Abstract
Healthy subjects present higher summer than winter S100B protein concentrations. There is no available information regarding if schizophrenia patients present the same pattern. The aim of this research is to study if patients with schizophrenia present seasonal changes in serum S100B concentrations
ABSTRACT
Introduction
Healthy subjects present higher summer than winter S100B protein concentrations. There is no available information regarding if schizophrenia patients present the same pattern. The aim of this research is to study if patients with schizophrenia present seasonal changes in serum S100B concentrations.
Introduction
Healthy subjects present higher summer than winter S100B protein concentrations. There is no available information regarding if schizophrenia patients present the same pattern. The aim of this research is to study if patients with schizophrenia present seasonal changes in serum S100B concentrations.
Methods
In fifty-two Caucasian schizophrenia paranoid inpatients meeting DSM-IV criteria, serum S100B protein was measured at 12:00 h and 00:00 h the next day after admission. Patients were recruited for a period of nine months (July-March) and were grouped as summer, autumn or winter group according to the date of admission. Serum S100B levels were measured with an enzyme-linked immunoassay (ELISA) kit.
Results
Patients admitted in winter had significantly higher serum S100B concentrations at 12:00 h and 00:00 h than patients admitted in summer (12:00, winter: 287.5±264.9 vs. summer: 33.7±22.6, p<0.05; 00:00, winter: 171.2±143.8 vs. summer: 23.3±18.6, p<0.05). Autumn serum S100B concentrations were not significantly different from the summer or winter concentrations (12:00: 128.7±208.8, 00:00: 102.2±153.2). There were no significant differences between 12:00 and 00:00 serum S100B concentrations in any season.
Conclusions
Acutely relapsed paranoid schizophrenia inpatients present significantly higher serum S100B concentrations in winter than summer, the opposite pattern
INTRODUCTION
Schizophrenia is a disease characterized by delusions, hallucinations, disorganized speech and behaviour, and other symptoms that cause social or occupational dysfunction. Its diagnosis is still based on the clinical interview, so the investigation of biological markers to help with the diagnosis should be a paramount concern (1). Bioanalysis results rely on the accuracy of laboratory techniques, including aspects of sensitivity and specificity among other parameters. The human subject from whom the biological sample is taken, sometimes, is not considered a source of variability in the biological parameters´ results. Human biomarkers research is a difficult task because some biological variables present values that depend on demographic and individual factors such as gender, ethnic group or chronotype (2)-(3), among others. High inter-individual and low intra-individual variability have been reported in biological measures (2)-(4). Non-individual related variables, such as circadian and seasonal changes (5)-(6), have also been reported to affect some biological markers.
S100B, a calcium-binding protein that has been proposed as a marker of astrocyte activation and brain dysfunction, has been used as a biological marker in schizophrenia (7). S100B exerts both autocrine and paracrine functions on neurons and glial cells (8).In in vitro studies, S100B promotes neural growth and survival at nanomolar concentrations (9) but at micromolar concentrations induces apoptosis (10).
In general, schizophrenia patients present higher S100B levels compared to healthy subjects 11 14. In healthy subjects, serum S100B do not present circadian changes but present significantly higher summer than winter serum levels 15. In a previous paper published by our research group we have reported that at admission acute paranoid schizophrenia inpatients present a higher day than night serum S100B levels, but at discharge there was no difference between day and night serum concentrations, matching what happened in the control group 12. It is not known if S100B protein in schizophrenia patients present seasonal changes as it happens in healthy subjects.
The aim of this research is to explore if patients with schizophrenia present seasonal changes in serum S100B concentrations.
METHODS
Subjects
Fifty-two Caucasian paranoid schizophrenic inpatients meeting DSM-IV criteria participated in the study. For a period of nine months (July 2006-March 2007), acutely relapsed patients were recruited from the psychiatric ward of the University Hospital of the Canary Islands. All patients were independently diagnosed by two clinical psychiatrists. Patients were grouped as summer, autumn or winter season according to the date of admission. Patients with alcohol or substance abuse, physical illness, pregnancy, physical trauma and intellectual disability were excluded. The study protocol was carried out following the Helsinki Declaration and patients or their relatives gave written informed consent before inclusion. The protocol was approved by the Ethics and Investigation Committee of the University Hospital of the Canary Islands.
Psychopathological assessment
Psychopathology was measured with the Positive and Negative Syndrome Scale (PANSS)(16). The positive, negative and general subscales scores were used as marker of clinical symptoms severity. The PANSS was administered within the 24 hours after admission.
S100B measurement
Blood was collected the day after admission. Samples were collected at 12:00 and 00:00 h. After blood was extracted, samples were placed in vacutainer tubes without anticoagulant and centrifuged at 3000 rpm for 5 minutes, then serum was aliquoted in Eppendorf tubes, and stored frozen at -70º C until analysis. To minimize the assay variance all serum samples were analysed by the technician the same day with the same laboratory batch. The technician was blind with respect to the samples pertaining to summer, autumn or winter season as well as to the moment of the day the sample was collected.
Serum S100B levels were measured with an enzyme-linked immunoassay (ELISA) kit according to the manufacturer instructions (BioVendor, Candler, USA). The BioVendor Human S100B ELISA uses a polyclonal anti-cow S100B coated in microtitration wells. The absorbance of the resulting yellow colour product was measured spectrophotometrically at 450 nm in a microplate spectrophotometer reader (Benchmark Plus, Bio-Rad, Hercules, CA, USA). In this ELISA, the lowest detection limit was 17.0 pg./ml. Coefficients of variation were 3.92 % and 5.03 % for intraand inter-assay variabilities, respectively
Statistical analyses
Data were analysed using the 21st version of the SPSS statistical package (SPSS, Chicago, Illinois, USA). An analyses of variance (ANOVA) was applied to examine the summer-autumn-winter serum S100B differences. If ANOVA results were significant a post-hoc Bonferroni´s comparison was applied. A paired t-test was applied to compare midday vs midnight serum S100B concentrations. Chi-square was applied to study the association between qualitative variables.
All antipsychotic treatments were transformed into chlorpromazine antipsychotic equivalent doses (CAED)(17) to control the possible effect of the different antipsychotics on S100B concentration.
All statistical tests were two-tailed. Statistical significance level was set at 0.05.
RESULTS
Comparison of demographic and clinical variables according to the season of the patient’s admission
The comparison of the demographic and clinical variables according to the season of the patient admission is presented in Table 1 and Table 2 . The three seasonal patient samples were similar according to age and gender distribution. Patients admitted in summer, autumn or winter did not differ significantly in the clinical variables.
1. Table 1.
Comparison of demographic variables according to the season of the patient’s admission
| Variables | Summer | Autumn | Winter | F or X2 | P |
| Age | 39.4±9.8 | 35.2±10.3 | 36.4±9.1 | 0.89 | 0.42 |
| Gender male/female | 12/8 | 10/4 | 14/4 | 4.0 | 0.13 |
2. Table 2.
Comparison of clinical variables according to the season of the patient’s admission
| Variables | Summer | Autumn | Winter | F | P |
| CAED | 673.5±408.9 | 795.2±407.8 | 797.1±407.5 | 0.31 | 0.74 |
| Positive scale | 19.6±4.1 | 19.2±5.3 | 21.3±5.4 | 0.52 | 0.59 |
| Negative scale | 14.9±6.2 | 13.3 ±5.3 | 15.4±6.3 | 0.22 | 0.81 |
| General scale | 24.3±4.9 | 24.1±3.7 | 23.7±3.9 | 0.04 | 0.96 |
| Age of illness onset | 21.5±5.5 | 24.5±9.2 | 25.9±6.6 | 0.86 | 0.43 |
| Illness duration | 16.6±13.0 | 9.3±7.2 | 11.7±8.9 | 1.58 | 0.22 |
| NPA | 4.0±3.2 | 4.8±3.9 | 3.0±2.3 | 0.82 | 0.45 |
| Body Mass Index | 28.9±6.4 | 26.1±3.2 | 26.4±4.9 | 1.20 | 0.32 |
CAED: Chlorpromazine Antipsychotic Equivalent Dose; NPA: Number of Previous Admissions
Comparison of serum S100B concentrations according to the season of the patient’s admission
Table 3 presents the result of the ANOVA comparing the serum S100B concentrations by the season of patient´s admission. The comparison of serum S100B concentrations by season elicited a significant effect of season on serum S100B at 12:00 and 00:00 h. Figure 1 represents the post-hoc Bonferroni´s comparison according to the seasons. Serum S100B concentrations were significantly higher in winter than summer both at 12:00 and 00:00 h. Autumn serum S100B concentrations were not significantly different from summer or winter concentrations.
3. Table 3.
Comparison of serum S100B levels according to the season of the patient’s admission
| Variable | Summer | Autumn | Winter | F | P |
| S100B 12:00 | 33.7±22.6 | 128.9±208.5 | 287.5±264.9 | 4.13 | 0.02 |
| S100B 00:00 | 23.3±18.6 | 102.2±153.2 | 171.2±143.8 | 3.35 | 0.04 |
Figure 1.
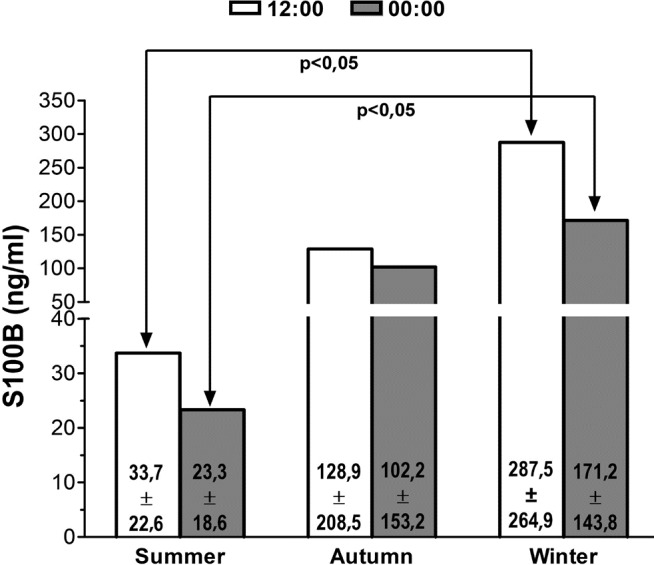
Post-hoc Bonferronis comparison of serum S100B concentration by season at midday and midnight
Comparison of serum S100B concentrations according to the blood sampling time of the day by season
The t-test S100B comparison of 12:00 h vs 00:00 h blood sampling by season did not elicit significant differences Table 4 .
4. Table 4.
Comparison of serum S100B levels according to the time of the day in each season
| Season | S100B 12:00 | S100B 00:00 | T | P |
| Summer | 33.7±22.6 | 23.3±18.6 | 1.29 | 0.23 |
| Autumn | 128.9±208.5 | 102.2±153.2 | 0.77 | 0.46 |
| Winter | 287.5±264.9 | 171.2±143.8 | 1.91 | 0.93 |
DISCUSSION
To the best of our knowledge, this is the first time that a summer-winter change in the serum concentrations of the S100B protein is reported in acutely relapsed paranoid schizophrenia inpatients. Patients had significantly higher serum S100B concentrations in winter than summer, being the autumn concentrations higher than the summer concentrations but lower than the winter ones. This seasonal pattern, higher in winter than summer, is the opposite pattern reported in healthy subjects, this is, higher in summer than winter(15)
Several medical conditions, such as, heart attack mortality(18) , blood pressure in diabetes(19) or psychiatric admissions(20) have a seasonal distribution. Some biological parameters such as melatonin(5), total antioxidant capacity(21) or vitamin D(22) among others, have seasonal changes. In a previous paper we speculated if in healthy subjects the difference in temperature between summer and winter could be a possible explanation for this change(15).
Hyperpermeability of the BBB has been reported in psychosis(25), but our question would remain unsolved, because the increased permeability of the BBB would explain the increased levels of S100B in schizophrenia subjects compared to healthy subjects, but it would not be a valid explanation for the summer-winter difference of serum S100B concentrations in schizophrenia patients because they presented the opposite pattern.
An alternative and complementary explanation may stem from the study of the genes linked to seasonality(26) . Several genes linked to seasonality have been reported, the ARNTL and NPAS2(27), the CRY1 and CRY2(28) and the PERIOD3(29), among others. Seasonal genes may modulate the expression of the protein production. However, no associations have been described between S100B genes and seasonal genes.
The main strength of our study stems from the fact that the three seasonal samples were similar with respect to the demographic and clinical variables making the results not attributable to those differences.
In our opinion the main limitation of our study is the small size of the sample. It might be possible that the difference between 12:00 and 00:00 h serum S100B concentrations had achieved statistical significance if our seasonal samples had been bigger.
To conclude, acutely relapsed paranoid schizophrenia inpatients present significantly higher serum S100B protein concentrations at 12:00 and 00:00 h in winter than summer, just the opposite result that had been reported in healthy subjects. Autumn S100B concentrations are in the middle between the winter and summer values, not being significantly different from the summer or winter values. Despite that the reason of this alteration is not known, it is strongly advisable to include the season of S100B sampling in the study design in order to control this bias.
ACKNOWLEDGEMENTS
We would like to thank to Dr. Aram Morera-Mesa (freelance translator) for his assistance in the translation of this article.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
References
- 1.Morera AL, Henry M, García-Hernández A, Fernandez-López L. Acute phase proteins as biological markers of negative psychopathology in paranoid schizophrenia. Actas Esp Psiquiatr. 2007 [PubMed] [Google Scholar]
- 2.Yue Q, Svensson J, Alm C, Sjoqvist F, Sawe J. Interindividual and interethnic differences in the demethylation and glucuronidation of codeine. Br J Clin Pharmacol. 1989 doi: 10.1111/j.1365-2125.1989.tb03555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morera-Fumero AL, Abreu-González P, Henry-Benitez M, Diaz-Mesa E, Yelmo-Cruz S, Gracia-Marco R. Chronotype as modulator of morning serum melatonin levels. Actas Esp Psiquiatr. 2013 [PubMed] [Google Scholar]
- 4.Cope EL, Shrubsole MJ, Cohen SS, Cai Q, Wu J, Ueland PM. Intraindividual variation in one-carbon metabolism plasma biomarkers. Cancer Epidemiol Biomarkers Prev. 2013 doi: 10.1158/1055-9965.EPI-13-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergiannaki JD, Paparrigopoulos TJ, Stefanis CN. Seasonal pattern of melatonin excretion in humans: Relationship to daylength variation rate and geom. Experientia. 1996 doi: 10.1007/BF01920718. [DOI] [PubMed] [Google Scholar]
- 6.Morera AL, Abreu P. Daytime/night-time and summer/winter melatonin and malondialdehyde rhythms: an inverse relationship. J Pineal Res. 2007 doi: 10.1111/j.1600-079X.2007.00467.x. [DOI] [PubMed] [Google Scholar]
- 7.Yelmo-Cruz S, Morera-Fumero AL, Abreu-González P. S100B and schizophrenia. Psychiatry Clin Neurosci. 2013 doi: 10.1111/pcn.12024. [DOI] [PubMed] [Google Scholar]
- 8.Donato R. Intracellular and extracellular roles of S100 proteins. Microsc Res Tech. 2003 doi: 10.1002/jemt.10296. [DOI] [PubMed] [Google Scholar]
- 9.Alexanian AR, Bamburg JR. Neuronal survival activity of S100ββ is enhanced by calcineurin inhibitors and requires a. FASEB J. 1999 doi: 10.1096/fasebj.13.12.1611. [DOI] [PubMed] [Google Scholar]
- 10.Mariggió MA, Fulle S, Calissano P, Nicoletti I, Fanó G. The brain protein S-100ab induces apoptosis in PC12 cells. Neuroscience. 1994 doi: 10.1016/0306-4522(94)90201-1. [DOI] [PubMed] [Google Scholar]
- 11.Chen S, Tian L, Chen N, Xiu M, Wang Z, Yang G. Cognitive dysfunction correlates with elevated serum S100B concentration in drug-free acutely relaps. Psychiatry Res. 2017 doi: 10.1016/j.psychres.2016.09.029. [DOI] [PubMed] [Google Scholar]
- 12.Morera-Fumero AL, Díaz-Mesa E, Abreu-Gonzalez P, Fernandez-Lopez L, Cejas-Mendez M del R. Day/night changes in serum S100B protein concentrations in acute paranoid schizophrenia. Prog Neuro-Psychopharmacology Biol Psychiatry. 2017 doi: 10.1016/j.pnpbp.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 13.Deng H, Kahlon RS, Mohite S, Amin PA, Zunta-Soares G, Colpo GD. Elevated Plasma S100B, Psychotic Symptoms, and Cognition in Schizophrenia. Psychiatr Q. 2018 doi: 10.1007/s11126-017-9514-y. [DOI] [PubMed] [Google Scholar]
- 14.Schmitt A, Bertsch T, Henning U, Tost H, Klimke A, Henn FA. Increased serum S100B in elderly, chronic schizophrenic patients: Negative correlation with deficit . Schizophr Res. 2005 doi: 10.1016/j.schres.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 15.Morera-Fumero AL, Abreu-Gonzalez P, Henry-Benitez M, Yelmo-Cruz S, Diaz-Mesa E. Summer/winter changes in serum S100B protein concentration as a source of research variance. J Psychiatr Res. 2013 doi: 10.1016/j.jpsychires.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 16.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987 doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 17.Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003 doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]
- 18.Kriszbacher I, Bódis J, Boncz I, Koppan Á, Koppan M. The time of sunrise and the number of hours with daylight may influence the diurnal rhythm of acute . Int J Cardiol. 2008 doi: 10.1016/j.ijcard.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 19.Hermann JM, Rosenbauer J, Dost A, Steigleder-Schweiger C, Kiess W, Schöfl C. Seasonal Variation in Blood Pressure in 162,135 Patients With Type 1 or Type 2 Diabetes Mellitus. J Clin Hypertens. 2016 doi: 10.1111/jch.12743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aguglia A, Borsotti A, Cuniberti F, Serafini G, Amore M, Maina G. The influence of sunlight exposure on hospitalization in emergency psychiatry. . Chronobiol Int. 2017 doi: 10.1080/07420528.2017.1374286. [DOI] [PubMed] [Google Scholar]
- 21.Morera-Fumero AL, Abreu-Gonzalez P, Henry-Benitez M, Fernandez-Lopez L, Diaz-Mesa E, del Rosario Cejas-Mendez M. Day/night and summer/winter changes in serum total antioxidant capacity. Med Chem . 2018 doi: 10.2174/1573406413666171002123158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kasahara AK, Singh RJ, Noymer A. Vitamin D (25OHD) Serum Seasonality in the United States. PLoS One. 2013 doi: 10.1371/journal.pone.0065785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kiyatkin EA, Sharma HS. Permeability of the blood-brain barrier depends on brain temperature. Neuroscience. Neuroscience. 2009 doi: 10.1016/j.neuroscience.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu YC, Lee Y Da, Wang HL, Liao KH, Chen KB, Poon KS. Anesthesia-induced hypothermia attenuates early-phase blood-brain barrier disruption but not infarct. PLoS One. . 2017 doi: 10.1371/journal.pone.0170682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pollak TA, Drndarski S, Stone JM, David AS, McGuire P, Abbott NJ. The blood–brain barrier in psychosis. The Lancet Psychiatry. 2018 doi: 10.1016/S2215-0366(17)30293-6. [DOI] [PubMed] [Google Scholar]
- 26.Dopico XC, Evangelou M, Ferreira RC, Guo H, Pekalski ML, Smyth DJ. Widespread seasonal gene expression reveals annual differences in human immunity and physiology. Nat Commun. 2015 doi: 10.1038/ncomms8000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kovanen L, Saarikoski ST, Aromaa A, Lönnqvist J, Partonen T. ARNTL (BMAL1) and NPAS2 gene variants contribute to fertility and seasonality. PLoS One. 2010 doi: 10.1371/journal.pone.0010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kovanen L, Donner K, Kaunisto M, Partonen T. CRY1 and CRY2 genetic variants in seasonality: A longitudinal and cross-sectional study. Psychiatry Res. 2016 doi: 10.1016/j.psychres.2016.05.044. [DOI] [PubMed] [Google Scholar]
- 29.Zhang L, Hirano A, Hsu P-K, Jones CR, Sakai N, Okuro M. A PERIOD3 variant causes a circadian phenotype and is associated with a seasonal mood trait. Proc Natl Acad Sci. 2016 doi: 10.1073/pnas.1600039113. [DOI] [PMC free article] [PubMed] [Google Scholar]


