Abstract
Yersinia enterocolitica 29930 (biogroup 1A; serogroup O:7,8) produces a bacteriocin, designated enterocoliticin, that shows inhibitory activity against enteropathogenic strains of Y. enterocolitica belonging to serogroups O:3, O:5,27 and O:9. Enterocoliticin was purified, and electron micrographs of enterocoliticin preparations revealed the presence of phage tail-like particles. The particles did not contain nucleic acids and showed contraction upon contact with susceptible bacteria. Enterocoliticin addition to logarithmic-phase cultures of susceptible bacterial strains led to a rapid dose-dependent reduction in CFU. Calorimetric measurements of the heat output of cultures of sensitive bacteria showed a complete loss of cellular metabolic activity immediately upon addition of enterocoliticin. Furthermore, a dose-dependent efflux of K+ ions into the medium was determined, indicating that enterocoliticin has channel-forming activity.
Bacteriocins have traditionally been defined as proteinaceous compounds produced by bacteria that inhibit or kill closely related bacteria (16). A special group of bacteriocins are high-molecular-weight particles, which can be sedimented by ultracentrifugation and are resolved by electron microscopy as phage tail-like particles (8, 13). These particles have been regarded as defective bacteriophages, which might have arisen from temperate phages by several successive mutations (8, 13). Bacteriocins of this type have been found in cultures of several gram-negative bacteria, including members of the families Enterobacteriaceae, Vibrionaceae, and Pseudomonadaceae (8, 10).
The most thoroughly studied phage tail-like bacteriocins are the F-type and R-type pyocins produced by Pseudomonas aeruginosa. The F-type pyocins resemble flexible but noncontractile tail structures of bacteriophages, whereas the R-type pyocins are similar to contractile but nonflexible tails (26). The bactericidal activity of the R-type pyocins is caused by the depolarization of the cytoplasmic membranes of sensitive bacteria (33). The pyocin biosynthesis genes were chromosomally located, and the gene organization clearly demonstrated that pyocins possess an ancestral origin common with bacteriophages. It was suggested that the pyocins have evolutionarily specialized as phage tails, rather than being just simple defective phages (26).
As the increase in bacteria resistant to a wide range of antibiotics has become a major public health problem, alternative strategies are being looked for to counter bacterial infections. In recent years the approach of using bacteriophages for the treatment of bacterial infections has come into focus again (2, 5, 23), as the advantage of phages as therapeutic agents is the high specificity for their target organisms, which would enable the selective elimination of phage-susceptible bacteria from a bacterial community (20). Bacteriocins are comparable to bacteriophages in terms of specificity for target bacteria, and given the morphological similarity, the use of phage tail-like bacteriocins for therapeutic use is conceivable, although bacteriocins lack the ability of phages to multiply during infection of target bacteria.
In the genus Yersinia, the production of phage tail-like particles by strains of Y. kristensenii, Y. frederiksenii, and Y. intermedia has been reported (9). These particles were used as diagnostic tools for typing Yersinia strains. Another early report about a phage tail produced by a Y. enterocolitica strain consisted mainly of morphological data (18). By studying a number of Yersinia isolates (19, 25) we found a food-borne strain of Y. enterocolitica which produced a bacteriocin-like substance with an inhibitory activity against serogroups O:3, O:5,27 and O:9 of Y. enterocolitica, which are the dominating pathogenic serogroups for humans (7, 21). The aim of our study was to characterize structural and functional features of this bacteriocin, which was designated enterocoliticin according to the usual nomenclature of bacteriocins (16), by studying its inhibitory activity against a pathogenic Y. enterocolitica O:3 strain.
MATERIALS AND METHODS
Bacterial strains and growth medium.
Y. enterocolitica 29930 (serogroup O:7,8, biogroup 1A), a food-borne isolate (19), was the strain producing enterocoliticin. Y. enterocolitica 13169 (serogroup O:3, biogroup 4) (25) and its derivative 13169-2, which had been cured of the Yop virulon plasmid, were used as sensitive indicator strains for most experiments. Y. enterocolitica 29807 (serogroup O:5, biogroup 1A) (30) was used as the nonsusceptible control strain. Y. enterocolitica Frederiksen P413 (biogroup 1A, serogroup O:7,8), a guinea pig isolate, was obtained from the Institute Pasteur, Paris, France.
Yersinia strains used for the determination of the inhibitory activity of enterocoliticin (Table 1) were taken from the strain collection of the Robert Koch Institute and include several strains initially obtained from the Institute Pasteur. The Enterobacteriaceae strains used (see Table 1) were obtained from L. Beutin and H. Steinrück, Robert Koch Institute. Bacterial strains were grown in Luria-Bertani (LB) medium (liquid cultures and agar) at 37°C. The susceptibility of strains to enterocoliticin was tested at 37°C.
TABLE 1.
Inhibitory spectrum of enterocoliticin
| Organism | Sourcea | Strain properties | No. of strainsb
|
|
|---|---|---|---|---|
| Tested | Susceptible | |||
| Y. enterocolitica | RKI, IP | Serogroup O:3 | 37 | 37 |
| Y. enterocolitica | RKI, IP | Serogroup O:5,27 | 5 | 5 |
| Y. enterocolitica | RKI, IP | Serogroup O:9 | 10 | 10 |
| Y. enterocolitica | RKI, IP | Serogroup O:8 | 7 | 0 |
| Y. enterocolitica | RKI, IP | Biogroup 1A, various serogroups, n.t.c | 10 | 2 |
| Y. pseudotuberculosis | RKI, IP | Serogroups I–VII | 10 | 0 |
| Y. intermedia | RKI, IP | Various serogroups, n.t. | 13 | 2 |
| Y. kristensenii | RKI, IP | Various serogroups, n.t. | 10 | 1 |
| Y. frederiksenii | RKI, IP | Various serogroups, n.t. | 10 | 4 |
| Y. mollaretii | RKI, IP | Various serogroups, n.t. | 13 | 3 |
| Escherichia coli | RKI | K-12, various serogroups | 28 | 0 |
| Salmonella enterica serovar Typhimurium | RKI | LT1, LT2, LT3 | 3 | 0 |
| Salmonella enterica serovar Enteritidis | RKI | 1 | 0 | |
| Salmonella enterica serovar Derby | RKI | 1 | 0 | |
| Proteus mirabilis | RKI | Tetracycline resistant | 1 | 0 |
| Shigella dysenteriae | RKI | Type 2 | 1 | 0 |
| Citrobacter freundii | RKI | 1 | 0 | |
| Shigella sonnei | RKI | 1 | 0 | |
| Enterobacter cloacae | RKI | 1 | 0 | |
| Vibrio harveyi | DSM 2332, DSM 6904 | 2 | 0 | |
| Pseudomonas fluorescens | ATCC 6538 | 1 | 0 | |
| Pseudomonas aeruginosa | ATCC 15492, ATCC 27853 | 2 | 0 | |
| Micrococcus luteus | ATCC 4698 | 1 | 0 | |
| Staphylococcus aureus | ATCC 25923, ATCC 6538 | 2 | 0 | |
| Bacillus subtilis | ATCC 9372 | 1 | 0 | |
| Streptococcus faecium | DSM 2146 | 1 | 0 | |
Abbreviations: RKI, strain collection of the Robert Koch Institute; IP, strains from the Institute Pasteur, Paris, France.
Tests were repeated three times for Yersinia strains by spotting a twofold serial dilution of enterocoliticin and incubating at 37°C. (The highest enterocoliticin activity was 8.8 × 10−5 AU ml−1, determined on the reference strain Y. enterocolitica 13169). All other bacteria were tested twice.
Various serogroups, n.t., strains belonging to various serotypes, some strains not typeable with standard sera.
Production and purification of enterocoliticin.
Y. enterocolitica 29930 was grown for 10 h at 28°C in 120 ml of LB broth and harvested at 8,000 × g. The cell pellet was resuspended in 30 ml of M9 medium with 1% glucose, and three flasks containing 790 ml of the same medium were inoculated with 10 ml of the resuspended cells. Mitomycin C (Sigma-Aldrich, Munich, Germany) was added at a final concentration of 1 μg/ml, and the cultures were stirred at 20°C for 14 h. The cultures were centrifuged at 10,000 × g for 30 min at 4°C, and the supernatants were passed through a 0.2-μm filter and pelleted at 230,000 × g for 2 h. The combined pellets were resuspended in 8 ml of water and applied to a CsCl step gradient (1.3 to 1.7 g/ml) and centrifuged at 28,000 rpm (141,000 × g) at 10°C for 16 h. Enterocoliticin was collected by puncturing the tube with a needle and dialyzed against water. Preparations were stored at 4°C for months without loss of activity.
Gel filtration.
A chromatography column (diameter, 1.6 cm) packed with 60 ml of Sephacryl S-400 HR (Pharmacia Biotech, Freiburg, Germany) was equilibrated with 0.07 M Tris–0.05 M BisTris, pH 8.0. For calibration of the column, retention times were determined with cytochrome c (12 kDa) (Boehringer, Mannheim, Germany) and thyroglobin (669 kDa), and the void volume was determined with blue dextran (ca. 2,000 kDa) (Pharmacia Biotech, Freiburg, Germany). Dialyzed aliquots of CsCl-purified enterocoliticin preparations were applied to the column, and protein elution from the column was monitored by absorbance at 280 nm. Fractions (2 ml) were collected, and enterocoliticin activity was quantified as described below.
Quantification of antimicrobial activity.
The antimicrobial activity of enterocoliticin was determined by a spot test. Serial dilutions (twofold or fourfold) of enterocoliticin preparations in water (27) were made, and 20 μl of each dilution was spotted on LB plates which had been overlaid with LB soft agar with a thickness of 2 mm containing approximately 106 CFU of susceptible indicator bacteria per ml. Plates were incubated at 37°C overnight. The reciprocal of the highest dilution that formed a visible inhibition zone was defined as the relative activity in activity units (AU) of enterocoliticin. Enterocoliticin titers are expressed as activity units per milliliter.
Treatment of cell pellets isolated from enterocoliticin-producing cultures of Y. enterocolitica 29930.
Aliquots of an enterocoliticin-producing culture were taken after 14 h (see above) and centrifuged at 10,000 × g. Supernatants were kept, and cell pellets were treated in two ways. (i) Pellets were resuspended in 2 ml of phosphate-buffered saline (PBS) and sonicated and the supernatant was adjusted to 10 ml. (ii) Cells were centrifuged and resuspended in 10 ml of PBS, and 1 ml of CHCl3 was added. The activity of the initial supernatant was compared to the activity of the supernatant of the treated cell pellets.
Nucleic acid contents of enterocoliticin preparations and stability.
A 1-ml amount of an enterocoliticin solution (activity, 1.3 × 107 AU ml−1) was extracted with 500 μl of phenol. The supernatant was transferred to another tube and extracted with phenol-CHCl3. The aqueous supernatant was precipitated with ethanol and resuspended in 20 μl of H2O. Fluorometric measurement of the DNA concentration was performed on a fluorometer (DYNA Quant 200; Pharmacia Biotech, Freiburg, Germany) according to the manufacturer's recommendation with Hoechst 33258 dye. Additionally, DNA preparations were analyzed on 0.8% agarose gels.
Physical and chemical stability of enterocoliticin was tested by incubating different concentrations of enterocoliticin (highest concentration was 1.3 × 106 AU ml−1) in 0.1 M sodium phosphate buffer (pH 7.2) and determining the activity after treatment by the spot test. Trypsin (Sigma-Aldrich, Munich, Germany) was added at a final concentration of 0.5 mg ml−1 and incubated with serial dilutions of enterocoliticin for 1 h at 37°C. Proteinase K (Roche Diagnostics, Mannheim, Germany) treatments were performed by incubating enterocoliticin dilutions in 50 mM Tris-HCl, pH 7.5 (final concentration of proteinase K, 0.2 mg ml−1), for 1 h at 37°C.
Electron microscopy.
Bacteriocin suspension (20 μl) was used to coat Formvar carbon-coated copper grids which had been treated with 0.1% bacitracin (wt/vol). After the bacteriocin suspension had dried, negative staining was performed with a solution of 1% uranyl acetic acid or 1% phosphotungstic acid, pH 6.8. Bacteriocin was visualized with a Philips 400 electron microscope.
Adsorption of enterocoliticin was performed by mixing 5 × 107 bacteria suspended in 100 μl of sodium cacodylate buffer with 400 μl of an enterocoliticin preparation containing approximately 4 × 1011 ± 0.8 × 1011 particles ml−1 (activity of the undiluted enterocoliticin preparation was 1.3 × 107 AU ml−1). Negative staining was performed as described above.
MIC determination and bactericidal activity of enterocoliticin.
The MIC of enterocoliticin was determined in LB medium following a protocol described for other bacteriocins (6). Cultures of susceptible strains 13169 and 13169-2 were grown at 25 and 37°C to an optical density at 588 nm (OD588) of 0.55. Aliquots of the cultures were diluted (at a ratio of 1:9) in a volume of 200 μl of LB containing a twofold serial dilution of enterocoliticin in 96-well microtiter plates. The plates were incubated at either 25 or at 37°C. The OD620 was read after 6 h by a microtiter reader (Easyrider EAR 400 AT; SLT-Labinstruments, Grödig, Austria), and the results were confirmed by visual control. The experiments were repeated three times for each temperature. The MIC corresponds to the lowest concentration of enterocoliticin that suppressed growth of the indicator strain completely.
Determination of CFU after addition of enterocoliticin was done as follows. A culture of 90 ml was grown at 37°C to an OD588 of 0.55 and divided into 15-ml aliquots. Enterocoliticin was added to give final concentrations of 2.2 × 105, 2.2 × 104, 2.2 × 103, and 2.2 × 102 AU ml−1 and portions of the treated cultures were removed after 1, 3, 5, 10, 20, 30, and 60 min. The bacteria were immediately washed once with 0.9% (wt/vol) NaCl, and CFU were determined on LB plates. Growth inhibition of cultures after addition of enterocoliticin was also determined by measuring the OD588.
Microcalorimetry.
Calorimetric measurements were performed on a flow microcalorimeter LKB 2107 (LKB, Bromma, Sweden) as described previously (34). A flow rate of 40 ml h−1 was maintained by a peristaltic pump. The incubation flask (100 ml) containing 60 ml of LB medium was inoculated with 1 ml of the bacterial culture grown overnight, shaken (175 rpm) at 37°C, and connected to the flow microcalorimeter. The bacterial suspension was pumped through the measuring coil (0.7 ml) of the microcalorimeter, and the outflow was returned to the incubation flask. The heat output (microwatts) of the culture was recorded continuously and included a short thermal equilibration period (about 10 min). The OD588 was determined throughout the experiment by removing 1 ml of culture every 30 min. Enterocoliticin was added at a final concentration of 4.4 × 104 AU ml−1 after 2.5 h of growth of the bacterial culture.
Determination of extracellular K+ ion concentration.
A 90-ml culture of Y. enterocolitica 13169 was grown on a shaker (135 rpm) to an OD588 of 0.55 at 37°C. The culture was harvested, washed three times with 0.9% (wt/vol) NaCl with change of centrifuge tubes, and finally resuspended in 90 ml of the same solution. Enterocoliticin was added to aliquots of the cultures at final concentrations of 2.2 × 105, 2.2 × 104, 2.2 × 103, and 2.2 × 102 AU ml−1. Samples (1 ml) were taken after 1, 3, 5, 10, 20, 30, 60, and 120 min and centrifuged immediately at 15,000 × g, and the supernatants were frozen in liquid nitrogen. Controls were (i) no addition of enterocoliticin and (ii) heat treatment of cultures (10 min at 100°C) to release intracellular K+ ions. Samples (1 ml) of the enterocoliticin-free control were taken at 0, 60, and 120 min and treated as described above. The K+ ion concentration was determined on an Atomic Adsorbance spectrometer (Perkin Elmer 3100; Perkin Elmer, Boston, Mass.). The determination was performed at 766.5 nm, and each sample was measured twice. Calibrations were done with K+ standards in the range from 0.2 to 2.0 mg ml−1. The K+ background of the enterocoliticin preparation was very low and was subtracted.
RESULTS
Enterocoliticin production.
In the supernatant of Y. enterocolitica 29930, a bacteriocin, designated enterocoliticin, that inhibited the growth of the pathogenic O:3 strain Y. enterocolitica 13169 was detected. Significant amounts of enterocoliticin were produced by the bacteriocinogenic strain in a temperature range between 12 and 27°C, whereas higher temperatures led to a significant reduction in enterocoliticin production. At 37°C no inhibitory activity was detectable in cultures of the producing strain (data not shown).
At permissive temperatures, production of enterocoliticin was stimulated by UV induction and was also increased by the addition of mitomycin C (up to a final concentration of 1 μg/ml) to cultures in the early logarithmic growth phase. Higher concentrations of mitomycin C did not further increase enterocoliticin synthesis. Optimal production was achieved by cultivating the producing strain at 20°C in minimal medium (see Materials and Methods).
To test if enterocoliticin was bound to cells of the producing strain, cell pellets were harvested and subjected to physical and chemical treatments (PBS-CHCl3 treatment and sonication). The total activity of enterocoliticin released from cell pellets was less than 1% of the activity present in the supernatant and thus negligible.
Ultrastructure of enterocoliticin particles.
Initial attempts to isolate enterocoliticin by combining different chromatographic methods (ion-exchange chromatography, gel filtration, hydrophobic-interaction chromatography) did not give satisfactory results. However, by applying phage isolation protocols, purification of enterocoliticin was achieved by ultracentrifugation techniques (see Materials and Methods). Enterocoliticin activity was isolated from a visible band in a CsCl step gradient. Enterocoliticin activity was adjusted to 1.3 × 107 AU ml−1, and preparations of 5 to 8 ml were obtained routinely from cultures with a total volume of 2.4 liters.
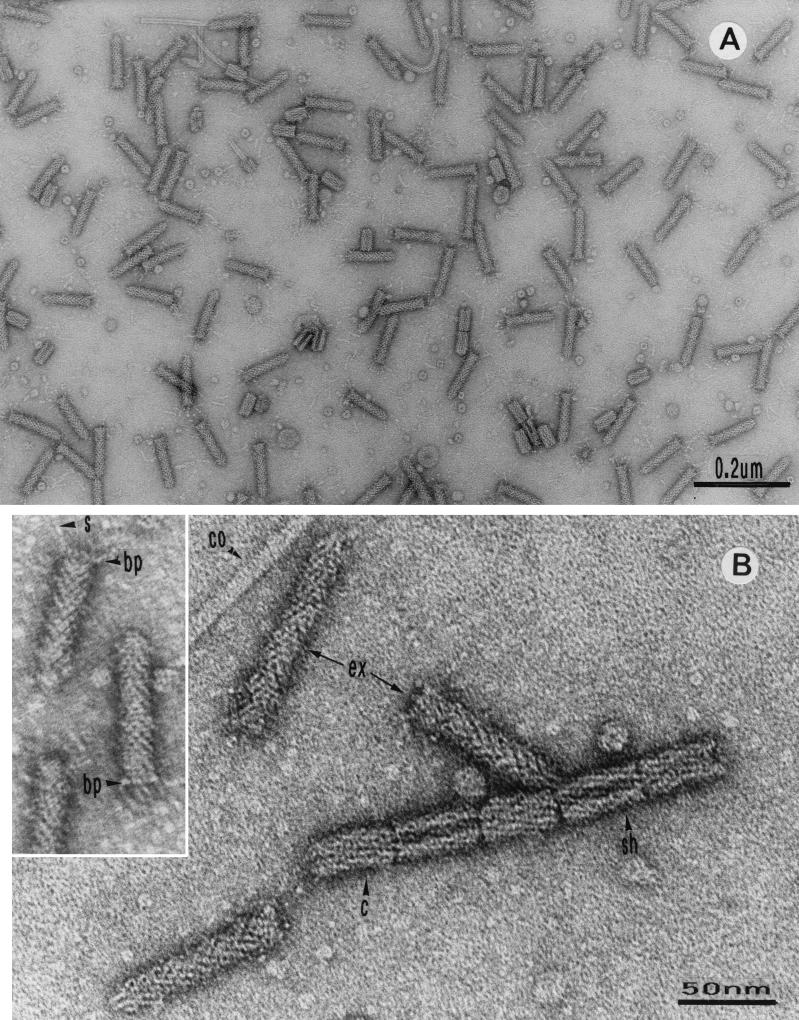
Electron micrographs of enterocoliticin preparations revealed the presence of phage tail-like particles without contamination with other particle structures (Fig. 1A). Typical structural elements of phage tails were identified, such as extended tails, contracted tails, sheath, and core structures (Fig. 1B). In some particles a base plate with spikes was visible (Fig. 1B, inset). The length of the extended particles was 80 ± 5 nm and the diameter was 15 ± 1 nm. The contracted structures were 35 ± 2 nm in longitudinal length and 20 ± 2 nm in diameter. Core particles had a diameter of 5 ± 1 nm.
FIG. 1.
Electron micrographs of enterocoliticin particles after negative staining. (A) Extended and contracted phage tail-like structures. (B) Higher magnification of enterocoliticin particles with different structural elements, showing the similarity to the architecture of phage tails (ex, extended particles; c, contracted particles; sh, sheath; co, core). (Inset) Particles with base plate (bp) and spikes (s).
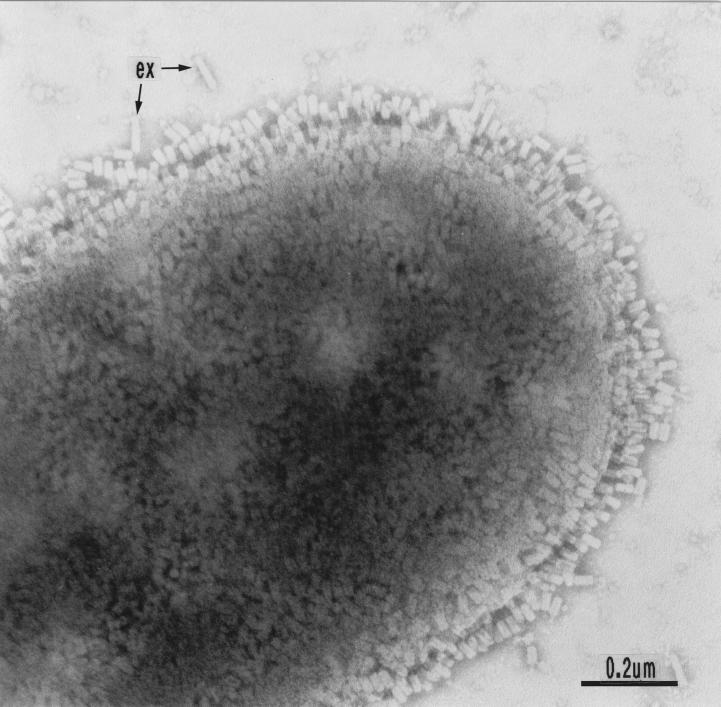
In Fig. 2 adsorption of enterocoliticin particles to a sensitive bacterium is shown, revealing contraction of the particles upon contact with the surface of the bacterium. Electron micrographs were taken from appropriate dilutions of enterocoliticin preparations spotted on grids with a defined mesh size. After determining the numbers of particles, it was concluded that an activity of 1.3 × 107 AU ml−1 corresponds to 2 × 1012 ± 0.4 × 1012 particles ml−1. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of enterocoliticin preparations revealed the presence of approximately 15 proteins of between 10 and 60 kDa (data not shown).
FIG. 2.
Negatively stained cells of Y. enterocolitica strain 13169. The surface of the sensitive cell is covered with contracted enterocoliticin particles (ex, extended particles not adsorbed to bacterial cell).
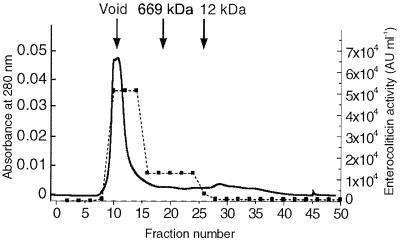
An aliquot of a CsCl-purified enterocoliticin preparation was subsequently purified on a size exclusion column to demonstrate that the inhibitory activity copurified with the phage tails (Fig. 3). More than 90% of the activity was eluted in fractions with a molecular mass over 669 kDa (Fig. 3). Electron micrographs taken from peak fractions also revealed the presence of phage tails.
FIG. 3.
Gel filtration analysis of an enterocoliticin preparation. The elution profile of an aliquot of a CsCl-purified preparation of enterocoliticin (total activity, 1.3 × 106 AU) was measured at OD280 (solid line). Enteroliticin activity was determined by serial dilution spot test (fraction size, 2 ml; dotted line).
Nucleic acid content and chemical stability of enterocoliticin.
Enterocoliticin preparations containing about 1012 particles (1.3 × 107 AU ml−1) were examined for nucleic acid content following standard protocols for phage DNA isolation. However, DNA was never detected in such preparations (estimated detection limit for DNA was 0.5 ng). To further determine the chemical stability, enterocoliticin preparations were incubated for 10 min at different temperatures. No loss of activity was detected at incubation temperatures of up to 50°C; in contrast, more than 90% of the activity was lost at 55°C, and no activity was retained after incubation at 60°C. Incubation in the presence of trypsin for 1 h did not affect enterocoliticin activity; however, proteinase K treatment reduced enterocoliticin activity to less than 1%.
Comparison of enterocoliticin with a phage tail particle produced by Y. enterocolitica Frederiksen P413.
Hamon et al. (18) described a bacteriocin-like substance from a Y. enterocolitica strain (Frederiksen P413) that was active against a number of Y. enterocolitica strains. The substance was reported to consist of particles resembling phage tails with a length of 80 nm, and in a contracted form a length of 40 nm and a diameter of 23 nm. The diameter of the central core was given as 6 nm. The strain Frederiksen P413 was kindly provided by E. Carniel, Institute Pasteur, Paris, who characterized the strain as serogroup O:7,8, biogroup 1A.
Electron micrographs of the phage tail-like particles that we isolated from the supernatant of strain Frederiksen P413 revealed a close resemblance to enterocoliticin particles (data not shown). Additionally, by testing a number of Yersinia strains, we did not discover a difference in the inhibition spectrum between enterocoliticin and the bacteriocin-like substance of strain Y. enterocolitica Frederiksen P413 (see below). Although both strains belong to the same bio- and serogroup as the enterocoliticin-producing strain, our strain 29330 harbored plasmids, while strain Frederiksen P413 did not.
MIC determination and inhibitory action of enterocoliticin.
The MIC of enterocoliticin was determined at 25 and 37°C for the susceptible strains Y. enterocolitica 13169 and its derivative 13169-2, which had been cured of the virulence plasmid. The MIC determined for the two strains was 8.2 × 103 ± 0.4 × 103 AU ml−1 at both growth temperatures, indicating that none of the genes located on the virulence plasmid influenced susceptibility.
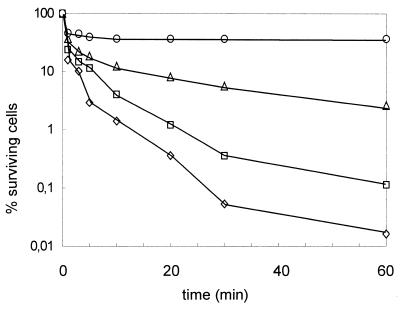
The bactericidal activity of enterocoliticin was demonstrated by adding enterocoliticin to logarithmic-phase cultures of the susceptible O:3 indicator strain Y. enterocolitica 13169. The highest concentration used (2.2 × 105 AU ml−1), corresponding to approximately 25 times the MIC, reduced the CFU by nearly 10,000-fold within 60 min, while an enterocoliticin concentration of 2.5 times the MIC reduced the CFU titer by 1,000-fold (more than 99% killing) (Fig. 4).
FIG. 4.
Killing of Y. enterocolitica 13169 after addition of enterocoliticin at final concentrations of (⋄) 2.2 × 105 AU ml−1, (□) 2.2 × 104 AU ml−1, (▵) 2.2 × 103 AU ml−1, and (○) 2.2 × 102 AU ml−1. Initial cell concentrations were 7.0 × 108 ± 0.3 × 108 CFU ml−1, and the detection limit was 10 CFU ml−1. CFU were determined in triplicate, and the mean deviation was less than 10%. The experiment was repeated twice with similar results.
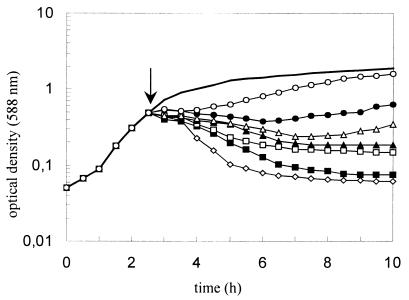
The lysis of the susceptible strain was determined by following the decrease in the OD588 upon addition of various concentrations of the enterocoliticin to a logarithmic culture. A significant decrease in optical density started 30 to 60 min after addition of enterocoliticin at a concentration above the MIC (Fig. 5). The highest concentration of enterocoliticin chosen was 2.2 × 104 AU ml−1, which killed more than 99% of the cells. Concentrations of enterocoliticin below the MIC (5.5 × 103 AU ml−1 and below) lysed the culture only partially, while the lowest concentration used in this test delayed the growth of the culture for 90 min (0.3 × 103 AU ml−1). Based on these data, the following calculation was performed: Enterocoliticin preparations with an activity of 1.3 × 107 AU ml−1 contain 2 × 1012 ± 0.4 × 1012 particles ml−1, which means that a final concentration of enterocoliticin of 5.5 × 103 AU ml−1 corresponds to ca. 8 × 108 ± 1.6 × 108 particles ml−1. A drop in OD588 from 0.55 to 0.2 corresponds to a decrease from 5.5 × 108 ± 0.5 × 108 CFU ml−1 to 2 × 108 ± 0.5 × 108 CFU ml−1. The calculation indicates that very few and probably even a single enterocoliticin particle is sufficient to kill a bacterial cell.
FIG. 5.
Inhibition of Y. enterocolitica 13169 after addition of enterocoliticin (↓) at final concentrations of (⋄) 2.2 × 104 AU ml−1, (▪) 1.1 × 104 AU ml−1, (□) 5.5 × 103 AU ml−1, (▴) 2.6 × 103 AU ml−1, (▵) 1.3 × 103 AU ml−1, (●) 0.7 × 103 AU ml−1, and (○) 0.3 × 103 AU ml−1. Solid curve shows the untreated control. The experiment was repeated twice with similar results.
The experiment described above was repeated with enteropathogenic Y. enterocolitica strains belonging to serogroups O:8, O:9, and O:5,27. The addition of serial dilutions of enterocoliticin to O:9 and O:5,27 strains resulted in a decrease in the OD588 to the same extent as shown for the O:3 strain in Fig. 5, whereas growth of the O:8 strain was not affected (see also below).
Action of enterocoliticin on growing cultures determined by microcalorimetric investigation.
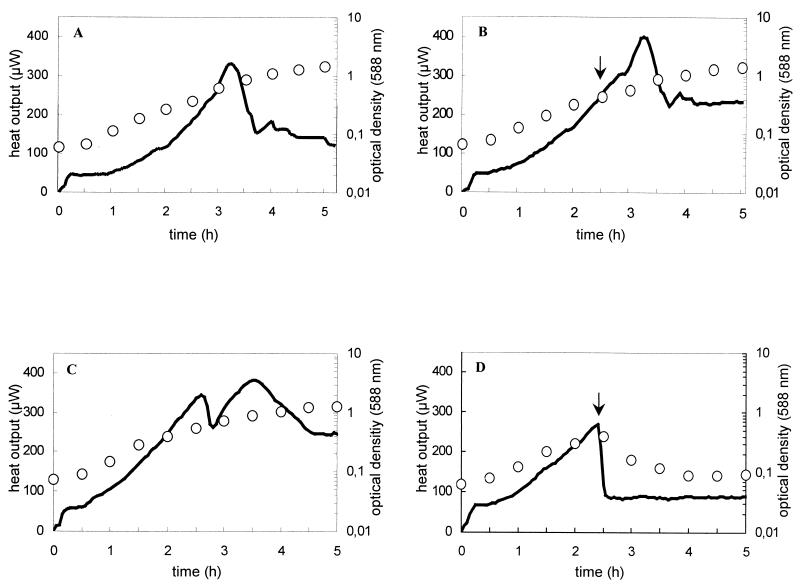
The effect of enterocoliticin on the metabolic activity of the sensitive indicator strain Y. enterocolitica 13169 was determined by measuring the heat output of a growing culture after addition of enterocoliticin. The nonsusceptible Y. enterocolitica strain 29807 was used as the control. The thermograms of the untreated bacterial cultures are shown in Fig. 6A and 6C. After an initial equilibration phase of 10 min, the heat output, which reflects metabolic activity, increased with the growth of the cultures and decreased when they entered the stationary phase. The thermograms of cultures of the same strains which were treated with enterocoliticin (5.5 times the MIC) are shown in Fig. 6B and 6D. The heat output of the culture of the sensitive strain 13169 declined rapidly after addition of enterocoliticin, while the metabolic activity of the nonsusceptible strain 29807 was not affected. The immediate cessation of metabolic activity in the sensitive strain is characteristic of a bactericidal effect.
FIG. 6.
Thermograms and growth curves of Y. enterocolitica 29807 (A and B) and Y. enterocolitica 13169 (C and D) without (A and C) and with addition of enterocoliticin at a final concentration of 4.4 × 104 AU ml−1(B and D). Solid lines show the recorded heat output of the bacterial suspension, and open symbols depict the growth of the culture (OD588). Arrows indicate the time point of addition of enterocoliticin. The experiments shown in A, B, and C were repeated once and the experiment shown in D was repeated twice with similar results.
Efflux of K+ ions after addition of enterocoliticin.
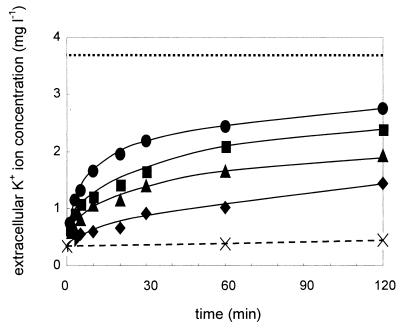
The experiments described above indicated that the immediate breakdown of the metabolic activity of the sensitive strain Y. enterocolitica 13169 after addition of enterocoliticin might be the result of the formation of channels across the bacterial cell envelope. Such channels might be induced by the adsorption and contraction of enterocoliticin particles to susceptible cells and might cause a rapid efflux of metabolites. To test this hypothesis, we measured the efflux of K+ ions after addition of various concentrations of enterocoliticin (Fig. 7). Within a few minutes, a dose-dependent and rapid increase in K+ ions in the extracellular medium was observed, supporting this hypothesis.
FIG. 7.
Increase in K+ ion concentration in the extracellular medium after addition of enterocoliticin to Y. enterocolitica 13169 cultures at 37°C. Final concentrations of enterocoliticin were (●) 2.2 × 105 AU ml−1, (▪) 2.2 × 104 AU ml−1, (▴) 2.2 × 103 AU ml−1, and (♦) 2.2 × 102 AU ml−1. Maximal K+ ion release from heated samples (dotted line) and untreated control culture (dashed line) is shown. Each K+ value was determined twice (the deviation between two values was less than 1%). The experiment was repeated twice with similar results.
Inhibitory spectrum of enterocoliticin.
The inhibitory spectrum of enterocoliticin was determined for a number of Yersinia strains and related bacteria of the family Enterobacteriaceae. Furthermore, some other gram-negative and gram-positive bacteria were included in the test (Table 1). The inhibitory spectrum was determined by spotting a twofold serial dilution of enterocoliticin on agar seeded with bacteria (see Materials and Methods).
The main focus of the determination of the inhibitory spectrum was the enteropathogenic Yersinia strains. While Y. pseudotuberculosis and strains of the pathogenic Y. enterocolitica serogroup O:8 were not sensitive to enterocoliticin, all investigated strains of the pathogenic Y. enterocolitica serogroups O:3, O:5,27, and O:9 were susceptible. Among the nonpathogenic Yersinia strains (Y. enterocolitica biogroup 1A, Y. kristensenii, Y. frederiksenii, and Y. intermedia), some strains were also susceptible. Remarkably, almost all Yersinia strains were inhibited up to the same final concentration of enterocoliticin in the serial dilution spot test, indicating that all sensitive Yersinia strains were susceptible to the same degree as the control strain Y. enterocolitica 13169. Only one Y. frederiksenii strain exhibited an intermediate sensitivity and was not investigated further. None of the remaining bacterial strains investigated which do not belong to the genus Yersinia were susceptible to enterocoliticin.
The bacteriocin-like substance of Y. enterocolitica Frederiksen P413 was also tested on Yersinia strains by spot tests. It was found that the inhibitory activity of this bacteriocin was identical to that of enterocoliticin, indicating that the substances are identical or closely related.
DISCUSSION
Bacteriocins with a phage tail-like structure have been found in different families of gram-negative bacteria, including Enterobacteriaceae, Vibrionaceae, and Pseudomonadaceae. The longitudinal length of enterocoliticin particles (ca. 80 nm) was clearly smaller than described for other particles of Enterobacteriacea, i.e., the length of carotovericin of Erwinia carotovora Er was 184 nm (27), the length of xenorhabdicin of Xenorhabdus nematophilus was 170 nm (31), and the proteocins of Proteus vulgaris had a length of 128 nm (11). The ultrastructural details of the enterocoliticin particles, including the measurements of tail length and diameter, resembled those of bacteriophages of the T-even phages (1) or coliphage Mu (32).
The production of bacteriocins with a phage tail structure seems to occur with a certain frequency in environmental Yersinia isolates, as bacteriocins of this type were isolated from 5% (12 of 236 strains) of cultures of Y. kristensenii, Y. frederiksenii, and Y. intermedia strains, although no detailed morphological and functional analysis was performed (9). As these bacteriocins did not clearly discriminate between pathogenic and environmental Yersinia isolates, they were not used in further studies for diagnostic purposes. The inhibitory activity of enterocoliticin was also not restricted to the enteropathogenic Y. enterocolitica strains, but all tested strains of serogroups O:3, O:5,27, and O:9 were highly susceptible, whereas the pathogenic O:8 strains and Y. pseudotuberculosis strains were not.
Electron micrographs (Fig. 2) showed a great number of adsorbed and contracted enterocoliticin particles on the cell wall of susceptible Y. enterocolitica strain 13169. Similar electron micrographs depicting adsorbed R pyocins on P. aeruginosa (17) and proteocins on the surface of Proteus mirabilis and Proteus vulgaris cells (3) have been published. In both cases, lipopolysaccharide components of the cell wall were identified as receptors for the phage tails (15, 29).
Most phage tail-like bacteriocins were reported to be resistant to trypsin and to be labile to heat treatment above 50°C (3, 13), which was also confirmed for enterocoliticin. Attempts to find DNA in enterocoliticin particles were not successful. Most of the described phage tail-like bacteriocins do not contain DNA (8, 13). However, a recent publication reported for the first time that in the R pyocins of P. aeruginosa strain C, two small DNA molecules, whose function remained unclear, could be isolated from the particles (24).
The enteropathogenic Y. enterocolitica O:3 strain 13169 was used to investigate the inhibitory activity of enterocoliticin, as this serogroup is a common cause of yersinial infection in humans worldwide (7) and seems to be the most abundant in swine (21, 28), which serve as a major reservoir for the human-pathogenic Y. enterocolitica strains (7). Following protocols for determining the MICs of bacteriocins (6), the MICs of enterocoliticin were determined for wild-type strain 13169 and its derivative 13169-2, which had been cured of the virulence plasmid. The MICs were identical, indicating that traits encoded by the Yop virulon did not play a role in enterocoliticin susceptibility. Presumably, the MICs of all sensitive strains are identical or very similar, as the serial dilution test performed with enterocoliticin preparations of defined activity always showed inhibition to the same extent as the reference strain 13169. Only one Y. frederiksenii strain showed a lower susceptibility in the serial dilution test; we did not investigate this strain further.
To gain more insight into the function of enterocoliticin, we performed cell viability assays by measuring the heat output of cultures after addition of enterocoliticin. The metabolic heat production measured by flow microcalorimetry has been proven to be a very sensitive indicator of metabolic disturbances after addition of antibiotics (22, 34). We showed that treatment of the sensitive strain immediately resulted in a complete loss of metabolic activity. The very rapid action of enterocoliticin might indicate that channels are formed through the bacterial surface, as described for other phage tail-like bacteriocins.
In the case of the pyocins, the mechanism of killing is by pore formation in the membrane causing disruption of the membrane potential (33). The vibriocins of Vibrio cholerae were also shown to form pores and led to an increased efflux of K+ ions (10). If bacteriocins with a phage tail-like structure are to be considered defective bacteriophages, it is conceivable to compare adsorption to sensitive cells with the infection process of bacteriophages. For bacteriophages, it is strongly believed that the phage DNA is transported via channels into the bacterial cells induced by the action of the tails. A short transient efflux of K+ ions after adsorption to sensitive cells was measured and was remarkably prolonged in defective headless phage ghosts (for a review, see reference 14). The rapid increase in potassium ions in the extracellular medium after addition of enterocoliticin to sensitive bacteria (Fig. 7) might be mechanistically similar to the effects reported for bacteriophages and thus supports the hypothesis of channel formation.
The increase in bacteria resistant to a large number of antibiotics has renewed the scientific discussion about the use of bacteriophages to counter bacterial infections. The specific activity of enterocoliticin against the most common pathogenic serogroups of Y. enterocolitica makes it conceivable to test phage tails as a possible therapeutic agent. Enteropathogenic Y. enterocolitica strains should be an appropriate model pathogen for therapeutic application of phage tail-like particles, since anatomopathological examinations have shown that the pathogen remains mainly extracellular in eukaryotic hosts and forms microcolonies in the gastrointestinal tract and the associated mesenteric lymphatic tissue (12). Thus, it should be accessible to the phage tail particles.
The efficacy of enterocoliticin as an antimicrobial agent will be tested in future experiments in a mouse infection model. The infection of mice with Y. enterocolitica belonging to the so-called low-pathogenicity strains of serogroups O:3, O:5,27, and O:9 is not fatal for the animals, but results in prolonged colonization (4). It resembles the infection of swine, which transmit the pathogen to humans via the food chain. If enterocoliticin application leads to fast elimination of the enteropathogen in the mouse infection model, one could envisage the use of enterocoliticin in swine populations which are infected with enteropathogenic Y. enterocolitica strains.
ACKNOWLEDGMENTS
We thank E. Carniel, Institute Pasteur, France, for providing strain Y. enterocolitica Frederiksen P413 and Gudrun Hultsch and Dorothea Knabner for serotyping bacterial strains.
REFERENCES
- 1.Ackermann H W, Berthiaume L. Atlas of virus diagrams. Boca Raton, Fla: CRC Press; 1995. [Google Scholar]
- 2.Alisky J, Iczkowski K, Rapoport A, Troitsky N. Bacteriophages show promise as antimicrobial agents. J Infect. 1998;36:5–15. doi: 10.1016/s0163-4453(98)92874-2. [DOI] [PubMed] [Google Scholar]
- 3.al-Jumaili I J. Physical properties and the fine structure of proteocines. Zentralbl Bakteriol. 1976;235:421–432. [PubMed] [Google Scholar]
- 4.Bakour R, Balligand G, Laroche Y, Cornelis G, Wauters G. A simple adult-mouse test for tissue invasiveness in Yersinia enterocolitica strains of low experimental virulence. J Med Microbiol. 1985;19:237–246. doi: 10.1099/00222615-19-2-237. [DOI] [PubMed] [Google Scholar]
- 5.Barrow P A, Soothill J S. Bacteriophage therapy and prophylaxis: rediscovery and renewed assessment of potential. Trends Microbiol. 1997;5:268–271. doi: 10.1016/S0966-842X(97)01054-8. [DOI] [PubMed] [Google Scholar]
- 6.Bennik M H, Verheul A, Abee T, Naaktgeboren-Stoffels G, Gorris L G, Smid E J. Interactions of nisin and pediocin PA-1 with closely related lactic acid bacteria that manifest over 100-fold differences in bacteriocin sensitivity. Appl Environ Microbiol. 1997;63:3628–3636. doi: 10.1128/aem.63.9.3628-3636.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bottone E J. Yersinia enterocolitica: the charisma continues. Clin Microbiol Rev. 1997;1O:257–276. doi: 10.1128/cmr.10.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bradley D E. Ultrastructure of bacteriophage and bacteriocins. Bacteriol Rev. 1967;31:230–314. doi: 10.1128/br.31.4.230-314.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calvo C, Brault J, Ramos-Cormenzana A, Mollaret H H. Production of bacteriocin-like substances by Yersinia frederiksenii, Y. kristensenii, and Y. intermedia strains. Folia Microbiol (Praha) 1986;31:177–186. doi: 10.1007/BF02927998. [DOI] [PubMed] [Google Scholar]
- 10.Chatterjee S N, Maiti M. Vibriophages and vibriocins: physical, chemical, and biological properties. Adv Virus Res. 1984;29:263–312. doi: 10.1016/s0065-3527(08)60411-x. [DOI] [PubMed] [Google Scholar]
- 11.Coetzee H L, De Klerk H C, Coetzee J N, Smit J A. Bacteriophage-tail-like particles associated with intraspecies killing of Proteus vulgaris. J Gen Virol. 1968;2:29–36. doi: 10.1099/0022-1317-2-1-29. [DOI] [PubMed] [Google Scholar]
- 12.Cornelis G R, Boland A, Boyd A P, Geuijen C, Iriarte M, Neyt C, Sory M P, Stainier I. The virulence plasmid of Yersinia, an antihost genome. Microbiol Mol Biol Rev. 1998;62:1315–1352. doi: 10.1128/mmbr.62.4.1315-1352.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daw M A, Falkiner F R. Bacteriocins: nature, function and structure. Micron. 1996;27:467–479. doi: 10.1016/s0968-4328(96)00028-5. [DOI] [PubMed] [Google Scholar]
- 14.Dreiseikelmann B. Translocation of DNA across bacterial membranes. Microbiol Rev. 1994;58:293–316. doi: 10.1128/mr.58.3.293-316.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dyke J, Berk R S. Growth inhibition and pyocin receptor properties of endotoxin from Pseudomonas aeruginosa. Proc Soc Exp Biol Med. 1974;145:1405–1408. doi: 10.3181/00379727-145-38023. [DOI] [PubMed] [Google Scholar]
- 16.Dykes G A. Bacteriocins: ecological and evolutionary significance. Trends Ecol Evol. 1995;1O:186–189. doi: 10.1016/s0169-5347(00)89049-7. [DOI] [PubMed] [Google Scholar]
- 17.Govan J R. Studies on the pyocins of Pseudomonas aeruginosa: morphology and mode of action of contractile pyocins. J Gen Microbiol. 1974;8O:1–15. doi: 10.1099/00221287-80-1-1. [DOI] [PubMed] [Google Scholar]
- 18.Hamon Y, Nicolle P, Vieu J F, Mollaret H. Recherche de la bacteriocinogenie parmi les souches de Yersinia enterocolitica. Ann Inst Pasteur. 1966;111:368–372. [PubMed] [Google Scholar]
- 19.Hoffmann B, Strauch E, Gewinner C, Nattermann H, Appel B. Characterization of plasmid regions of foodborne Yersinia enterocolitica biogroup 1A strains hybridizing to the Yersinia enterocolitica virulence plasmid. Syst Appl Microbiol. 1998;21:201–211. doi: 10.1016/S0723-2020(98)80024-6. [DOI] [PubMed] [Google Scholar]
- 20.Holzman D. Reassessment of medicinal phage. ASM News. 1998;64:620–622. [Google Scholar]
- 21.Kapperud G. Yersinia enterocolitica in food hygiene. Int J Food Microbiol. 1991;12:53–65. doi: 10.1016/0168-1605(91)90047-s. [DOI] [PubMed] [Google Scholar]
- 22.Krueger D, Giesbrecht P. Flow microcalorimetry as a tool for an improved analysis of antibiotic activity: the different stages of chloramphenicol action. Experientia. 1989;45:322–325. doi: 10.1007/BF01957463. [DOI] [PubMed] [Google Scholar]
- 23.Lederberg J. Smaller fleas . . . ad infinitum: therapeutic bacteriophage redux. Proc Natl Acad Sci USA. 1996;93:3167–3168. doi: 10.1073/pnas.93.8.3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee F K, Dudas K C, Hanson J A, Nelson M B, LoVerde P T, Apicella M A. The R-type pyocin of Pseudomonas aeruginosa C is a bacteriophage tail-like particle that contains single-stranded DNA. Infect Immun. 1999;67:717–725. doi: 10.1128/iai.67.2.717-725.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lewin A, Strauch E, Hertwig S, Hoffmann B, Nattermann H, Appel B. Comparison of plasmids of strains of Yersinia enterocolitica biovar 1A with the virulence plasmid of a pathogenic Y. enterocolitica strain. Zentralbl Bakteriol. 1996;285:52–63. doi: 10.1016/s0934-8840(96)80022-3. [DOI] [PubMed] [Google Scholar]
- 26.Nakayama K, Takashima K, Ishihara H, Shinomiya T, Kageyama M, Kanaya S, Ohnishi M, Murata T, Mori H, Hayashi T. The R-type pyocin of Pseudomonas aeruginosa is related to P2 phage, and the F-type is related to lambda phage. Mol Microbiol. 2000;38:213–231. doi: 10.1046/j.1365-2958.2000.02135.x. [DOI] [PubMed] [Google Scholar]
- 27.Nguyen A H, Tomita T, Hirota M, Sato T, Kamio Y. A simple purification method and morphology and component analyses for carotovoricin Er, a phage-tail-like bacteriocin from the plant pathogen Erwinia carotovora Er. Biosci Biotechnol Biochem. 1999;63:1360–1369. doi: 10.1271/bbb.63.1360. [DOI] [PubMed] [Google Scholar]
- 28.Pilon J, Higgins R, Quessy S. Epidemiological study of Yersinia enterocolitica in swine herds in Quebec. Can Vet J. 2000;41:383–387. [PMC free article] [PubMed] [Google Scholar]
- 29.Smit J A, Hugo N, De Klerk H C. A receptor for a Proteus vulgaris bacteriocin. J Gen Virol. 1969;5:33–37. doi: 10.1099/0022-1317-5-1-33. [DOI] [PubMed] [Google Scholar]
- 30.Strauch E, Voigt I, Broll H, Appel B. Use of a plasmid of a Yersinia enterocolitica biogroup 1A strain for the construction of cloning vectors. J Biotechnol. 2000;79:63–72. doi: 10.1016/s0168-1656(00)00216-9. [DOI] [PubMed] [Google Scholar]
- 31.Thaler J O, Baghdiguian S, Boemare N. Purification and characterization of xenorhabdicin, a phage tail-like bacteriocin, from the lysogenic strain F1 of Xenorhabdus nematophilus. Appl Environ Microbiol. 1995;61:2049–2052. doi: 10.1128/aem.61.5.2049-2052.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.To C M, Eisenstark A, Toreci H. Structure of mutator phage Mu1 of Escherichia coli. J Ultrastruct Res. 1966;14:441–448. doi: 10.1016/s0022-5320(66)80074-6. [DOI] [PubMed] [Google Scholar]
- 33.Uratani Y, Hoshino T. Pyocin R1 inhibits active transport in Pseudomonas aeruginosa and depolarizes membrane potential. J Bacteriol. 1984;157:632–636. doi: 10.1128/jb.157.2.632-636.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wecke J, Madela K, Fischer W. The absence of d-alanine from lipoteichoic acid and wall teichoic acid alters surface charge, enhances autolysis and increases susceptibility to methicillin in Bacillus subtilis. Microbiology. 1997;143:2593–2960. doi: 10.1099/00221287-143-9-2953. [DOI] [PubMed] [Google Scholar]