Abstract
Lung cancer is one of the leading causes of cancer incidence and cancer-related deaths in the world. Early diagnosis of pulmonary tumors results in improved survival compared to diagnosis with more advanced disease, yet early disease is not reliably indicated by symptoms. Despite of the improved testing and monitoring techniques for lung cancer in the past decades, most diagnostic tests, such as sputum cytology or tissue biopsies, are invasive and risky, rendering them unfeasible for large population screening. The non-invasive analysis of exhaled breath has gained attentions as an innovative screening method to measure chemical alterations within the human volatilome profile as a result of oncogenesis. More importantly, volatile organic compounds (VOCs) have been correlated to the pathophysiology of disease since the source of volatile compounds relies mostly on endogenous metabolic processes that are altered as a result of disease onset. Therefore, studying VOCs emitted from human breath may assist lung cancer diagnosis, treatment monitoring, and other surveillance of this devastating disease. In this mini review, we evaluated recent human studies that have attempted to identify lung cancer-derived volatiles in exhaled breath of patients. We also examined reported volatiles in cell cultures of lung cancer to better understand the origins of cancer-associated VOCs. We highlight the metabolic processes of lung cancer that could be responsible for the endogenous synthesis of these VOCs and pinpoint the protein-encoding genes involved in these pathways. Finally, we highlight the potential value of a breath test in lung cancer and propose prominent areas for future research required for the incorporation of VOCs-based testing into clinical settings.
Keywords: volatile organic compounds, lung cancer, breathomics, metabolic deregulation
Impact Statement
Lung cancer is a debilitating disease with an average 5-year survival rate of about 10–20%. As a result of its rising global incidence, lung cancer has become the most common cause of cancer deaths. The development of novel diagnostic methods and tools for early disease detection is critical to increase the rate of successful treatment. Recently, the detection of cancer-derived volatile organic compounds (VOCs) in human breath has been proposed to provide insight into the endogenous metabolic processes of cancer. Due to the non-invasive nature of sample collection, monitoring VOCs and exhaled breath volatiles as cancer biomarkers has gained traction for the potential to be used for disease diagnostics. Advancements in analytical platforms have allowed the screening of hundreds of volatile compounds; it is thus critical to evaluate the biological origins of these compounds in the context of lung cancer to pave the way for early diagnosis, disease monitoring, and therapy outcomes.
Introduction
Lung cancer is a debilitating disease with an average 5-year survival rate ranging between 10% and 20%. 1 As a result of its rising global incidence, lung cancer has become the second most frequently diagnosed cancer and responsible for 18% of the total cancer deaths. 2 Lung cancer patients are generally diagnosed at the age of 65 years or above with late-stage disease, 3 where the histotypes of these tumors are commonly divided into small cell lung carcinoma (SCLC) and non–small cell carcinoma (NSCLC) with specific DNA mutations permitting further molecular stratification. 4 Smoking is still considered the primary culprit responsible for most respiratory cancers, including esophageal adenocarcinoma and esophagogastric junctional adenocarcinoma.5,6 Early diagnosis of lung cancer results in improved survival compared to diagnosis with more advanced disease, as increased accessibility to positron emission tomography with computed tomography (PET-CT) scanning and endobronchial ultrasound for the sampling of mediastinal lymph node have improved the accuracy of staging for lung cancers. 7 Lower stages of lung cancer (I–III) are treated with surgical resection since the tumors have yet to metastasize. Surgery affords a 5-year survival rate of 70–90% for localized tumors. 8 Patients with early-stage disease exhibit general symptoms, such as fatigue and chest pains, 9 thus the cancer is not reliably indicated by symptoms. Therefore, up to 75% of patients present advanced disease at the time of diagnosis. 10 Most of the currently used diagnostics for lung cancers, such as tissue biopsy and sputum cytology, have yet to meet the standards of screening, as they are insufficiently accurate, invasive, and risky, 11 rendering them impracticable for large population screening. Meanwhile, advancements in high-throughput analytical technologies have greatly enhanced our understanding of cancer itself. Substantial efforts have been focused toward uncovering the relationship between the volatile signatures of the human body and the presence of cancer.12,13 VOCs emitted through the endogenous metabolic processes of cells have been hypothesized to be released into the blood where they subsequently diffuse across the pulmonary alveolar membrane and are exhaled through breath. 14 Non-invasive breath analysis has been proposed as an innovative means to study some of the hundreds of VOCs exhaled by humans, as exhaled breath contains many reported VOCs that can reflect biochemical processes are deregulated as a result of biological activities, such as oncogenesis, oxidative stress, or inflammation. 14 Since the source of volatile compounds relies mostly on endogenous metabolic processes primarily related to oxidative stress, 15 which are tightly coupled with oncogenesis, 16 VOCs analyses have been considered to provide unique markers for the detection of cancer.
Case–control studies have attempted to analyze specific VOCs in exhaled breath of cancer patients and healthy individuals, with promising results identifying cancer-specific VOCs in liver and breast cancers.17,18 In addition, pioneering work from Nakhleh et al. 19 achieved 813 independent diagnoses of 1 of 17 different diseases, including colon, prostate, and esophageal cancers, using exhaled breath samples from 1404 subjects and 591 control subjects. Evidence from several published studies in lung cancer patients highlights abnormal VOCs in exhaled breath.13,20,21 This has been confirmed with many analytical methods, such as gas chromatography–mass spectrometry (GC-MS).4,22,23 In addition, exhaled breath analysis revealed altered VOC profiles in lung cancer patients after tumor resection. 24 Thus, it can be postulated that in addition to disease diagnosis; volatilomics can also offer a non-invasive method to detect drug treatment response in patients. Promising evidence for this method has emerged where drug metabolites have been reportedly detected in exhaled breath condensates. 25 Taken together, breath volatiles appear to bolster a potential utility as part of the screening protocols for the evaluation of lung cancer onset in patients.
Over the past decade, researchers have reported numerous candidate compounds as diverse as toluene, acetaldehyde, benzene derivatives, and alcohols to serve as potential biomarkers of lung cancer. 22 It has now become the time to place a greater emphasis on using this gained knowledge of lung cancer-associated volatiles to enhance our understanding of their potential biological origins and involvement in cancer pathogenesis. The goal of this mini review is to discuss some highlights of the recent analytical methods and research progress made in the analysis of lung cancer-derived VOCs in human and cell studies. Since the potential endogenous origins have yet to be elucidated, we probed publicly available databases to identify endogenous metabolic pathways in which the identified VOCs can be generated based on several clinical studies; we also conducted a secondary analysis of the available data to highlight the biochemical pathways in vivo.
Analytical requirements and emerging technologies for human breath volatile analysis
Detection of breath volatiles requires robust analytical techniques that allow chemical identification of low abundance volatiles, which can range in concentrations between parts-per-million (ppmv) and parts-per-trillion by volume (pptv). 26 Among several analytical approaches that have been implemented for the assessment of exhaled VOCs, the two mainstream ones are as follows: (1) semi-selective analytical sensors aimed at pattern recognition to uncover disease fingerprints19,27 and (2) high-throughput mass spectrometry practices that seek to detect and quantify the emitted VOCs in complex biological matrices.28,29 Utilizing analytical sensors provides an advantage since analyses are conducted on a preselected subset of compounds. These sensors can include colorimetric or electronic chemical sensor arrays, which are akin to targeted analyses in mass spectrometry platforms, where only a subset of analytes is considered in the investigation. Upon detecting the desired analytes, the electronic chemical sensors will determine the signal as a shift in electrical current. 30 Conversely, colorimetric sensors entail chemically reactive colorants printed on a disposable cartridge which change color when interacting with the desired analytes. 31 While this analytic method is convenient and portable, it does include some exhaled breath from nasal airways. Thus, it is important to standardize sampling protocols to fully capture the VOCs excreted by the lungs.
In the case of mass spectrometry-based analyses, several analytical platforms have been used for VOC identification, including selective-ion flow-tube mass spectrometry (SIFT-MS) 32 and proton-transfer reaction mass spectrometry (PTR-MS), 33 while GC-MS 20 has been deemed the gold standard. In addition to increased sensitivity of volatile analyte detection, mass spectrometry-based analyses offer a unique advantage in the ability to detect and identify new analytes that may not have been previously reported 34 as they are able to detect a wider array of VOCs reported by untargeted analyses to unravel prominent VOCs as biomarkers. Untargeted analyses have exhibited better discriminatory ability in cancer patients, 35 which is attributed to the broader range of chemicals detected. In addition, the semi-quantitative nature of mass spectrometry analyses also affords a unique advantage in the form of comparative analyses to monitor changes in lung cancer-derived volatiles in response to drug-based therapies. Emerging methods for volatile analyses in exhaled breath have been reviewed elsewhere.36–38 While the constantly evolving technologies for volatile analyses empower great research discoveries, this review seeks to highlight the detected volatiles in lung cancer studies and their biological and clinical relevance.
The composition of lung cancer volatilome
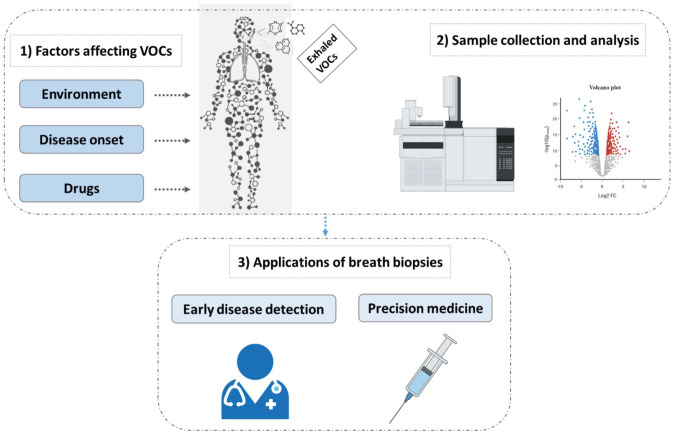
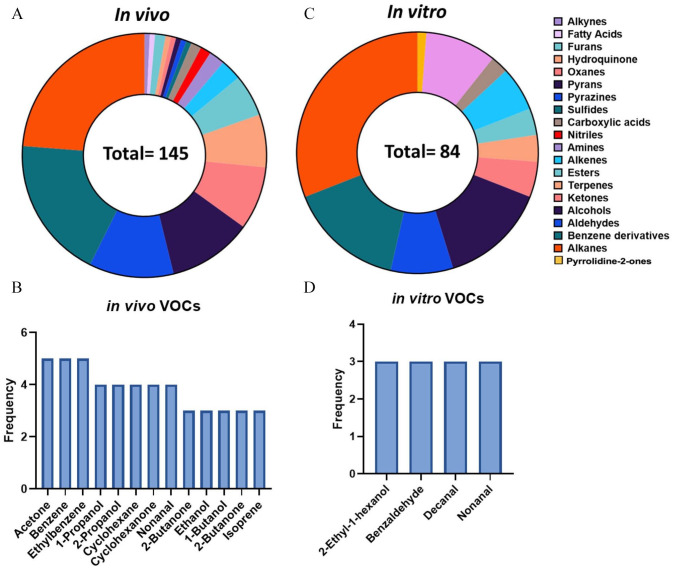
Exhaled breath contains thousands of trace volatiles that are generated endogenously within the body or absorbed from the environment (Figure 1). Exhaled breath is proposed to consist of three phases: 39 Phase I, II, and III. Phase I, deemed dead space, consists of exogenous volatiles from the environment and offers little clinical insight. This is followed by Phase II which represents the transition phase as it is a mixture of exogenous and endogenous volatiles from the upper respiratory tract. These VOCs may provide clinical relevance, yet analyses must be taken with background influence in mind. Phase III, termed alveolar phase, consists of the biologically relevant volatiles that originate from endogenous metabolic processes. Collectively, VOC patterns in breath are considered to mirror a person’s health condition since the volatiles can be products of many metabolic pathways. 40 To better understand the chemical composition of the lung cancer volatilome, we sought to first summarize the volatiles reportedly deregulated in breath of lung cancer patients. We probed the literature for studies using mass spectrometry-based methods to analyze volatiles in either lung cancer patients or cell lines in vitro ranging from 2015 to 2021. Herein, it was evident that GC-MS-based methods were the most frequently used (Table 1). In lung cancer patients, it was apparent that 145 unique VOCs encompassing 20 unique compounds classes were reportedly deregulated in exhaled breath of lung cancer patients with respect to healthy controls (Figure 2(A)).
Figure 1.
General schematic of breathomics workflow and clinical applications. (A color version of this figure is available in the online journal.)
Table 1.
Summary of the study populations and analytical methods in the selected relevant literature.
| Study | Chen et al. | Huang et al. | Koureas et al. | Sakumura et al. | Schallschmidt et al. | Song et al. | Sun et al. | Tsou et al. | |
|---|---|---|---|---|---|---|---|---|---|
| Sample size (age) | Control | 122 (49.58 ± 7.71) | 30 (59) | 53 (66.8 ± 13.2) | 29 (n/a) | 23 (61.4) | 43 (47.9 ± 6.5) | 30 (34.27 ± 9.38) | 168 (31.4 ± 10.4) |
| Cancer | 160 (61.19 ± 9.91) | 20 (70) | 51 (70.9 ± 8.1) | 107 (n/a) | 37 (65.9) | 41 (58.1 ± 8.1) | 30 (52.35 ± 15.5 and 59.40 ± 8.97) | 148 (64.5 ± 11) | |
| Smoking status | Smoking history | 171 | N/A | 86 | 70 | 25 | 21 | 24 | 57 |
| Non-smoker | 131 | N/A | 18 | 66 | 35 | 63 | 36 | 259 | |
| Disease stage | I: 56, II: 18, III: 22, IV: 58, N/A: 6 | N/A | N/A | I: 55, II: 15, III: 28, IV: 9 | N/A | I: 13, II: 7, III: 6, IV: 17 | N/A | I: 1, II: 4, III: 35, IV: 105 | |
| Analytical tools | GC-MS | SPME GC-MS | SPME GC-MS | GC-MS | SPME GC-MS | SPME GC-MS | PTR-MS | SIFT-MS | |
| Reference | Choueiry and Zhu 41 | Phillips et al. 42 | Graff et al. 43 | de Vries et al. 44 | Dillon et al. 45 | Chen et al. 25 | Sun et al. 78 | Bianchi et al. 28 |
GC-MS: gas chromatography–mass spectrometry; PTR-MS: proton-transfer reaction mass spectrometry; SIFT-MS: selective-ion flow-tube mass spectrometry; SPME: solid-phase microextraction.
Figure 2.
Classes and frequency of lung cancer-derived VOCs reported in in vivo and in vitro studies. A total of 20 unique compound classes were reported in the literature, where 84 distinct VOCs were identified in (A) in vitro cell lines compared to (B) 143 reported in vivo from exhaled breath. (C) In total, 4 VOCs were reported in three or more cell line studies compared to (D) 13 VOCs in three or more breath studies. (A color version of this figure is available in the online journal.)
Detection of acetonitrile, toluene, and some benzene derivatives in exhaled breath, for example, is proposed to be mainly associated with smoking, 46 yet these volatiles were also reported in non-smokers, suggesting they can also arise from other environmental exposure to gas pollutants or secondhand smoking. 47 Nonetheless, 13 VOCs were reported in at least three independent studies which screened different population of patients (Figure 2(B)). Acetone, benzene, and ethylbenzene were the most frequently reported VOCs as they were shown to be elevated in lung cancer patients in five distinct studies (Table 1). Four studies reported 1-propanol, 2-propanol, cyclohexane, cyclohexanone, and nonanal. Benzene and its derivatives are most commonly reported in breath of smokers but have also been proposed as lung cancer markers in breath. 48 Detection of ketones, such as acetone, is also common since it is a by-product of acetyl-CoA decarboxylation, a process catalyzed by the enzyme acetyl-CoA carboxylase. Acetyl-CoA carboxylase is involved in fatty acid synthesis mediated by the expression of the ACC gene, which is upregulated in lung cancer cells. 49 Propanol has been proposed to be formed endogenously in humans when ethanol is present. 50 Ethanol is also reported in breath of lung cancer patients and is a downstream product of glycolysis. 51 Increased glycolytic activity in pulmonary tumors could explain this finding. Collectively, multiple reports of the same VOCs across different studies increase the confidence that these volatiles are truly associated with lung cancer incidence. A full list of the reported VOCs is summarized in Table 2 and encompasses all the reported classes, including alcohols, aldehydes, and benzene derivatives, among others.
Table 2.
VOCs and their respected classes reported in the selected literature.
| Class | VOC |
|---|---|
| Alcohols | Dimethylbenzyl alcohol* + , 1-butanol*, 1-pentanol*, 1-propanol*, 2-ethyl-1-hexanol* + †, 2-methylpropanol*, 2-propanol*, 3-methyl-1-butanol*, cedrol*, cyclohexanol* + †, dodecanol*, ethanol* †, methanol*, n-butanol*, n-nonanol*, n-octanol alcohol*, trans-2-hexenol + , 1-decanol + , 1-nonanol + , 1-undecanol + , 2,4-decadien-1-ol + , 2-butanol + , 2-ethyl-1-hexanol + |
| Aldehydes | (E)-2-heptenal*, 2-ethylhexanal*, 2-methyl-2-propenal*, acrolein*, benzaldehyde* + , ethanal* †, ethanedial*, hexanal* + , n-butanal*, n-decanal* + , n-hexanal* †, n-octanal*, nonanal* + , n-pentanal*, octanal*, propanalaldehyde*, 1-dodecanal + , undecanal + , 4-hydroxynonenal + , propanal + |
| Alkanes | 1,5-diaminopentane*, 2,2,4-trimethylpentane*, 2,2-dimethylpentane*, 2,2-dinethybutane*, 2,3-dimethylpentane*, 2,4-dimethylheptane*, 2-methylhexane*, 2-methylpentane* †, 3-methylhexane*, 3-methylpentane* †, 4-methyloctane*, 4-methylheptane*, butane*, chloroform*, cyclohexane*, dichloromethane* †, dodecane*, ethylcyclohexane*, heptane*, 3-ethyl-3-methylheptane*, hexamethylcyclotrisiloxane*, hexane*, methylcyclopentane* †, methylcyclohexane*, methylpentane*, n-heptane* †, n-hexane* †, n-nonane nonyl hydride*, n-octane*, n-pentane*, octane*, pentanal*, propylcyclohexane*, trichloromethane* †, undecane + , pentadecane + , hexadecane + , heptadecane + , octadecane + , tridecane + , 2-methylpentane + , 2,4-dimethyldodecane + , 2-bromododecane + , 2-methyl-5-ethylheptane + , 4,6-dimethyldodecane + , n-dodecyl chloride + , heneicosane + , tetradecane + , trimethyl[4(trimethylsilyl)butoxy]silane + |
| Alkenes | 1-butene*, 1-heptene*, 1-hexene*, 2,4-dimethyl-1-heptene*, 2,4-dimethyl-2-pentene, 1-decene + , 1-dodecene + , heptamethylnonene + |
| Alkynes | 1-butyne* |
| Amines | 1-methylpyrrolidine*, isopropylamine*, trimethylamine* |
| Benzene derivatives | 1,2,3,4-etrahydronaphthalene*, 1-methylindan*, 2,6-butylated hydroxytoluene* †, 4,7-methano-1H-indene*, octahydro-, benzene* †, benzene*, 4-ethyl-1,2-dimethyl, phenol*, 1,2,3-trimethylbenzene*, 1,2,4-trimethylbenzene* + , 1-methyl-3-propylbenzene*, 2-ethyl-p-xylene*, 4-ethylbenzamide*, benzoic acid*, chlorobenzene*, cymene*, dichlorobenzene*, ethylbenzene* †, n-propylbenzene*, n-propylbenzene*, o-Xylene*, p-Cymene*, propylbenzene* + , pyridine*, styrene* + , tetramethylbenzene* + †, toluene* + †, xylene* + †, phenol + , benzene + , 1,3-bis(1,1-dimethylethyl)-, 2-ethyl-1,3-dimethyl-benzene + |
| Benzofurans | 3,7-dimethyl, hexahydro-benzofuranone* |
| Carboxylic acids | Formic acid*, propanoic acid* |
| Esters | 2,2,4-trimethyl-3-carboxyisopropyl pentanoic acid isobutyl ester* †, butylacetate*, DL-sec-butyl acetate*, ethylacetate*, ethylbutyrate*, methylacetate*, methyldodecanoate*, methylmyristate*, propylacetate*, 2-ethylhexyl benzoate + , methyl salicylate + |
| Fatty acids | Palmitate*, propionate + , 3-methyl pentanoate + , caproate + , heptanoate + , acetate + , formate + , isobutyrate + , butyrate + , valerate + , methyl acetate†, ethyl acetate† |
| Furans | 2,5-dimethylfuran*, tetrahydrofuran* |
| Hydroquinones | 2,6-bis(1,1-dimethylethyl)-1,4-benzenediol* |
| Ketones | 2,3-butanedione*, 2-butanone*, 2-pentanone* †, 3-hydroxy-2-butanone*, 4-heptanone*, acetoin*, acetone* + †, acetophenone* + , camphor*, cyclohexanone* †, cyclopentane*, 2-dodecanone + , 2-pentadecanone + |
| Nitriles | Acetonitrile*, acrylonitrile* |
| Oxanes | 2-hydroxy cineole* |
| Pyrans | 2,3-dehydro-1,8-cineole* |
| Pyrazines | 2-methylpyrazine* |
| Pyrrolidine-2-ones | 1-methyl-2-pyrrolidinone |
| Sulfides | Dimethylsulfide* |
| Terpenes | 3-methylbutanal*, 4-isopropyl toluene*, 5-isopropenyl-2-methyl-7-oxabicyclo heptan-2-ol*, alpha-pinene*, alpha-terpinene*, beta-caryophyllene*, eucalyptol*, isoprene*, limonene* + , pinene*, 3-carene + |
VOC: volatile organic compounds.
in vivo, + in vitro, † Huang et al.
To better understand which volatile results strictly from cancer metabolism compared to other metabolic processes, we next screened the literature for in vitro volatiles of cultured lung cancer cells in efforts of excluding the contribution of systemic individual metabolism on the chemical composition of the volatilome. It was apparent that 84 unique VOCs were detected in lung cancer cell cultures and pertain to 11 distinct chemical classes (Figure 2(C)). Alkanes, benzene derivatives, and alcohols were among the most notable compound classes identified. A summary of the reported volatiles emitted from in vitro cell cultures is outlined in Table 2. Among these volatiles, 2-ethyl-1-hexanol, benzaldehyde, decanal, and nonanal were the most prevalently identified volatiles in culture headspace (Figure 2(D)). These findings are not surprising since alkanes were the most frequently reported compound classes in both the in vitro and in vivo literature.
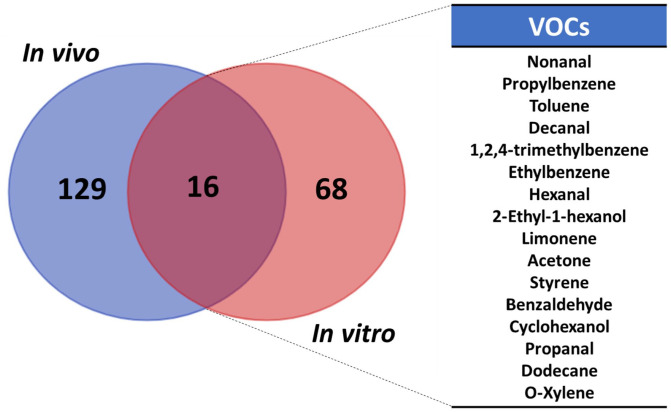
As of yet, there exists no unanimous approach to probe VOCs, as evident by the various analytical techniques and sample matrices explored. This results in convoluted lists of identified VOCs, with no mutually agreed on volatiles as putative markers. Thus, it may be beneficial to compare VOCs in the headspace of cell cultures and exhaled breath of lung cancer patients to identify overlapping volatiles as potential markers for disease. We highlighted 16 reported VOCs in both breath and the headspace of cell cultures (Figure 3). Namely, these compounds are nonanal, propylbenzene, toluene, decanal, 1,2,4-trimethylbenzene, ethylbenzene, hexanal, 2-ethyl-1-hexanol, limonene, acetone, styrene, benzaldehyde, cyclohexanol, propanal, dodecane, and o-Xylene. Detection of benzene derivatives from the headspace of cell cultures was a little surprising since metabolic pathways responsible for their endogenous synthesis have not been described. It is thus highly probable that some of the reported aromatic derivatives (e.g. trimethylbenzene and 2,6-butylated hydroxytoluene), halogenated volatiles (e.g. dichloromethane and chlorobenzene), and silylated compounds (bis(trimethylsilyl)ester and hexamethylcyclotrisiloxane) are a result of background contamination during sample collection or the reflection of recent human exposures to airborne, food, or water contaminants. In addition, these compounds are generally associated with smoking and environmental exposure. 52 Thus, one must be cautious before suggesting benzene derivatives solely as lung cancer markers. Alcohols and aldehydes, such as 2-ethyl-1-hexanol and benzaldehyde, were also among the most reported compound classes. Since lung cancers heavily rely on glycolysis for sustained proliferation, 53 it is suspected that some of these VOCs are downstream products of endogenous energy metabolism. Additional validation experiments, such as metabolic flux analysis, could be further performed. Therefore, it is imperative to continue building knowledge of metabolic alterations in lung cancers to uncover the biochemical origin of exhaled volatiles in efforts of cementing VOCs as critical markers for lung cancer diagnosis.
Figure 3.
Overlap of VOCs detected both in vivo from exhaled breath of lung cancer patients and in vitro in headspace of lung cancer cell cultures. (A color version of this figure is available in the online journal.)
Endogenous origins of lung cancer VOCs
The source of the VOCs and semi-volatile VOCs in breath is largely under-investigated and hard to trace. 54 While endogenous volatiles are assumed to be related to changes to oxidative stress and metabolic activities, exogenous VOCs can be attributed to smoking habits or air pollution. 46 As reported before, the vast majority of VOCs exhaled by lung cancer patients pertain to classes of alkanes, alcohols, aldehydes, ketones, nitriles, and aromatic compounds. 55 This was also commensurate with our literature summary for this mini review. To better understand the metabolic origins of VOCs in humans, we explored the Kyoto Encyclopedia of Genes and Genomes (KEGG) for the identified compounds in metabolic pathway databases and attempted to link the volatiles to biochemical alterations that occur in lung cancer (Table 3). In total, 11 metabolic pathways were identified that assisted our interpretation of biological origins of seven lung cancer-associated volatiles. Those metabolic processes include glycolysis/gluconeogenesis, pyruvate metabolism, glycerophospholipid metabolism, propanoate metabolism, glutathione metabolism, glyoxylate and dicarboxylate metabolism, biosynthesis of unsaturated fatty acids, fatty acid elongation, fatty acid degradation, fatty acid biosynthesis, and steroid hormone biosynthesis. It was also apparent that hexadecanoate and acetaldehyde were the most involved compounds, as they were reported in four and three pathways, respectively. Hexadecanoate is a fatty acid anion and the conjugate base of palmitic acid, the primary product of the fatty acid synthase, and an integral part of cell membrane structure. 56 It is not surprising that it was frequently identified as an intermediate of fatty acid biosynthesis, elongation, and degradation. Studies have demonstrated that unbalanced redox environment in which cancer cells grow induces oxidative stress. 18 Peroxidation of polyunsaturated fatty acids (PUFA) comprising membranes of organelles can form alkanes, leading to the degradation of phospholipids. 57 Elevated lipid contents of the cellular membranes in lung cancer cells coupled with increased oxidative stress render this hypothesis plausible.
Table 3.
KEGG pathways that contained one of the identified breath VOCs.
| Compound | KEGG entry ID | Metabolic pathway | KEGG pathway ID |
|---|---|---|---|
| Acetaldehyde | C00084 | Glycolysis/gluconeogenesis | hsa00010 |
| Pyruvate metabolism | hsa00620 | ||
| Glycerophospholipid metabolism | hsa00564 | ||
| Ethanol | C00469 | Glycolysis/gluconeogenesis | |
| Propanoate | C00163 | Propanoate metabolism | hsa00640 |
| 1,5-Diaminopentane | C01672 | Glutathione metabolism | hsa00480 |
| Formate | C00058 | Glyoxylate and dicarboxylate metabolism | hsa00630 |
| Hexadecanoate | C00249 | Biosynthesis of unsaturated fatty acids | hsa01040 |
| Fatty acid elongation | hsa00062 | ||
| Fatty acid degradation | hsa00071 | ||
| Fatty acid biosynthesis | hsa00061 | ||
| 4-Methylpentanal | C02373 | Steroid hormone biosynthesis | hsa00140 |
KEGG: Kyoto Encyclopedia of Genes and Genomes.
Furthermore, peroxidation of lipids in cancer can lead to the endogenic production of volatile ketones, alkanes, aldehydes, and furans in the body.24,58 Ketones and alcohols can also be generated when the glycolytic pathway takes place in hypoxic environments of proliferating tumors. 59 Among the reported ketones, acetone was found both in breath and cell culture headspace. In humans, hepatocytes can produce considerable quantities of acetoacetate, the simplest keto acid and prominent cellular fuel supply. 60 Decarboxylation of acetoacetate, which is achieved in both fatty acid degradation and the metabolism of ketogenic amino acids, yields endogenous acetone. 61 In addition, increased oxidative stress could impair glutathione metabolism since glutathione is an active antioxidant in the cell. However, 1,5-diaminopentane was reportedly involved in this glutathione metabolism, and it serves a role in amino acid export. 62
Degradation of membrane lipids has been postulated in several reports to be the endogenous origin of alkanes and other hydrocarbons in lung cancer patients.15,55 Cancers of various histological origins are responsible for the increased levels of circulating alkanes. 42 It is hypothesized that CYP450 may be a contributing factor for this phenomenon and responsible for the increased levels of methylaklanes. 63 Metabolism of hydrocarbons can also generate alcohols. Circulating levels of alcohols differ as a result of intake. In addition, endogenous acetaldehyde can serve as a precursor for ethanol in anaerobic conditions. CYP450 isoenzymes contribute to alcohol metabolism as well, by the hydroxylation of lipid peroxidation biomarkers. In particular, CYP2E1 can convert alcohol into acetaldehyde and produce more reactive oxygen species to increase oxidative stress in the tumor environment. 64 Cancers have also been thought to metabolize alcohols in vitro since 2-ethyl-1-hexanol has been reported both in human breath and headspace of lung cancer cell cultures. 65
Digging deeper into the origins of VOCs from humans, we conducted a secondary analysis on the reported VOCs by Huang et al. 66 In that report, pleural effusions were collected from 50 elderly individuals divided into two groups: malignant population consisting of 20 diagnosed cancer patients and benign inflammatory population encompassing 30 patients with tuberculous pleurisy or other diseases (Table 1). The study reported a total of 76 identified volatiles from the effusions, with a subset of 24 deemed to be associated with cancer (Table 2). While this dataset does not represent the whole lung cancer volatilome, it provides vital information to determine the metabolic origins of these VOCs. Global pathway analysis using MetaboAnalyst’s established pathway module was able to attribute the origins of three cancer-associated VOCs to four metabolic pathways. Acetaldehyde was determined to be involved in glycolysis/gluconeogenesis, glycerophospholipid metabolism, and pyruvate metabolism. In addition, pathway analysis confirmed glycolytic origins of endogenous ethanol. Steroid hormone biosynthesis was also responsible for the formation of 4-methylpentanal in cancer cells. However, 4-methylpentanal is a by-product of cholesterol metabolism to pregnenolone which is catalyzed by the enzyme cholesterol side-chain cleavage enzyme in the mitochondria. It can also serve as a substrate for aldehyde formation in cells.
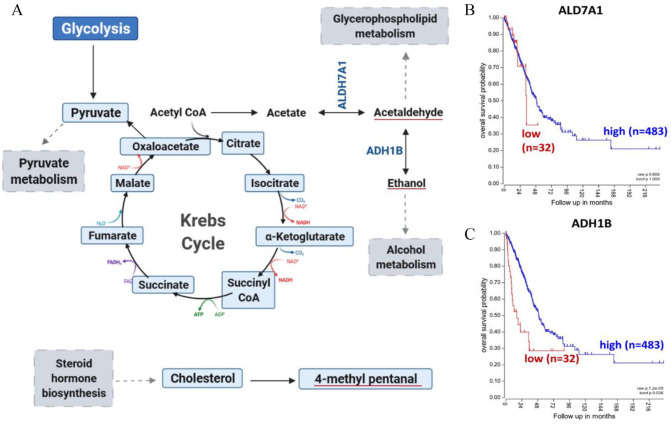
In humans, alcohol metabolism is primarily catalyzed by alcohol dehydrogenase (ADH). 67 For instance, endogenous ethanol can be derived from metabolism of hydrocarbons, another group of cancer-associated VOCs, by ADHs. 67 In addition, alcohol can serve as a precursor for acetaldehyde. In the mitochondria, acetaldehyde is rapidly oxidized to acetate by aldehyde dehydrogenase (ALDH) to free up carbon sources for carbon dioxide or fatty acid synthesis. 68 This downstream metabolic process of glucose metabolism is visualized in Figure 4(A). Endogenous acetate can be synthesized in the body through acetyl-CoA hydrolysis. Acetate can then be converted by acetaldehyde through ALDH7A1 among other ALDHs. 69 Aldehydes can subsequently be reduced to their corresponding alcohols through ADHs. 70 Elevated expression of both ALDH7A1 (Figure 4(B)) and ADH1B (Figure 4(C)) was associated with worse survival outcomes in lung cancer patients. 71 Increased expression of these genes would catalyze the reactions of ethanol and acetaldehyde, which could lead to their accumulation in the tumor. Upregulation of a certain gene would reflect in increased synthesis of the product metabolite since increasing levels of the enzyme catalyzing the reaction would be present. 72 This would provide greater abundance for these compounds to transfer to exhaled breath. Thus, the detection of these compounds in breath could be used to reflect on the genetic anomalies in lung cancers and assist in identifying disease severity. 73 Collectively, reports of these VOCs are of particular interest since 13 C-glucose labeling studies in lung cancer patients have highlighted glycolytic dependence of pulmonary tumors. 74 13 C-enrichment of lactate and succinate in malignant tissues corroborates reliance of lung cancers on glycolysis and pyruvate carboxylation for sustained energy. 75 As these metabolic processes funnel through to acetyl-coA and acetaldehyde metabolism, the detection of these VOCs in breath could indicate disease onset. Taken together, these findings suggest the importance of understanding the biochemical origins of breath VOCs so we can improve early diagnosis of pulmonary carcinomas in efforts of mitigating cancer progression using breath analysis.
Figure 4.
Secondary analysis of reported VOCs in Huang et al. with clinical relevance. (A) Endogenous synthesis of acetaldehyde and ethanol in humans, downstream of glycolysis, as outlined in KEGG (B and C) Kaplan–Meier in lung adenocarcinoma patients revealing lower survival of patients with elevated expression of genes involved in endogenous VOC synthesis. (A color version of this figure is available in the online journal.)
Novel advancements for VOC-based research in clinical settings
Since VOCs are tightly linked to lung cancer’s metabolic deregulations, their abundance in breath varies accordingly to the disease status. 73 Thus, exhaled volatiles can serve as prospective markers where changes in the detected levels could be used to determine disease stage or patient’s response to treatment. 73 Here, we explore the diverse applications of VOC analyses in the clinical settings. For instance, the inferred correlation between lung cancer progression and endogenously produced VOCs in humans suggests that volatilomics in breath has the potential to detect tumor formation at the very early stages of the disease. Meanwhile, reports have suggested that the concentration of some exhaled VOCs, such as cyclohexane and xylene, increases in breath as lung cancer progresses to later stages. 76 This could reflect the increased metabolic rate of tumors to sustain growth, which in turns produces more volatile by-products that could be detected in breath. In addition, abundance of VOCs was shown to be varied between patients exhibiting different histological types of lung cancer. 76
In addition to assisting lung cancer diagnosis, exhaled VOCs analysis also has the great potential to be utilized for the monitor and evaluation of lung cancer treatment efficacy. As chemotherapy can disrupt tumor growth, it is expected that the underlying metabolic mechanisms responsible for VOC production will be inherently interrupted. Accordingly, after identifying prominent volatiles for lung cancer diagnosis, it would be of particular interest to monitor the changes in their abundance throughout treatment regimens. Thus, the development of a novel screening method to monitor patient responses to treatment is a vital clinical concern. Reports have shown the detection of 29 altered volatile compounds in lung cancer patients after tumor resection, 77 of which 12 were statistically significant. The significantly deregulated VOCs included butyl glycol and cyclohexanone where the levels were found to be decreased with a fold change of −0.85 and −1.75, respectively. The abundance of cyclooctanemethanol was the most noticeably increased, with a reported fold change of 10.7. In addition, significant changes to VOC levels were revealed after subjecting patients to systemic therapy cycles. 23 The levels of α-phellandrene and 4-methyldodecane were decreased in exhaled breath of lung cancer patients compared to baseline controls. After treatment, the exhaled levels of these volatiles were restored to the levels similar to that of the baseline. Conversely, styrene exhibited an inverse trend where baseline levels were higher than progressive lung cancer patients and levels were decreased after treatment. Drug efficacy was assessed using computerized tomography scan and corroborated the results of the exhaled breath analysis. Treatment monitoring using headspace volatiles was also possible in cell cultures. Using online sampling, creatinine, methyl-3-hydroxybutyric acid, 2-phenylacetamide, L-histidine, and decyl acetate were identified in the headspace of lung cancer cell cultures. 41 After cisplatin treatment, the levels of all but creatinine were increased in headspace gas. Decreased levels of histidine were reported in cultured lung cancer cells and consistent with the reports of occult metastases in patients. 43 Increased levels of histidine in the headspace of in vitro cell cultures after treatment suggest that VOCs can also provide valuable information for treatment responses in exhaled patient breath.
Another exciting finding highlighting the diverse utility of VOCs in the clinical setting was the prediction of non–small cell lung cancer patients’ response to immunotherapy by the analysis of exhaled breath in treatment-naïve patients. 44 Lung cancer patients were subjected to routine standard clinical care, including computed tomography (CT) scans and blood tests prior to immunotherapy regiments. Patient response to anti-PD-1 therapy was monitored throughout the course of the study along with the collections of exhaled breath. After training a linear discriminant analysis-based model using 92 patients, the results were validated in an independent cohort of 51 lung cancer patients. After validating the findings, the exhaled breath profiles of anti-PD-L1 responders were unique from the breath of non-responders with an area under the curve (AUC) of 0.85 (95% CI: 0.7–0.96). Strikingly, 12 out of the 51 patients in the validation set (24%) were predicted and subsequently proven to be non-responders to immunotherapy. These promising findings corroborate the unique exhaled molecular fingerprint of lung cancers and highlight the potential of VOC-based diagnostics.
Current clinical applications of these breathomics screenings are hindered by the availability of expensive instruments, such as mass spectrometers. Yet, they are the primary cost to run breath analyses as sample cost is considerably low. While PET-CT scans are currently effective, the elevated cost per screening may be a financial burden for patients. Besides, if a panel of known lung cancer VOC biomarkers is established, the technology can be afforded for the masses by implementing this biomarker panel into cheaper and portable breathalyzers. Exhaled breath analyses of volatiles have been demonstrated to (1) identify unique exhaled breath VOC patterns associated with lung cancer, (2) monitor changes in VOC abundance to monitor patients’ response to treatment, and (3) predict patients’ response to treatment prior to therapy. If done correctly with proper exclusion of environmental volatiles, exhaled breath prints of lung cancer patients could possibly be utilized in conjunction with the current clinical standards of care, such as CT scans or blood tests, for disease diagnosis and monitoring in efforts of affording patients rapid and individualized treatments.
Conclusion and future directions
Analyses of VOCs in exhaled breath and cell cultures have been considered as promising and non-invasive tools for evaluating the disease status and treatment outcomes of lung cancer in patients. Endogenously derived volatiles can mirror the metabolic process that is deregulated by oncogenesis. The literature is abundant with studies highlighting unique lung cancer-associated volatiles in breath of patients that could reflect early disease onset. While the endogenous origins of VOCs remain elusive, the curated knowledge of metabolic mechanisms and gene functions could facilitate our understanding of the effect of cancer on exhaled VOCs. In addition, our review has highlighted the potential use of volatiles to pinpoint genes used for patient survival prediction. Advancements in breath analyses could also offer an alternative to the current screening methods, which are incapable of detecting lung cancer in the early stages. However, it is acknowledged that VOC analysis is not mature enough to be broadly applied in clinical settings. Standardization of exhaled breath collection and analytical analyses is currently lacking and could explain the variation in proposed lung cancer biomarkers. Another limitation arises due to the ambiguity surrounding the metabolic processing responsible for many volatiles. Putative validation of the deregulated metabolic processes responsible for VOCs would provide invaluable information for scientists and clinicians investigating cancer-related VOCs. Nonetheless, research highlighted in this mini review suggests that exhaled volatiles can play a prominent role in future clinics for lung cancer-related applications. The rise of more accurate and sensitive methods, such as secondary electrospray ionization mass spectrometry, 45 for VOC detections could validate the utility of these volatile biomarkers in reflecting the efficacy of treatment in lung cancer patients. Moving forward, we believe that the advancement of exhaled breath analysis will yield great results that will lay the foundation for the use of VOCs as markers for cancer diseases and for monitoring disease progression. The non-invasive collection of this limitless sample is likely to facilitate better patient compliance and increase willingness to get tested. We hope this mini review will stimulate new quantitative experimental and theoretical studies of cancer-related VOCs to provide most personalize treatment options for lung cancer patients.
Footnotes
Authors’ Contributions: JZ contributed in conceptualization, resources, writing – review and editing, project administration, and funding acquisition; JZ and FC participated in methodology and investigation; FC performed the formal analysis and contributed in writing – original draft preparation; FC and AB participated in data curation. All authors have read and agreed to the submitted version of the article.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award No. R35GM133510 for JZ and the Taber-Thompson award for FC. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
ORCID iD: Jiangjiang Zhu  https://orcid.org/0000-0002-4548-8949
https://orcid.org/0000-0002-4548-8949
References
- 1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209–49 [DOI] [PubMed] [Google Scholar]
- 2. Clark SB, Alsubait S. Non small cell lung cancer. In StatPearls. Treasure Island, FL: StatPearls Publishing, 2021, https://www.ncbi.nlm.nih.gov/books/NBK562307/#:~:text=Non%2Dsmall%20cell%20lung%20cancer,of%20patients%20with%20this%20condition. [Google Scholar]
- 3. Jacob S, Rahbari K, Tegtmeyer K, Zhao J, Tran S, Helenowski I, Zhang H, Walunas T, Varga J, Dematte J, Villaflor V. Lung cancer survival in patients with autoimmune disease. JAMA Netw Open 2020;3:e2029917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Peled N, Hakim M, Bunn PA, Jr, Miller YE, Kennedy TC, Mattei J, Mitchell JD, Hirsch FR, Haick H. Non-invasive breath analysis of pulmonary nodules. J Thorac Oncol 2012;7:1528–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brown LM, Silverman DT, Pottern LM, Schoenberg JB, Greenberg RS, Swanson GM, Liff JM, Schwartz AG, Hayes RB, Blot WJ. Adenocarcinoma of the esophagus and esophagogastric junction in white men in the United States: alcohol, tobacco, and socioeconomic factors. Cancer Causes Control 1994;5:333–40 [DOI] [PubMed] [Google Scholar]
- 6. Veugelers PJ, Porter GA, Guernsey DL, Casson AG. Obesity and lifestyle risk factors for gastroesophageal reflux disease, Barrett esophagus and esophageal adenocarcinoma. Dis Esophagus 2006;19:321–8 [DOI] [PubMed] [Google Scholar]
- 7. Jones GS, Baldwin DR. Recent advances in the management of lung cancer. Clin Med (Lond) 2018;18:s41–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WE, Nicholson AG, Groome P, Mitchell A, Bolejack V. The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol 2016;11:39–51 [DOI] [PubMed] [Google Scholar]
- 9. Collins LG, Haines C, Perkel R, Enck RE. Lung cancer: diagnosis and management. Am Fam Physician 2007;75:56–63 [PubMed] [Google Scholar]
- 10. Walters S, Maringe C, Coleman MP, Peake MD, Butler J, Young N, Bergström S, Hanna L, Jakobsen E, Kölbeck K, Sundstrøm S, Engholm G, Gavin A, Gjerstorff ML, Hatcher J, Johannesen TB, Linklater KM, McGahan CE, Steward J, Tracey E, Turner D, Richards MA, Rachet B, ICBP Module 1 Working Group. Lung cancer survival and stage at diagnosis in Australia, Canada, Denmark, Norway, Sweden and the UK: a population-based study, 2004-2007. Thorax 2013;68:551–64 [DOI] [PubMed] [Google Scholar]
- 11. Latimer KMMT. Lung cancer: diagnosis, treatment principles, and screening. Am Fam Physician 2015;91:250–6 [PubMed] [Google Scholar]
- 12. Mazzone PJ, Hammel J, Dweik R, Na J, Czich C, Laskowski D, Mekhail T. Diagnosis of lung cancer by the analysis of exhaled breath with a colorimetric sensor array. Thorax 2007;62:565–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dragonieri S, Annema JT, Schot R, van der Schee MP, Spanevello A, Carratú P, Resta O, Rabe KF, Sterk PJ. An electronic nose in the discrimination of patients with non-small cell lung cancer and COPD. Lung Cancer 2009;64:166–70 [DOI] [PubMed] [Google Scholar]
- 14. van de Kant KD, van der Sande LJ, Jöbsis Q, van Schayck OC, Dompeling E. Clinical use of exhaled volatile organic compounds in pulmonary diseases: a systematic review. Respir Res 2012;13:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Janfaza S, Khorsand B, Nikkhah M, Zahiri J. Digging deeper into volatile organic compounds associated with cancer. Biol Methods Protoc 2019;4:bpz014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hitosugi T, Chen J. Post-translational modifications and the Warburg effect. Oncogene 2014;33:4279–85 [DOI] [PubMed] [Google Scholar]
- 17. Qin T, Liu H, Song Q, Song G, Wang HZ, Pan YY, Xiong FX, Gu KS, Sun GP, Chen ZD. The screening of volatile markers for hepatocellular carcinoma. Cancer Epidemiol Biomarkers Prev 2010;19:2247–53 [DOI] [PubMed] [Google Scholar]
- 18. Li J, Peng Y, Liu Y, Li W, Jin Y, Tang Z, Duan Y. Investigation of potential breath biomarkers for the early diagnosis of breast cancer using gas chromatography-mass spectrometry. Clin Chim Acta 2014; 436:59–67 [DOI] [PubMed] [Google Scholar]
- 19. Nakhleh MK, Amal H, Jeries R, Broza YY, Aboud M, Gharra A, Ivgi H, Khatib S, Badarneh S, Har-Shai L, Glass-Marmor L, Lejbkowicz I, Miller A, Badarny S, Winer R, Finberg J, Cohen-Kaminsky S, Perros F, Montani D, Girerd B, Garcia G, Simonneau G, Nakhoul F, Baram S, Salim R, Hakim M, Gruber M, Ronen O, Marshak T, Doweck I, Nativ O, Bahouth Z, Shi DY, Zhang W, Hua QL, Pan YY, Tao L, Liu H, Karban A, Koifman E, Rainis T, Skapars R, Sivins A, Ancans G, Liepniece-Karele I, Kikuste I, Lasina I, Tolmanis I, Johnson D, Millstone SZ, Fulton J, Wells JW, Wilf LH, Humbert M, Leja M, Peled N, Haick H. Diagnosis and classification of 17 diseases from 1404 subjects via pattern analysis of exhaled molecules. ACS Nano 2017;11:112–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Phillips M, Bauer TL, Pass HI. A volatile biomarker in breath predicts lung cancer and pulmonary nodules. J Breath Res 2019;13:036013. [DOI] [PubMed] [Google Scholar]
- 21. Chen X, Xu F, Wang Y, Pan Y, Lu D, Wang P, Ying K, Chen E, Zhang W. A study of the volatile organic compounds exhaled by lung cancer cells in vitro for breath diagnosis. Cancer 2007;110:835–44 [DOI] [PubMed] [Google Scholar]
- 22. Jia Z, Patra A, Kutty VK, Venkatesan T. Critical review of volatile organic compound analysis in breath and in vitro cell culture for detection of lung cancer. Metabolites 2019;9:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nardi-Agmon I, Abud-Hawa M, Liran O, Gai-Mor N, Ilouze M, Onn A, Bar J, Shlomi D, Haick H, Peled N. Exhaled breath analysis for monitoring response to treatment in advanced lung cancer. J Thorac Oncol 2016;11:827–37 [DOI] [PubMed] [Google Scholar]
- 24. Broza YY, Kremer R, Tisch U, Gevorkyan A, Shiban A, Best LA, Haick H. A nanomaterial-based breath test for short-term follow-up after lung tumor resection. Nanomedicine 2013;9:15–21 [DOI] [PubMed] [Google Scholar]
- 25. Chen X, Zhang K, Yin Z, Fang M, Pu W, Liu Z, Li L, Sinues P, Dallmann R, Zhou Z, Li X. Online real-time monitoring of exhaled breath particles reveals unnoticed transport of nonvolatile drugs from blood to breath. Anal Chem 2021;93:5005–8 [DOI] [PubMed] [Google Scholar]
- 26. Ghosh C, Singh V, Grandy J, Pawliszyn J. Recent advances in breath analysis to track human health by new enrichment technologies. J Sep Sci 2020;43:226–40 [DOI] [PubMed] [Google Scholar]
- 27. Obermeier J, Trefz P, Wex K, Sabel B, Schubert JK, Miekisch W. Electrochemical sensor system for breath analysis of aldehydes, CO and NO. J Breath Res 2015;9:016008. [DOI] [PubMed] [Google Scholar]
- 28. Bianchi F, Riboni N, Carbognani P, Gnetti L, Dalcanale E, Ampollini L, Careri M. Solid-phase microextraction coupled to gas chromatography-mass spectrometry followed by multivariate data analysis for the identification of volatile organic compounds as possible biomarkers in lung cancer tissues. J Pharm Biomed Anal 2017;146:329–33 [DOI] [PubMed] [Google Scholar]
- 29. Song G, Qin T, Liu H, Xu GB, Pan YY, Xiong FX, Gu KS, Sun GP, Chen ZD. Quantitative breath analysis of volatile organic compounds of lung cancer patients. Lung Cancer 2010;67:227–31 [DOI] [PubMed] [Google Scholar]
- 30. Albert KJ, Lewis NS, Schauer CL, Sotzing GA, Stitzel SE, Vaid TP, Walt DR. Cross-reactive chemical sensor arrays. Chem Rev 2000;100: 2595–626 [DOI] [PubMed] [Google Scholar]
- 31. Li Z, Askim JR, Suslick KS. The optoelectronic nose: colorimetric and fluorometric sensor arrays. Chem Rev 2019;119:231–92 [DOI] [PubMed] [Google Scholar]
- 32. Tsou PH, Lin ZL, Pan YC, Yang HC, Chang CJ, Liang SK, Wen YF, Chang CH, Chang LY, Yu KL, Liu CJ, Keng LT, Lee MR, Ko JC, Huang GH, Li YK. Exploring volatile organic compounds in breath for high-accuracy prediction of lung cancer. Cancers (Basel) 2021;13:1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cristescu SM, Gietema HA, Blanchet L, Kruitwagen CL, Munnik P, van Klaveren RJ, Lammers JW, Buydens L, Harren FJ, Zanen P. Screening for emphysema via exhaled volatile organic compounds. J Breath Res 2011;5:046009. [DOI] [PubMed] [Google Scholar]
- 34. Schrimpe-Rutledge AC, Codreanu SG, Sherrod SD, McLean JA. Untargeted metabolomics strategies-challenges and emerging directions. J Am Soc Mass Spectrom 2016;27:1897–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Koureas M, Kalompatsios D, Amoutzias GD, Hadjichristodoulou C, Gourgoulianis K, Tsakalof A. Comparison of targeted and untargeted approaches in breath analysis for the discrimination of lung cancer from benign pulmonary diseases and healthy persons. Molecules 2021;26:2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dent AG, Sutedja TG, Zimmerman PV. Exhaled breath analysis for lung cancer. J Thorac Dis 2013;5:S540–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nardi-Agmon I, Peled N. Exhaled breath analysis for the early detection of lung cancer: recent developments and future prospects. Lung Cancer (Auckl) 2017;8:31–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pereira J, Porto-Figueira P, Cavaco C, Taunk K, Rapole S, Dhakne R, Nagarajaram H, Câmara JS. Breath analysis as a potential and non-invasive frontier in disease diagnosis: an overview. Metabolites 2015;5:3–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lawal O, Ahmed WM, Nijsen TME, Goodacre R, Fowler SJ. Exhaled breath analysis: a review of “breath-taking” methods for off-line analysis. Metabolomics 2017;13:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ajibola OA, Smith D, Spaněl P, Ferns GA. Effects of dietary nutrients on volatile breath metabolites. J Nutr Sci 2013;2:e34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Choueiry F, Zhu J. Secondary electrospray ionization-high resolution mass spectrometry (SESI-HRMS) fingerprinting enabled treatment monitoring of pulmonary carcinoma cells in real time. Anal Chim Acta 2021;1189: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Phillips M, Cataneo RN, Saunders C, Hope P, Schmitt P, Wai J. Volatile biomarkers in the breath of women with breast cancer. J Breath Res 2010;4:026003. [DOI] [PubMed] [Google Scholar]
- 43. Graff L, Frungieri M, Zanner R, Pohlinger A, Prinz C, Gratzl M. Expression of histidine decarboxylase and synthesis of histamine by human small cell lung carcinoma. Am J Pathol 2002;160:1561–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. de Vries R, Muller M, van der Noort V, Theelen W, Schouten RD, Hummelink K, Muller SH, Wolf-Lansdorf M, Dagelet JWF, Monkhorst K, Maitland-van der Zee AH, Baas P, Sterk PJ, van den Heuvel MM. Prediction of response to anti-PD-1 therapy in patients with non-small-cell lung cancer by electronic nose analysis of exhaled breath. Ann Oncol 2019;30:1660–6 [DOI] [PubMed] [Google Scholar]
- 45. Dillon LA, Stone VN, Croasdell LA, Fielden PR, Goddard NJ, Thomas CL. Optimisation of secondary electrospray ionisation (SESI) for the trace determination of gas-phase volatile organic compounds. Analyst 2010;135:306–14 [DOI] [PubMed] [Google Scholar]
- 46. Bajtarevic A, Ager C, Pienz M, Klieber M, Schwarz K, Ligor M, Ligor T, Filipiak W, Denz H, Fiegl M, Hilbe W, Weiss W, Lukas P, Jamnig H, Hackl M, Haidenberger A, Buszewski B, Miekisch W, Schubert J, Amann A. Noninvasive detection of lung cancer by analysis of exhaled breath. BMC Cancer 2009;9:348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gashimova E, Temerdashev A, Porkhanov V, Polyakov I, Perunov D, Azaryan A, Dmitrieva E. Investigation of different approaches for exhaled breath and tumor tissue analyses to identify lung cancer biomarkers. Heliyon 2020;6:e04224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Phillips M, Gleeson K, Hughes JM, Greenberg J, Cataneo RN, Baker L, McVay WP. Volatile organic compounds in breath as markers of lung cancer: a cross-sectional study. Lancet 1999;353:1930–3 [DOI] [PubMed] [Google Scholar]
- 49. Svensson RU, Parker SJ, Eichner LJ, Kolar MJ, Wallace M, Brun SN, Lombardo PS, Van Nostrand JL, Hutchins A, Vera L, Gerken L, Greenwood J, Bhat S, Harriman G, Westlin WF, Harwood HJ, Jr, Saghatelian A, Kapeller R, Metallo CM, Shaw RJ. Inhibition of acetyl-CoA carboxylase suppresses fatty acid synthesis and tumor growth of non-small-cell lung cancer in preclinical models. Nat Med 2016;22:1108–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wunder C, Weber C, Paulke A, Koelzer SC, Holz F, Toennes SW. Endogenous formation of 1-propanol and methanol after consumption of alcoholic beverages. Forensic Sci Int 2021;325:110905. [DOI] [PubMed] [Google Scholar]
- 51. Lunt SY, Vander Heiden MG. Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu Rev Cell Dev Biol 2011;27: 441–64 [DOI] [PubMed] [Google Scholar]
- 52. Wester RC, Maibach HI, Gruenke LD, Craig JC. Benzene levels in ambient air and breath of smokers and nonsmokers in urban and pristine environments. J Toxicol Environ Health 1986;18:567–73 [DOI] [PubMed] [Google Scholar]
- 53. Smolle E, Leko P, Stacher-Priehse E, Brcic L, El-Heliebi A, Hofmann L, Quehenberger F, Hrzenjak A, Popper HH, Olschewski H, Leithner K. Distribution and prognostic significance of gluconeogenesis and glycolysis in lung cancer. Mol Oncol 2020;14:2853–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pleil JD, Stiegel MA, Risby TH. Clinical breath analysis: discriminating between human endogenous compounds and exogenous (environmental) chemical confounders. J Breath Res 2013;7:017107. [DOI] [PubMed] [Google Scholar]
- 55. Hakim M, Broza YY, Barash O, Peled N, Phillips M, Amann A, Haick H. Volatile organic compounds of lung cancer and possible biochemical pathways. Chem Rev 2012;112:5949–66 [DOI] [PubMed] [Google Scholar]
- 56. Carta G, Murru E, Banni S, Manca C. Palmitic acid: physiological role, metabolism and nutritional implications. Front Physiol 2017;8:902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Phillips M, Cataneo RN, Greenberg J, Gunawardena R, Naidu A, Rahbari-Oskoui F. Effect of age on the breath methylated alkane contour, a display of apparent new markers of oxidative stress. J Lab Clin Med 2000;136:243–9 [DOI] [PubMed] [Google Scholar]
- 58. Zabłocka-Słowińska K, Płaczkowska S, Skórska K, Prescha A, Pawełczyk K, Porębska I, Kosacka M, Grajeta H. Oxidative stress in lung cancer patients is associated with altered serum markers of lipid metabolism. PLoS ONE 2019;14:e0215246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Deng C, Zhang X, Li N. Investigation of volatile biomarkers in lung cancer blood using solid-phase microextraction and capillary gas chromatography-mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 2004;808:269–77 [DOI] [PubMed] [Google Scholar]
- 60. Rui L. Energy metabolism in the liver. Compr Physiol 2014;4:177–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Grabacka M, Pierzchalska M, Dean M, Reiss K. Regulation of ketone body metabolism and the role of PPARα. Int J Mol Sci 2016;17:2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hawel L, 3rd, Tjandrawinata RR, Fukumoto GH, Byus CV. Biosynthesis and selective export of 1,5-diaminopentane (cadaverine) in mycoplasma-free cultured mammalian cells. J Biol Chem 1994;269:7412–8 [PubMed] [Google Scholar]
- 63. Mangler M, Freitag C, Lanowska M, Staeck O, Schneider A, Speiser D. Volatile organic compounds (VOCs) in exhaled breath of patients with breast cancer in a clinical setting. Ginekol Pol 2012;83:730–6. [PubMed] [Google Scholar]
- 64. Le Daré B, Lagente V, Gicquel T. Ethanol and its metabolites: update on toxicity, benefits, and focus on immunomodulatory effects. Drug Metab Rev 2019;51:545–61 [DOI] [PubMed] [Google Scholar]
- 65. Sponring A, Filipiak W, Mikoviny T, Ager C, Schubert J, Miekisch W, Amann A, Troppmair J. Release of volatile organic compounds from the lung cancer cell line NCI-H2087 in vitro. Anticancer Res 2009;29:419–26 [PubMed] [Google Scholar]
- 66. Huang Z, Zhang J, Zhang P, Wang H, Pan Z, Wang L. Analysis of volatile organic compounds in pleural effusions by headspace solid-phase microextraction coupled with cryotrap gas chromatography and mass spectrometry. J Sep Sci 2016;39:2544–52 [DOI] [PubMed] [Google Scholar]
- 67. Matejcic M, Gunter MJ, Ferrari P. Alcohol metabolism and oesophageal cancer: a systematic review of the evidence. Carcinogenesis 2017;38:859–72 [DOI] [PubMed] [Google Scholar]
- 68. Cederbaum AI. Alcohol metabolism. Clin Liver Dis 2012;16:667–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Klyosov AA. Kinetics and specificity of human liver aldehyde dehydrogenases toward aliphatic, aromatic, and fused polycyclic aldehydes. Biochemistry 1996;35:4457–67 [DOI] [PubMed] [Google Scholar]
- 70. Crabb DW, Matsumoto M, Chang D, You M. Overview of the role of alcohol dehydrogenase and aldehyde dehydrogenase and their variants in the genesis of alcohol-related pathology. Proc Nutr Soc 2004;63:49–63 [DOI] [PubMed] [Google Scholar]
- 71. Deng M, Brägelmann J, Schultze JL, Perner S. Web-TCGA: an online platform for integrated analysis of molecular cancer data sets. BMC Bioinformatics 2016;17:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Rothman DL, Stearns SC, Shulman RG. Gene expression regulates metabolite homeostasis during the Crabtree effect: implications for the adaptation and evolution of Metabolism. Proc Natl Acad Sci USA 2021;118:e2014013118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Belizário JE, Faintuch J, Malpartida MG. Breath biopsy and discovery of exclusive volatile organic compounds for diagnosis of infectious diseases. Front Cell Infect Microbiol 2020;10:564194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hensley CT, Faubert B, Yuan Q, Lev-Cohain N, Jin E, Kim J, Jiang L, Ko B, Skelton R, Loudat L, Wodzak M, Klimko C, McMillan E, Butt Y, Ni M, Oliver D, Torrealba J, Malloy CR, Kernstine K, Lenkinski RE, DeBerardinis RJ. Metabolic heterogeneity in human lung tumors. Cell 2016;164:681–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Fan TW, Lane AN, Higashi RM, Farag MA, Gao H, Bousamra M, Miller DM. Altered regulation of metabolic pathways in human lung cancer discerned by (13)C stable isotope-resolved metabolomics (SIRM). Mol Cancer 2009;8:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Oguma T, Nagaoka T, Kurahashi M, Kobayashi N, Yamamori S, Tsuji C, Takiguchi H, Niimi K, Tomomatsu H, Tomomatsu K, Hayama N, Aoki T, Urano T, Magatani K, Takeda S, Abe T, Asano K. Clinical contributions of exhaled volatile organic compounds in the diagnosis of lung cancer. PLoS ONE 2017;12:e0174802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wang C, Dong R, Wang X, Lian A, Chi C, Ke C, Guo L, Liu S, Zhao W, Xu G, Li E. Exhaled volatile organic compounds as lung cancer biomarkers during one-lung ventilation. Sci Rep 2014;4:7312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Sun Y, Chen Y, Sun C, Liu H, Wang Y, Jiang X. Analysis of volatile organic compounds from patients and cell lines for the validation of lung cancer biomarkers by proton-transfer-reaction mass spectrometry. Anal Methods 2019;11:3188–97 [Google Scholar]