Abstract
COVID-19 pandemic revolutionized how cancer patients are treated worldwide. Regarding neuro-oncological patients, usually considered frail and with lower life expectancy than other oncological patients, the international scientific community had to urgently reorganize the treatment approach to minimize the risk of in-hospital contagious. For GBM patients, adjuvant treatments have been evaluated with even much more attention to the expected efficacy. As a consequence, a hypofractionated radiotherapy regimen has been preferred to reduce the daily hospital accesses and, especially in pMGMT unmethylated patients, chemotherapy with Temozolomide was avoided. Here, we made a comprehensive evaluation of the neurooncological community suggestions regarding GBM treatment in the pre-vaccine era of the COVID-19 pandemic. (www.actabiomedica.it)
Keywords: Glioblastoma Multiforme, Hypofractioned radiotherapy, Covid-19, In-hospital contagion, multimodal therapy
Introduction
Coronavirus disease 2019 (COVID-19) has severely threatened public health worldwide and changed how cancer patients (pts) are treated during the spread of the pandemic. In particular, during the first spread of the covid-19 pandemic, pts usually avoided in-hospital access with a consecutive delay in diagnosis, and interruption of therapy and follow-up. As a consequence, oncological pts were at risk of an increased incidence of mortality. On the other hand, due to immunodepression related to tumor disease and anti-cancer treatments, cancer pts are more vulnerable to COVID-19 and have a higher risk of severe complications entailing intensive care (1).
So, medical oncologists had to balance the survival benefit derived from oncological treatments and the risk of exposure because of in-hospital accesses to therapy administration and the possible contact with infected subjects. In addition, other risk factors are associated with the onset of serious symptoms such as older age of oncological pts, advanced stage of cancer, and cardiological and pulmonary comorbidities (for example the presence of lung metastases) (2,3). So, the entire oncological therapeutic approach changed.
Focusing on neurooncological pts, changes in therapeutic strategies involved especially GBM pts, considered frail and at risk of critical events from SARS-COV-2 infection due to several specific factors: age-related comorbidities, thromboembolism propensity due to cancer-related factors, chemotherapy-induced immunodepression, prolonged immobilization for sickness and disability, use of steroid therapy which increase the patient’s immunodepression making him more defenseless to infection. Furthermore, considering the poor life expectancy of these pts (4), in the pandemic era, the risk of contagion and related death has been carefully evaluated by the neuro-oncological scientific community to formulate guidelines to help clinicians. To date, despite this aim, whether to discontinue cancer treatment (such as chemotherapy) or not during the SARS-CoV-2 pandemic is still debated (1). In this regard, during the first spread of the pandemic, in the pre-vaccine era, there was an unmet need for class I evidence about modulating treatment and achieving the difficult aim of reducing the risk of contagion without compromising the oncological therapeutic efficacy (5,6).
GBM treatment during the pandemic in the pre-vaccine era
Surgical approach
Regarding surgical indications, maximal safe surgery should still be considered a priority due to the major improvement in survival determined by a maximum extent of resection (EOR). The EOR is usually verifiable within 48 hours after surgery with neuroimaging techniques (contrast-enhanced Magnetic Resonance Imaging, MRI). The goal of surgery should be to completely resect the tumor mass or at least to ensure the resection of more than 80%, as the percentage of mass removed is related to increased survival. On other hand, extended resection could lead to neurological deficits and compromise a patient ‘s quality of life. Therefore, the prognostic characteristics of the patient must be carefully evaluated to decide the best therapeutic strategy, considering that multimodal therapy in GBM consists of surgery integrated with radio-chemotherapy. To date, patients with neuroimaging features suggestive of GBM were directed to a neurosurgical evaluation even during the spread of the pandemic, except elderly patients for which biopsy can be omitted (6). In younger adults with good performance status, a delay of tumor exeresis could increase the risk of impairment of neurological status, quality of life, and survival. Moreover, surgery provides neoplastic tissue for both histological and molecular diagnoses. The knowledge of the O6-methylguanine DNA methyltransferase promoter (pMGMT) status can be critical in deciding who would benefit from TMZ chemotherapy, especially in a pandemic scenario (7). However, during the pandemic, surgery was aimed at controlling symptoms in pts with GMB aged more than 65 years, postponing asymptomatic elderly pts to avoid long stays in hospitalization (8).
Post-surgical management
Post-surgical management is guided by the prognostic evaluation that takes into account the molecular features of GBM, the EOR, the patient’s age, and performance status. This comprehensive evaluation helps clinicians in predicting the potential survival benefit derived from adjuvant treatments, thus justifying the risk of in-hospital contagion (6). Hence, newly diagnosed GBM pts without the methylation of pMGMT, who performed only biopsy or limited resection, aged more than 70 years with Karnofsky Performance Status (KPS) less than 70, should be directed to best supportive care (BSC), without performing active oncological treatments (9).
On the other hand, also pts suitable for oncological treatment have to be carefully evaluated to define the most adequate strategy. As well known, adjuvant treatment for GBM comprises multimodal therapy, consisting of concomitant radio-chemotherapy (fractionated total dose of 60 Gy, performed daily for six weeks) with concomitant orally administration of Temozolomide (TMZ) 75 mg/mq daily followed by adjuvant chemotherapy phase with TMZ at a higher dose (150 mg/mq for cycle 1 then, if well tolerated, 200 mg/mq) for 6-12 cycles, defined “Stupp regimen” (10). In elderly pts, commonly, the choice of a hypofractionated RT treatment (total dose of 40 Gy, administered in 15 daily fractions over three weeks) with concomitant and adjuvant TMZ maintains an oncological efficacy with a reduction of RT-related side effects (11,12). Shorter-course RT regimens of 35 Gy in 10 fractions have successful results but the addition of TMZ in these schedules has not been tested in randomized trials, unlike the 40 Gy in 15 fraction regimen and TMZ, which is associated with a survival advantage. Recommendations of Neuro-Oncology management suggest standard treatment for patients younger than 60–65 years with ECOG 0-1, and MGMT hypermethylated tumors. In selected cases of the non-extensive disease, Ultra-short Fractionation (25 Gy in 5 Fractions) can be considered despite limited experience (8). During pandemics, it may be challenging to maintain treatments over multiple weeks, and there is greater interest in hypofractionated regimens. To date, in an emergency scenario, there is a lack of scientific evidence concerning the treatment modulation, especially if focusing on GBM treatment. The only available data derive from previous disasters such as Hurricane Maria in Puerto Rico, a category 4 hurricane that led to a lack of electricity resulting in the longest blackout in US history, with an estimated $94 billion in damages. American Society for Radiation Oncology (ASTRO) cancer experts and radiation oncologists of Puerto Rico formulated a recommendation template based on the framework: PCOC (“Prepare, Communicate, Operate, Compensate”). In that circumstance, cancer pts who underwent daily radiotherapy should not have stopped it for a long time, to ensure locoregional control of the disease. Wherefore, radiation oncologists (RO) used 4 steps of PCOC in responding to the catastrophe. In the preparatory and communication phases RO appointed a Coordinator of Emergency Operations (EOC), a non-medical figure with a detailed knowledge of hospital administration and local resources. EOC was the primary point of contact with emergency management personnel, and then enabled communication between RO and patients. The patient has received his medical record in digital format and images (CT scan or MRI) to guarantee the continuation of treatment at another center. In successive phases, minimizing the gap in RT treatment was provided, about the stage of the disease, through altered fractionation or dose escalation (13). Nevertheless, the number of examined patients is too low to make scientific evidence-based recommendations but this work represents a measure to lessen the medical impact of a disaster (14). Furthermore, it has been taken into count that, during pandemics, the aim of oncological treatment and in particular RT, can not only be the improvement of survival but it is mandatory to consider the risk of contagion. RT could increase the risk of contagion due to the daily hospital access. Furthermore, the patient must be moved to a radiation facility either alone or with a proxy, or with a transportation service, and may need to be hospitalized. Moreover, most centers are restricting all visitors, which can add difficulty to GBM pts who have often cognitive and motor dysfunctions. In addition, elderly pts are more vulnerable and the risk of contagion and severe complications of COVID-19 should be balanced with the expected survival benefit from treatment. However, for GBM patients with rapidly progressing tumors, the risk of delaying or avoiding treatments must be counterbalanced with the risk of SARS-CoV-2 exposure or infection. Thus, to minimize exposure, reduce the risk of infection and increase the chance of completing a course of RT, hypofractionated schedules should be used as a standard in older and/or frail poor performance status patients (9). Regarding the indications of chemotherapy during the COVID-19 pandemic, the omission of concurrent and adjuvant temozolomide should be discussed in newly diagnosed IDH1/2 wild-type pMGMT unmethylated GBM pts (8). However, the classification of pMGMT unmethylated and pMGMT methylated GBM is too simplistic. Another prognostic score of pMGMT methylation (high, medium or low) is related to outcomes of patients treated with TMZ. The higher methylation score is associated with a greater benefit, the medium score (so-called “gray area”) is related with a partial benefit, while the lower score has no benefit from TMZ therapy. Nevertheless, in clinical practice TMZ is administered in pMGMT unmethylated GBM because in the binary system described above, partially methylated GBMs (which represent the majority of GBM) are placed in the group of pMGMT unmethylated GBMs. In randomized trials, these patients are included in the tails of the survival curves, showing a limited advantage in survival and justifying the prescription of TMZ for also pMGMT unmethylated GBM. Moreover, data from the CATNON trial (which compared the use of TMZ plus RT with observation in anaplastic astrocytoma) fail to find an improved survival after adjuvant therapy in IDH wild type GBM. Hence, in an era of a pandemic can be justified not to treat pts with IDH 1/2 wild-type pMGMT unmethylated due to the well-known minimum survival benefit added by TMZ, in subjects with a median survival of 1 year (15). To date, this subset of pts, if treated with TMZ, could be exposed to Sars-Cov-2 contagion due to hospital visits and, in the case of chemo-induced immunosuppression, to severe form of infectious, without gaining survival benefit (8). Another solution could be to shift infusional chemotherapies to oral therapies (such as TMZ), adopted in patients with Oligodendroglial tumors. In this setting, procarbazine/lomustine/vincristine (PCV) regimen and RT are the standard of care, but may cause severe side effects. Pulmonary fibrosis associated with procarbazine or lomustine could worsen SASARS-Cov-2-related lung fibrosis. In addition, PVC-induced hahematologicaloxicity implicates hospital visits for transfusions, not recommended in an era of limited resources pandemics (8).
Figure 1.
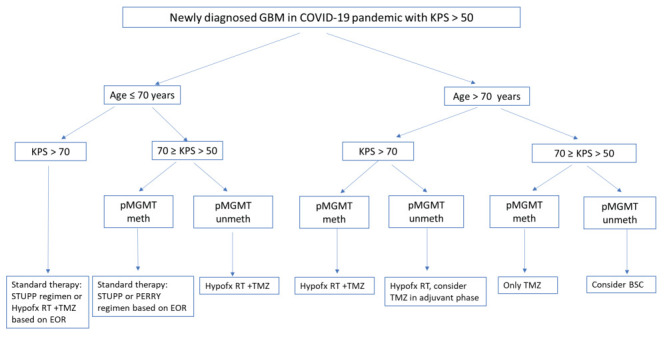
Therapeutic algorithm of newly diagnosed GBM pts in COVID-19 pandemic with KPS >50
Abbreviations: hypofx, hypofractionated; meth, methylated; TMZ, temozolomide, RT, radiotherapy
Another questioned topic is the use of steroids, especially in neuro-oncological pts. A great percentage of GBM pts needs steroid therapy to mitigate neurological symptoms mainly caused by cerebral edema and/or infiltrations of brain areas. Many studies show a detrimental effect of chronic dexamethasone on GBM outcomes Although data are inconclusive and mainly retrospective, steroids should be used as needed, but with caution during the SARS-CoV-2 pandemic. The beneficial anti-inflammatory and anti-oedematous effects should be weighed against the potentially detrimental effects of inhibiting antiviral immunity and immunosuppression (8). If, on one hand corticosteroids suppress the inflammatory pulmonary response, on other hand, they hinder the immune response contributing to serious clinical manifestations: bacterial and fungal superinfections needing mechanical ventilation or dialysis and often hesitate in septic or vasoplegic shock. In these cases, steroids are not helpful. Furthermore, survivors develop avascular necrosis, psychosis, and worsening diabetes (if preexisting). Thus, corticosteroid use in SARS-CoV-2 respiratory disease should be performed within clinical trials (16). However, independent of the pandemic, it is recommended to use the lowest dose useful for symptom control in neuro-oncological pts (8).
Discussion
In conclusion, the scientific community, in the pre-vaccine era of the COVID-19 pandemic, recommended standard treatments for patients younger than 65 years with good performance status and pMGMT methylated tumors. In elderly patients, a short course of RT (40.5 Gy/15 fractions) administered over 3 weeks, represents the treatment of choice, with or without concomitant and adjuvant TMZ, depending on the patient’s Karnofsky Performance Status score and pMGMT methylation status. Challenging topics include determining which patients require early treatment, how treatment can be delayed and how we can offer optimal care while minimizing the potential risk of infections. Maintaining physical distance, limiting unnecessary interactions between patients physicians can or nurses using teleconsultations, the existence of triage points the s at the entrance of hospitals and departments, wearing FFP2 for medical staff and patients during interviews (accordingly to World Health Organization indications), adherence to a protocol for hand hygiene and sanitizing surfaces are essential precaution to reduce the risk of contagion and the spread of the virus. the clinical practice changed when pandemics started in March 2020. Clinical research accrual ceased, and trials have ended prematurely, due to a lack of financial or institutional support. However, according to a survey on the impact of the COVID-19 pandemic on the field of neuro-oncology (8), the patient-doctor relationship is improved through telemedicine: better communication reduced waiting periods, decreased travel for caregivers, and patients with loss of autonomy. Nonetheless, the role of telemedicine should be assessed through a survey of older or lower-class patients. In the future, we could also understand benefits in terms of financial consequences for the health system, psychological satisfaction of the patient,and, above all, equate medical care. In oncology, even in the pandemic scenario, it is critical to define the aim of each treatment option and then design the strategy tailored to the individual patient, based on clinical characteristics and molecular features.
Acknowledgments:
The authors are grateful to the Pandora for Research, ONLUS Association for Oncology
Conflicts of interest:
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement, etc.) that might pose a conflict of interest in connection with the submitted article.
References
- Peng L, Zagorac S, Stebbing J. Managing patients with cancer in the COVID-19 era. Eur J Cancer. 2020;132:5–7. doi: 10.1016/j.ejca.2020.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Zhao Y, Okwan-Duodu D, Basho R. Cui X COVID-19 in cancer patients: risk, clinical features, and management. Cancer Biol Med. 2020;17(3):519–527. doi: 10.20892/j.issn.2095-3941.2020.0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazzato G, Foti C, Colagrande A, et al. Skin Manifestation of SARS-CoV-2: The Italian Experience J Clin. Med. 2021;10:566. doi: 10.3390/jcm10081566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado-López PD. Corrales-García EM Survival in glioblastoma: a review on the impact of treatment modalities. Clin Transl Oncol. 2016;18(11):1062–1071. doi: 10.1007/s12094-016-1497-x. [DOI] [PubMed] [Google Scholar]
- Weinkove R, McQuilten ZK, Adler J, et al. Managing hematology and oncology patients during the COVID-19 pandemic: interim consensus guidance. Med J Aust. 2020;212(10):481–489. doi: 10.5694/mja2.50607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessina F, Navarria P, Bellu L, et al. Treatment of patients with glioma during the COVID-19 pandemic: what we learned and what we take home for the future. Neurosurg Focus. 2020;49(6):E10. doi: 10.3171/2020.9.FOCUS20704. [DOI] [PubMed] [Google Scholar]
- Weller M, Preusser M. How we treat patients with a brain tumour during the COVID-19 pandemic. ESMO Open. 2020;4(Suppl 2):e000789. doi: 10.1136/esmoopen-2020-000789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt D, Wick W, Weiss SE, et al. Neuro-oncology Management During the COVID-19 Pandemic With a Focus on WHO Grade III and IV Gliomas [published online ahead of print, 2020 May 5] Neuro Oncol. 2020;22(7):928–935. doi: 10.1093/neuonc/noaa113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrugala MM, Ostrom QT, Pressley SM, et al. The state of neuro-oncology during the COVID-19 pandemic: a worldwide assessment. Neurooncol Adv. 2021;3(1):vdab035. doi: 10.1093/noajnl/vdab035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- Perry JR, Laperriere N, O’Callaghan CJ, et al. Short-Course Radiation plus Temozolomide in Elderly Patients with Glioblastoma. N Engl J Med. 2017;376(11):1027–1037. doi: 10.1056/NEJMoa1611977. [DOI] [PubMed] [Google Scholar]
- Pellerino A, Bruno F, Internò V, Rudà R, Soffietti R. Current clinical management of elderly patients with glioma. Expert Rev Anticancer Ther. 2020;20(12):1037–1048. doi: 10.1080/14737140.2020.1828867. [DOI] [PubMed] [Google Scholar]
- Gay HA, Santiago R, Gil B, et al. Lessons Learned From Hurricane Maria in Puerto Rico: Practical Measures to Mitigate the Impact of a Catastrophic Natural Disaster on Radiation Oncology Patients. Pract Radiat Oncol. 2019;9(5):305–321. doi: 10.1016/j.prro.2019.03.007. [DOI] [PubMed] [Google Scholar]
- Hegi ME, Stupp R. Withholding temozolomide in glioblastoma patients with unmethylated MGMT promoter-still a dilemma? Neuro Oncol. 2015;17(11):1425–1427. doi: 10.1093/neuonc/nov198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamson DO, Grossman SA. The Role of Temozolomide in Patients With Newly Diagnosed Wild-Type IDH, Unmethylated MGMTp Glioblastoma During the COVID-19 Pandemic. JAMA Oncol. 2021;7(5):675–676. doi: 10.1001/jamaoncol.2020.6732. [DOI] [PubMed] [Google Scholar]
- Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395(10223):473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


