Abstract
Several foreign antigens such as those derived from viruses and bacteria have been linked to long-term deleterious effects on the brain and other organs; yet, health outcomes subsequent to foreign antigen exposure vary depending in large part on the host’s immune system, in general, and on human leukocyte antigen (HLA) composition, in particular. Here we first provide a brief description of 3 conditions characterized by persistent long-term symptoms, namely long-COVID-19, myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), and Gulf War Illness (GWI), followed by a brief overview of the role of HLA in the immune response to foreign antigens. We then discuss our Persistent Antigen (PA) hypothesis and highlight associations between antigen persistence due to HLA-antigen incongruence and chronic health conditions in general and the 3 “long” diseases above in particular. This review is not intended to cover the breadth and depth of symptomatology of those diseases but is specifically focused on the hypothesis that the presence of persistent antigens underlies their pathogenesis.
Keywords: Human leukocyte antigen, Gulf War illness, long-COVID, myalgic encephalomyelitis/chronic fatigue syndrome, persistent antigen
Introduction
Long-COVID-19, myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and Gulf War Illness (GWI) all share the following attributes. (a) They present with chronic symptomatology arising from involvement of various organ systems, including the brain; (b) they appear following an “event”: long-COVID-19 after a COVID-19 infection with the SARS-Cov-2 virus, ME/CFS after an infection with any of various viruses (“postviral syndrome”), and GWI after exposure to potentially toxic chemicals and biologics (vaccine antigens) while serving in the first Gulf War of 1990-91; and (c) in all 3 conditions, women are more adversely affected than men (in long-COVID-19, ME/CFS, and GWI - below). We attribute the development of long-COVID-19, ME/CFS and GWI to the presence of persistent antigen(s) to which patients were exposed and which could not be eliminated. We further attribute the varied symptomatology to (a) continuous direct tissue damage by the offending antigen, (b) chronic inflammation accompanying tissue damage, (c) diverse immune reactions depending on the antigen itself, the organs affected, and the sex of the patients, and (d) potential autoimmunity stemming from chronic breakdown of tissue proteins and/or antigen fragments. All of the above are the result of the persistence of offending antigen(s); hence we called this idea the “Persistent Antigen” (PA) hypothesis. 1 We attribute this antigen persistence (in otherwise immunocompetent people) to ineffective elimination of the offending antigen due primarily to a mismatch between the antigen and the patient’s Human Leukocyte Antigen (HLA) genetic capacity.
The 3 “Long” Diseases
Long-COVID-19
Over 483 million people worldwide are estimated to have been infected with SARS-CoV-2, the virus responsible for the ongoing coronavirus-19 (COVID-19) pandemic, and over 6 million people have died as a result of infection. 2 Although the cases of SARS-CoV-2 have decreased rapidly over the last several months, the long-term effects of COVID infection are just beginning to take shape. Studies have documented that nearly 80% of patients develop one or more long-term symptoms, 3 commonly referred to as long-COVID or Post-Acute Sequelae of SARS-CoV-2 (PASC), and as many continue to experience symptoms 12 months after acute infection.3,4 An even higher percentage of patients hospitalized with COVID experience persistent symptoms, 5 yet sizable numbers of individuals with mild or even asymptomatic cases experience long-COVID6-8 as well as vaccinated individuals following breakthrough COVID-19 infection. 9
Several systems are affected by long-COVID including respiratory, cardiac, cutaneous, and CNS, 10 with the most common persistent symptoms (for the initial strain) including fatigue and post-exertional malaise, headaches, dyspnea, joint and chest pain, brain fog and memory impairment.3,5,7,11 These effects have resulted in sustained impairment in daily functioning including inability to return to work months after infection. 11
While the lungs are the primary site of acute COVID infection, several other systemic effects have been associated with long-COVID. Long-term effects of COVID infection, even among those with mild COVID cases, include “COVID-heart” which refers to dysrhythmias, cerebrovascular disorders, heart disease, myocarditis, heart failure, and thromboembolic disease well after acute infection, even among those with mild cases of COVID 12 ; new onset Type 2 diabetes after recovery 13 ; neurological events including Bell’s Palsy, encephalomyelitis, Guillain-Barre syndrome 14 ; autoimmune diseases15,16; and significant multi-organ impairment. 17 In addition, long-COVID is associated with brain gray matter loss especially in olfactory areas, whole brain atrophy, and cognitive disruptions associated with cerebellar atrophy. 18 Furthermore, patients with pneumonia associated SARS-CoV-2 infection have 30% higher odds of new onset dementia compared to other pneumonias. 19 Indeed, the effects on the brain are so substantial that some have referred to the commonly reported brain fog, difficulty concentrating, dizziness, and fatigue characteristic of long-COVID as “neuro-COVID.” 20
ME/CFS
ME/CFS is an umbrella term used to characterize a condition of uncertain etiology defined by extreme fatigue, neurocognitive problems, and autonomic dysfunction. 21 Similarities between long-COVID/PASC and ME/CFS have been widely recognized11,22,23 as has overlap between GWI and ME/CFS.24,25 Like GWI and long-COVID, ME/CFS lacks a definitive case definition though generally involves debilitating chronic systemic symptoms including fatigue, post-exertional malaise, unrefreshing sleep, cognitive impairment, orthostatic intolerance, and musculoskeletal pain. 21 Approximately 1% of the population suffers from ME/CFS with more women affected than men though the prevalence varies widely depending on case definition. 26 Among those affected, ME/CFS results in significant impacts on quality of life including significant social and occupational impairment and disability.21,27,28
GWI
GWI appeared in a large number of otherwise healthy veterans after the 1990-91 Persian Gulf War. Of the 693 826 veterans enlisted then, 29 about 1/3 (25%-32%, 173 456-222 021 veterans) reported symptoms from multiple organs, which have since been24,30 and are even currently a major health problem, 29 especially in women veterans.31,32 This Gulf War Illness (GWI) is a condition characterized by chronic and diffuse symptoms including muscle and joint pain, fatigue, neurological/cognitive/mood impairment, skin rashes, respiratory complaints, and gastrointestinal problems.33,34 GWI symptoms prominently involve the brain, and numerous structural and functional brain anomalies have been associated with GWI,35-40 yet symptoms span several organ systems suggesting broad systemic effects. No definitive objective indicator of GWI exists; rather, symptoms are evaluated via self-report and case status is determined according to 2 primary case definitions recommended by the Institute of Medicine. 41 There is currently no cure for GWI and treatments are aimed at alleviating symptoms piecemeal. 30 years after the Gulf War, veterans with GWI have continued to experience deteriorating health resulting in significant impairment.24,42 In addition to GWI, GW veterans are at higher risk for several chronic health conditions including numerous infectious diseases as well as irritable bowel syndrome, chronic fatigue syndrome, and amyotrophic lateral sclerosis. 43
The etiology of GWI is uncertain although numerous biological and chemical exposures have been implicated. In theater exposures such as burning pits, smoke from oil well fires, pesticides, organophosphates, sarin/cyclosarin nerve agents, pyridostigmine bromide and others have been associated with GWI. 44 However, GWI symptoms have also been reported among Gulf War era veterans who did not deploy (albeit to a lesser extent) 35 suggesting involvement of exposures not tied to service in theater. Whereas early evaluations documented significantly more GWI symptoms among deployed compared to non-deployed veterans, recent research demonstrated that as the Gulf War era veteran population ages, up to 80% of non-deployed veterans meet criteria for at least 1 case definition of GWI, 42 suggesting that Gulf War veterans, regardless of deployment status, are at high risk of poor health outcomes for reasons that are not entirely certain but appear to extend beyond deployment theater.
In addition to deployment-related exposures, several investigators have considered the role of vaccines as contributing to GWI. To limit effects of potential biological warfare agents, Gulf War veterans were administered the anthrax, botulinum toxin, and plague vaccines in addition to a spate of routine and geographic-specific immunobiologics including cholera, measles, meningitis, rabies, rubella, tetanus, yellow fever, and typhoid vaccines. 44 Altogether, up to 17 antigens were administered via vaccination in the first 2 weeks of training with additional vaccinations to follow. 45 Since the Gulf War, questions have been raised about the role of various vaccines in GWI; however, vaccination records for Gulf War veterans are limited rendering direct links between vaccine administration and GWI challenging to establish. 46 Nonetheless, there appears to be evidence linking vaccines administered to Gulf War veterans with GWI, either due to the direct effects of the vaccine antigen(s)47-52 or to vaccine adjuvants such as squalene.53,54
Effects of sex on prevalence and severity of the 3 “long” diseases
Sex has significant effects on the prevalence and/or severity of all 3 “long” diseases above. More specifically, (a) women are more likely to develop long-COVID-19 and are more adversely affected than men,55-57 (b) women are more frequently and more severely affected with ME/CFS than men, 58 and (c) women veterans of the Gulf War are in worse health than men.31,32
Immune Response to Foreign Antigens
The human immune system has evolved to provide a range of defenses starting with mechanical and chemical barriers to protect against foreign antigens such as viruses and bacteria. 59 If those barriers are breached, several the innate immune system 60 pathways are promptly triggered to inhibit infection, protect cells, and eliminate infected cells. The initial line of defense involves the mobilization at the site of infection of macrophages and polymorphonuclear phagocytes (neutrophils, basophils, and eosinophils) to contain the infection. The involvement of adaptive immune mechanisms follows soon thereafter.
Adaptive immunity: HLA
Adaptive immune mechanisms are slower, starting about 4 to 10 days following infection, and include T-cell mediated immunity to resolve infection and B-cell mediated production of antibodies to clear lingering pathogens and protect against reinfection. If the infection is not controlled due to immune system disruption or evasion, antigen persistence can occur, the outcome of which can range from persistent infection with low viral load (eg, cytomegalovirus [CMV] and Epstein-Barr virus [EBV]) to latency with periods of reactivation (eg, herpes simplex viruses) to persistent viremia (eg, human immunodeficiency virus [HIV], hepatitis B virus, hepatitis C virus).61-63 A central component of human immune defenses involves HLA. HLA genes, located on chromosome 6, code for cell-surface glycoproteins that play an essential role in host protection from foreign antigens (eg, viruses, bacteria, cancer neoantigens) by facilitating immune surveillance and initiating an immune response to eliminate foreign antigens. 64 There are 2 primary classes of HLA that work in concert via distinct mechanisms.
HLA Class I: Cytotoxic immunity
HLA Class I molecules (HLA-A, B, C), which are expressed on nucleated cells, export small peptides (8-10 amino acid residues) from proteolytically degraded cytosolic viruses, bacteria, and tumors to the cell surface for presentation to CD8 + cytotoxic T cells to signal cell destruction.
HLA Class II: Humoral immunity
Class II HLA molecules (HLA-DPB1, DQB1, DRB1), which are expressed on lymphocytes and professional antigen presenting cells (APC; eg, macrophages, dendritic cells, and monocytes), present larger peptides (12-22 amino acid residues) derived from endocytosed exogenous antigens such as viruses and bacteria to CD4 + T cells, facilitating antibody production and adaptive immunity. The success of antigen elimination requires, as an initial step, a match between epitopes derived from foreign antigens and the HLA receptor binding groove. Even a single amino acid difference can alter the binding groove, 65 thereby changing the landscape of antigens that a given HLA molecule can bind for presentation to T cells. HLA is the most highly polymorphic region of the human genome; each individual carries 12 HLA alleles (6 from Class I and 6 from Class II) inherited in a Mendelian fashion, determining the antigens that each individual can successfully bind to and eliminate. To that end, HLA variation is known to contribute to variation in disease susceptibility. 66
Relations between sex and immunity
There are well established differences between men and women with respect to native, cellular and humoral immune response to pathogens and vaccines, as reviewed succinctly recently.67,68 Briefly, women mount stronger immune responses to viruses and vaccines than men but also suffer more serious illness when infected and show more serious adverse effects to vaccination. These differences are attributed to a combination of hormonal and genetic factors, namely the differential effects of sex hormones (estrogen, progesterone, testosterone) on the immune response and to the genetic coding of several immune-related proteins on the X chromosome. It is thought that the more rigorous immune response protects women better than men from viral infections but, at the same time, renders them more susceptible to potential autoimmunity.
The Persistent Antigen Hypothesis
Since a match between antigen epitopes and proteins of the HLA receptor binding groove is the critical initial step in antigen presentation and elimination, and since each individual has a limited repertoire of HLA molecules, what then happens in the event of an HLA-antigen mismatch? This is the crux of the persistent antigen hypothesis 1 which posits that, in the absence of HLA with sufficient affinity and immunogenicity, the antigens persist, contributing to downstream deleterious effects. Such effects comprise 4 major categories: (a) a persistent antigen continues to cause direct tissue damage, (b) this damage induces chronic inflammation produced by a persistent immune response, including cytokine production, (c) ongoing breakdown of proteins can lead to autoimmunity, and (d) antigen persistence can alter differentiation of pathogen-specific CD8 + T cells resulting in functional exhaustion of effector T cells and defective immunological memory.69,70 At the root of this lies a HLA-antigen mismatch in that HLA-antigen binding is critically involved in antigen presentation to T cells: a HLA-antigen mismatch leads to the breakdown of antigen elimination pathways and antigen persistence. Several common viruses have been associated with antigen persistence including HIV, hepatitis B virus, hepatitis C virus, influenza, several human herpes viruses including EBV and CMV 3 and, more recently, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).22,71-75 Persistent antigens stimulate immune pathways aimed at their elimination, the downsides of which include chronic inflammation and tissue damage which may impact multiple organ systems.
Antigen persistence: Systemic effects
Many foreign antigens ultimately impact brain function; however, other organs are often the primary target. For example, hepatitis B and C viruses affect primarily the liver but systemic effects involving the eye, gut, kidney, thyroid, cardiovascular system, and central nervous system (CNS) have been associated with infection. 76 Influenza primarily affects respiratory epithelial cells, yet is also associated with neurological complications and CNS disease due to cross-reactivity with human brain tissue.77,78 Similar systemic effects are seen in relation to other infections such as dengue fever for which viral antigens have been documented in the liver, spleen, lymph node, thymus, kidney, lung, skin, and in monocytes and lymphocytes, 79 and similarly for bacterial infections. 80
Although the brain is protected by the blood-brain barrier (BBB), there is substantial evidence that the defenses conferred by the BBB are readily breached by foreign antigens either via peripheral nerves or via the blood supply through several mechanisms including infection of the endothelial cells lining the BBB permitting direct access to the brain, the “Trojan Horse” approach, or access through the choroid plexus which is relatively unprotected.81,82 Inflammation, a common immune response to foreign antigens, has been found to increase BBB permeability, 83 permitting entry of foreign antigens into the brain. Indeed, evidence of persistence in brain tissue and associated neurocognitive disruptions have been documented for several neurotropic viruses including hepatitis C, 84 HIV, 85 herpes simplex virus, 86 influenza, 87 and zika virus, 88 among others.
Antigen persistence and chronic conditions
Numerous chronic conditions have been associated with viral or bacterial persistence. EBV infection, for instance, has been associated with systemic lupus erythematosus, Sjögren’s syndrome, rheumatoid arthritis, multiple sclerosis, and other diseases.89,90 Herpes simplex virus and hepatitis B have been linked to Alzheimer’s disease.86,91 Hepatitis C virus has been linked with insulin resistance and type 2 diabetes as well as rheumatic diseases, 76 and several human herpes viruses (eg, CMV, EBV, HHV6) have been associated with brain tumors. 92 Bacterial infections have also been linked to long-term sequelae. 93 For example, helicobacter pylori (H. pylori) infections are thought to cause severe gastroduodenal disorders, 94 bacterial infections have been implicated in chronic obstructive pulmonary disease, 95 and oral bacterial infections are associated with several non-oral systemic conditions including cardiovascular disease, diabetes mellitus, stroke, inflammatory bowel disease, and others.96,97 These varied insults result in remarkably similar systemic effects indicative of equifinality related to antigen persistence.
In summary, the host immune system has evolved to maximize host health via elimination of pathogens; however, pathogen elimination in an otherwise immunocompetent host relies on congruence between an individual’s HLA composition and foreign antigen epitopes, thereby constraining the pathogens one can successfully eliminate. In the absence of HLA-antigen congruence with sufficient binding affinity and immunogenicity, the antigen may persist to various degrees resulting in long-term sequelae including chronic low-grade inflammation, autoimmunity, and chronic disease. In the next section we provide an overview of 3 chronic conditions—Gulf War Illness, Long-COVID, and chronic fatigue syndrome—and highlight evidence suggesting a contributory role of antigen persistence.
Antigen persistence in long-COVID-19
Of the 3 diseases reviewed above, long-COVID is by definition a postviral syndrome, ME/CFS is possibly a postviral syndrome, 98 but GWI has not been associated with any infection. Accordingly, SARS-CoV-2 RNA or protein have been found to persist in the olfactory mucosa for at least 6 months 71 and have been found in the brainstem,99,100 presumably having entered the brain retrogradely via olfactory sensory neurons. 71 SARS-CoV-2 RNA was found to persist in various organs up to 230 days, 72 and SARS-CoV-2 nucleic acids and immunoreactivity in the small bowel individuals 4 months after the onset of symptoms. 73 Finally, with respect to long-COVID-19, the offending antigen has been found in the cerebrospinal fluid (CSF), 101 and has been shown to persist 102 and be associated with adverse clinical outcomes101,102
Antigen persistence in ME/CFS
Similarly to GWI and long-COVID, ME/CFS is associated with immune system disruption 103 including systemic inflammation104,105 and neuroinflammation.106-108 Numerous infectious agents including several human herpes viruses (eg, EBV, HHV6, CMV) have long been suspected in the pathogenesis of ME/CFS 109-113; indeed, CFS was historically known as “chronic Epstein-Barr virus syndrome.” 114 Several lines of research suggest that viral reactivation or viral persistence underlies ME/CFS,98,112 including evidence of HHV-6 antigen in peripheral blood mononuclear cells in patients with CFS, 115 evidence of active and latent HHV-6/HHV-7 infection in plasma samples of CFS patients, 116 and deficient EBV-specific B- and T-cell memory response indicative of impaired ability to control early steps involved in EBV reactivation in CFS patients. 117 ME/CFS is also associated with autoimmunity,118,119 and recent evidence suggest that molecular mimicry between viral and human proteins may contribute to the condition. 120 Finally, both Class I and Class II HLA have been implicated in ME/CFS, linking immune-mediated pathways targeting foreign pathogens in the pathogenesis of the chronic, debilitating condition. 121
Although several brain anomalies are associated with ME/CFS, 122 brainstem abnormalities have been the most consistently documented.108,122-124 The brainstem, a central hub in inflammation neurocircuitry, 125 is implicated in sickness behaviors such as malaise, lassitude, fatigue, numbness, coldness, muscle and joint aches, and reduced appetite. 126 Like ME/CFS, both long-COVID/PASC22,127 and GWI37,128 have been linked to brainstem anomalies. Since sickness behavior is triggered by proinflammatory cytokines resulting from activation of the innate immune system in response to pathogens, 126 it is likely that the overlapping sickness behavior symptoms associated with ME/CFS, GWI, and long-COVID may be similarly driven by exposure to pathogens; although notable differences between the conditions129,130 suggest potential involvement of distinct pathogens.
Antigen persistence in GWI
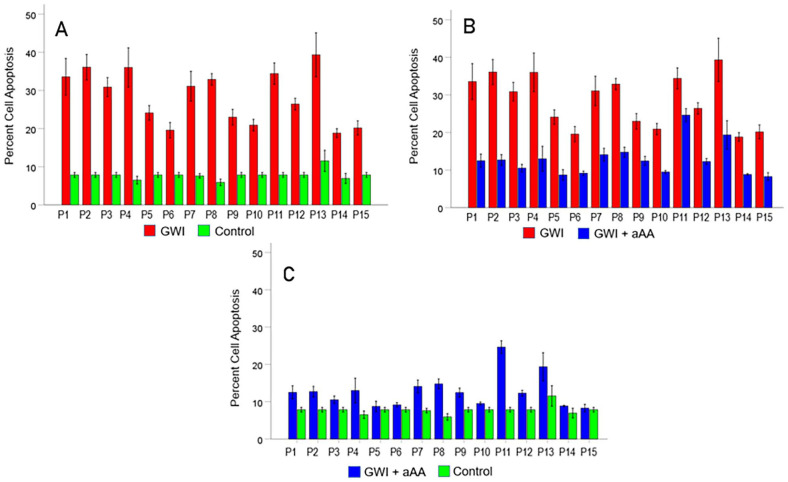
Unlike long-COVID and ME/CFS where viral components (nucleic acids and/or proteins) have been identified or implicated as persistent antigens, no such connection exists for GWI. However, research during the past 5 years has produced direct evidence for the presence of persistent neurotoxic antigens in the blood of GWI patients.50-52 Using an in vitro system of testing the effect of serum from GWI patients on the spreading and apoptosis of cells in N2A neuroblastoma cultures, it was found (a) that GWI serum has deleterious effects on the culture by reducing cell spreading and inducing cell death (apoptosis), 131 and (b) that these negative effects are prevented by the concomitant addition of serum from heathy GW veterans 131 and, partly, by the addition of pooled human IgG. 132 These findings documented the presence of harmful substances in GWI serum which can be neutralized by healthy GW serum and partly by IgG. A plausible hypothesis to explain these findings would be to attribute the adverse effects on circulating persistent antigens. Since every enlisted GW veteran was administered at least 17 vaccines, including vaccines against anthrax, botulinum toxin and plague, and since GWI appeared in all GW veterans, we entertained the hypothesis that such harmful persistent antigens in GWI serum are components of antigens contained in the administered vaccines. We then tested this hypothesis by evaluating the effect of specific antibodies against the various GW vaccine antigens and found that, indeed, such antibodies substantially reduced or even reversed the detrimental effect of GWI serum on N2A cells.50-52 The most notable beneficial effect was that of the antibody against the anthrax antigen contained in the vaccine, “protective antigen 63” (PA63),50,51 as illustrated in Figure 1 for 15 GWI patients, as follows. The serum of each patient increased substantially cell apoptosis compared to heathy GW serum (Figure 1A), an effect that was very ameliorated by the addition of anti-anthrax PA63 antibody (Figure 1B). However, the apoptosis was not fully reduced to control level (Figure 1C), due to fact that other vaccine antigens also induced increased apoptosis. 52 These findings suggest that (a) vaccine-related neurotoxic antigens circulate in the blood of veterans with GWI, (b) the presence of these antigens may contribute to systemic GWI symptoms affecting multiple organs, and (c) deleterious effects can be neutralized by serum from healthy GW veterans suggesting promising therapeutics aimed at elimination of the persistent harmful antigens. Interestingly, the connection of GWI with vaccine antigens and the fact that GW women veterans are in poorer health overall than GW male veterans31,32 are in keeping with the fact that women have more serious and prolonged side effects to vaccines.67,68
Figure 1.
Evidence for the presence of anthrax protective antigen (PA63) in the serum of veterans with GWI. Data are from 15 GWI patients (P1-P15) compared to a healthy GW veteran control. Ordinate is percent apoptosis (cell death) in neuroblastoma N2A cultures following incubation with the stated treatment. (A) much higher percent apoptosis in incubation with each GWI patient serum vs. control. (B) systematic beneficial effect (reduction in apoptosis) in each patient when antibody against anthrax PA63 was added to the patient’s serum. (C) apoptosis in GWI + anti anthrax antibody vs. apoptosis in control; the apoptosis in GWI + anti anthrax antibody is still higher than in the control presumably due to the presence of other persistent antigens in GWI. 59 The figure is a composite of figures 12, 13 and 14 in Bai et al. 57
Finally, it should be noted that our persistent antigen hypothesis (and evidence for it) is not incompatible with an adverse effect of stress, inflammation and toxic exposures in GWI. In fact, as we argued in detail elsewhere, 37 those factors have been shown in various studies133-141 to break and/or enhance permeability of the blood brain barrier, thus effectively allowing entrance to the brain of harmful persistent antigens. Such BBB breakdown has been specifically described in an animal model of GWI. 133 In that view, the combined effect of toxic exposures, stress and inflammation (of diverse origin) is to make the brain vulnerable by breaking the blood brain barrier and thus enable and/or potentiate the brain damage inflicted by otherwise circulating persistent antigens (eg, anthrax PA63 and other vaccine-related antigens) by allowing their entry to the brain. It should be noted that that mechanism is fairly general and does not apply only to GWI but to other disorders as well, including ME/CFS and long-COVID.
Antigen persistence and HLA
As mentioned above, HLA is essential for eliminating offending antigens shortly after infection (through CD8+ T cell cytotoxicity mediated by HLA Class I molecules) and preventing future infection (through CD4+ T cell initiation of antibody production by B cells). Assuming immunocompetence along the T cell immune response pathways, the first and crucial step in eliminating an offending antigen is a good match between epitopes of the antigen and HLA molecules. A mismatch with HLA Class I molecules will result in defective cytotoxicity of infected cells mediated by CD8+ T cells, whereas a mismatch with HLA Class II molecules will result in defective antibody production initiated by CD4+ T cells. Absence of both HLA Class I and II molecules (primary immunodeficiency disorder) is fatal due to overwhelming, mostly viral, infections. 142 (A similarly serious outcome comes from loss of innate immunity, as seen, eg, in agranulocytosis.)
Long-COVID-19 and HLA
Studies have documented that sex, age, and pre-existing conditions are associated with increased risk of long-COVID.143,144 Clinical heterogeneity in post-infection outcomes may also be partially driven by variability in host immune-mediated responses including variability in HLA composition. Indeed, HLA has been implicated in COVID course and severity.145-151 In silico studies have documented Class I147,148 and Class II HLA 151 alleles with high and low binding affinity to SARS-CoV-2 peptides, presumably resulting in, respectively, enhanced and reduced ability to present viral antigens to immune cells. To our knowledge no studies have documented HLA-associations with long-COVID; however, we have posited that reduced binding affinity due to HLA-antigen incongruence may contribute to persistent COVID antigens and subsequent downstream long-COVID sequelae. 150 Several lines of evidence document immune system disruption that may be associated with HLA including lower levels of certain antibodies soon after infection, suggesting deficient adaptive immune system response, 152 and evidence of SARS-CoV-2 viral antigen in the gut 73 and in several other extrapulmonary tissues including the brain, up to 7 months after symptom onset. 72 Since antigen persistence is known to result in immune system disruption and inflammation, it is possible that the prolonged inflammation in patients with long-COVID3,153-155 may be partially attributable to viral antigen persistence.
ME/CFS and HLA
Early, small scale attempts to identify ME/CFS and HLA associations yielded mixed and inconclusive results. However, a recent study on 426 adults suffering from ME/CFS and 4511 healthy controls provided the first clear association of this disorder with specific HLA alleles. 28 All participants were genotyped for the 6 classical HLA genes (Class I: A, B, C; Class II: DBP1, DQB1, DRB1) at high, 4-digit resolution and the HLA composition between patients and controls compared. Two risk alleles were identified, one from HLA Class I (C*07:04) and another from Class II (DQB1 * 03:03), as well as one protective allele from Class I (B*08:01). This latter allele frequently occurs with 3 other alleles on the haplotype C*07:01-B*08:01-DRB1 * 03:01-DQB1*02:01, which was also found to be less prevalent among the ME/CFS patients studied, as compared to the controls. Although the emphasis of that study is on the risk alleles, the identification of protective alleles is more interesting from the perspective of our persistent antigen hypothesis, because it is their presence that may be instrumental in eliminating the offending pathogens and thus preventing the disease. In our view, in the absence of those alleles, chronic inflammation due to the persistence of the offending antigen(s) and accompanying tissue breakdown would trigger autoimmunity (mediated by C*07:04 and/or DQB1 * 03:03) and perpetuate the symptomatology of the disorder.
GWI and HLA
An intriguing issue is why serum from healthy GW veterans exerts the same neutralizing effects as antibodies against anthrax antigen? We have previously surmised that healthy Gulf War veterans were able to make antibodies against the antigens in vaccines administered to them whereas GWI veterans were unable to do so due to lack of immunogenetic protection, resulting in the persistence of antigens.1,47 Since antibody production hinges on HLA Class II molecules, we compared HLA composition in GWI veterans and healthy Gulf War veterans and found that veterans with GWI can be distinguished from healthy Gulf War veterans by the lower prevalence of 6 HLA Class II alleles in GWI veterans, indicating lack of protection against GWI 47 (DPB1 * 01:01, DPB1 * 06:01, DQB1 * 02:02, DRB1 * 01:01, DRB1 * 13:02). This protective effect was further documented by the finding that the severity of GWI symptoms was negatively associated with the frequency of these alleles such that lower allele frequency was associated with greater symptom severity. 47 The 6 Class II HLA alleles shown to protect against GWI (ie, those that were present in healthy Gulf War veterans but not GWI veterans) were shown to bind with high affinity to antigens from vaccines administered to Gulf War veterans. 156 As such, it was concluded that healthy Gulf War veterans were able to mount a sufficient immune response to vaccine antigens whereas individuals lacking immunogenetic protection against those vaccine antigens are unable to mount a sufficient immune response resulting in antigen persistence. 1
Downstream effects of antigen persistence include inflammation, autoimmunity, and cell death.44,81,82 Numerous studies have documented immune system disruption47,157-159 and altered brain function36,160 in genetically vulnerable Gulf War veterans. 47 In addition, studies have documented elevated peripheral inflammation in GWI.161,162 Furthermore, C-reactive protein, a marker of inflammation, is associated with cortical thinning, hippocampal atrophy, and decreased white matter integrity in veterans with GWI.38,39 With regard to autoimmunity, one-quarter of veterans with GWI are positive for lupus anticoagulant (LAC), an autoantibody against phospholipids or phospholipid-binding proteins that results in formation of blood clots in vivo. 163 Furthermore, LAC-positivity is associated with significant brain atrophy in GWI veterans. 164 Remarkably, the presence of specific Class II HLA has been shown to spare brain atrophy, 165 a characteristic of GWI, 37 and prevent inflammation and cortical thinning. 40 Finally, evidence indicates that brain function in veterans with GWI is indistinguishable from that of classic autoimmune disorders. 166 The latter, coupled with evidence of immune system disruption and a large number of symptoms involving the brain, has led to the conclusion that GWI is most accurately considered a neuroimmune disorder.166-168
In summary, persistent harmful antigens do not have to be confined to viruses or other pathogens but they can come from other sources, such as the anthrax vaccine. Interestingly, there is a parallel in the mechanism of antigen persistence that, at least partly, underlies that persistence - namely a diminished (or lack of) protection by the HLA system due to a pathogen effect or genetic makeup. For example, viruses (eg, HIV, HHV, influenza) downregulate the expression of HLA molecules,169-173 whereas an individual may lack specific HLA molecules possessing high binding affinity to specific harmful antigens (given that 1 carries only 12 classical HLA alleles), therefore becoming vulnerable to those antigens. The latter case is exemplified in GWI where the lack of 6 protective HLA Class II alleles has been documented, 47 among which DRB1 * 01:01 and DRB1 * 13:02 have been shown to have very high affinity of binding to anthrax vaccine antigen PA63. 156
Summary and Implications
Here we briefly reviewed evidence suggesting that 3 distinct chronic conditions (long-COVID, ME/CFS, and GWI) are associated with immune system dysfunction and result in similar symptoms involving multiple organ systems including the brain. While long-COVID is attributable to infection with the SARS-CoV-2 virus, the etiology of GWI and ME/CFS are uncertain though infectious agents have been implicated. We suggest that all 3 chronic conditions are, in part, associated with persistent antigens resulting from HLA-antigen incongruence. We discuss this point further in that what follows.
Why do pathogen antigens persist?
As we reviewed above, pathogenic antigens that persist are at the root of many chronic disease conditions affecting the brain and other organs. A fundamental question then is, why do such antigens persist? Obviously they entered the body, typically as component parts of a pathogen (eg, virus or other microbe), but could not be eradicated from it. Such entrance triggers an immune response at basically 3 different levels, including (a) activation of the innate immune system to mobilize white blood cells (macrophages and polymorphonuclear cells) and demarcate infection by initiating local inflammatory response, (b) engagement of HLA Class I molecules (present in nucleated cells) which bind to small peptide fragments (9-10 AA length) of the cleaved offending protein, and migrate to the cell surface to activate CD8+ cytotoxic lymphocytes which kill the infected cell, and (c) engagement of HLA Class II molecules (present in antigen presenting cells – APC) which bind to longer peptides (12-22 AA length) of the offending protein, and migrate to the cell surface to activate CD4+ lymphocytes which transport the peptide(s) to the B cells and initiate antibody production. This defense scheme aims (a) to immediately delimit the infection, (b) to kill quickly (apoptosis) the infected cells, and (c) to initiate production of antibodies which will inactivate persisting offending antigens (after a couple of weeks) and prevent reinfection in the future by the same pathogen. All these 3 lines of defense are crucial for dealing successfully with infections, as evidenced by the severe detrimental consequences when these defenses fail, as discussed above.
Between the 2 extremes of a perfectly healthy organism and a severely immunosuppressed one, there lies a wide spectrum of conditions where immune defenses are not at their best and the organism is at relative risk. For example, suppression of white cell formation of various degrees (and consequently low white blood cell count) can occur as a side effect of pharmacotherapy of various diseases, reduction of CD4+ T lymphocytes is caused by human immunodeficiency virus, suppression of lymphocyte function can be caused by substances produced in tumor cells, etc. Such cases are collectively labeled as “immunocompromised,” in contrast to the term “immunocompetent” denoting a healthy immune system. It is not surprising that antigens can, and usually do, persist in immunocompromised individuals simply because the immune mechanisms to eliminate them are deficient and hence only partially successful in that aspect. However, antigens can persist in fully immunocompetent individuals—but why? There are 2 main reasons for this, not mutually exclusive, namely (a) properties of the pathogen, and (b) genetic makeup of the host. With respect to pathogens, a typical case concerns human herpesviruses (HHV) which notoriously can persist in the body in a latent state for a long time due to various mechanisms. For example, HHV1 invades sensory nerves at the periphery shortly after infection, it is transported retrogradely to the cell soma where it can remain latent for long periods of time, and get reactivated periodically (due to various reasons), in which case it is transported anterogradely to the periphery, exits the axon and infects epithelial cells. 174 In a different “strategy,” human betaherpesviruses HHV6A and HHV6 evade the adaptive immune response by downregulating the production of HLA Class I and Class II molecules,169,170 among other mechanisms. 175 This mechanism targeting the production of HLA molecules is particularly interesting because its effect is essentially the same as the lack of specific HLA molecules for genetic reasons. As mentioned above, an individual carries 6 HLA Class I and 6 Class II classical alleles. Each one of these alleles has a very specific structure, such that it can connect with high affinity only to peptides with amino acid sequence that matches that in the groove of the HLA molecule. Given that specificity, it is not surprising that a given HLA molecule can match with high affinity only to a limited number of peptide structures, ultimately coming from a limited number of pathogens. The vast polymorphism of the HLA system assures the evolutionary benefit conferred to the population but it means little to the individual who carries only 12 HLA alleles. In contrast to the HHV6 downregulation of HLA molecules indiscriminately, the genetic HLA makeup of the individual essentially protects only against certain pathogens and, conversely, renders the individual susceptible to other pathogens for which HLA specificity is absent (lack of protection). Given the large variety of pathogens (mostly viruses) and the very limited coverage afforded by 12 HLA alleles, it is not surprising that a good number of pathogens may persist after infection simply because of a mismatch of their proteins to the specific set of 12 HLA alleles the individual possesses. In fact, this is the main point of this review, namely that antigen persistence may be attributed, to a major degree, to the lack of HLA molecules suitably specific to match with those antigens. In that sense, in our view, antigen persistence, with all associated manifestations such as chronic inflammation, is due in part to the lack of protection afforded by HLA against those antigens. This is illustrated schematically in Figures 2 and 3. It is noteworthy that lack of HLA protection in GWI has been documented 47 and associated brain mechanisms identified.160,166 Conversely, it was found that the presence of the GWI-protective HLA allele DRB1 * 13:02 protects from subcortical brain atrophy in GWI. 165
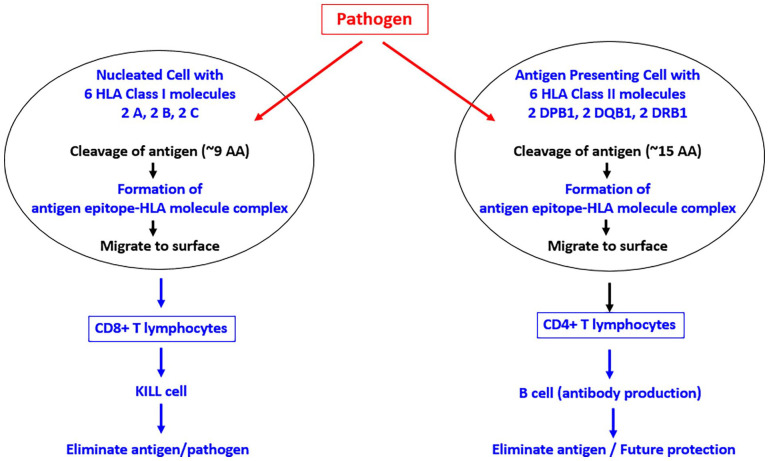
Figure 2.
Schematic diagram to illustrate the normal HLA mechanism of antigen processing. See text for details.
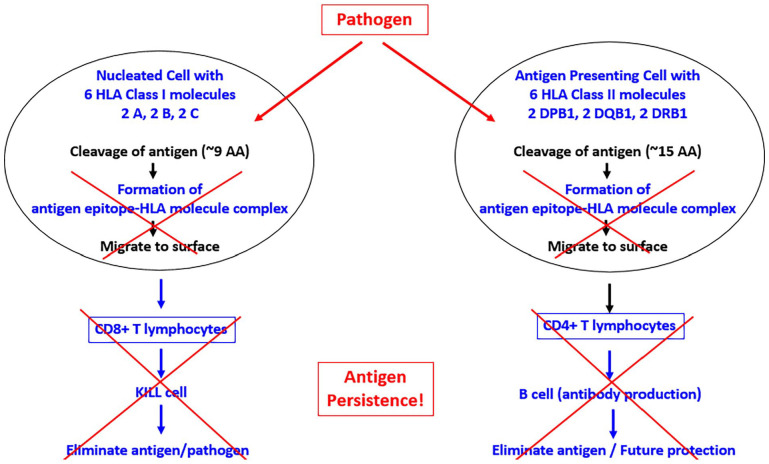
Figure 3.
Schematic diagram to illustrate the altered antigen processing in the absence of HLA match. See text for details.
Can persistent antigens alone cause disease?
The cell culture studies on GWI reviewed above have demonstrated the presence of vaccine-related persistent antigens in the blood of veterans with GWI 30 years after their enlistment. One question is to what extent the harmful effects of these persistent protein antigens on the N2A neuroblastoma culture are associated with symptom severity in those patients. We found that this is indeed the case 132 ; specifically, the severity of neurocognitive-mood GWI symptom was positively associated with apoptosis, an effect that was highly statistically significantly (r = .837, P < .001). 132 This result validates the in vitro culture testing approach which could be applied to other disease conditions. Interestingly, the possible pathogenicity of pure viral protein fragments (without nucleic acids) was reported recently for SARS-CoV-2 176 where it was shown that intratracheally instillation of the S1 subunit of SARS-CoV-2 spike protein (S1SP) in K18-hACE2 transgenic mice that overexpress human ACE2 and examined signs of COVID-19-associated lung injury 72 hours later. Indeed, mice instilled with S1SP exhibited a decline in body weight, dramatically increased white blood cells and protein concentrations in bronchoalveolar lavage fluid (BALF), upregulation of multiple inflammatory cytokines in BALF and serum, histological evidence of lung injury. In contrast, K18-hACE2 mice that received either saline or SP exhibited little or no evidence of lung injury. This is the first demonstration of a COVID-19-like response by an essential virus-encoded protein by SARS-CoV-2 in vivo and is in keeping with the finding that SARS-CoV-2 spike glycoprotein is proinflammatory. 177 Given the considerations above concerning the potential of persistent protein antigens to produce disease, it would be important to know whether such antigens can be transmitted from person-to-person. In this context, it is interesting that a multisystem syndrome resembling GWI has been reported in spouses of veterans with GWI, 178 in a prevalence independent of the deployment status of the GWI spouse. This finding raises the issue of potential transmissibility of GWI persistent antigens.
Potential interventions
Current treatments for the 3 chronic diseases discussed above are essentially focused on the treatment of symptoms. The ideal treatment would be the eradication of the offending persistent antigens to arrest the ongoing damage and prevent future damage. (a) With respect to long-COVID-19, the obvious objective is to eradicate the SARS-CoV-2 virus from the body, a feat waiting to be accomplished. (b) With respect to ME/CFS, the pathogenic persistent antigens are suspected but unknown and, therefore, no radical intervention at removing them is possible. Interestingly, an early attempt at intervention by administering pooled human IgG did not have a beneficial effect. 179 (b) With respect to GWI, the case is different for GWI because persistent antigens have been identified circulating in the blood of GWI patients.50-52 Theoretically, these antigens could be eliminated by administering specific antibodies but this is infeasible, especially since these antigens include the anthrax PA63 antigen. The addition of pooled IgG to the N2A culture did reduce the apoptosis induced by GWI serum but did not fully eliminate it (Figure 4). This was expected since pooled IgG from the general population is not likely to contain antibodies against anthrax, botulinum toxin, plague, and, perhaps, against cholera, yellow fever and rabies, 52 that is for rare diseases for which no routine vaccines are given. Nevertheless, there is a potentially beneficial intervention aiming at the removal of those antigens from the blood, namely plasma exchange. Since disease symptoms are likely due to chronic inflammation (induced and maintained by the persistent antigens) and associated immune reaction(s) (eg, release of cytokines), it is reasonable to expect that the removal of the offending antigens would be beneficial in ameliorating the symptoms and reduce the persistent antigen load. As mentioned above, plasma exchange would be most beneficial in GWI, where, for example, the harmful anthrax antigen was introduced and persisted because of genetic HLA vulnerability but which did not replicate. On the other hand, this procedure would be only temporarily beneficial in cases where a virus becomes latent by “hiding,” as is the case of HHV1 which invades nerve cells and does not circulate in the absence of overt infection. Therapeutic Plasma Exchange is a relatively safe procedure, which is performed in certain diseases, notably in myasthenia gravis, 180 where it effectively clears the blood from circulating autoantibodies against the neuromuscular junction. 181 It is an invasive and expensive procedure, and with potential serious side effects, as reported in early studies.182,183 However, recent applications of TPE in severe COVID-19 cases has proved beneficial and without significant side effects184-188 probably due to improvements in the procedure (eg, intravenous calcium replacement and administration of chlorpheniramine during TPE to reduce side effects). 188
Figure 4.
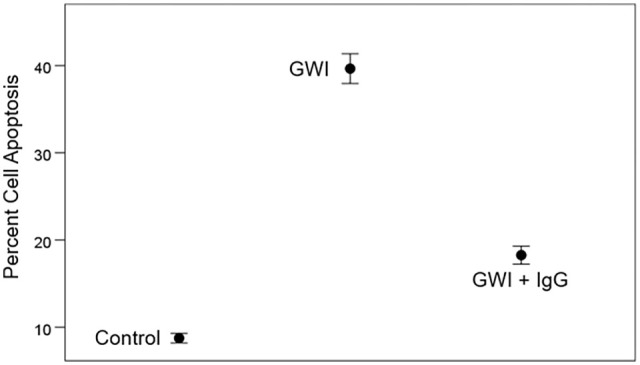
Beneficial but limited effect of added pooled human IgG to GWI serum in reducing apoptosis. The limited effect is presumably due to the expected lack of antibodies in IgG of rare diseases (anthrax, botulism, plague, rabies, etc.) to which the general population is not typically exposed and for which no vaccines are given routinely. Bars are SEM. From Rouse and Kaistha. 63
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Partial funding for this study was provided by the University of Minnesota (the Anita Kunin Chair in Women’s Healthy Brain Aging, the Brain and Genomics Fund, the McKnight Presidential Chair of Cognitive Neuroscience, and the American Legion Brain Sciences Chair) and the U.S. Department of Veterans Affairs. The sponsors had no role in the current study design, analysis or interpretation, or in the writing of this paper. The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: APG and LMJ contributed equally to writing and editing the paper.
Significance Statement: In this review we highlight the influence of persistent antigens and lack of human leukocyte antigen protection as contributing to three conditions characterized by persistent long-term symptoms affecting the brain and other systems.
ORCID iD: Apostolos P Georgopoulos  https://orcid.org/0000-0003-4412-725X
https://orcid.org/0000-0003-4412-725X
References
- 1. James L, Georgopoulos A. Persistent antigens hypothesis: the human leukocyte antigen (HLA) connection. J Neurol Neuromed. 2018;3:27-31. [Google Scholar]
- 2. WHO Coronavirus (COVID-19) Dashboard | Geneva: World Helath Organization, 2020. Accessed March 31, 2022. Available https://covid19.who.int/
- 3. Lopez-Leon S, Wegman-Ostrosky T, Perelman C, et al. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci Rep. 2021;11:16144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Seeßle J, Waterboer T, Hippchen T, et al. Persistent symptoms in adult patients 1 year after Coronavirus Disease 2019 (COVID-19): a Prospective Cohort Study. Clin Infect Dis. 2022;74:1191-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Carfì A, Bernabei R, Landi F. Persistent symptoms in patients after acute covid-19. JAMA. 2020;324:603-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Logue JK, Franko NM, McCulloch DJ, et al. Sequelae in adults at 6 months after COVID-19 infection. JAMA Netw Open. 2021;4:e210830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tenforde MW, Kim SS, Lindsell CJ, et al. Symptom duration and risk factors for delayed return to usual health among outpatients with COVID-19 in a multistate health care systems network — United States, March–June 2020. MMWR Morb Mortal Wkly Rep. 2020;69:993-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Townsend L, Dowds J, O’Brien K, Martin-Loeches I, Nadarajan P, Bannan C. Reply: the relation between persistent poor health after COVID-19 and respiratory complications or initial disease severity. Ann Am Thorac Soc. 2021;18:1431-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Al-Aly Z, Bowe B, Xie Y. Long COVID after breakthrough SARS-CoV-2 infection. Nat Med. Published online 25 May 2022. doi: 10.1038/s41591-022-01840-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Garg M, Maralakunte M, Garg S, et al. The conundrum of ‘long-COVID-19’: a narrative review. Int J Gen Med. 2021;14:2491-2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Davis HE, Assaf GS, McCorkell L, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021;38:101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Xie Y, Xu E, Bowe B, Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat Med. 2022;28:583-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rathmann W, Kuss O, Kostev K. Incidence of newly diagnosed diabetes after Covid-19. Diabetologia. 2022;65:949-954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li X, Raventós B, Roel E, et al. Association between covid-19 vaccination, SARS-CoV-2 infection, and risk of immune mediated neurological events: population based cohort and self-controlled case series analysis. BMJ. 2022;e068373. 10.1136/bmj-2021-068373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu Y, Sawalha AH, Lu Q. COVID-19 and autoimmune diseases. Curr Opin Rheumatol. 2021;33:155-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ehrenfeld M, Tincani A, Andreoli L, et al. Covid-19 and autoimmunity. Autoimmun Rev. 2020;19:102597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dennis A, Wamil M, Alberts J, et al.; COVERSCAN study investigators. Multiorgan impairment in low-risk individuals with post-COVID-19 syndrome: a prospective, community-based study. BMJ Open. 2021;11:e048391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Douaud G, Lee S, Alfaro-Almagro F, et al. SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature. 2022;604:697-707. Published online Mar 7, 2022. doi: 10.1038/s41586-022-04569-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Qureshi AI, Baskett WI, Huang W, Naqvi SH, Shyu CR. New-onset dementia among survivors of pneumonia associated with severe acute respiratory syndrome coronavirus 2 infection. Open Forum Infect Dis. 2022;9:ofac115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Baig AM. Deleterious outcomes in long-hauler COVID-19: the effects of SARS-CoV-2 on the CNS in Chronic COVID syndrome. ACS Chem Neurosci. 2020;11:4017-4020. [DOI] [PubMed] [Google Scholar]
- 21. Institute of Medicine. Beyond Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Redefining an Illness. Washington, DC: The National Academies Press, 2015. 10.17226/19012. [DOI] [PubMed] [Google Scholar]
- 22. Proal AD, VanElzakker MB. Long COVID or post-acute sequelae of COVID-19 (PASC): an overview of biological factors that may contribute to persistent symptoms. Front Microbiol. 2021;12:698169. 1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Komaroff AL, Lipkin WI. Insights from myalgic encephalomyelitis/chronic fatigue syndrome may help unravel the pathogenesis of postacute COVID-19 syndrome. Trends Mol Med. 2021;27:895-906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Eisen SA. Gulf war veterans' health: medical evaluation of a US cohort. Ann Intern Med. 2005;142:881-890. [DOI] [PubMed] [Google Scholar]
- 25. Kipen HM, Hallman W, Kang H, Fiedler N, Natelson BH. Prevalence of chronic fatigue and chemical sensitivities in Gulf registry veterans. Arch Environ Health. 1999;54:313-318. [DOI] [PubMed] [Google Scholar]
- 26. Lim EJ, Ahn YC, Jang ES, Lee SW, Lee SH, Son CG. Systematic review and meta-analysis of the prevalence of chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME). J Transl Med. 2020;18:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Falk Hvidberg M, Brinth LS, Olesen AV, Petersen KD, Ehlers L. The health-related quality of life for patients with myalgic encephalomyelitis / Chronic Fatigue Syndrome (ME/CFS). PLoS One. 2015;10:e0132421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lande A, Fluge Strand E.B, et al. Human leukocyte antigen alleles associated with myalgic encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS). Sci Rep. 2020;10:5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fappiano CM, Baraniuk JN. Gulf war illness symptom severity and onset: A cross-sectional survey. Mil Med. 2020;185:e1120-e1127. [DOI] [PubMed] [Google Scholar]
- 30. Kang HK, Li B, Mahan CM, Eisen SA, Engel CC. Health of US veterans of 1991 Gulf war: a follow-up survey in 10 years. J Occup Environ Med. 2009;51:401-410. [DOI] [PubMed] [Google Scholar]
- 31. Dursa EK, Barth SK, Porter BW, Schneiderman AI. Health status of female and male Gulf war and Gulf Era Veterans: A population-based study. Womens Health Issues. 2019;29 Suppl 1:S39-S46. [DOI] [PubMed] [Google Scholar]
- 32. Brown MC, Sims KJ, Gifford EJ, et al. Gender-based differences among 1990-1991 Gulf war era veterans: demographics, lifestyle behaviors, and health conditions. Womens Health Issues. 2019;29 Suppl 1:S47-S55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fukuda K, Nisenbaum R, Stewart G, et al. Chronic multisymptom illness affecting Air Force veterans of the Gulf war. JAMA. 1998;280:981-988. [DOI] [PubMed] [Google Scholar]
- 34. Steele L. Prevalence and patterns of Gulf war illness in Kansas veterans: association of symptoms with characteristics of person, place, and time of military service. Am J Epidemiol. 2000;152:992-1002. [DOI] [PubMed] [Google Scholar]
- 35. White RF, Steele L, O’Callaghan JP, et al. Recent research on Gulf war illness and other health problems in veterans of the 1991 Gulf war: Effects of toxicant exposures during deployment. Cortex. 2016;74:449-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Engdahl BE, James LM, Miller RD, et al. A magnetoencephalographic (MEG) study of Gulf War Illness (GWI). EBioMedicine. 2016;12:127-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Christova P, James LM, Engdahl BE, Lewis SM, Carpenter AF, Georgopoulos AP. Subcortical brain atrophy in Gulf war Illness. Exp Brain Res. 2017;235:2777-2786. [DOI] [PubMed] [Google Scholar]
- 38. Christova P, James L, Carpenter A, et al. C-reactive protein is associated with brain white matter anomalies in Gulf war Illness. J Neurol Neuromed. 2020;5:55-62. [Google Scholar]
- 39. Christova P, James L, Carpenter A, et al. Gulf war Illness: C-reactive protein is associated with reduction of the volume of hippocampus and decreased fractional anisotropy of the fornix. J Neurol Neuromed. 2020;5:6-15. [Google Scholar]
- 40. Christova P, James L, Carpenter A, Lewis S, Engdahl B, Georgopoulos A. Human leukocyte antigen (HLA) alleles prevent metabolically-induced inflammation and cerebrocortical thinning in Gulf war Illness. J Neurol Neuromed. 2020;5:16-27. [Google Scholar]
- 41. Institute of Medicine. Chronic Multisymptom Illness in Gulf War Veterans: Case Definitions Reexamined. Washington, DC: The National Academies Press; 2014. [PubMed] [Google Scholar]
- 42. Gifford EJ, Vahey J, Hauser ER, et al. Gulf war illness in the Gulf war era cohort and biorepository: the Kansas and Centers for Disease Control definitions. Life Sci. 2021;278:119454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. National Academies of Sciences, Engineering, and Medicine. Gulf War and Health: Volume 10: Update of Health Effects of Serving in the Gulf War, 2016. Washington, DC: The National Academies Press; 2016. [Google Scholar]
- 44. Institute of Medicine National Research Council. Gulf War and Health: Volume 1. Depleted Uranium, Pyridostigmine Bromide, Sarin, and Vaccines. National Academies Press; 2000. [PubMed] [Google Scholar]
- 45. Takafuji ET, Russell PK. Military immunizations: Past, present, and future prospects. Infect Dis Clin North Am. 1990;4:143-158. [PubMed] [Google Scholar]
- 46. OSAGWI (Office of the Special Assistant for Gulf War Illnesses). Military Medical Recordkeeping During and After the Gulf War: Interim Report. U.S. Department of Defense; 1999. [Google Scholar]
- 47. Georgopoulos AP, James LM, Mahan MY, Joseph J, Georgopoulos A, Engdahl BE. Reduced human leukocyte antigen (HLA) protection in Gulf War Illness (GWI). EBioMedicine. 2016;3:79-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hotopf M, David A, Hull L, Ismail K, Unwin C, Wessely S. Role of vaccinations as risk factors for ill health in veterans of the Gulf war: cross sectional study. BMJ. 2000;320:1363-1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Unwin C, Blatchley N, Coker W, et al. Health of UK servicemen who served in Persian Gulf War. Lancet. 1999;353:169-178. [DOI] [PubMed] [Google Scholar]
- 50. Tsilibary EP, Souto E, Kratzke M, James L, Engdahl B, Georgopoulos A. Anthrax and Gulf War Illness (GWI): Evidence for the presence of harmful anthrax antigen PA63 in the serum of veterans with GWI. J Neurol Neuromed. 2019;4:1-9. [Google Scholar]
- 51. Tsilibary EC, Souto EP, Kratzke M, James LM, Engdahl BE, Georgopoulos AP. Anthrax protective antigen 63 (PA63): Toxic effects in neural cultures and role in Gulf War Illness (GWI). Neurosci Insights. 2020;15:2633105520931966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tsilibary EC, Souto EP, Kratzke M, James LM, Engdahl BE, Georgopoulos AP. Vaccine-induced adverse effects in cultured neuroblastoma 2A (N2A) cells duplicate toxicity of serum from patients with Gulf war Illness (GWI) and are prevented in the presence of specific anti-vaccine antibodies. Vaccines. 2020;8:232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Israeli E. Gulf war syndrome as a part of the autoimmune (autoinflammatory) syndrome induced by adjuvant (ASIA). Lupus. 2012;21:190-194. [DOI] [PubMed] [Google Scholar]
- 54. Toubi E. ASIA—Autoimmune syndromes induced by adjuvants: rare, but worth considering. Israel Med Assoc J. 2012;14:121. [PubMed] [Google Scholar]
- 55. Pelà G, Goldoni M, Solinas E, et al. Sex-related differences in long-COVID-19 syndrome. J Womens Health. 2022;31:620-630. [DOI] [PubMed] [Google Scholar]
- 56. PHOSP-COVID Collaborative Group. Clinical characteristics with inflammation profiling of long COVID and association with 1-year recovery following hospitalisation in the UK: a prospective observational study. Lancet Respir Med. 2022:S2213-2600(22)00127-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bai F, Tomasoni D, Falcinella C, et al. Female gender is associated with long COVID syndrome: a prospective cohort study. Clin Microbiol Infect. 2022;28:611.e9-611.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Faro M, Sàez-Francás N, Castro-Marrero J, Aliste L, Fernández de, Sevilla T, Alegre J. Gender differences in chronic fatigue syndrome. Reumatol Clin. 2016;12:72-77. [DOI] [PubMed] [Google Scholar]
- 59. Mueller SN, Rouse BT. Immune responses to viruses. Clin Immunol. 2008;421-431. [Google Scholar]
- 60. Beutler B. Innate immunity: an overview. Mol Immunol. 2004;40:845-859. [DOI] [PubMed] [Google Scholar]
- 61. Klenerman P, Hill A. T cells and viral persistence: lessons from diverse infections. Nat Immunol. 2005;6:873-879. [DOI] [PubMed] [Google Scholar]
- 62. Eligio P, Delia R, Valeria G. EBV chronic infections. Mediterr J Hematol Infect Dis. 2010;2:e2010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Rouse BT, Kaistha SD. A tale of 2 alpha-herpesviruses: lessons for vaccinologists. Clin Infect Dis. 2006;42:810-817. [DOI] [PubMed] [Google Scholar]
- 64. Meuer SC, Hussey RE, Hodgdon JC, Hercend T, Schlossman SF, Reinherz EL. Surface structures involved in target recognition by human cytotoxic T lymphocytes. Science. 1982;218:471-473. [DOI] [PubMed] [Google Scholar]
- 65. Hov JR, Kosmoliaptsis V, Traherne JA, et al. Electrostatic modifications of the human leukocyte antigen-DR P9 peptide-binding pocket and susceptibility to primary sclerosing cholangitis. Hepatology. 2011;53:1967-1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Dendrou CA, Petersen J, Rossjohn J, Fugger L. HLA variation and disease. Nat Rev Immunol. 2018;18:325-339. [DOI] [PubMed] [Google Scholar]
- 67. Ruggieri A, Anticoli S, D’Ambrosio A, Giordani L, Viora M. The influence of sex and gender on immunity, infection and vaccination. Ann Ist Super Sanita. 2016;52:198-204. [DOI] [PubMed] [Google Scholar]
- 68. Fischinger S, Boudreau CM, Butler AL, Streeck H, Alter G. Sex differences in vaccine-induced humoral immunity. Semin Immunopathol. 2019;41:239-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Shin H, Wherry EJ. CD8 T cell dysfunction during chronic viral infection. Curr Opin Immunol. 2007;19:408-415. [DOI] [PubMed] [Google Scholar]
- 70. Hope JL, Stairiker CJ, Bae EA, Otero DC, Bradley LM. Striking a balance-cellular and molecular drivers of memory T cell development and responses to chronic stimulation. Front Immunol. 2019;10:1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. de Melo GD, Lazarini F, Levallois S, et al. COVID-19-related anosmia is associated with viral persistence and inflammation in human olfactory epithelium and brain infection in hamsters. Sci Transl Med. 2021;13:eabf8396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Chertow D, Stein S, Ramelli S, et al. SARS-CoV-2 infection and persistence throughout the human body and brain. Research Square. 2021. Preprint posted online December 20, 2021. 10.21203/rs.3.rs-1139035/v1 [DOI] [Google Scholar]
- 73. Gaebler C, Wang Z, Lorenzi JCC, et al. Evolution of antibody immunity to SARS-CoV-2. Nature. 2021;591:639-644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Vibholm LK, Nielsen SSF, Pahus MH, et al. SARS-CoV-2 persistence is associated with antigen-specific CD8 T-cell responses. EBioMedicine. 2021;64:103230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Jarrah SA, Kmetiuk LB, de Carvalho OV, et al. Persistent SARS-CoV-2 antigen presence in multiple organs of a naturally infected cat from Brazil. J Venom Anim Toxins Incl Trop Dis. 2022;28:e20210074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Mathew S, Faheem M, Ibrahim SM, et al. Hepatitis C virus and neurological damage. World J Hepatol. 2016;8:545-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Guo CY, Tang YG, Qi ZL, et al. Development and characterization of a panel of cross-reactive monoclonal antibodies generated using H1N1 influenza virus. Immunobiology. 2015;220:941-946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Sarkanen TO, Alakuijala APE, Dauvilliers YA, Partinen MM. Incidence of narcolepsy after H1N1 influenza and vaccinations: systematic review and meta-analysis. Sleep Med Rev. 2018;38:177-186. [DOI] [PubMed] [Google Scholar]
- 79. Jessie K, Fong MY, Devi S, Lam SK, Wong KT. Localization of dengue virus in naturally infected human tissues, by immunohistochemistry and in situ hybridization. J Infect Dis. 2004;189:1411-1418. [DOI] [PubMed] [Google Scholar]
- 80. Bravo D, Hoare A, Soto C, Valenzuela MA, Quest AF. Helicobacter pyloriin human health and disease: mechanisms for local gastric and systemic effects. World J Gastroenterol. 2018;24:3071-3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Getts DR, Chastain EM, Terry RL, Miller SD. Virus infection, antiviral immunity, and autoimmunity. Immunol Rev. 2013;255:197-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Swanson Pa, 2nd, McGavern DB. Viral diseases of the central nervous system. Curr Opin Virol. 2015;11:44-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Haruwaka K, Ikegami A, Tachibana Y, et al. Dual microglia effects on blood brain barrier permeability induced by systemic inflammation. Nat Commun. 2019;10:5816-5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Fletcher NF, McKeating JA. Hepatitis C virus and the brain. J Viral Hepat. 2012;19:301-306. [DOI] [PubMed] [Google Scholar]
- 85. Koenig S, Gendelman HE, Orenstein JM, et al. Detection of AIDS virus in macrophages in brain tissue from AIDS patients with encephalopathy. Science. 1986;233:1089-1093. [DOI] [PubMed] [Google Scholar]
- 86. Marcocci ME, Napoletani G, Protto V, et al. Herpes simplex virus-1 in the brain: the dark side of a sneaky infection. Trends Microbiol. 2020;28:808-820. [DOI] [PubMed] [Google Scholar]
- 87. Tesoriero C, Codita A, Zhang MD, et al. H1N1 influenza virus induces narcolepsy-like sleep disruption and targets sleep-wake regulatory neurons in mice. PNAS. 2016;113:E368-E377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Martines RB, Bhatnagar J, de Oliveira Ramos AM, et al. Pathology of congenital Zika syndrome in Brazil: a case series. Lancet. 2016;388:898-904. [DOI] [PubMed] [Google Scholar]
- 89. Houen G, Trier NH. Epstein-barr virus and systemic autoimmune diseases. Front Immunol. 2021;11:587380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Bjornevik K, Cortese M, Healy BC, et al. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science. 2022;375:296-301. [DOI] [PubMed] [Google Scholar]
- 91. Mastroeni D, Nolz J, Sekar S, et al. Laser-captured microglia in the Alzheimer's and Parkinson's brain reveal unique regional expression profiles and suggest a potential role for hepatitis B in the Alzheimer’s brain. Neurobiol Aging. 2018;63:12-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Athanasiou E, Gargalionis AN, Boufidou F, Tsakris A. The association of human herpesviruses with malignant brain tumor pathology and therapy: two sides of a coin. Int J Mol Sci. 2021;22:2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Young D, Hussell T, Dougan G. Chronic bacterial infections: living with unwanted guests. Nat Immunol. 2002;3:1026-1032. [DOI] [PubMed] [Google Scholar]
- 94. Salama NR, Hartung ML, Müller A. Life in the human stomach: persistence strategies of the bacterial pathogen Helicobacter pylori. Nat Rev Microbiol. 2013;11:385-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. New Engl J Med. 2008;359:2355-2365. [DOI] [PubMed] [Google Scholar]
- 96. Hayashi C, Gudino CV, Gibson Fc, 3rd, Genco CA. Review: pathogen-induced inflammation at sites distant from oral infection: bacterial persistence and induction of cell-specific innate immune inflammatory pathways. Mol Oral Microbiol. 2010;25:305-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Li X, Kolltveit KM, Tronstad L, Olsen I. Systemic diseases caused by oral infection. Clin Microbiol Rev. 2000;13:547-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Hickie I, Davenport T, Wakefield D, et al.; Dubbo Infection Outcomes Study Group. Post-infective and chronic fatigue syndromes precipitated by viral and non-viral pathogens: prospective cohort study. BMJ. 2006;333:575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Matschke J, Lütgehetmann M, Hagel C, et al. Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet Neurol. 2020;19:919-929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Meinhardt J, Radke J, Dittmayer C, et al. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat Neurosci. 2021;24:168-175. [DOI] [PubMed] [Google Scholar]
- 101. Edén A, Grahn A, Bremell D, et al. Viral antigen and inflammatory biomarkers in cerebrospinal fluid in patients with COVID-19 infection and neurologic symptoms compared with control participants without infection or neurologic symptoms. JAMA Netw Open. 2022;5:e2213253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Batra A, Clark JR, Kang AK, et al. Persistent viral RNA shedding of SARS-CoV-2 is associated with delirium incidence and six-month mortality in hospitalized COVID-19 patients. GeroScience. 2022;44:1241-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Lorusso L, Mikhaylova SV, Capelli E, Ferrari D, Ngonga GK, Ricevuti G. Immunological aspects of chronic fatigue syndrome. Autoimmun Rev. 2009;8:287-291. [DOI] [PubMed] [Google Scholar]
- 104. Montoya JG, Holmes TH, Anderson JN, et al. Cytokine signature associated with disease severity in chronic fatigue syndrome patients. PNAS. 2017;114:E7150-E7158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Raison CL, Lin JMS, Reeves WC. Association of peripheral inflammatory markers with chronic fatigue in a population-based sample. Brain Behav Immun. 2009;23:327-337. [DOI] [PubMed] [Google Scholar]
- 106. Chaves-Filho AJM, Macedo DS, de Lucena DF, Maes M. Shared microglial mechanisms underpinning depression and chronic fatigue syndrome and their comorbidities. Behav Brain Res. 2019;372:111975. [DOI] [PubMed] [Google Scholar]
- 107. Nakatomi Y, Mizuno K, Ishii A, et al. Neuroinflammation in patients with chronic fatigue syndrome/myalgic encephalomyelitis: an ¹¹C-(R)-PK11195 PET study. J Nucl Med. 2014;55:945-950. [DOI] [PubMed] [Google Scholar]
- 108. VanElzakker MB, Brumfield SA, Lara Mejia PS. Corrigendum: neuroinflammation and cytokines in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): a critical review of research methods. Front Neurol. 2019;10:316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Komaroff AL. Chronic fatigue syndromes: relationship to chronic viral infections. J Virol Methods. 1988;21:3-10. [DOI] [PubMed] [Google Scholar]
- 110. Komaroff AL. Is human herpesvirus-6 a trigger for chronic fatigue syndrome? J Clin Virol. 2006;37:S39-S46. [DOI] [PubMed] [Google Scholar]
- 111. Blomberg J, Gottfries CG, Elfaitouri A, Rizwan M, Rosén A. Infection elicited autoimmunity and myalgic encephalomyelitis/chronic fatigue syndrome: an explanatory model. Front Immunol. 2018;9:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Rasa S, Nora-Krukle Z, Henning N, et al. Chronic viral infections in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). J Transl Med. 2018;16:268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Underhill RA. Myalgic encephalomyelitis, chronic fatigue syndrome: an infectious disease. Med Hypotheses. 2015;85:765-773. [DOI] [PubMed] [Google Scholar]
- 114. Holmes GP, Kaplan JE, Stewart JA, Hunt B, Pinsky PF, Schonberger LB. A cluster of patients with a chronic mononucleosis-like syndrome. Is Epstein-barr virus the cause? JAMA. 1987;257:2297-2302. [PubMed] [Google Scholar]
- 115. Ablashi DV, Eastman HB, Owen CB, et al. Frequent HHV-6 reactivation in multiple sclerosis (MS) and chronic fatigue syndrome (CFS) patients. J Clin Virol. 2000;16:179-191. [DOI] [PubMed] [Google Scholar]
- 116. Chapenko S, Krumina A, Logina I, et al. Association of active human herpesvirus-6, -7 and parvovirus B19 infection with clinical outcomes in patients with myalgic encephalomyelitis/chronic fatigue syndrome. Adv Virol. 2012;2012:1-7. Article ID 205085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Loebel M, Strohschein K, Giannini C, et al. Deficient EBV-specific B- and T-cell response in patients with chronic fatigue syndrome. PLoS One. 2014;9:e85387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Morris G, Berk M, Galecki P, Maes M. The emerging role of autoimmunity in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). Mol Neurobiol. 2014;49:741-756. [DOI] [PubMed] [Google Scholar]
- 119. Sotzny F, Blanco J, Capelli E, et al. Myalgic encephalomyelitis/chronic fatigue syndrome - evidence for an autoimmune disease. Autoimmun Rev. 2018;17:601-609. [DOI] [PubMed] [Google Scholar]
- 120. Phelan J, Grabowska AD, Sepúlveda N. A potential antigenic mimicry between viral and human proteins linking myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) with autoimmunity: the case of HPV immunization. Autoimmun Rev. 2020;19:102487. [DOI] [PubMed] [Google Scholar]
- 121. Hajdarevic R, Lande A, Rekeland I, et al. Fine mapping of the major histocompatibility complex (MHC) in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) suggests involvement of both HLA class I and class II loci. Brain Behav Immun. 2021;98:101-109. [DOI] [PubMed] [Google Scholar]
- 122. Shan ZY, Barnden LR, Kwiatek RA, Bhuta S, Hermens DF, Lagopoulos J. Neuroimaging characteristics of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): a systematic review. J Transl Med. 2020;18:335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Barnden LR, Kwiatek R, Crouch B, Burnet R, Del Fante P. Autonomic correlations with MRI are abnormal in the brainstem vasomotor centre in Chronic Fatigue Syndrome. NeuroImage Clin. 2016;11:530-537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Nelson T, Zhang LX, Guo H, Nacul L, Song X. Brainstem abnormalities in myalgic encephalomyelitis/chronic fatigue syndrome: A scoping review and evaluation of magnetic resonance imaging findings. Front Neurol. 2021;12:769511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Kraynak TE, Marsland AL, Wager TD, Gianaros PJ. Functional neuroanatomy of peripheral inflammatory physiology: a meta-analysis of human neuroimaging studies. Neurosci Biobehav Rev. 2018;94:76-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Yong SJ. Persistent brainstem dysfunction in long-COVID: a hypothesis. ACS Chem Neurosci. 2021;12:573-580. [DOI] [PubMed] [Google Scholar]
- 128. Haley RW, Marshall WW, McDonald GG, Daugherty MA, Petty F, Fleckenstein JL. Brain abnormalities in Gulf war syndrome: evaluation with 1H MR spectroscopy. Radiology. 2000;215:807-817. [DOI] [PubMed] [Google Scholar]
- 129. Baraniuk JN, Shivapurkar N. Exercise – induced changes in cerebrospinal fluid miRNAs in Gulf war Illness, chronic fatigue syndrome and sedentary control subjects. Sci Rep. 2017;7:15338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Halpin P, Williams MV, Klimas NG, Fletcher MA, Barnes Z, Ariza ME. Myalgic encephalomyelitis/chronic fatigue syndrome and gulf war illness patients exhibit increased humoral responses to the herpesviruses-encoded dUTPase: implications in disease pathophysiology. J Med Virol. 2017;89:1636-1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Georgopoulos AP, Tsilibary EC, Souto EP, James LM, Engdahl BE, Georgopoulso A. Adverse effects of Gulf war illness (GWI) serum on neural cultures and their prevention by healthy serum. J Neurol Neuromed. 2018;3:19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Tsilibary EC, Souto EP, James LM, Engdahl BE, Georgopoulos AP. Human immunoglobulin G (IgG) neutralizes adverse effects of Gulf war illness (GWI) serum in neural cultures: paving the way to immunotherapy for GWI. J Neurol Neuromed. 2018;3:23-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Abdel-Rahman A, Shetty AK, Abou-Donia MB. Disruption of the blood–brain barrier and neuronal cell death in cingulate cortex, dentate gyrus, thalamus, and hypothalamus in a rat model of Gulf-war syndrome. Neurobiol Dis. 2002;10:306-326. [DOI] [PubMed] [Google Scholar]
- 134. Esposito P, Gheorghe D, Kandere K, et al. Acute stress increases permeability of the blood-brain-barrier through activation of brain mast cells. Brain Res. 2001;888:117-127. [DOI] [PubMed] [Google Scholar]
- 135. Fabis MJ, Phares TW, Kean RB, Koprowski H, Hooper DC. Blood-brain barrier changes and cell invasion differ between therapeutic immune clearance of neurotrophic virus and CNS autoimmunity. Proc Natl Acad Sci USA. 2008;105:15511-15516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Muller DM, Pender MP, Greer JM. Blood-brain barrier disruption and lesion localisation in experimental autoimmune encephalomyelitis with predominant cerebellar and brainstem involvement. J Neuroimmunol. 2005;160:162-169. [DOI] [PubMed] [Google Scholar]
- 137. Phares TW, Kean RB, Mikheeva T, Hooper DC. Regional differences in blood-brain barrier permeability changes and inflammation in the apathogenic clearance of virus from the central nervous system. J Immunol. 2006;176:7666-7675. [DOI] [PubMed] [Google Scholar]
- 138. Welcome MO, Mastorakis NE. Stress-induced blood brain barrier disruption: molecular mechanisms and signaling pathways. Pharmacol Res. 2020;157:104769. [DOI] [PubMed] [Google Scholar]
- 139. Vahabi Z, Etesam F, Zandifar A, Badrfam R. Psychosocial stress, blood brain barrier and the development of anti N-methyl-D-aspartate receptor (NMDAR) encephalitis. Multiple Scler Relat Disord. 2021;50:102876. [DOI] [PubMed] [Google Scholar]
- 140. Welcome MO. Cellular mechanisms and molecular signaling pathways in stress-induced anxiety, depression, and blood-brain barrier inflammation and leakage. Inflammopharmacology. 2020;28:643-665. [DOI] [PubMed] [Google Scholar]
- 141. Sanchez-Cano F, Hernández-Kelly LC, Ortega A. The blood–brain barrier: much more than a selective access to the brain. Neurotox Res. 2021;39:2154-2174. [DOI] [PubMed] [Google Scholar]
- 142. Hanna S, Etzioni A. MHC class I and II deficiencies. J Allergy. 2014;134:269-275. [DOI] [PubMed] [Google Scholar]
- 143. Sudre CH, Murray B, Varsavsky T, et al. Attributes and predictors of long COVID. Nat Med. 2021;27:626-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Yomogida K, Zhu S, Rubino F, Figueroa W, Balanji N, Holman E. Post-acute sequelae of SARS-CoV-2 infection among adults aged ⩾18 years — Long Beach, California, April 1–December 10, 2020. MMWR Morb Mortal Wkly Rep. 2021;70:1274-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Tavasolian F, Rashidi M, Hatam GR, et al. HLA, immune response, and susceptibility to COVID-19. Front Immunol. 2022;11:601886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Langton DJ, Bourke SC, Lie BA, et al. The influence of HLA genotype on the severity of COVID-19 infection. HLA. 2021;98:14-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Nguyen A, David JK, Maden SK, et al. Human leukocyte antigen susceptibility map for severe acute respiratory syndrome coronavirus 2. J Virol. 2020;94:e00510-e00520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Charonis SA, Tsilibary EP, Georgopoulos AP. In silico investigation of binding affinities between human leukocyte antigen class I molecules and SARS-CoV-2 virus spike and ORF1ab proteins. Explor Immunol. 2021;1:16-26. [Google Scholar]
- 149. Pisanti S, Deelen J, Gallina AM, et al. Correlation of the two most frequent HLA haplotypes in the Italian population to the differential regional incidence of covid-19. J Transl Med. 2020;18:352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. James L, Tsilibary EP, Charonis S, Georgopoulos A. Commentary: COVID-19 and the path to Immunity. J Immunological Sci. 2020;4:31-33. [Google Scholar]
- 151. Charonis S, Tsilibary EP, Georgopoulos A. SARS-CoV-2 virus and Human Leukocyte Antigen (HLA) Class II: Investigation in silico of binding affinities for COVID-19 protection and vaccine development. J Immunological Sci. 2020;4:12-23. [Google Scholar]
- 152. Cervia C, Zurbuchen Y, Taeschler P, et al. Immunoglobulin signature predicts risk of post-acute COVID-19 syndrome. Nat Commun. 2022;13:446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Bohnacker S, Hartung F, Henkel F, et al. Mild COVID-19 imprints a long-term inflammatory eicosanoid- and chemokine memory in monocyte-derived macrophages. Mucosal Immunol. 2022;15:515-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Ahearn-Ford S, Lunjani N, McSharry B, et al. Long-term disruption of cytokine signalling networks is evident in patients who required hospitalization for SARS-CoV-2 infection. Allergy. 2021;76:2910-2913. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Schmidt C. Publisher Correction: COVID-19 long haulers. Nat Biotechnol. 2021;39:1017-1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Charonis S, James L, Georgopoulos A. In silico analysis of the binding affinities of antigenic epitopes of vaccines administered to Gulf war veterans to specific HLA Class II alleles protective for Gulf war Illness. J Neurol Neuromed. 2019;4:23-30. [Google Scholar]
- 157. Whistler T, Fletcher MA, Lonergan W, et al. Impaired immune function in Gulf war illness. BMC Med Genomics. 2009;2:12-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Parkitny L, Middleton S, Baker K, Younger J. Evidence for abnormal cytokine expression in Gulf war Illness: A preliminary analysis of daily immune monitoring data. BMC Immunol. 2015;16:57-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Skowera A, Hotopf M, Sawicka E, et al. Cellular immune activation in Gulf war Veterans. J Clin Immunol. 2004;24:66-73. [DOI] [PubMed] [Google Scholar]
- 160. James LM, Engdahl BE, Leuthold AC, Georgopoulos AP. Brain correlates of human leukocyte antigen (HLA) protection in Gulf war Illness (GWI). EBioMedicine. 2016;13:72-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. James L, Engdahl B, Johnson R, Georgopoulos A. Gulf war illness and inflammation: Association of symptom severity with C-reactive protein. J Neurol Neuromed. 2019;4:15-19. [Google Scholar]
- 162. Johnson GJ, Slater BC, Leis LA, Rector TS, Bach RR. Blood biomarkers of chronic inflammation in Gulf war Illness. PLoS One. 2016;11:e0157855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. James L, Johnson R, Lewis S, et al. Lupus anticoagulant in Gulf war illness and autoimmune disorders: A common pathway toward autoimmunity. J Immunological Sci. 2021;5:14-18. [Google Scholar]
- 164. James LM, Christova P, Johnson RA, et al. Association of lupus anticoagulant with brain atrophy in Gulf war Illness (GWI). J Immunological Sci. 2021;5:47-50. [Google Scholar]
- 165. James LM, Christova P, Engdahl BE, Lewis SM, Carpenter AF, Georgopoulos AP. Human leukocyte antigen (HLA) and Gulf war Illness (GWI): HLA-drb1*13:02 spares subcortical atrophy in Gulf war veterans. EBioMedicine. 2017;26:126-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Georgopoulos AP, James LM, Carpenter AF, Engdahl BE, Leuthold AC, Lewis SM. Gulf war illness (GWI) as a neuroimmune disease. Exp Brain Res. 2017;235:3217-3225. [DOI] [PubMed] [Google Scholar]
- 167. Coughlin SS. A neuroimmune model of Gulf war Illness. J Environ Health Sci. 2017;3:1-11. 10.15436/2378-6841.17.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. O’Callaghan JP, Michalovicz LT, Kelly KA. Supporting a neuroimmune basis of Gulf war Illness. EBioMedicine. 2016;13:5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Ota M, Serada S, Naka T, Mori Y. MHC class I molecules are incorporated into human herpesvirus-6 viral particles and released into the extracellular environment. Microbiol Immunol. 2014;58:119-125. [DOI] [PubMed] [Google Scholar]
- 170. Glosson NL, Hudson AW. Human herpesvirus-6A and -6b encode viral immunoevasins that downregulate class I MHC molecules. Virology. 2007;365:125-135. [DOI] [PubMed] [Google Scholar]
- 171. Compeer EB, Flinsenberg TW, van der Grein SG, Boes M. Antigen processing and remodeling of the endosomal pathway: requirements for antigen cross-presentation. Front Immunol. 2012;3:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Petersen JL, Morris CR, Solheim JC. Virus evasion of MHC class I molecule presentation. J Immunol. 2003;171:4473-4478. [DOI] [PubMed] [Google Scholar]
- 173. Koutsakos M, McWilliam HEG, Aktepe TE, et al. Downregulation of MHC class I expression by influenza A and B viruses. Front Immunol. 2019;10:1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Nicoll MP, Proença JT, Efstathiou S. The molecular basis of herpes simplex virus latency. FEMS Microbiol Rev. 2012;36:684-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Sausen DG, Reed KM, Bhutta MS, Gallo ES, Borenstein R. Evasion of the host immune response by Betaherpesviruses. Int J Mol Sci. 2021;22:7503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Colunga Biancatelli RML, Solopov PA, Sharlow ER, Lazo JS, Marik PE, Catravas JD. The SARS-CoV-2 spike protein subunit S1 induces COVID-19-like acute lung injury in Κ18-hACE2 transgenic mice and barrier dysfunction in human endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2021;321:L477-L484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Shirato K, Kizaki T. SARS-CoV-2 spike protein S1 subunit induces pro-inflammatory responses via toll-like receptor 4 signaling in murine and human macrophages. Heliyon. 2021;7:e06187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Blanchard M, Toomey R, Karlinsky J, Reda D, Alpern R, Xue L. Increased risk of chronic multisymptom illness in spouses of Gulf war era veterans. Mil Med. 2017;182:e1648-e1656. [DOI] [PubMed] [Google Scholar]
- 179. Peterson PK, Shepard J, Macres M, et al. A controlled trial of intravenous immunoglobulin G in chronic fatigue syndrome. Am J Med. 1990;89:554-560. [DOI] [PubMed] [Google Scholar]
- 180. Sanders DB, Wolfe GI, Benatar M, et al. International consensus guidance for management of myasthenia gravis: Executive summary. Neurology. 2016;87:419-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Lazaridis K, Tzartos SJ. Autoantibody specificities in myasthenia gravis; implications for improved diagnostics and therapeutics. Front Immunol. 2020;11:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Rock GA, Tricklebank GW, Kasaboski CA. Plasma exchange in Canada. The Canadian Apheresis Study Group. CMAJ. 1990;142:557-562. [PMC free article] [PubMed] [Google Scholar]
- 183. Sutton DM, Nair RC, Rock G. Complications of plasma exchange. Transfusion. 1989;29:124-127. [DOI] [PubMed] [Google Scholar]
- 184. Faqihi F, Alharthy A, Abdulaziz S, et al. Therapeutic plasma exchange in patients with life-threatening COVID-19: a randomised controlled clinical trial. Int J Antimicrob Agents. 2021;57:106334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Roshandel E, Sankanian G, Salimi M, et al. Plasma exchange followed by convalescent plasma transfusion in COVID-19 patients. Transfus Apher Sci. 2021;60:103141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Arulkumaran N, Thomas M, Brealey D, et al. Plasma exchange for COVID-19 thrombo-inflammatory disease. EJHaem. 2021;2:26-32. 10.1002/jha2.140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Truong AD, Auld SC, Barker NA, et al. Therapeutic plasma exchange for COVID-19-associated hyperviscosity. Transfusion. 2021;61:1029-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Alharthy A, Faqihi F, Balhamar A, Memish ZA, Karakitsos D. Life-threatening COVID-19 presenting as stroke with antiphospholipid antibodies and low ADAMTS-13 activity, and the role of therapeutic plasma exchange: a case series. SAGE Open Med Case Rep. 2020;8:2050313X20964089. [DOI] [PMC free article] [PubMed] [Google Scholar]