Abstract
Septic shock is a main cause of morbidity and mortality in neonates. Septic shock evolves from compensated to uncompensated through 3 distinct phases. Prompt diagnosis is challenging, since neonatal septic shock may overlap with the physiological changes occurring at birth. The outcome of septic shock depends on a prompt recognition of symptoms and a strict adherence to cardiopulmonary resuscitation guidelines. Fluid administration plays a major role in the initial management of septic shock. If there is no response to volume filling, inotropes must be infused within one hour of onset (dopamine, dobutamine, adrenaline). Life-threatening infections require immediate and aggressive empiric use of antimicrobials. In the pediatric age, delay in antibiotic initiation for treating septic shock is associated with poor outcome and increased risk of mortality. There is a gap regarding first line interventions in neonatal septic shock. This review addresses initial interventions in the treatment of neonatal septic shock and discusses currently available evidences., These interventions may allow to improve the outcome if they are promptly carried out. (www.actabiomedica.it)
Keywords: sepsis, newborn, septic shock, hemodynamic support, antibiotics
Introduction
Sepsis is a life-threatening organ dysfunction due to dysregulated host response to infection. Neonatal sepsis is categorized in early- (EOS) and late-onset sepsis (LOS) defined as a positive blood or cerebro-spinal fluid culture obtained within or beyond 3 days of age, respectively. (1) Neonates born before 28 weeks’ gestation or with a birth weight under 1500 g are particularly susceptible to sepsis. (2-5) Sepsis-associated mortality rates varies according to the severity of clinical symptoms, risk factors, geographical location. Two recent Italian area-based studies showed that mortality was also strictly associated with low gestational age (EOS: OR 0.71 [0.60–0.83]; LOS: OR 2.3 [1.2-4.7]) or the need of catecholamine support for treating septic shock (EOS: OR 21.3 [6.58-68]; LOS: OR 12.6 [1.6-101.9]). (6,7)
Pathogens involved in septic shock may vary according to neonatal age, site of infection and pre-existent underlying diseases. (8,9) Main pathogens causing EOS are bacteria that colonize the maternal genital tract, i.e. Group B streptococcus (GBS), Escherichia coli and Listeria monocitogenes. (7,10,11) Pathogens may be transmitted during the labor or after membrane rupture. In some high-income countries using extensively intrapartum antibiotic prophylaxis for preventing Group B Streptococcus, E. coli is now the first pathogen in EOS. (10,12,13)
LOS is more commonly due to Gram positive (Coagulase-negative stafilococci, S. aureus, GBS) or Gram negative pathogens (E. Coli, Enterobacteriacee, Pseudomonas aeruginosa). (7,14,15) CONS are the most common nosocomial pathogens causing LOS, whereas Candida albicans is the main pathogen among fungi in VLBW neonates with LOS. (1) In contrast, some pathogens may be more commonly acquired in community. (7) Late onset group B streptococcus is often acquired from maternal sources either at delivery or in the post-partum, after discharge from the nursery. Pathogens can also be acquired from non-maternal sources. Prolonged hospital stay in Neonatal Intensive Care Unit (NICU) and use of wide spectrum antibiotics increase the risk of neonatal colonization with multidrug resistant pathogens, (16,17) and neonates admitted to NICU may suffer from one or more episodes of LOS during their hospitalization. (7)
This review focuses on initial interventions for treating septic shock. These interventions improve the neonatal outcome when promptly carried out.
Definition of sepsis and septic shock
Shock is a generalized, rapidly establishing tissue hypoperfusion. Shock results from multiple and related mechanisms that lead to severe cellular impairment due to an imbalance between cellular nutrition and removal of toxic metabolites. (18) The definition of sepsis and septic shock in the pediatric age is controversial. Three different definitions have been suggested and subsequently adapted to premature neonates by Wynn. (3,4,19,20) These definitions recognize three subsequent phases: systemic inflammatory response syndrome: SIRS, sepsis and septic shock.
However, these pediatric sepsis definitions have limited value to bedside clinicians for identifying cases of sepsis, since they have poor predictive value and have not been validated. (21,22) Recently, the adult definition of sepsis has been updated by highlighting the central role of organ impairment; septic shock occurs in a subset of patients with sepsis in whom circulatory, cellular or metabolic impairment is profound enough to increase the risk of mortality. (23)
During the first weeks of life, organs are immature and physiological changes occur in the functions of the vital systems. As a consequence, reference values and variations from the norm of some vital parameters are not well defined. Therefore, the concept of organ dysfunction as a diagnostic criterium is unfrequently used in neonates. (20) Further studies are required to assess criteria for neonates, possibly by adapting those established in the adult population. (24)
Physiopathology
Once the pathogen has entered the body, monocytes and macrophages recognize molecules expressed (PAMPS: pathogen-associated molecular patterns) through surface receptors, including TLRs (Toll Like Receptors). As consequence, this interaction generates the activation of second messengers and intracellular cascades leading to the release of cytokines and chemokines that amplify the immune response (INFγ, IL1β, IL6, IL8, IL12, IL18, MCP, MIP, TNFα) and activate the endothelium. Other receptors involved are intracytoplasmic receptors (NOD-like receptors [NLRs], RIG-like receptors [RLRs]); they have a role in the creation of the inflammasome, contributing to the activation of lymphocyte and complement function, especially CR3 receptor. In full-term infants, in the first month of life, a reduced expression of CR3 receptor and L-selectin predisposes to the risk of sepsis. If the infection and inflammation are not controlled, a generalized endothelial inflammatory state can lead to septic shock. The damage and generalized activation of the endothelium, associated with the systemic spread of the pathogen, cause damage to the microcirculation with tissue hypoxia, acidosis and hypotension. (18,25) Parallel to the inflammatory state, the body also produces a number of anti-inflammatory molecules (TNF2, IL6-receptor, IL1 receptor antagonist) in order to attenuate the intensity of the systemic inflammatory response. For the same purpose there is an increase in endogenous cortisol. Preterm infants have relative adrenal insufficiency which contributes to hemodynamic instability and hypotension. (3)
Clinical aspects of septic shock
Sepsis may present with non-specific symptoms (tachycardia, respiratory sings, poor feeding, hypotonia, lethargy, pale or cyanotic skin, decreased urine output), that may rapidly evolve to septic shock. Prompt diagnosis is challenging, since neonatal sepsis may overlap with the physiological changes occurring at birth. These changes may affect the normal course of neonatal adaptation, and complicate this precarious equilibrium, making difficult to assess the burden of organ compromise associated with septic shock. (2) Septic shock can progress through three consecutive phases, ranging from compensated to uncompensated. Each one of these phases has specific neuroendocrine compensatory mechanisms and clinical signs (table 1). (3,6)
Table 1.
Neuroendocrine compensatory mechanisms and clinical signs of septic shock
| Compensated shock | Neuroendocrine compensatory mechanisms Signs: increased heart rate, decreased urine output, poor perfusion, cold extremities delayed refill time |
| Uncompensated shock | Failure of neuroendocrine compensatory mechanisms Signs: systemic hypotension, metabolic acidosis |
| Irreversible shock | Failure of microcirculation, irreparable cell damage and cell death. Signs: Multiorgan failure |
Furthermore, septic shock is distinguished in warm and cold. Table 2 shows hemodynamic changes and clinical presentations during warm and cold septic shock.
Table 2.
Haemodynamic changes and clinical presentations during warm and cold shock
| Haemodynamic changes | Clinical presentations | |
|---|---|---|
| Warm shock |
|
|
| Cold shock |
|
|
Vasodilation with reduced vascular resistances initially prevails in warm shock, while the cardiac output increases. During the subsequent cold shock, cardiac compensation fails, while vascular resistances increase, due to vasoconstriction.
In adults, septic shock usually presents with myocardial dysfunction and decreased cardiac ejection fraction. In contrast, severe hypovolemia is predominant in the pediatric age, as shown by the improvement observed after volume filling. (3) The neonatal myocardium is not able to compensate for the raised needs through an increase in the ejection volume (that is relatively fixed, due to the small ventricular size and reduced compliance), nor with a sustained increase in the heart rate, that is already high. Persistent tachycardia also worsens the perfusion of the myocardium itself that occurs during diastole and myocardial O2 consumption, with the risk of ischemia. (3)
Sepsis leads to metabolic acidosis and hypoxia with consequent increase in pulmonary resistances and persistence of fetal circulation, characterized by right to left intra and extra cardiac shunts. The increase in pulmonary vascular resistances and their persistence cause right ventricular failure which affects left ventricular function. In the newborn, it is also important to distinguish septic from cardiogenic shock due to the closure of the arterial ductus in complex ductus-dependent congenital heart diseases. Prostaglandin treatment should be started immediately in any neonate with shock and hepatomegaly, cyanosis, heart murmur, difference in blood pressure and / or peripheral pulses between the upper and lower limbs. Prostaglandin treatment should be discontinued once ductus-dependent heart diseases have been ruled out. Early phases of inborn metabolic errors can also simulate septic shock. (20)
Therapy
The outcome of septic shock depends on a prompt recognition of symptoms suggesting sepsis and a strict adherence to cardiopulmonary resuscitation guidelines, protocols and algorithms according to PALS guidelines. (27,28,29) Outcome varies also according to the timeliness by which the following 3 targets are achieved: a) Recovery from the shock, as demonstrated by the normalization of both capillary refill time (< 3”) and blood pressure; b) Infusion of broad-spectrum antimicrobials and c) Infusion of fluids (crystalloids) within 1 hour from the onset of symptoms. (30) Figure 1 shows initial steps in the treatment of septic shock in the newborn.
Figure 1.
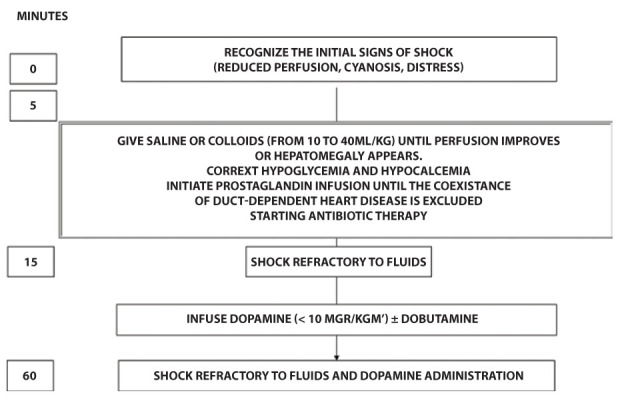
Initial steps in the treatment of septic shock in the newborn (modified from Ref. 30)
Fluid administration plays a major role in the initial management of septic shock. The administration of colloids (5% albumin) remains controversial, (31,32,33) whereas crystalloids (saline for neonates, Lactated Ringer or saline for children) are usually the first line fluids. A prospective observational study enrolled 49,153 children (aged <18 years) with severe sepsis. The study was carried out in 43 US hospitals from 2004 to 2012. Among 36,908 children who were analyzed for fluid resuscitation, 2398 (median age 3.2 years) received only balanced crystalloids (i.e. Lactated Ringer’s) and 30,166 (median age 5.8 years) received only unbalanced fluids. Mortality rates were reduced (12.5% vs 15.9%; p = 0.007, OR 0.76; 95%, CI 0.62-0.93) when balanced fluids rather than saline were administered. Furthermore, author suggested to avoid fluid overload, since it was associated with worse outcomes. (32)
A retrospective cohort study was carried out in 12,529 children (aged <18 years) with severe sepsis or septic shock in 382 US hospitals (from 2000 to 2013). Ringer lactate and saline were administered to 2150 and 10,379 children respectively. A sample of 2117 children was selected from each group (median age 8 and 7 years for Ringer lactate and saline infusion respectively). No difference in mortality was found between the groups (RR 0.99, 95%, CI 0.98, 1.01, p =0.2). (31)
In addition, the duration of i.v. fluids’ infusion is also controversial. Sankar enrolled 96 children aged less than 18 years receiving fluid boluses with normal saline. Fluid boluses were administered in 45 children (median age 6.5 years) within 15-20 minutes and in 51 children (median age 6 years) within 5-10 minutes. Mechanical ventilation or increase in oxygenation index after fluid resuscitation were less frequent among children receiving fluid boluses within 15–20 minutes in the first 6 hours (36% vs 57%; relative risk, 0.62; 95% CI, 0.39–0.99) and 24 hours of septic shock (43% vs 68%; relative risk, 0.63; 95% CI, 0.42–0.93). This study supports a longer duration of fluid boluses and a change in current practice, (34) although, critically reread by others, these data led to opposite conclusions, namely that fluids should be administered within 10 minutes. (35)
It is preferable to use a central venous line; however, in emergency conditions, an intraosseous (if the body weight is over 3 kg) or a peripheral venous access can be used. In the newborn the umbilical venous access can be inserted up to 15 days of life. (30)
Fluids (usually saline in neonates) should be administered with caution in neonates, especially in preterm infant with persistent patent ductus arteriosus, because of risks of congestive heart failure, pulmonary edema or reopening of a previously closed arterial ductus. Furthermore, large and rapid administration of fluids in preterm infants may lead to intraventricular hemorrhage, whereas prolonged cerebral hypoperfusion, consequent to septic shock, can lead to periventricular leukomalacia. (19,30,35) Although there are only a few data, fluid boluses (saline) of 10 mL/kg (up to 40 mL/kg in the first hour) can be administered in neonates. (3,30) Fluid infusion should always be titrated to clinical markers of cardiac output (i.e. hepatomegaly, or increased work of breathing). They should be discontinued if signs of fluid overload develop. (27,30)
In hypotensive preterm neonates, some investigators recommend that a single bolus of saline (10–20 ml/kg over 30–60 minutes) be given and if further intervention is necessary, to begin vasoactive medications. (36)
Volume filling is indicated before intubation in cases of severe septic shock, since analgo-sedation could precipitate severe hemodynamic instability. (30) If there is no response to volume filling, inotropes must be infused within one hour of onset (dopamine, dobutamine, epinephrine). Recent guidelines recommend the preferential use of epinephrine or norepinephrine (compared to dopamine) as first-line catecholamines in children with septic shock, since the inotropic and vasopressor effect is higher, and useful in the treatment of fluid-refractory septic shock. (27)
There is a great gap of studies regarding the use of amines in the neonatal period. Norepinephrine is currently the inotropic first line drug in septic shock of the child. (37,38,39) A double-blind randomized controlled trial (40) was carried out in 40 neonates (of which 18 had ≤ 30 and 22 had ≥ 31 weeks’ gestation respectively) with fluid refractory septic shock. Epinephrine was more effective than dopamine in reversing shock and haemodynamic instability in premature neonates under ≤ 30 weeks’ gestation, whereas epinephrine and norepinephrine had comparable safety and efficacy (50% vs 30%; RR 1,67 (95% CI 0,75-3,71) in infants with higher gestational age (≥ 31 weeks’). (40)
A retrospective observational study during a 10-year period included 279 preterm infants (≤ 32 weeks’ gestation) with LOS, of which 25 progressed to septic shock. Indications for fluid bolus and vasopressors administration were blood pressure < 20% and 41% below to what was found before the onset of disease symptoms, respectively. Authors found no significant hemodynamic changes when the first fluid bolus was given, but the start of vasopressors significantly increased blood pressure and heart rate (p<0.01). This study shows that vasopressors, not fluid bolus, significantly increased blood pressure and heart rate. (41)
Life-threatening infections require immediate and aggressive empiric use of bactericidal antibiotics. Delay in antibiotic initiation for treating septic shock has been associated with poor outcome and increased risk of mortality. In adults with hypotensive septic shock, survival was found to decreases by 7.6% for each hour of delay in antibiotic administration. (42) Despite a very low quality of evidence, recent guidelines for the management of septic shock and sepsis-associated organ dysfunction in children suggest administering antimicrobials as soon as possible, but within 1 h of recognition. (27) Two retrospective observational studies investigated the association between early administration of antibiotics and decrease in sepsis-related mortality in the pediatric age. (43,44) The first study (43) was carried out in 130 septic children (median age of 7.7 years), of which 103 had septic shock. Median time from sepsis recognition to initial antimicrobial administration was 140 minutes. The risk of mortality increased when antimicrobials were given ≥ 3 hours of sepsis recognition (unadjusted OR 3.92, 95% C.I. 1.3-12.1). A larger study was carried out (from 2014 to 2016) in 54 USA hospitals. Children with sepsis or septic shock (mean age 7.2 years; admitted to emergency department or already in hospital were 1179. Among them, 811 (68.8%) were diagnosed as having shock, and 139 (11.8%) died. A lower risk-adjusted odds of in-hospital mortality (OR 0.59, 95% CI, 0.38-0.93, P=0.02) was found when all three elements of the 1-hour sepsis bundle were achieved (blood culture collected prior to antibiotic administration, broad-spectrum antibiotics administered and completion of at least 20 ml/kg crystalloid fluid bolus). (44) Unfortunately, data regarding associations between neonatal outcome and early administration of antibiotics are very scanty. Schmatz and co-workers investigated timing of initiation of antibiotics and 7-, 14- and 30-days mortality in 113 neonates with culture proven sepsis. Although the patient population was very heterogeneous and a clear time cutoff could not be defined, investigators demonstrated an association between delayed time to antibiotics administration and increased risk of both, mortality and prolonged cardiovascular dysfunction. (45)
Broad-spectrum antibiotics against the most likely pathogens of EOS and LOS should be promptly given in septic shock. A combination of ampicillin and gentamicin remain an appropriate empiric therapy for EOS. A semisynthetic penicillin (oxacillin or nafcillin), in combination with an aminoglycoside is an appropriate empiric therapy for LOS. Third generation cephalosporins should be added when meningitis is suspected. Lumbar puncture remains an essential tool for diagnosing sepsis or meningitis, guiding appropriate antibiotic therapies. However, it is often necessary to defer lumbar puncture if the infant has hemodynamic instability. (46) Therefore, even more so in such cases, empirical antibiotic therapies must have a particularly broad spectrum. In fact, they must be promptly effective in more severe cases. Piperacillin-tazobactam, carbapenem or cefepime should be used for Gram-negative coverage, in infants known to be colonized with resistant Gram-negative organisms. Empirical therapy should be re-evaluated within 48 hours; antibiotics with the narrowest spectrum (de-escalation) and distributing to the infected body site should be administered according to the pathogen recovered from cultures. Antibiotics should be discontinued if the neonate has improved and systemic cultures are sterile. Antimicrobial stewardship programs may guide the optimal choice of drugs, dosage, and duration of therapies. (47,48)
In conclusion, prompt recognition and treatment of sepsis and septic shock improves prognosis, and initial approaches are key to improving the outcome. Recently, approaches for treating the adult septic shock have been defined, but strong evidences regarding neonates are still lacking. There is a need to carry out well-designed studies to clarify these aspects in the neonatal period.
Conflict of Interest:
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article
References
- Aneja RK, Varughese-Aneja R, Vetterly CG, et al. Antibiotic therapy in neonatal and pediatric septic shock. Curr Infect Dis Rep. 2011;13:433–41. doi: 10.1007/s11908-011-0197-5. [DOI] [PubMed] [Google Scholar]
- Agyeman PKA, Schlapbach LJ, Giannoni E, et al. Pediatric Sepsis Study. Epidemiology of blood culture-proven bacterial sepsis in children in Switzerland: a population-based cohort study. Lancet Child Adolesc Health. 2017;1:124–133. doi: 10.1016/S2352-4642(17)30010-X. [DOI] [PubMed] [Google Scholar]
- Wynn JL, Wong HR. Pathophysiology and treatment of septic shock in neonates. Clin Perinatol. 2010;37:439–79. doi: 10.1016/j.clp.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn JL. Defining neonatal sepsis. Curr Opin Pediatr. 2016;28:135–40. doi: 10.1097/MOP.0000000000000315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shane AL, Sánchez PJ, Stoll BJ. Neonatal sepsis. Lancet. 2017;14(390):1770–1780. doi: 10.1016/S0140-6736(17)31002-4. [DOI] [PubMed] [Google Scholar]
- Berardi A, Baroni L, Bacchi Reggiani ML, et al. GBS Prevention Working Group Emilia-Romagna. The burden of early-onset sepsis in Emilia-Romagna (Italy): a 4-year, population-based study. J Matern Fetal Neonatal Med. 2016;29:3126–31. doi: 10.3109/14767058.2015.1114093. [DOI] [PubMed] [Google Scholar]
- Berardi A, Sforza F, Baroni L, et al. Epidemiology and complications of late-onset sepsis: an Italian area-based study. PLoS One. 2019;14:e0225407. doi: 10.1371/journal.pone.0225407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berardi A, Ficara M, Pietrella E, et al. Stewardship antimicrobica nel neonato e nel piccolo lattante. Perché e come praticarla. Medico e Bambino. 2017;36:493–501. [Google Scholar]
- Wilson CB, Nizet V, Maldonado YA, et al. Infecious diseases of the fetus and newborn infant. Elsevier; 2016. [Google Scholar]
- Barbara M, Stoll J, Karen M. P, Puopolo M, Nellie M, Hansen I, et al. Early-Onset Neonatal Sepsis 2015 to 2017, the Rise of Escherichia coli, and the Need for Novel Prevention Strategies. JAMA Pediatrics. 2020:E1–E12. doi: 10.1001/jamapediatrics.2020.0593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrag SJ, Farley MM, Petit S, Reingold A, Weston EJ, et al. Epidemiology of Invasive Early-Onset Neonatal Sepsis, 2005 to 2014. Pediatrics. 2016;138:e20162013. doi: 10.1542/peds.2016-2013. [DOI] [PubMed] [Google Scholar]
- Berardi A, Di Fazzio G, Gavioli S, et al. GBS Prevention Working Group, Emilia-Romagna. Universal antenatal screening for group B streptococcus in Emilia-Romagna. J Med Screen. 2011;18:60–4. doi: 10.1258/jms.2011.011023. [DOI] [PubMed] [Google Scholar]
- Van Dyke MK, Phares CR, Schrag SJ, et al. Evaluation of universal antenatal screening for group B streptococcus. N Engl J Med. 2009;360:2626–36. doi: 10.1056/NEJMoa0806820. [DOI] [PubMed] [Google Scholar]
- Vergnano S, Menson E, Kennea , et al. Neonatal infections in England: the NeonIN surveillance network. Arch Dis Child Fetal Neonatal Ed. 2011;96:F9–F14. doi: 10.1136/adc.2009.178798. [DOI] [PubMed] [Google Scholar]
- Cailes B, Kortsalioudaki C, Buttery J, et al. Epidemiology of UK neonatal infections: the neonIN infection surveillance network. Arch Dis Child Fetal Neonatal Ed. 2018;103:F547–F553. doi: 10.1136/archdischild-2017-313203. [DOI] [PubMed] [Google Scholar]
- Pammi M, Weisman LE, et al. Late-onset sepsis in preterm infants: update on strategies for therapy and prevention. Expert Rev Anti Infect Ther. 2015;13:487–504. doi: 10.1586/14787210.2015.1008450. [DOI] [PubMed] [Google Scholar]
- Ficara M, Pietrella E, Spada C, et al. Changes of intestinal microbiota in early life. J Matern Fetal Neonatal Med. 2020;33:1036–1043. doi: 10.1080/14767058.2018.1506760. [DOI] [PubMed] [Google Scholar]
- Polin Richard, Abman Steven. Fetal and Neonatal Physiology. Fifth Edition. Vol. 1. Elsevier: 2016. pp. 1536–1551. [Google Scholar]
- Goldstein B, Giroir B, Randolph A, et al. International Consensus Conference on Pediatric Sepsis. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005;6:2–8. doi: 10.1097/01.PCC.0000149131.72248.E6. [DOI] [PubMed] [Google Scholar]
- McGovern M, Giannoni E, Kuester H, et al. Infection, Inflammation, Immunology and Immunisation (I4) section of the ESPR. Challenges in developing a consensus definition of neonatal sepsis. Pediatr Res. 2020;88:14–26. doi: 10.1038/s41390-020-0785-x. [DOI] [PubMed] [Google Scholar]
- Schlapbach LJ, MacLaren G, Festa M, et al. Australian & New Zealand Intensive Care Society (ANZICS) Centre for Outcomes & Resource Evaluation (CORE) and Australian & New Zealand Intensive Care Society (ANZICS) Paediatric Study Group. Prediction of pediatric sepsis mortality within 1 h of intensive care admission. Intensive Care Med. 2017;43:1085–1096. doi: 10.1007/s00134-017-4701-8. [DOI] [PubMed] [Google Scholar]
- Schlapbach LJ, Kissoon N. Defining Pediatric Sepsis. JAMA Pediatr. 2018;172:312–314. doi: 10.1001/jamapediatrics.2017.5208. [DOI] [PubMed] [Google Scholar]
- Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315:801–10. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlapbach LJ, Straney L, Bellomo R, et al. Prognostic accuracy of age-adapted SOFA, SIRS, PELOD-2, and qSOFA for in-hospital mortality among children with suspected infection admitted to the intensive care unit. Intensive Care Med. 2018;44:179–188. doi: 10.1007/s00134-017-5021-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369:2063. doi: 10.1056/NEJMc1312359. [DOI] [PubMed] [Google Scholar]
- Wynn JL, Polin RA. Progress in the management of neonatal sepsis: the importance of a consensus definition. Pediatr Res. 2018;83:13–15. doi: 10.1038/pr.2017.224. [DOI] [PubMed] [Google Scholar]
- Weiss SL, Peters MJ, Alhazzani W, et al. Surviving Sepsis Campaign International Guidelines for the Management of Septic Shock and Sepsis-Associated Organ Dysfunction in Children. Pediatr Crit Care Med. 2020;21:e52–e106. doi: 10.1097/PCC.0000000000002198. [DOI] [PubMed] [Google Scholar]
- Luthander J, Bennet R, Giske CG, et al. Trends of Pediatric Bloodstream Infections in Stockholm, Sweden: A 20-year Retrospective Study. Pediatr Infect Dis J. 2020;39(12):1069–1074. doi: 10.1097/INF.0000000000002850. [DOI] [PubMed] [Google Scholar]
- Carcillo JA. A synopsis of 2007 ACCM clinical practice parameters for hemodynamic support of term newborn and infant septic shock. Early Hum Dev. 2014:S45–7. doi: 10.1016/S0378-3782(14)70015-5. [DOI] [PubMed] [Google Scholar]
- Davis AL, Carcillo JA, Aneja RK, et al. American College of Critical Care Medicine Clinical Practice Parameters for Hemodynamic Support of Pediatric and Neonatal Septic Shock. Crit Care Med. 2017;45:1061–1093. doi: 10.1097/CCM.0000000000002425. [DOI] [PubMed] [Google Scholar]
- Weiss SL, Keele L, Balamuth F, et al. Crystalloid Fluid Choice and Clinical Outcomes in Pediatric Sepsis: A Matched Retrospective Cohort Study. J Pediatr. 2017;182:304–310.e10. doi: 10.1016/j.jpeds.2016.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emrath ET, Fortenberry JD, Travers C, et al. Resuscitation With Balanced Fluids Is Associated With Improved Survival in Pediatric Severe Sepsis. Crit Care Med. 2017;45:1177–1183. doi: 10.1097/CCM.0000000000002365. [DOI] [PubMed] [Google Scholar]
- Arikan AA, Zappitelli M, Goldstein SL, et al. Fluid overload is associated with impaired oxygenation and morbidity in critically ill children. Pediatr Crit Care Med. 2012;13:253–8. doi: 10.1097/PCC.0b013e31822882a3. [DOI] [PubMed] [Google Scholar]
- Sankar J, Ismail J, Sankar MJ, et al. Fluid Bolus Over 15-20 Versus 5-10 Minutes Each in the First Hour of Resuscitation in Children With Septic Shock: A Randomized Controlled Trial. Pediatr Crit Care Med. 2017;18:e435–e445. doi: 10.1097/PCC.0000000000001269. [DOI] [PubMed] [Google Scholar]
- Russell MJ, Kanthimathinathan HK. Is There an Optimum Duration of Fluid Bolus in Pediatric Septic Shock? A Critical Appraisal of “Fluid Bolus Over 15-20 Versus 5-10 Minutes Each in the First Hour of Resuscitation in Children With Septic Shock: A Randomized Controlled Trial” by Sankar et al (Pediatr Crit Care Med 2017; 18:e435-e445) Pediatr Crit Care Med. 2018;19:369–371. doi: 10.1097/PCC.0000000000001459. [DOI] [PubMed] [Google Scholar]
- Seri I, Evans J. Controversies in the diagnosis and management of hypotension in the newborn infant. Curr Opin Pediatr. 2001;13:116–23. doi: 10.1097/00008480-200104000-00005. [DOI] [PubMed] [Google Scholar]
- Gamper G, Havel C, Arrich J, et al. Vasopressors for hypotensive shock. Cochrane Database Syst Rev. 2016;2:CD003709. doi: 10.1002/14651858.CD003709.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Backer D, Biston P, Devriendt J, et al. SOAP II Investigators. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med. 2010;362:779–89. doi: 10.1056/NEJMoa0907118. [DOI] [PubMed] [Google Scholar]
- Avni T, Lador A, Lev S, et al. Vasopressors for the Treatment of Septic Shock: Systematic Review and Meta-Analysis. PLoS One. 2015;10:e0129305. doi: 10.1371/journal.pone.0129305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baske K, Saini SS, Dutta S, et al. Epinephrine versus dopamine in neonatal septic shock: a double-blind randomized controlled trial. Eur J Pediatr. 2018;177:1335–1342. doi: 10.1007/s00431-018-3195-x. [DOI] [PubMed] [Google Scholar]
- Gorantiwar S, de Waal K. Progression from sepsis to septic shock and time to treatments in preterm infants with late-onset sepsis. J Paediatr Child Health. 2021 doi: 10.1111/jpc.15606. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34:1589–96. doi: 10.1097/01.CCM.0000217961.75225.E9. [DOI] [PubMed] [Google Scholar]
- Weiss SL, Fitzgerald JC, Balamuth F, et al. Delayed antimicrobial therapy increases mortality and organ dysfunction duration in pediatric sepsis. Crit Care Med. 2014;42:2409–17. doi: 10.1097/CCM.0000000000000509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans IVR, Phillips GS, Alpern ER, et al. Association Between the New York Sepsis Care Mandate and In-Hospital Mortality for Pediatric Sepsis. JAMA. 2018;320:358–367. doi: 10.1001/jama.2018.9071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmatz M, Srinivasan L, Grundmeier RW, et al. Surviving Sepsis in a Referral Neonatal Intensive Care Unit: Association between Time to Antibiotic Administration and In-Hospital Outcomes. J Pediatr. 2020;217:59–65.e1. doi: 10.1016/j.jpeds.2019.08.023. [DOI] [PubMed] [Google Scholar]
- Bedetti L, Lugli L, Marrozzini L, et al. Safety and Success of Lumbar Puncture in Young Infants: A Prospective Observational Study. Front Pediatr. 2021;9:692652. doi: 10.3389/fped.2021.692652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berardi A, Zinani I, Rossi C, et al. Antibiotic Use in Very Low Birth Weight Neonates After an Antimicrobial Stewardship Program. Antibiotics (Basel) 2021;10:411. doi: 10.3390/antibiotics10040411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantey JB, Patel SJ. Antimicrobial stewardship in the NICU. Infect Dis Clin North Am. 2014;28:247–61. doi: 10.1016/j.idc.2014.01.005. [DOI] [PubMed] [Google Scholar]


