Abstract
The aim of this study was to determine the relationship between physical fitness, cardiopulmonary function and patient-reported severity of symptoms in people with post-COVID-19 condition. We examined ambulatory patients (n = 72) with post-COVID-19 condition who had a chronic symptomatic phase lasting > 12 weeks from the onset of symptoms, but had not been hospitalized for acute COVID-19. A comprehensive medical screening was conducted, including clinical history, symptomatology, comorbidities, body composition and physical activity levels. We then identified the relationship between physical fitness (cardiorespiratory fitness and muscular strength), cardiopulmonary function (echocardiographic and spirometry parameters) and patient-reported severity of symptoms (fatigue, dyspnea, health-related quality of life, anxiety, and depression). Age, body mass index, sex, number of comorbidities and duration of symptoms were included as potential confounders. Results showed that greater physical fitness and cardiopulmonary function were associated with lower severity of symptoms in people with post-COVID-19 condition. Cardiorespiratory fitness, lower-limb muscle strength, maximal voluntary ventilation and left ventricular ejection fraction account for reducing fatigue and dyspnea. Greater physical activity levels were associated with fewer symptoms and less-severe fatigue and dyspnea. In conclusion, preserving better cardiopulmonary health and physical condition during the course of the disease—even in mild cases—was related to a lower intensity of symptoms in non-hospitalized people with post-COVID-19 condition. It is probable that exercise and physical conditioning are valuable pre- and post-COVID-19 countermeasures that could help decrease the severity, not only of acute infection, but of post-COVID-19 persistent symptoms and prognosis.
Keywords: Fatigue, Breathlessness, Post-exercise malaise, Long COVID-19, Post-COVID-19 syndrome, Rehabilitation
Introduction
The post-COVID-19 condition refers to individuals who have a history of probable or confirmed SARS-CoV-2 infection, usually 3 months from the onset of COVID-19, whose symptoms last for at least 2 months and cannot be explained by an alternative diagnosis [1]. This condition applies to a syndrome that includes a plethora of symptoms, such as post-exertional fatigue, dyspnea, headache, central nervous system, and neurocognitive impairment [2]. Consequently, people with post-COVID-19 condition suffer from a decreased quality of life [3] and physical activity levels [4], mostly due to intense daily fatigue that can occur even during light activities. Estimates on the breadth of this health problem are difficult since data on the prevalence of the disease vary depending on the diagnosis definition and patient characteristics [5], but the risk of persistent symptoms is present in both hospitalized [6] and non-hospitalized [7] patients.
Mechanisms underlying the post-COVID-19 condition are not completely understood [8, 9] and there is not sufficient evidence to fully and confidently support emerging pharmacological treatments [10]. Therefore, until a treatment is found, effective symptom-maintenance strategies should be adopted to help protect people from developing pertinacious symptoms and related health and socioeconomic complications after acute SARS-CoV-2 infection.
Although not much research exists on preventive strategies, it is suggested that physical exercise may offer protection in case of COVID-19 infection by improving many of the symptoms and reducing the long-term effects of the virus [11]. Among other mechanisms, exercise stimulates a systemic anti-inflammatory environment by decreasing circulating inflammatory molecules [12]. In addition, exercise improves immune function by improving immunosurveillance against viral and bacterial infections [13]. Furthermore, data suggest that exercise may augment vaccine efficacy by increasing the antibody titter response as it does in influenza vaccines [14].
Along the same line of thought, physical inactivity and low exercise capacity are associated with a higher risk for severe COVID-19 outcomes [15, 16]. These findings support the fact that people with better physical capacity might present an important advantage in preserving their health status and suffering from less-severe acute manifestations after contracting the virus. Furthermore, in the absence of structural or functional organ damage [17], the chronic nature of symptoms may be explained by impaired exercise capacity, reduced muscle mass, and alterations in skeletal muscle metabolism [18, 19]. Therefore, improving skeletal muscle function and increasing physical activity levels, exercise capacity, and cardiorespiratory fitness before contracting COVID-19 and soon after the acute phase of the infection, may help better manage the severity of post-COVID-19 condition.
In this study, we evaluated non-hospitalized people with post-COVID-19 condition to identify the relationship between physical fitness (physical activity levels, cardiorespiratory fitness, and muscular strength), cardiopulmonary function (echocardiographic and spirometry parameters), and patient-reported severity of symptoms (fatigue, dyspnea, health-related quality of life, anxiety, and depression).
Materials and methods
Study design and recruitment
This observational cross-sectional study has been conducted according to the STROBE statement. Participants were recruited through advertisements on social media or via recommendations from general practitioners. Inclusion criteria were; subjects aged over 18 years old who had a confirmed microbiological diagnosis of COVID-19 by SARS-CoV2 reverse transcription-polymerase chain reaction on an oropharyngeal-nasopharyngeal swab or a positive rapid antigen test, who presented a chronic symptomatic phase lasting > 12 weeks from the onset of symptoms and had not been hospitalized because of the acute COVID-19 infection. In case of reinfection, patients were recruited if symptoms consistent with a post-COVID-19 condition persisted after a further 12 weeks. Those with evidence of COVID-19 pneumonia needed a Brixia score ≤ 5 [20] and to show total recovery of pulmonary function and radiological follow-up. We excluded pregnant patients, and those who had acute or unstable chronic diseases such as unstable myocardiopathy, ischemic heart disease, uncontrolled hypertension, uncontrolled chronic obstructive pulmonary disease (COPD), or major surgery in the past 3 months. Written informed consent was obtained from all study participants. The study was approved by an ethical review board (Murcia University Ethics Committee No. 3447/2021).
Measurements
Baseline characteristics
Participants initially completed a clinical evaluation that included an interview, physical examination and standardized questionnaire on medical history, conducted by an internal medicine specialist (infectious diseases consultant) and a cardiology team. The participants were asked about the presence of the 22 most frequent symptoms reported in the largest population survey of patients with long-term COVID-19 carried out in Spain [21]. Body composition and body mass index (BMI) were measured by a multi-frequency segmental body composition analyzer (Tanita MC-780U, Tokyo, Japan). Blood tests (cardiac and muscle injury markers, coagulation and inflammatory markers), spirometry, resting electrocardiogram, and echocardiography were also performed to rule out any potential major cardiopulmonary issues. Physical activity levels were recorded by patient-reported Global Physical Activity Questionnaire [22], and compared with the most recent World Health Organization guidelines for adults [23].
Severity of symptoms
Patient-reported outcomes (PROs) included: Health-related quality of life by the 12-item Short Form Survey (SF-12) [24], calculating the mental component (MCS) and physical component scores (PCS). Population-based norms of the regional population [25] were used for secondary analyses. Anxiety and Depression, by the General Anxiety Disorder 7 Questionnaire GAD-7 [26] and the Patient Health Questionnaire-9 (PHQ-9) [27]. A cut-off score for moderate-severe depression and anxiety ≥ 10 points was considered for secondary analyses. Dyspnea, by the Modified Medical Research Council Dyspnea l Scale (mMRC) [28]. A cut-off score for severe breathlessness ≥ 2 was considered for secondary analyses. Fatigue, by the Chalder Fatigue Scale (CFQ-11) with the bimodal scoring system [29] and the Fatigue Severity Scale (FSS) [30]. Scores of ≥ 4 indicate severe fatigue [31]. The DePaul Symptom Questionnaire Short Form (DSQ-14 short form) [32] was used to screen myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) symptoms by measuring its frequency and severity over the past 6 months. Functional limitations after COVID-19, by the Post-COVID-19 Functional Status (PCFS) scale [33].
Physical fitness
Participants completed a submaximal multistage and individualized cardiopulmonary exercise test on a cycle ergometer (Ergoline, Ergoselect 200) while wearing heart rate (HR) monitors (Polar V800, Kempele, Finland) and reporting their rate of perceived exertion (RPE 6–20) according to the Ekblom-Bak protocol [34]. Mean heart rate during the last minute at the higher work rate was recorded. Maximal oxygen consumption (VO2max) was estimated by sex-specific equations and compared against the population norms, further adjusted by sex and age [35].
Muscular strength measures included a handgrip test using a calibrated digital dynamometer (Takei 5401-C, Shinagawa-Ku, Tokyo), the 5-time sit-to-stand test, a 3 s isometric knee extension test at 110° of knee flexion angle using a force sensor (Chronojump, BoscoSystem, Barcelona) recording in Newtons (N) [36], and a progressive submaximal loading test in Smith machine for the bench press (BP) and half squat (HSQ) exercises using a linear velocity transducer (T-Force, Ergotech Consulting, Murcia, Spain) [37]. The progressive loading tests were performed from a starting load of 5 kg and increasing up to reaching a target mean propulsive velocity corresponding to ~ 50% of the 1-repetition maximum effort (1RM).
Cardiopulmonary function
A resting echocardiogram was performed following standard procedures [38] by a team of cardiologists, using the same ultrasound system (Philips CX50; Guilford, England), methodology, and interpretation in all patients. Left ventricular ejection fraction (LVEF) and end-diastolic volumes (LVEDV), tricuspid annular plane systolic excursion (TAPSE), and the peak early diastolic velocity and late diastolic velocity ratio (E/A) measures were considered for secondary analyses [19]. Participants completed a forced spirometry test (MetaLyzer 3B-R3, Cortex Biophysik GmbH, Leipzig, Germany) following standardized procedures [39]. Data from the forced vital capacity (FVC), forced expiratory volume at the end of the first second (FEV1), forced expiratory flow rate at the mid-portion of FVC (FEV 25–75%), and maximum voluntary ventilation (MVV) were collected.
Statistical analysis
The required sample size was determined based on the population affected by SARS-CoV2 infection in the Region of Murcia in October 2020 (21,198 cases), for an estimated prevalence of post-COVID-19 syndrome in the non-hospitalized population of 1% [40] and assuming a confidence interval of 95% and margin of error of 10%. The estimated size was 67 participants. Continuous variables were presented as mean and standard deviation (SD) or median and interquartile range (IQR). Binary and categorical variables were presented as counts and percentages. When possible, all values were compared against the population norms, adjusted for sex, age, BMI, and/or height. The exploratory analysis included Chi-Squared tests and correlations to examine the associations between variables, and Student’s t tests were used to compare mean differences. Effect size was calculated according to Cohen’s d (large 0.80, medium 0.50, and low 0.20) and Cramer’s V (large 0.50, medium 0.30, and low 0.10). Multiple linear regression analyses were conducted to identify the associations of symptom severity with physical fitness and cardiopulmonary function outcomes. Regression models (enter method) were adjusted for age, sex, BMI, number of symptoms and number of comorbidities. Multicollinearity was determined by calculating the variance inflation factors, whereby data was excluded if > 5. The level of significance was set at p < 0.05. Calculations were performed using SPSS 24.0 (IBM Corp., Armonk, NY) for Windows.
Results
From 157 participants who expressed interest in the study, 72 met the inclusion/exclusion criteria and were enrolled in the study from February to November 2021. Participant characteristics, clinical history, and symptomatology are presented in Table 1. Since vaccination in Spain on non-priority groups was initiated on June 2021, 40 out of 48 (83.3%) recruited participants from that time were vaccinated with at least one dose.
Table 1.
Characteristics, medical screening, clinical history and symptomatology of people with post-COVID-19 condition (n = 72)
| Characteristics | Value | Characteristics | Value |
|---|---|---|---|
| Age (years) | 45.5 ± 9.0 | Symptom’s length (weeks) | 36.3 ± 21.1 |
| Sex | Number of symptoms (n) | 7.7 ± 3.4 a,b | |
| Men | 25 (34.7) | Symptoms (n) | |
| Women | 47 (65.3) | Fatigue | 60 (83.3) |
| Occupational status (n) | Dyspnea | 49 (68.0) | |
| Employed | 38 (52.8) | Memory problems or confusion | 44 (61.1) |
| Sick leave | 25 (34.7) | Insomnia or sleep disturbances | 42 (58.3) |
| Unemployed | 9 (12.5) | Lack of concentration | 41 (56.9) |
| Body composition | Brain fog | 40 (55.5) | |
| Body mass (kg) | 74.7 ± 14.3 | Myalgia | 40 (55.5)c |
| Height (cm) | 166.4 ± 9.0 | Low mood | 38 (52.7)d |
| BMI (kg·m−2) | 26.9 ± 4.8 | Headache | 34 (47.2) |
| Fat mass (%) | 31.7 ± 8.2 | Anxiety | 31 (43.0)d |
| Lean body mass (kg) | 49.8 ± 10.5 | Loss of smell/taste | 25 (34.7) a |
| Toxic Habits (n) | Hair loss | 21 (29.1) a | |
| Alcohol | 8 (11.1) | Dizziness | 19 (26.3) |
| Drink units (SDU) | 3.75 (1.9) | Chest pain | 19 (26.3) a |
| Active smoker | 4 (8.0) | Palpitations | 17 (23.6) |
| Former smoker | 22 (30.5) | Abdominal pain | 12 (16.6) |
| Comorbidity (n) | Diarrhea | 11 (15.2) | |
| Any comorbidity | 42 (58.3) | Loss of appetite | 11 (15.2) |
| Psychiatric conditions | 28 (38.8) | Low grade fever | 10 (13.8) |
| Asthma | 11 (15.2) | Weight loss | 10 (13.8) |
| Hypertension | 6 (8.3) | Cough | 10 (13.8) a |
| Structural heart disease | 4 (5.5) | Nausea and/or vomiting | 3 (4.1) |
| Diabetes | 3 (4.1) | Evolution (n) | |
| COPD | 2 (2.7) | Fluctuating course | 36 (50.0) |
| Medication (n) | Progressive improvement | 53 (73.6) | |
| Taking medication | 49 (69.0) | Vaccination | 40 (56.6) |
| Antidepressants | 21 (29.1) | Reinfection | 7 (9.7) |
| Benzodiazepines | 21 (29.1) | Physical activity (min·wk-1) | |
| Bronchodilators | 15 (20.8) | Moderate | 268 ± 343 |
| Thorax X-ray at diagnosis (n) | 52 (72.2) | Vigorous | 83 ± 213 |
| Pneumonia (n) | 6 (8.3) | Meet OMS recommendations | 42 (58.3) |
| Sedentary (hours) | 6.5 ± 3.6 |
aSignificant sex differences (p < 0.05)
bSignificant association with physical activity levels (p < 0.05)
cSignificant association with vaccination (p < 0.05)
dSignificant association with previous psychiatric conditions (p < 0.05). Data are means and standard deviation (M ± SD) or frequencies and percentages (n (%))
BMI Body mass index, SDU (Standard Drink Unit) Spanish standard unit is equivalent to 10 g of alcohol, COPD Chronic Obstructive Pulmonary Disease
Women presented a larger number of symptoms (8.7 ± 3.4 vs. 6.0 ± 2.6, p < 0.001, d = 0.90) and more prevalence of loss of smell/taste (47.0% vs 12.0%, p = 0.005, V = 0.27), hair loss (43.0% vs 4.0%, p = 0.002, V = 0.37), chest pain (36.0 vs. 8.0%, p = 0.021, V = 0.33) and cough (21.0 vs. 0%, p = 0.001, V = 0.30) than men. Those participants who had previous psychopathology, presented significantly more frequency of mood disorders (anxiety and low mood, p < 0.01), as well as a tendency to neurocognitive limitation in the domains consulted [lack of concentration (p = 0.055), poor speech skills (p = 0.051), memory loss (p = 0.082) and sleep disorders (p = 0.055)]. Thirty-two participants (44%) met the WHO physical activity guidelines and presented a lower number of symptoms compared to those who did not meet the recommendations (6.2 ± 3.2 vs. 9.1 ± 3.2, p < 0.001, d = 0.89).
The prevalence of clinically significant punctuations of depression, anxiety and severe breathlessness were 68.1, 47.2 and 43.1%, respectively. Severe fatigue by means of CSF and FSS scales was presented in 90.3 and 81.7% of participants, respectively. Quality of life (physical and mental health domains) were under population values in 87.5 and 91.7% of the cases respectively. When compared to the population norm, 49.2% of cases were below the mean in VO2max and 42.2% in hand grip strength. Regarding the pulmonary function, FVC, FEF1 and MVV were 66.7, 27.8 and 60.6% under the expected values.
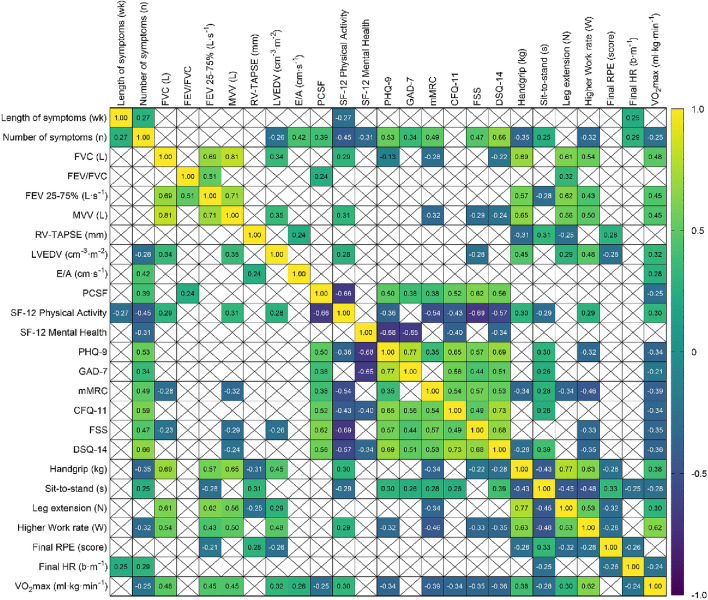
Results from physical fitness, cardiopulmonary function, and severity of symptom outcomes are presented in Table 2. Patients with a larger number of symptoms reported greater severity in all the PROs examined (r from 0.31 to 0.67; p < 0.003). In turn, people with higher physical activity levels reported a lower severity (p < 0.025); in particular, the greater the number of moderate and vigorous physical activity minutes, the lower the scores on the fatigue scales (CFQ-11, r = − 0.27 and − 0.31), dyspnea (mMRC, r = − 0.27 and − 0.24), and depression (PHQ-9, r = − 0.27 and − 0.30). Correlations between variables are shown in Fig. 1.
Table 2.
Physical fitness, cardiopulmonary function and severity of symptoms in people with post-COVID-19 condition (n = 72)
| Physical fitness | Value | Cardiopulmonary function | Value | Severity of symptoms | Value |
|---|---|---|---|---|---|
| Initial work rate (W) | 73.4 ± 23.7 | FVC (L) | 3.7 ± 0.9 | CFQ-11 | 8.0 ± 3.0 |
| Higher work rate (W) | 98.6 ± 25.9 | % FVC | 95.8 ± 14 | FSS | 5.3 ± 1.4 |
| Initial RPE | 12.5 ± 2.0 | FEV-1 (L) | 3.1 ± 0.8 | DSQ-14 | 55.3 ± 18.4 |
| Initial HR (b·m−1) | 121.3 ± 13.9 | % FEV-1 | 104.8 ± 17.1 | PCFS | 2.4 ± 0.9 |
| Final HR (b·m−1) | 143.6 ± 16.0 | FEV/FVC | 84.5 ± 7.2 | mMRC | 1.4 ± 0.9 |
| Final RPE | 15.1 ± 1.5 | FEV 25–75% (L·s−1) | 3.7 ± 1.2 | SF-12 PCS | 35.9 ± 11.1 |
| VO2max (ml·kg·min−1) | 35.8 ± 10.4 | MVV (L) | 102.2 ± 38.4 | SF-12 MCS | 41.8 ± 12.0 |
| Sit-to-stand (s) | 7.6 ± 2.8 | % MVV | 87.2 ± 23.1 | PHQ-9 | 12.1 ± 5.6 |
| Handgrip (kg) | 32.8 ± 10.1 | LVEF (%) | 61.6 ± 5.1 | GAD-7 | 9.4 ± 5.2 |
| BP-50% 1RM (kg) | 17.3 ± 9.3 | RV-TAPSE (mm) | 24.0 ± 3.1 | ||
| HSQ-50% 1RM (kg) | 17.8 ± 14.2 | LVEDV (cm−3m−2) | 48.4 ± 10.1 | ||
| Leg extension (N) | 416.9 ± 148.9 | E/A (cms−1) | 1.3 ± 0.5 |
Data are means and standard deviation (M ± SD) or frequencies and percentages (n (%))
HR Heart rate. VO2max: Maximum oxygen uptake estimated by sex-specific equations [34]. HG Handgrip, BP Bench Press, HSQ Half Squat, FVC Forced ventilatory capacity, FEV Forced expiratory volume, MVV Maximum voluntary ventilation, LVEF Left Ventricular Ejection Fraction, RV-TAPSE Right ventricular- Tricuspid Annular Plane Systolic Excursion, LVEDV Left Ventricular End Diastolic Volume, E/A ratio early diastolic velocity (E), late diastolic velocity (A), CFQ-11 Chalder Fatigue Scale, FSS Fatigue Severity Scale, DSQ-14 The DePaul Symptom Questionnaire, PCFS Post-COVID-19 Functional Status scale, mMRC Modified Medical Research Council dyspnea scale, PCS Physical health component score, MCS Mental health component score, PHQ Patient Health Questionnaire, GAD Generalized Anxiety Disorder scale
Fig. 1.
Heatmap shows the correlation between severity of symptoms, physical fitness, and cardiopulmonary function in people with post-COVID-19 condition (n = 72). Crossed (X) squares mean non-significant associations (p > 0.05)
Linear regression analyses adjusted for sex, age, BMI, number of comorbidities and number of symptoms (Table 3) identified significant negative associations (p < 0.024, R2 > 0.28) between physical fitness, cardiopulmonary function, and the severity of particular symptoms. mMRC was negatively associated with VO2max, the final HR of the Ekblom-Bak test, MVV, and LVEF%. FSS was associated with HSQ-50%1RM, and MVV. Bimodal CFQ-11 was associated with final RPE score and FVC. DSQ-14 was associated with RPE, MVV, and LVEF. The analysis of cofounder variables confirmed the exploratory correlation results, showing that the larger the number of symptoms, the greater the severity in all the models explored (p < 0.037). No significant relationships were found for anxiety (PHQ-9) or depression (GAD-7) scales.
Table 3.
Multiple linear regression examining the associations of symptoms severity with physical fitness, and cardiopulmonary function in people with post-COVID-19 condition (n = 72)
| Dyspnea | Fatigue | |||||||
|---|---|---|---|---|---|---|---|---|
| mMRC | CFQ-11 | FSS | DSQ-14 | |||||
| β | p | β | p | β | p | β | p | |
| Physical fitness | ||||||||
| Higher Work rate (W) | − 0.04 | 0.869 | 0.07 | 0.770 | − 0.21 | 0.364 | − 0.17 | 0.377 |
| Final HR (b·m−1) | − 0.67 | 0.019* | − 0.11 | 0.700 | − 0.41 | 0.138 | − 0.34 | 0.151 |
| Final RPE (score) | − 0.24 | 0.211 | − 0.37 | 0.053 | − 0.21 | 0.240 | − 0.31 | 0.048* |
| VO2max (ml·kg·min−1) | − 1.12 | 0.027* | − 0.14 | 0.762 | − 0.36 | 0.445 | − 0.01 | 0.980 |
| Sit-to-stand (s) | 0.01 | 0.943 | 0.25 | 0.221 | − 0.08 | 0.674 | 0.21 | 0.202 |
| Handgrip (kg) | − 0.37 | 0.286 | 0.29 | 0.382 | − 0.32 | 0.338 | 0.14 | 0.616 |
| BP-50% 1RM (kg) | − 0.14 | 0.783 | − 0.72 | 0.150 | 0.22 | 0.648 | − 0.09 | 0.820 |
| HSQ-50% 1RM (kg) | − 0.18 | 0.578 | 0.39 | 0.220 | − 0.64 | 0.046* | − 0.14 | 0.587 |
| Leg extension (N) | − 0.05 | 0.847 | 0.08 | 0.749 | 0.37 | 0.118 | 0.24 | 0.237 |
| Adjusted R2 of the model | 0.28 | 0.30 | 0.33 | 0.51 | ||||
| Cardiopulmonary function | ||||||||
| FVC (L) | − 0.10 | 0.644 | − 0.57 | 0.021* | 0.30 | 0.133 | 0.22 | 0.260 |
| FEV-1 (L) | − 0.24 | 0.634 | − 0.47 | 0.376 | 0.44 | 0.345 | 0.40 | 0.373 |
| FEV/FVC | 0.04 | 0.768 | 0.16 | 0.278 | 0.21 | 0.112 | 0.06 | 0.620 |
| FEV 25–75% (L·s−1) | 0.31 | 0.112 | − 0.14 | 0.496 | 0.05 | 0.777 | − 0.06 | 0.710 |
| MVV (L) | − 0.57 | 0.013* | − 0.29 | 0.241 | − 0.76 | < 0.001* | − 0.46 | 0.024* |
| LVEF (%) | − 0.24 | 0.038* | − 0.01 | 0.986 | − 0.08 | 0.459 | − 0.21 | 0.049* |
| RV-TAPSE (mm) | − 0.05 | 0.717 | − 0.10 | 0.480 | 0.08 | 0.537 | 0.05 | 0.692 |
| LVEDV (cm−3·m−2) | 0.07 | 0.519 | 0.07 | 0.542 | − 0.01 | 0.945 | 0.15 | 0.125 |
| E/A (cm·s−1) | 0.16 | 0.294 | − 0.14 | 0.400 | − 0.19 | 0.184 | − 0.14 | 0.289 |
| Adjusted R2 of the model | 0.47 | 0.36 | 0.55 | 0.58 | ||||
β indicates standardized regression coefficients with significance levels of t. All the analyses were adjusted for age, body mass index, sex, number of comorbidities, and number of symptoms. All adjusted R2 values were significant; all P < 0.010
Variables excluded for collinearity (VIF > 10): Initial Work rate, Initial RPE, Initial HR, %FVC, FEV-1%, FEV-1, FEV/FVC, and %MVV
HR Heart rate, VO2max Maximum oxygen uptake estimated by sex-specific equations [34], HG Handgrip, BP Bench Press, HSQ Half Squat, FVC Forced ventilatory capacity, FEV Forced expiratory volume, MVV Maximum voluntary ventilation, LVEF Left Ventricular Ejection Fraction, RV-TAPSE Right ventricular-Tricuspid Annular Plane Systolic Excursion, LVEDV Left Ventricular End Diastolic Volume, E/A ratio early diastolic velocity (E), late diastolic velocity (A), CFQ-11 Chalder Fatigue Scale (bimodal), FSS Fatigue Severity Scale, DSQ-14 The DePaul Symptom Questionnaire (composite), mMRC Modified Medical Research Council) dyspnea scale
Discussion
This study examined the relationships between cardiorespiratory fitness and the severity of post-COVID-19 symptoms in an ambulatory cohort of people with persistent symptoms. The main contributions were: (1) better physical conditioning was associated with less-severe symptoms (VO2max and leg strength correlates with dyspnea and fatigue, respectively); (2) higher physical activity levels were associated with a smaller number of symptoms; (3) the number of symptoms influenced the perception of their intensity (the larger the number, the more the severity); (4) physical conditioning tests may provide a detailed evaluation of patients with a post-COVID-19 condition even if the usual complementary medical tests (spirometry, echocardiogram and chest X-ray) are normal.
The epidemiological characteristics of the patients participating in the study are comparable to the outpatients with post-COVID-19 condition previously described [7, 41, 42]. To date, our cohort includes out-of-hospital patients with longer-lived persistent symptoms. The female: male ratio and the mean number of symptoms are similar to those previously described [43]. This suggests that, regardless of the current epidemiological situation, of the characteristics of health systems, social and cultural influences, post-COVID-19 condition is understood in the same way all over the world.
One of the direct consequences of the persistence of symptoms is the undoubted impact on quality of life, since work activities (sick leave and unemployed represents almost a half of the sample), leisure activities, and daily life are interfered with. Physical and mental health domains were below the norm in most of participants (87.5 and 91.7% respectively), similar to earlier studies (83.3% reported moderate-to-poor self-reported health after 6 months) [44]. Physical activity, in particular, has been seriously compromised [45], especially during 2020, coinciding with the first and second waves of COVID-19, when 72% of our patients were diagnosed.
Our findings reflected that higher physical fitness and cardiopulmonary function were associated with lower severity of symptoms (Table 3). People with post-COVID-19 syndrome who met the global physical activity guidelines presented less symptoms. In turn, having fewer hours of moderate and/or vigorous physical activity increased the severity of manifestations. Because physical activity levels are conditioned by peoples’ physical and cardiopulmonary capacity, a worse physical condition seems to negatively affect the symptomatic perception, mainly in the case of dyspnea and post-exercise fatigue. Negative correlations and strength of associations between the number of symptoms and physical fitness, strength, and cardiopulmonary health might suggest a causal relationship. Therefore, people with mild COVID-19, who develop severe or limiting clinical symptoms in the persistent phase, would not necessarily be more physically limited by the symptoms themselves, but would be moderated by how fit they were when they acquired the disease. Ongoing randomized control trials [46] will try to prove that better pre-existing or acquired cardiopulmonary fitness and strength benefits from a lower intensity of symptoms.
In support of these findings, previous studies demonstrated that patients with mild forms of acute illness and post-COVID-19 condition presented evident physical restrictions on maximal [47] and submaximal [47, 48] exercise capacity and an increased ventilatory inefficiency [49]. The estimated loss of cardiopulmonary fitness (VO2max) in young, healthy, well-trained subjects with mild forms of COVID-19 has been estimated at approximately 10% 2 months after the acute phase [50]. Accordingly, dyspnea and fatigue in individuals with post-COVID-19 condition could be explained by a reduced oxygen diffusion in peripheral circulation even in the absence of cardiac, pulmonary, or major ventilatory limitations, as suggested earlier [18, 19, 49, 51]. Other mechanisms involved in the poor tolerance to effort have turned out to be the presence of chronotropic incompetence and insufficient increase in systolic volume during effort [51, 52].
In our cohort, lung function, on average, was within the expected normal limits, but we cannot rule out that ventilatory limitations may have partly influenced exercise intolerance, as over six out of ten patients were under normal MVV population norms. This suggests that physical deconditioning of the inspiratory pump and poor respiratory muscle endurance can justify the presence of dyspnea and fatigue as a moderately strong linear relationship exists between MVV and mMR and FSS respectively.
The perception of symptoms may be influenced by a deteriorated mental health status although we did not find this relationship in our linear regression models. Our study suggested that our non-hospitalized patients with post-COVID-19 condition are likely to suffer from moderate anxiety and depression and other neurocognitive symptoms, especially if they had a prior history of both conditions. A clinically significant level of depressive, anxiety, and post-traumatic stress symptoms related to several symptoms were reported by others in patients with mild COVID-19 [53].
The possibility that these patients present multisystem involvement (pulmonary, cardiac, muscular or central nervous system) advises a multidisciplinary approach. The high symptom burden and psychological and functional limitations of post-COVID-19 condition patients demonstrates that prompt and proactive identification of these individuals is warranted [9]. As aerobic capacity and lower extremity strength tests appear to be reliable markers of symptom burden and intensity of post-COVID-19 dyspnea and fatigue, we propose to incorporate physical fitness measures to assess health status in addition to conventional cardiopulmonary tests (spirometry and echocardiography) in these individuals.
As others have previously proposed [9], early and individualized rehabilitation is key to functional recovery after COVID-19. It is reasonable that a better physical condition may influence the evolution of the SARS CoV-2 infection, favoring a better prognosis, even in a complex syndrome like the post-COVID-19 condition. According to our findings and available literature, we propose an exercise program based on resistance and cardiovascular training, combined with inspiratory muscle exercises, to improve health markers that mostly determine the presence of symptoms. Hence, future studies are warranted to determine whether exercise may constitute a cost-efficient alternative for the treatment of the post-COVID-19 conditions sequelae.
Limitations
As it is a limited-size, single-center project, the results may not be directly applicable to other population cohorts. As such, we cannot generalize our results to all COVID-19 patients, irrespective of the age, disease severity or need for hospitalization. Furthermore, participants did not undergo an exercise stress test before infection, so changes from their baseline values were not assessed and the impact of pre-existing cardiopulmonary impairments is unknown. Although all patients had a follow-up chest X-ray, only one third (20/72, 27, 8%) underwent a chest CT-scan, therefore the residual lesions not previously appreciated could be partly responsible for the symptoms, in particular, dyspnea. However, none of the participants presented residual lesions in the chest CT performed.
Conclusions
Poor cardiopulmonary fitness and muscle strength were associated with greater severity of persistent symptoms in patients with post-COVID-19 condition. Higher levels of physical activity were associated with a smaller number of symptoms. Improving the physical condition and strength of patients through an early, tailored exercise intervention could be an effective strategy to better manage the post-COVID-19 condition.
Funding
This work was supported by the Hospital Médico Virgen de la Caridad (ID: 35110/2020).
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Statements
Study design and recruitment.
Footnotes
The original online version of this article was revised: The affiliation of author James J. Tufano has been corrected in the original article.
The article belongs to COVID 19.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
9/17/2022
A Correction to this paper has been published: 10.1007/s11739-022-03089-4
References
- 1.WHO Headquarters (HQ) (2021) A clinical case definition of post COVID-19 condition by a Delphi consensus [DOI] [PMC free article] [PubMed]
- 2.Nasserie T, Hittle M, Goodman SN. Assessment of the frequency and variety of persistent symptoms among patients with COVID-19: a systematic review. JAMA Netw Open. 2021;4:e2111417. doi: 10.1001/jamanetworkopen.2021.11417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Malik P, Patel K, Pinto C, et al. Post-acute COVID-19 syndrome (PCS) and health related quality of life (HRQoL)—A systematic review and meta-analysis. J Med Virol. 2021 doi: 10.1002/jmv.27309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Delbressine JM, Machado FVC, Goërtz YMJ, et al. The impact of post-covid-19 syndrome on self-reported physical activity. Int J Environ Res Public Health. 2021 doi: 10.3390/ijerph18116017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hayes LD, Ingram J, Sculthorpe NF. More than 100 persistent symptoms of SARS-CoV-2 (long COVID): a scoping review. Front Med. 2021 doi: 10.3389/fmed.2021.750378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang X, Wang F, Shen Y, et al. Symptoms and health outcomes among survivors of COVID-19 infection 1 year after discharge from hospitals in Wuhan, China. JAMA Netw Open. 2021;4:1–11. doi: 10.1001/jamanetworkopen.2021.27403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Søraas A, Kalleberg KT, Dahl JA, et al. Persisting symptoms three to eight months after non-hospitalized COVID-19, a prospective cohort study. PLoS ONE. 2021;16:1–13. doi: 10.1371/journal.pone.0256142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fogarty H, Townsend L, Morrin H, et al. Persistent endotheliopathy in the pathogenesis of long COVID syndrome. J Thromb Haemost. 2021;19:2546–2553. doi: 10.1111/jth.15490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yan Z, Yang M, Lai CL. Long COVID-19 syndrome: a comprehensive review of its effect on various organ systems and recommendation on rehabilitation plans. Biomedicines. 2021 doi: 10.3390/BIOMEDICINES9080966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Utrero-Rico A, Ruiz-Ruigómez M, Laguna-Goya R, et al. (2021) A short corticosteroid course reduces symptoms and immunological alterations underlying long-COVID. Biomedicines. 2021;9:1540. doi: 10.3390/BIOMEDICINES9111540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jimeno-Almazán A, Pallarés JG, Buendía-Romero Á, et al. Post-covid-19 syndrome and the potential benefits of exercise. Int J Environ Res Public Health. 2021;18:5329. doi: 10.3390/ijerph18105329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Severinsen MCK, Pedersen BK. Muscle-organ crosstalk: the emerging roles of myokines. Endocr Rev. 2020;41:594–609. doi: 10.1210/endrev/bnaa016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nieman DC. Exercise is medicine for immune function: implication for COVID-19. Curr Sports Med Rep. 2021;20:395–401. doi: 10.1249/JSR.0000000000000867. [DOI] [PubMed] [Google Scholar]
- 14.Hallam J, Jones T, Alley J, Kohut ML. Exercise after influenza or COVID-19 vaccination increases serum antibody without an increase in side effects. Brain Behav Immun. 2022;102:1–10. doi: 10.1016/j.bbi.2022.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sallis R, Young DR, Tartof SY, et al. Physical inactivity is associated with a higher risk for severe COVID-19 outcomes: a study in 48 440 adult patients. Br J Sports Med. 2021 doi: 10.1136/bjsports-2021-104080. [DOI] [PubMed] [Google Scholar]
- 16.Brawner CA, Ehrman JK, Bole S, et al. Inverse relationship of maximal exercise capacity to hospitalization secondary to coronavirus disease 2019. Mayo Clin Proc. 2021;96:32–39. doi: 10.1016/j.mayocp.2020.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cassar MP, Tunnicliffe EM, Petousi N, et al. Symptom persistence despite improvement in cardiopulmonary health–insights from longitudinal CMR, CPET and lung function testing post-COVID-19. EClinicalMedicine. 2021 doi: 10.1016/j.eclinm.2021.101159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lam GY, Befus AD, Damant RW, et al. Exertional intolerance and dyspnea with preserved lung function: an emerging long COVID phenotype? Respir Res. 2021 doi: 10.1186/s12931-021-01814-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baratto C, Caravita S, Faini A, et al. Impact of COVID-19 on exercise pathophysiology: A combined cardiopulmonary and echocardiographic exercise study. J Appl Physiol. 2021;130:1470–1478. doi: 10.1152/japplphysiol.00710.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borghesi A, Maroldi R. COVID-19 outbreak in Italy: experimental chest X-ray scoring system for quantifying and monitoring disease progression. Radiol Medica. 2020;125:509–513. doi: 10.1007/S11547-020-01200-3/FIGURES/3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodríguez Ledo P, Armenteros del Olmo L, Rodríguez Rodríguez E, Gómez AF. Descripción de los 201 síntomas de la afectación multiorgánica producida en los pacientes afectados por la COVID-19 persistente. Med Gen Y Fam. 2021;10:60–68. doi: 10.24038/MGYF.2021.016. [DOI] [Google Scholar]
- 22.Bull FC, Maslin TS, Armstrong T. Global physical activity questionnaire (GPAQ): nine country reliability and validity study. J Phys Act Heal. 2009;6:790–804. doi: 10.1123/jpah.6.6.790. [DOI] [PubMed] [Google Scholar]
- 23.Bull FC, Al-Ansari SS, Biddle S, et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med. 2020;54:1451–1462. doi: 10.1136/bjsports-2020-102955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gandek B, Ware JE, Aaronson NK, et al. Cross-validation of item selection and scoring for the SF-12 Health Survey in nine countries: Results from the IQOLA Project. J Clin Epidemiol. 1998;51:1171–1178. doi: 10.1016/S0895-4356(98)00109-7. [DOI] [PubMed] [Google Scholar]
- 25.Monteagudo Piqueras O, Hernando Arizaleta L, Palomar Rodríguez JA. Normas poblacionales de referencia de la versión española del SF-12V2 para la Región de Murcia. Gac Sanit. 2011;25:50–61. doi: 10.1016/j.gaceta.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 26.Spitzer RL, Kroenke K, Williams JBW, Löwe B. A brief measure for assessing generalized anxiety disorder: The GAD-7. Arch Intern Med. 2006;166:1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 27.Kroenke K, Spitzer RL, Williams JBW. The PHQ-9: Validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest. 1988;93:580–586. doi: 10.1378/chest.93.3.580. [DOI] [PubMed] [Google Scholar]
- 29.Jackson C. The Chalder Fatigue Scale (CFQ 11) Occup Med (Chic Ill) 2015;65:86. doi: 10.1093/occmed/kqu168. [DOI] [PubMed] [Google Scholar]
- 30.Krupp LB, Larocca NG, Muir Nash J, Steinberg AD. The fatigue severity scale: application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46:1121–1123. doi: 10.1001/archneur.1989.00520460115022. [DOI] [PubMed] [Google Scholar]
- 31.Cella M, Chalder T. Measuring fatigue in clinical and community settings. J Psychosom Res. 2010;69:17–22. doi: 10.1016/J.JPSYCHORES.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 32.Sunnquist M, Lazarus S, Jason LA. The development of a short form of the DePaul symptom questionnaire. Rehabil Psychol. 2019 doi: 10.1037/REP0000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klok FA, Boon GJAM, Barco S, et al. The post-COVID-19 functional status scale: a tool to measure functional status over time after COVID-19. Eur Respir J. 2020 doi: 10.1183/13993003.01494-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ekblom-Bak E, Björkman F, Hellenius ML, Ekblom B. A new submaximal cycle ergometer test for prediction of VO2max. Scand J Med Sci Sport. 2014;24:319–326. doi: 10.1111/sms.12014. [DOI] [PubMed] [Google Scholar]
- 35.Ekblom-Bak E (2020) Reference values for VO2max. Accessed July 15 2021. https://www.gih.se/Global/3_forskning/fysiologi/elinekblombak/Reference%20values%20EB-test_20201201_eng.pdf
- 36.Buendía-Romero Á, Hernández-Belmonte A, Martínez-Cava A, et al. Isometric knee extension test: a practical, repeatable, and suitable tool for lower-limb screening among institutionalized older adults. Exp Gerontol. 2021 doi: 10.1016/j.exger.2021.111575. [DOI] [PubMed] [Google Scholar]
- 37.Courel-Ibáñez J, Martínez-Cava A, Morán-Navarro R, et al. Reproducibility and repeatability of five different technologies for bar velocity measurement in resistance training. Ann Biomed Eng. 2019;47:1523–1538. doi: 10.1007/s10439-019-02265-6. [DOI] [PubMed] [Google Scholar]
- 38.Mitchell C, Rahko PS, Blauwet LA, et al. Guidelines for performing a comprehensive transthoracic echocardiographic examination in adults: recommendations from the American society of echocardiography. J Am Soc Echocardiogr. 2019;32:1–64. doi: 10.1016/j.echo.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 39.Graham BL, Steenbruggen I, Barjaktarevic IZ, et al. Standardization of spirometry 2019 update an official American Thoracic Society and European Respiratory Society technical statement. Am J Respir Crit Care Med. 2019;200:E70–E88. doi: 10.1164/rccm.201908-1590ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prevalence of ongoing symptoms following coronavirus (COVID-19) infection in the UK Statistical bulletins-Office for National Statistics. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/prevalenceofongoingsymptomsfollowingcoronaviruscovid19infectionintheuk/previousReleases?page=1. Accessed 10 May 2022
- 41.Augustin M, Schommers P, Stecher M, et al. Post-COVID syndrome in non-hospitalised patients with COVID-19: a longitudinal prospective cohort study. Lancet Reg Heal - Eur. 2021;6:1–8. doi: 10.1016/j.lanepe.2021.100122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fernández-de-las-Peñas C, Palacios-Ceña D, Gómez-Mayordomo V, et al. Prevalence of post-COVID-19 symptoms in hospitalized and non-hospitalized COVID-19 survivors: a systematic review and meta-analysis. Eur J Intern Med. 2021;92:55–70. doi: 10.1016/j.ejim.2021.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vanichkachorn G, Newcomb R, Cowl CT, et al. Post–COVID-19 Syndrome (long haul syndrome): description of a multidisciplinary clinic at mayo clinic and characteristics of the initial patient cohort. Mayo Clin Proc. 2021;96:1782–1791. doi: 10.1016/j.mayocp.2021.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vaes AW, Goërtz YMJ, Van HM, et al. Recovery from COVID-19: a sprint or marathon? 6-month follow-up data from online long COVID-19 support group members. ERJ Open Res. 2021;7:00141–02021. doi: 10.1183/23120541.00141-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wilke J, Mohr L, Tenforde AS, et al. A pandemic within the pandemic? Physical activity levels substantially decreased in countries affected by covid-19. Int J Environ Res Pub Health. 2021;18:1–12. doi: 10.3390/ijerph18052235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rehabilitation for Post-COVID-19 Syndrome Through a Supervised Exercise Intervention-Full Text View-ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT04718506. Accessed 12 May 2022
- 47.Vonbank K, Lehmann A, Bernitzky D, et al. Predictors of prolonged cardiopulmonary exercise impairment after covid-19 infection: a prospective observational study. Front Med. 2021;8:1–9. doi: 10.3389/fmed.2021.773788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pleguezuelos E, Del Carmen A, Llorensi G, et al. Severe loss of mechanical efficiency in COVID-19 patients. J Cachexia Sarcopenia Muscle. 2021;12:1056–1063. doi: 10.1002/jcsm.12739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Singh I, Joseph P, Heerdt PM, et al. Persistent Exertional Intolerance After COVID-19: Insights From Invasive Cardiopulmonary Exercise Testing. Chest. 2021 doi: 10.1016/j.chest.2021.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Crameri GAG, Bielecki M, Züst R, et al. Reduced maximal aerobic capacity after COVID-19 in young adult recruits, Switzerland, May 2020. Eurosurveillance. 2020;25:1. doi: 10.2807/1560-7917.ES.2020.25.36.2001542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Szekely Y, Lichter Y, Sadon S, et al. Cardiorespiratory abnormalities in patients recovering from coronavirus disease 2019. J Am Soc Echocardiogr. 2021 doi: 10.1016/j.echo.2021.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jimeno-Almazán A, Pallarés JG, Buendía-Romero Á, et al. Chronotropic incompetence in non-hospitalized patients with post-covid-19 syndrome. J Clin Med. 2021;10:1–10. doi: 10.3390/jcm10225434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ismael F, Bizario JCS, Battagin T, et al. Post-infection depressive, anxiety and post-traumatic stress symptoms: a prospective cohort study in patients with mild COVID-19. Prog Neuropsychopharmacol Biol Psychiatry. 2021 doi: 10.1016/J.PNPBP.2021.110341. [DOI] [PMC free article] [PubMed] [Google Scholar]