mahmod M. Symptom persistence despite improvement in cardiopulmonary health - insights from longitudinal CMR, CPET and lung function testing post-COVID-19. EClinicalMedicine. 2021:41. doi: 10.1016/J.ECLINM.2021.101159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Joy G., Artico J., Kurdi H., Seraphim A., Lau C., Thornton G.D., et al. Prospective case-control study of cardiovascular abnormalities 6 months following mild COVID-19 in healthcare workers. JACC Cardiovasc Imaging. 2021;14:2155–2166. doi: 10.1016/J.JCMG.2021.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Petersen E.L., Goßling A., Adam G., Aepfelbacher M., Behrendt C.A., Cavus E., et al. Multi-organ assessment in mainly non-hospitalized individuals after SARS-CoV-2 infection: the Hamburg City health study COVID programme. Eur Heart J. 2022;43:1124–1137. doi: 10.1093/EURHEARTJ/EHAB914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Haas E.J., Angulo F.J., McLaughlin J.M., Anis E., Singer S.R., Khan F., et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. The Lancet. 2021;397:1819–1829. doi: 10.1016/S0140-6736(21)00947-8/ATTACHMENT/687306D0-D11D-455C-BD73-1D4556C1F770/MMC1.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Su J.R., McNeil M.M., Welsh K.J., Marquez P.L., Ng C., Yan M., et al. Myopericarditis after vaccination, vaccine adverse event reporting system (VAERS), 1990–2018. Vaccine. 2021;39:839–845. doi: 10.1016/J.VACCINE.2020.12.046. [DOI] [PubMed] [Google Scholar]
- 92.Shimabukuro T.T., Nguyen M., Martin D., DeStefano F. Safety monitoring in the vaccine adverse event reporting system (VAERS) Vaccine. 2015;33:4398–4405. doi: 10.1016/J.VACCINE.2015.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Halsell J.S., Riddle J.R., Atwood J.E., Gardner P., Shope R., Poland G.A., et al. Myopericarditis following smallpox vaccination among vaccinia-naive US military personnel. JAMA. 2003;289:3283–3289. doi: 10.1001/JAMA.289.24.3283. [DOI] [PubMed] [Google Scholar]
- 94.Bozkurt B., Kamat I., Hotez P.J. Myocarditis with COVID-19 mRNA vaccines. Circulation. 2021;144:471–484. doi: 10.1161/CIRCULATIONAHA.121.056135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sinagra G., Porcari A., Merlo M., Barillà F., Basso C., Ciccone M.M., et al. Myocarditis and pericarditis following mRNA COVID-19 vaccination. Expert opinion of the italian Society of Cardiology. G Ital Cardiol (Rome) 2021;22:894–899. doi: 10.1714/3689.36747. [DOI] [PubMed] [Google Scholar]
- 96.Fronza M., Thavendiranathan P., Chan V., Karur G.R., Udell J.A., Wald R.M., et al. Myocardial injury pattern at MRI in COVID-19 vaccine-associated myocarditis. Radiology. 2022 doi: 10.1148/RADIOL.212559. [DOI] [PMC free article] [PubMed] [Google Scholar]