Abstract
Saccharomyces cerevisiae ferments hexoses efficiently but is unable to ferment xylose. When the bacterial enzyme xylose isomerase (XI) from Thermus thermophilus was produced in S. cerevisiae, xylose utilization and ethanol formation were demonstrated. In addition, xylitol and acetate were formed. An unspecific aldose reductase (AR) capable of reducing xylose to xylitol has been identified in S. cerevisiae. The GRE3 gene, encoding the AR enzyme, was deleted in S. cerevisiae CEN.PK2-1C, yielding YUSM1009a. XI from T. thermophilus was produced, and endogenous xylulokinase from S. cerevisiae was overproduced in S. cerevisiae CEN.PK2-1C and YUSM1009a. In recombinant strains from which the GRE3 gene was deleted, xylitol formation decreased twofold. Deletion of the GRE3 gene combined with expression of the xylA gene from T. thermophilus on a replicative plasmid generated recombinant xylose utilizing S. cerevisiae strain TMB3102, which produced ethanol from xylose with a yield of 0.28 mmol of C from ethanol/mmol of C from xylose. None of the recombinant strains grew on xylose.
Ethanol production from renewable lignocellulosic material represents an environmentally sustainable alternative to fossil-derived gasoline. In most lignocellulosic material, the second-most-common sugar is xylose (13). For an economically feasible fuel production process, both hexose and pentose sugars must be fermented to form ethanol (35). The yeast Saccharomyces cerevisiae is robust and well adapted for ethanol production, but it is unable to produce ethanol from xylose.
The initial metabolism of xylose in natural xylose-utilizing yeasts such as Pichia stipitis is catalyzed by xylose reductase (XR), which reduces xylose to xylitol, and xylitol dehydrogenase (XDH), which oxidizes xylitol to xylulose. Xylulose is then phosphorylated by xylulokinase (XK) to xylulose-5-phosphate that is further metabolized in the pentose phosphate pathway. P. stipitis requires low and carefully controlled oxygenation (28) and is sensitive to ethanol (8), which limits its use for industrial ethanol production. Recombinant Saccharomyces strains producing XR and XDH from P. stipitis in addition to overexpression of the homologous XKS1 gene encoding XK produce ethanol from xylose, with xylitol as a major by-product (9, 14, 36).
In bacteria, xylose isomerase (XI), encoded by the xylA gene, catalyzes the isomerization of xylose to xylulose. xylA genes from several bacteria have been cloned and transformed into S. cerevisiae, including xylA from Actinoplanes missouriensis (1), Bacillus subtilis (1), Clostridium thermosulfurigenes (19), Escherichia coli (15, 23), and Streptomyces rubiginosus (24). The use of rich-medium ethanol formation from xylose has been reported in recombinant Schizosaccharomyces pombe expressing xylA from E. coli (6). Ethanol formation from xylose in synthetic complete (SC) medium has so far been demonstrated only in recombinant S. cerevisiae expressing the xylA gene from Thermus thermophilus (37). A major by-product was xylitol, which inhibits XI (39) (Fig. 1). S. cerevisiae produces an unspecific aldose reductase (AR), encoded by the GRE3 gene on chromosome VIII (11), capable of reducing xylose to xylitol (17).
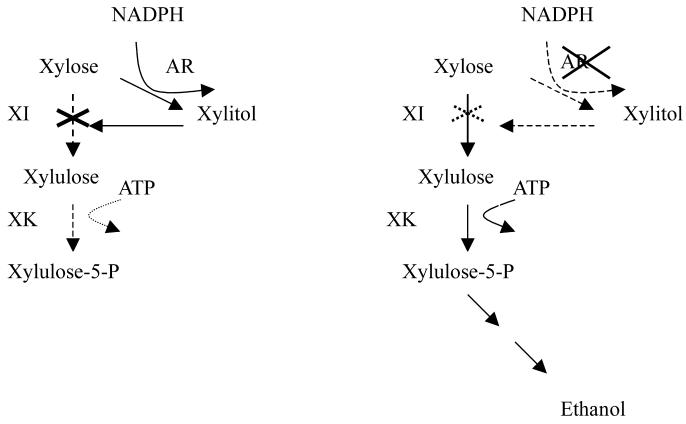
FIG. 1.
Model of xylose metabolism in recombinant S. cerevisiae expressing XI with (A) and without (B) the GRE3 gene.
In the present study, the GRE3 gene was deleted from chromosome VIII, yielding a recombinant S. cerevisiae strain with reduced xylitol formation. XI was introduced into the S. cerevisiae reference strain and in the Δgre3 strain to investigate the inhibition of XI by xylitol during fermentation of xylose. XK was overproduced in strains expressing xylA to increase the flux of xylulose into the central metabolism. The effects of glucose and oxygen supplementation were also studied.
MATERIALS AND METHODS
Strains and plasmids.
The genotypes of the microbial strains and plasmids used in the present study are summarized in Table 1. Plasmids were constructed and used to transform E. coli DH5α or JM109. Plasmid pUSM1006 was used to transform S. cerevisiae CEN.PK2-1C. Plasmids pBXI and pXks were used to transform YUSM1009a and CEN.PK2-1C. All strains were stored at −80°C.
TABLE 1.
Microbial strains and plasmids used in this study
| Strain or plasmid | Relevant feature(s) | Source or reference |
|---|---|---|
| Strains | ||
| E. coli | ||
| JM109 | F′ traD36 proA+proB+lacIq Δ(lacZ)M15 Δ(lac-proAB) supE44 hsdR17 recA1 gyrA96 thi-1 endA1 relA1 e14− λ− | 40 |
| DH5α | F− φ80dlacZ ΔM15 Δ(lacZYA-argF) U169 deoR recA1 endA1 hsdR17 (rK+ mK−) phoA supE44 λ−thi-1 gyrA96 relA1 | Life Technologies, Rockville, Md. |
| S. cerevisiae | ||
| CEN.PK2-1C | MATaleu2-3,112 his3-Δ1 ura3-52 trp1-289 MAL2-8(Con) MAL3 SUC3 | 10 |
| YUSM1006a | CEN.PK2-1C(pUSM1006) | This work |
| YUSM1009a | CEN.PK2-1C (Δgre3) | This work |
| TMB3101 | CEN.PK2-1C (xylA LEU2) | This work |
| TMB3102 | YUSM1009a (Δgre3 xylA LEU2) | This work |
| TMB3103 | CEN.PK2-1C (xylA LEU2 XKS1 TRP1) | This work |
| TMB3104 | YUSM1009a (Δgre3 xylA LEU2 XKS1 TRP1) | This work |
| Plasmids | ||
| pBR322 | bla tet | 3 |
| pGEM-T easy vector | bla | Promega Corporation, Madison, Wis. |
| pBluescript SK(−) | bla | GenBank (accession no. 52330) |
| pBXI | bla PGKp:xylA:PGKt LEU2 | 37 |
| pXks | bla PGKp:XKS1:PGKt TRP1 | 9 |
| pUSM1002 | bla GRE3t in pBR322 | This work |
| pUSM1003 | GRE3p in pGEM-T easy | This work |
| pUSM1004 | bla GRE3p | This work |
| pUSM1006 | bla URA3 GRE3p GRE3t | This work |
| pUSM1007 | pUC8/URA3 | This work |
| pUSM1008 | bla GRE3t in pBluescript SK(−) | This work |
Culture conditions.
S. cerevisiae CEN.PK2-1C was precultured in yeast extract-peptone-dextrose (26) or synthetic medium (20 g of glucose/liter and 6.7 g of yeast nitrogen base [YNB] without amino acids [Difco, Detroit, Mich.]/liter) supplemented with amino and nucleic acids (20 mg of tryptophan, uracil, and histidine per liter and 30 mg of leucine/liter, omitting those used as selection markers). The YNB medium was also used to make 5-fluoroorotic acid (5-FOA)-agar plates containing 1 g of 5-FOA (Sigma, Stockholm, Sweden)/liter for the selection of transformants that lost the URA3 marker with replica plating (38). In fermentation, a defined mineral medium (34) supplemented with amino acids including vitamins, trace elements, and citric acid buffer, pH 5.5, was used. The medium also contained 50 g of xylose/liter and 20 g of glucose/liter where appropriate. Bacterial strains were grown in Luria-Bertani medium (2), and transformants were selected with ampicillin (50 μg ml−1) after growth at 37°C. When cells were grown on solid media, 20 g of agar/liter was added.
DNA manipulations and amplifications.
Standard techniques for nucleic acid manipulations were used (22). Plasmids were prepared using a Qiagen Mini plasmid purification kit (Qiagen GmbH, Hilden, Germany). Restriction enzymes and other modifying enzymes were from Boehringer Mannheim Scandinavia AB (Bromma, Sweden). Plasmid transformations of E. coli were performed with the calcium chloride method (22). Yeast transformations were performed by the lithium acetate method (12).
The genomic DNA of S. cerevisiae CEN.PK2-1C was used as template for the PCR of the corresponding GRE3 promoter and terminator sequences. The oligonucleotides used for amplifying the promoter region were ARpL (left primer) (5′-GAT CGA ATT CTT TGT AAC TGT AAT TTC ACT CAT GC-3′ [EcoRI is underlined]) and ARpR (right primer) (5′-GAT CAA GCT TAA TCC ATA CTC AAC GAC CAT ATG-3′ [HindIII is underlined]). To amplify the terminator region, the primers used were ARtL (left primer) (5′-GTA CAA GCT TTT TCC AAT TTT ATT TTA CGA TTT-3′ [HindIII is underlined]) and ARtR (right primer) (5′-GTA AGG ATC CGC TCA TAT CTT GCT GTT G-3′ [BamHI is underlined]). These PCR primers were based on the published sequence of the GRE3 promoter and terminator regions of S. cerevisiae. This information was found at the Saccharomyces Genome Database website (http: //genome-www.stanford.edu/Saccharomyces/). All primers contained a restriction endonuclease site (Fig. 2A). For amplification of the DNA, Pfu polymerase (Stratagene, Capetown, Republic of South Africa) was used. The PCR mix contained PCR buffer with 2 mM MgSO4, 2 mM deoxynucleoside triphosphate, 0.5 μM concentrations of each primer, 0.1 μg of template, and 2.5 U of Pfu polymerase enzyme in a final volume of 50 μl. The thermocycler (Eppendorf 5330 plus; Analytical Instrument Recycle, Inc., Golden, Colo.) was used under the following conditions: 95°C for 5 min; 25 cycles of 95°C for 30 s, 54°C for 30 s, and 72°C for 1 min; 10 min at 72°C. Then the mixture was chilled to 4°C.
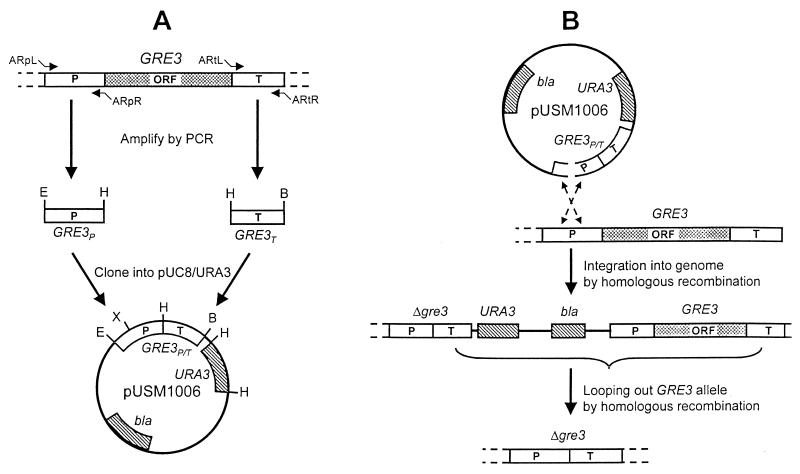
FIG. 2.
(A) Schematic summary of the construction of plasmid pUSM1006. The 5′ promoter (P) and 3′ terminator (T) regions of the corresponding GRE3 gene were amplified using oligonucleotide pairs ARpL-ARpR and ARtL-ARtR, respectively. The selectable markers bla and URA3 are indicated on the plasmid by striped boxes, and the GRE3 promoter (GRE3P) and terminator (GRE3T) sequences are indicated by open boxes. Sites for restriction endonucleases are indicated as follows: B, BamHI; E, EcoRI; H, HindIII; X, XbaI. (B) The plasmid pUSM1006 was linearized by cutting plasmid pUSM1006 with XbaI and integrating it into the genome by homologous recombination. The GRE3 allele was subsequently looped out by homologous recombination between the GRE3 terminator sequences. ORF, open reading frame.
Southern blot hybridization.
Total DNA was isolated from S. cerevisiae YUSM1006a and putative YUSM1009a, digested with HindIII, separated on a 1% agarose gel, and transferred to a Hybond-N membrane (Amersham Sweden AB, Stockholm, Sweden). Southern hybridizations (29) were performed as described by Sambrook et al. (22). The 0.4-kb promoter DNA region of GRE3 was used as the 32P-labeled probe.
Preparation of crude cell extracts for enzyme measurements.
Yeast cells were grown at 30°C in a YNB medium containing the required amino acids, 20 g of glucose/liter, and 50 g of xylose/liter. The cells were harvested in the stationary phase by centrifugation at 3,000 × g for 5 min and washed in 0.9% NaCl. The pellet was resuspended in disintegration buffer (100 mM triethanolamine [pH 7], 1.0 mM phenylmethylsulfonyl fluoride in dimethyl sulfoxide) and vortexed twice for 5 min at 4°C with an equal volume of glass beads (0.5 mm in diameter). The disintegrated cell mixture was centrifuged at 5,000 × g for 5 min at 4°C, and the supernatant was stored at −20°C until analyzed for protein concentration and enzyme activities. Protein concentrations were measured according to the method of Bradford (Bio-Rad, Rockford, Ill.) (4) with bovine serum albumin as the standard.
Enzyme assays.
Enzyme activities were measured with a U-2000 model spectrophotometer (Hitachi Ltd., Tokyo, Japan). In all assays, the decrease in NAD(P)H was monitored at 340 nm at 30°C. The activities were expressed in units per milligram of protein; 1 U is equivalent to the amount of enzyme required to reduce 1 μmol of substrate/min.
AR activity was determined as previously described (17) in a total volume of 1 ml.
XI activity was assayed in two steps (5). This assay is different from the one used previously (37), with the following modifications: crude cell extract was mixed in 700 mM xylose, 10 mM MnCl2, and 100 mM triethanolamine in a total volume of 500 μl. XI in the crude extract was allowed to convert xylose to xylulose for 10 min at 60°C. The reaction was stopped with 50% trichloric acid and subsequently neutralized with 2 M Na2CO3. In the second step, the decrease of NADH was measured when xylulose was converted to xylitol by sorbitol dehydrogenase (EC 1.1.1.14) in a mixture of neutralized sample, 10 μM NADH, 0.03 U of sorbitol dehydrogenase (Sigma-Aldrich Sweden AB), and 100 mM triethanolamine at 30°C and pH 7.0. The reaction was started by adding sorbitol dehydrogenase. The final assay volume was 1 ml. Standard curves were obtained with known concentrations of xylulose prepared as previously described (20).
XK activity measurements were based upon the method of Shamanna and Sanderson (25) and modified as described previously (9).
Fermentation conditions.
Fermentation was carried out in 25-ml closed bottles filled with 25 ml of defined mineral medium (34) containing citrate buffer (pH 5.5), amino acids, and xylose or xylose plus glucose. The bottles were plugged with rubber stoppers, and a gas outlet was secured by inserting a cannula. Fermentation was performed at 30°C with agitation by magnetic stirring. The initial cell mass concentration was 10 g (dry weight)/liter. Xylose fermentation was carried out in triplicate, and xylose plus glucose fermentation was carried out in duplicate.
Oxygen-supplemented fermentation was carried out in 1-liter baffled flasks filled with 500 ml of medium (27) containing citrate buffer (pH 5.5), 50 g of xylose/liter, and amino acids. The initial cell mass concentration was 2 g (dry weight)/liter. Fermentation was carried out in duplicate.
Analytical methods.
Concentrations of sugar substrates and fermentation products were determined using high-performance liquid chromatography (Beckman Instruments AB, Bromma, Sweden) with a hydrogen column (Aminex HPX-87H; Bio-Rad, Richmond, Calif.) at 45°C with 5 mM H2SO4 as the mobile phase with a flow rate of 0.6 ml min−1. The compounds were detected with a refractive-index detector (RID 6A; Shimadzu, Kyoto, Japan).
The dry weight of cells was determined by filtering a known volume of culture broth through a predried 0.45-μm-pore-size nitrocellulose filter (Gelman Sciences, Ann Arbor, Mich.). After washing with 3 volumes of double-distilled water (Millipore, Bedford, Mass.) and drying in a microwave oven (Whirlpool, Benton Harbor, Mich.) for 8 min at a level of 120 W, the filter was weighed. The dry weight of the cells was determined in duplicate.
Calculations.
Carbon balances, yields, and product formation were calculated using single carbon unit equivalents (moles of carbon) to permit comparison of hexose and pentose fermentation data (7). The theoretical yield of ethanol from xylose or glucose is 0.67 mol of C from ethanol/mol of C from xylose or glucose, which is equivalent to 0.51 g of ethanol/g of xylose or glucose. Yields of ethanol were calculated for total consumed sugars as well as for xylose. When calculating the ethanol yield from xylose with glucose as the cosubstrate, the theoretical amount of ethanol produced from glucose was subtracted from the total amount of ethanol. The carbon balance was calculated assuming that 1 mmol of C from CO2 was formed for every 2 mmol of C from ethanol and acetic acid.
RESULTS
Construction of Δgre3 strain expressing xylA and XKS1
The gene GRE3 was deleted from S. cerevisiae chromosome VIII. This recombinant S. cerevisiae strain was transformed with plasmids containing the xylA and XKS1 genes encoding XI and XK. The reference strain was transformed with the same plasmids.
The promoter and terminator sequences of the GRE3 gene were amplified by PCR (Fig. 2A). Plasmids pBR322, pGEM-T easy vector, pBluescript SK(−), pUSM1002 to -1005, and pUSM1007 and -1008 (Table 1) were used to generate pUSM1006 (Fig. 2A). pUSM1006 was cut with XbaI to linearize the fragment. The fragment was transformed into S. cerevisiae CEN.PK2-1C (Fig. 2B). Transformants with the plasmid cassette integrated into the promoter region of the GRE3 gene (YUSM1006a) were selected on SC−uracil agar plates. The transformants were then grown in yeast extract-peptone-dextrose medium to allow homologous recombination between terminator regions and loss of the URA3 marker gene and the GRE3 gene. Replica plating with SC plates containing 5-FOA screened for transformants that had lost the URA3 marker was performed. Transformants were generated by recombination in two ways. First, if the promoter regions combined, the original strain was obtained. Second, if the terminator regions combined, the whole plasmid cassette was lost as well as the GRE3 gene (Fig. 2B). Deletion of the AR gene was confirmed by Southern blot analysis (data not shown). Strain CEN.PK2-1C from which the GRE3 gene had been deleted was called YUSM1009a. S. cerevisiae CEN.PK2-1C and YUSM1009a were transformed with the replicative plasmid pBXI (37), resulting in TMB3101 and TMB3102, respectively. These two recombinant S. cerevisiae strains were subsequently transformed with the replicative plasmid pXks (9), resulting in TMB3103 and TMB1004, respectively.
Enzyme activities.
Stationary phase cells were harvested, and the specific AR, XI, and XK activities of CEN.PK2-1C, YUSM1009a, and TMB3101-4 were measured (Table 2). Cells were harvested in the stationary phase to mimic fermentation, which was carried out with nongrowing cells. AR activity was present in CEN.PK2-1C, TMB3101 (reference strain, XI), and TMB3103 (reference strain, XI plus XK), whereas no AR activity was detected when the GRE3 gene was deleted from YUSM1009a (Δgre3), TMB3102 (Δgre3, XI) and TMB3104 (Δgre3, XI plus XK). The XI activity was similar with or without the GRE3 gene. In TMB3101 and TMB3103, the specific XI activities were 0.90 and 0.55 U mg−1, respectively. In the GRE3-deletion strains TMB3102 and TMB3104, the specific XI activities were 1.0 and 0.42 U mg−1, respectively. XK activity was not detected in the reference strain CEN.PK2-1C, YUSM1009a, or TMB3101. In TMB3103 and TMB3104, the specific XK activities were 14 and 11 U mg−1, respectively.
TABLE 2.
Specific AR, XI, and XK activities in strains used in the present investigation
| Strain | Genotypea | AR activity (mU mg−1) (mean ± SD) | XI activity (U mg−1) (mean ± SD) | XK activity (U mg−1) (mean ± SD) |
|---|---|---|---|---|
| CEN.PK2-1C | Ref | 4.5 ± 0.5 | <0.05 | <0.01 |
| TMB3101 | Ref plus xylA | 4.3 ± 0.4 | 0.90 ± 0.16 | <0.01 |
| TMB3103 | Ref plus xylA plus XKS1 | 4.6 ± 0.5 | 0.55 ± 0.22 | 14 ± 1.5 |
| YUSM1009a | Δgre3 | <1 | <0.05 | <0.01 |
| TMB3102 | Δgre3 plus xylA | <1 | 1.01 ± 0.28 | 0.38 ± 0.12 |
| TMB3104 | Δgre3 plus xylA plus XKS1 | <1 | 0.42 ± 0.13 | 11 ± 1.1 |
Ref, reference strain.
Substrate consumption and product formation in xylose fermentation.
Six strains producing different levels of AR, XI, and XK were compared in batch fermentation (Table 3). None of the recombinant strains grew on xylose. Fermentation was performed under anaerobic conditions with 50 g of xylose/liter as the sole carbon source. For all six strains, the rate of xylose consumption was low, about 0.03 to 0.06 mmol of C/g of cells/h. Ethanol was produced only by TMB3102 (Δgre3, XI), in the amount of 11.6 mmol of C with a yield of 0.28 mmol of C from ethanol/mmol of C from consumed xylose. In strains from which the GRE3 gene was deleted, the xylitol production decreased by half. The acetic acid yield was similar in all strains and the glycerol yield increased twofold in Δgre3 strains. Overall carbon balances were calculated, and the consumed carbon was only recovered to 54 to 88%. No other products were detected by high-performance liquid chromatography.
TABLE 3.
Carbon balances after fermentation of 50 g of xylose/litera
| Strain | Amt of C (mmol) (mean ± SD) from:
|
Carbon recovery (%) | Yieldethanol/xylose (mmol of C from ethanol/mmol of C from xylose) (mean ± SD) | |||||
|---|---|---|---|---|---|---|---|---|
| Xylose (substrate) | Ethanol | Xylitol | Glycerol | Acetic acid | CO2b | |||
| CEN.PK2-1C | 36 ± 3 | NDc | 20 ± 1 | 0.62 ± 0.07 | 3.8 ± 0.03 | 1.9 | 75 | 0 |
| TMB3101 | 37 ± 4 | ND | 21 ± 5 | 0.52 ± 0.08 | 3.2 ± 0.01 | 1.6 | 72 | 0 |
| TMB3103 | 31 ± 7 | ND | 16 ± 4 | 0.27 ± 0.05 | 3.6 ± 0.03 | 1.8 | 70 | 0 |
| YUSM1009a | 21 ± 4 | ND | 10 ± 1 | 1.2 ± 0.02 | 3.0 ± 0.04 | 1.5 | 75 | 0 |
| TMB3102 | 42 ± 4 | 11.6 ± 1.1 | 13 ± 1 | 1.6 ± 0.02 | 3.3 ± 0.03 | 7.5 | 88 | 0.28 ± 0.02 |
| TMB3104 | 20 ± 5 | ND | 6.1 ± 1 | 0.68 ± 0.01 | 2.6 ± 0.03 | 1.3 | 54 | 0 |
Values are millimoles of C consumed or produced after 70 h, and they represent average values of fermentation based on triplicate experiments.
1 mmol of C from CO2 is assumed to be formed for every 2 mmol of C from ethanol and acetic acid.
ND, not detected.
Influence of glucose on anaerobic xylose fermentation.
Based on the results obtained from the xylose fermentation, the influence of glucose on xylose consumption and product formation was investigated next. The product formation during anaerobic batch fermentation with 50 g of xylose/liter was compared to that with 20 g of glucose/liter (Table 4). The rate of xylose consumption in these fermentations was higher than in those with xylose as the sole carbon source. This supports previous observations that a cosubstrate increases the uptake rate of xylose (18, 31, 33). The xylose uptake was between 1.5 and 2.8 mmol of C/g of cells/h for CEN.PK2-1C, TMB3101 (reference strain, XI), TMB3103 (reference strain, XI plus XK), and YUSM1009a (Δgre3). In TMB3102 (Δgre3, XI) and TMB3104 (Δgre3, XI plus XK), the xylose uptake increased to 6.4 and 10 mmol of C/g of cells/h, respectively. In Δgre3 strains, xylitol formation decreased two- to threefold. The xylitol yields on consumed xylose were low in TMB3102 and TMB3104: 0.09 and 0.06 mmol of C from xylitol/mmol of C from consumed xylose, respectively. Glycerol formation and acetate formation were similar for all strains except TMB3104, for which formation of both acetate and glycerol was higher. The ethanol yield on xylose was 0.23 mmol of C from ethanol/mmol of C from xylose in TMB3104. When calculating the ethanol yield from xylose, the theoretical amount of ethanol produced from glucose was subtracted from the total amount of ethanol. With this reasoning, ethanol formation from xylose was formed only in TMB3104. In TMB3102 the increased xylose uptake did not significantly decrease the ethanol yield from total sugars or increase the xylitol formed, suggesting that ethanol was also formed from xylose. Overall carbon balances were calculated, and the consumed carbon was recovered to 83 to 98%.
TABLE 4.
Carbon balances after fermentation of 50 g of xylose/liter and 20 g of glucose/litera
| Strain | Amt of C (mmol) (mean ± SD) fromb:
|
Carbon recovery (%) | Yieldethanol/xylose plus glucosec | Yieldethanol/xylosed | Amt of ethanol (g/liter) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Glucose | Xylose | Ethanol | Xylitol | Glycerol | Acetic acid | CO2e | |||||
| CEN.PK2-1C | 833 ± 18 | 46 ± 6 | 412 ± 30 | 46 ± 6 | 71 ± 6 | 20 ± 3 | 216 | 87 | 0.47 ± 0.02 | 0 | 9.5 ± 0.7 |
| TMB3101 | 640 ± 16 | 63 ± 7 | 323 ± 5 | 39 ± 3 | 64 ± 3 | 16 ± 3 | 170 | 87 | 0.46 ± 0.02 | 0 | 7.4 ± 0.1 |
| TMB3103 | 703 ± 14 | 84 ± 4 | 359 ± 11 | 59 ± 3 | 63 ± 3 | 24 ± 5 | 192 | 89 | 0.46 ± 0.03 | 0 | 8.3 ± 0.3 |
| YUSM1009a | 767 ± 17 | 58 ± 8 | 382 ± 5 | 13 ± 2 | 69 ± 2 | 18 ± 2 | 200 | 83 | 0.46 ± 0.02 | 0 | 8.8 ± 0.2 |
| TMB3102 | 700 ± 15 | 192 ± 18 | 402 ± 19 | 17 ± 5 | 56 ± 6 | 41 ± 15 | 221 | 83 | 0.45 ± 0.03 | 0 | 9.2 ± 0.5 |
| TMB3104 | 707 ± 14 | 502 ± 25 | 590 ± 24 | 30 ± 6 | 97 ± 3 | 114 ± 23 | 352 | 98 | 0.49 ± 0.02 | 0.23 ± 0.02 | 11.5 ± 0.6 |
Displayed values are millimoles of C consumed or produced at the maximum ethanol point, and they represent the average values of fermentation based on duplicate experiments.
Glucose and xylose were the substrates in the fermentation. Ethanol, xylitol, glycerol, acetic acid, and CO2 were the products of fermentation.
Results are millimoles of C from ethanol per millimole of C from xylose plus glucose. Results are means ± standard deviations.
Results are millimoles of C from ethanol per millimole of C from consumed xylose. Ethanol yield on xylose was estimated by subtracting the theoretical ethanol amount (0.67 mmol of C/mmol of C from glucose consumed) formed from glucose from the total amount of ethanol and dividing by the amount of consumed xylose. Results are means ± standard deviations.
1 mmol of C from CO2 is assumed to be formed for every 2 mmol of C from ethanol and acetic acid.
Influence of oxygen on xylose fermentation.
Oxygenation of xylose fermentation did not improve xylose consumption, and ethanol formation was not detected. After 70 h of fermentation, 4.9 ± 1.0 mmol of C from xylitol was produced by CEN.PK2-1C, TMB3101 (reference strain, XI), and TMB3103 (reference strain, XI plus XK), and 2.3 ± 0.2 mmol of C from xylitol was produced by YUSM1009a (Δgre3), TMB3102 (Δgre3, XI), and TMB3104 (Δgre3, XI plus XK). Small amounts of other products were also formed, about 0.9 mmol of C from glycerol for all six strains and 1.3 mmol of C from acetate for CEN.PK2-1C, TMB3101, and TMB3103. No acetate was detected in the Δgre3 strains.
DISCUSSION
Deletion of the GRE3 gene in S. cerevisiae decreased xylitol formation two- to threefold but not completely. During xylose fermentation, xylitol may also be formed from xylulose by an endogenous XDH (21). The equilibrium of this reaction favors xylitol formation. Xylitol could also be formed by putative ARs that have been identified in S. cerevisiae (11), although no AR activity was detected in the Δgre3 strains in this study.
So far only XI from T. thermophilus has been actively produced in S. cerevisiae (37). Ethanol formation from xylose was shown by this recombinant S. cerevisiae, whereas ethanol formation was not detected when TMB3101 (reference strain, XI) assimilated xylose. The difference is attributed to different fermentation temperatures (30°C compared to 38°C), host strains, and fermentation media (defined medium compared to SC medium). The XI activity is about twice as high at 38°C than at 30°C (37). S. cerevisiae H158 was used as the host strain in the previous study for heterologous XI production (37). This strain forms ethanol with a higher yield from xylose than the currently used CEN.PK strain when transformed with genes for xylose-metabolizing enzymes (16).
Xylitol inhibits the enzymatic isomerization of xylose to xylulose by XI (39), and less xylitol was produced in TMB3102 than in TMB3101 (reference strain, XI) (Fig. 1). Ethanol formation from xylose in Δgre3 strains TMB3102 (in xylose media) and TMB3104 (in xylose plus glucose media) was also favored by a reduced loss of carbon from xylitol, so the carbon flow was redirected towards the pentose phosphate pathway and the central metabolism.
TMB3104 produced no ethanol in xylose media. In this strain XK was overproduced. Metabolic modeling has suggested that a constitutively overproduced kinase in the beginning of a metabolic pathway depletes the intracellular ATP pool and causes intracellular accumulation of sugar phosphates (30). However, intracellular concentrations of ATP, xylulose, and xylulose-5-phosphate in strains overproducing XK did not differ from those in strains not overproducing XK (data not shown). On the contrary, reduced ATP levels were observed in a recombinant S. cerevisiae producing XR and XDH from P. stipitis and endogenous XK (32). Thus, an optimal level of XK seems to be important for efficient xylose fermentation.
ACKNOWLEDGMENTS
The Swedish Foundation for International Cooperation in Research (STINT) and Energimyndigheten (The Swedish National Energy Administration) financially supported this work.
Fredrik Levander is gratefully acknowledged for help with the high-performance anion-exchange chromatography system.
REFERENCES
- 1.Amore R, Wilhelm M, Hollenberg C P. The fermentation of xylose—an analysis of the expression of Bacillus and Actinoplanes xylose isomerase in yeast. Appl Microbiol Biotechnol. 1989;30:351–357. [Google Scholar]
- 2.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1995. [Google Scholar]
- 3.Bolivar F, Rodriguez R L, Greene P J, Betlach M C, Heyneker H L, Boyer H W. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2:95–113. [PubMed] [Google Scholar]
- 4.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 5.Callens M, Kersters-Hilderson H, van Opstal O, de Bruyne C K. Catalytic properties of D-xylose isomerase from Streptomyces violaceoruber. Enzyme Microb Technol. 1986;8:696–700. [Google Scholar]
- 6.Chan E, Ueng P, Chen L. D-xylose fermentation to ethanol by Schizosaccharomyces pombe cloned with xylose isomerase gene. Biotechnol Lett. 1986;8:231–234. [Google Scholar]
- 7.de Jong-Gubbels P, Vanrolleghem P, Heijnen S, van Dijken J P, Pronk J T. Regulation of carbon metabolism in chemostat cultures of Saccharomyces cerevisiae grown on mixtures of glucose and ethanol. Yeast. 1995;11:407–418. doi: 10.1002/yea.320110503. [DOI] [PubMed] [Google Scholar]
- 8.du Preez J C, Bosch M, Prior B A. Temperature profiles of growth and ethanol tolerance of the xylose fermenting yeasts Candida shehatae and Pichia stipitis. Appl Microbiol Biotechnol. 1987;25:521–525. [Google Scholar]
- 9.Eliasson A, Christensson C, Wahlbom C F, Hahn-Hägerdal B. Anaerobic xylose fermentation by recombinant Saccharomyces cerevisiae carrying XYL1, XYL2 and XKS1 in mineral medium chemostat cultures. Appl Environ Microbiol. 2000;66:3381–3386. doi: 10.1128/aem.66.8.3381-3386.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Entian K D, Kötter P, editors. Yeast gene analysis. Vol. 26. San Diego, Calif: Academic Press, Inc.; 1998. [Google Scholar]
- 11.Garay-Arroyo A, Covarrubias A A. Three genes whose expression is induced by stress in Saccharomyces cerevisiae. Yeast. 1999;15:879–892. doi: 10.1002/(SICI)1097-0061(199907)15:10A<879::AID-YEA428>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 12.Gietz R D, Schiestl R H, Willems A R, Woods R A. Studies on the transformation of intact yeast cells by the LiAc/SS-DNA/PEG procedure. Yeast. 1995;11:355–360. doi: 10.1002/yea.320110408. [DOI] [PubMed] [Google Scholar]
- 13.Hayn M, Steiner W, Klinger R, Steinmuller H, Sinner M, Esterbauer H. Basic research and pilot studies on the enzymatic conversion of lignocellulosics. In: Saddler J N, editor. Bioconversion of forest and agricultural plant residues. Wallington, United Kingdom: CAB International; 1993. pp. 33–72. [Google Scholar]
- 14.Ho N W Y, Chen Z, Brainard A P. Genetically engineered Saccharomyces yeast capable of effective cofermentation of glucose and xylose. Appl Environ Microbiol. 1998;64:1852–1859. doi: 10.1128/aem.64.5.1852-1859.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ho N W Y, Stevis P, Rosenfeld S, Tsao G T. Expression of the E. coli xylose isomerase gene by a yeast promoter. Biotechnol Bioeng Symp. 1983;13:245–250. [Google Scholar]
- 16.Johansson B, Christensson C, Hobley T, Hahn-Hägerdal B. Xylulokinase overexpression in two strains of Saccharomyces cerevisiae also expressing xylose reductase and xylitol dehydrogenase and its effect on fermentation of xylose and lignocellulosic hydrolysate. Appl Environ Microbiol. 2001;67:4249–4255. doi: 10.1128/AEM.67.9.4249-4255.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuhn A, van Zyl C, van Tonder A, Prior B A. Purification and partial characterization of an aldo-keto reductase from Saccharomyces cerevisiae. Appl Environ Microbiol. 1995;61:1580–1585. doi: 10.1128/aem.61.4.1580-1585.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meinander N Q, Hahn-Hägerdal B. Influence of cosubstrate concentration on xylose conversion by recombinant, XYL1-expressing Saccharomyces cerevisiae: a comparison of different sugars and ethanol as cosubstrates. Appl Environ Microbiol. 1997;63:1959–1964. doi: 10.1128/aem.63.5.1959-1964.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moes C J, Pretorius I S, van Zyl W H. Cloning and expression of the Clostridium thermosulfurogenes D-xylose isomerase gene (xylA) in Saccharomyces cerevisiae. Biotechnol Lett. 1996;18:269–274. [Google Scholar]
- 20.Olsson L, Lindén T, Hahn-Hägerdal B. A rapid chromatographic method for the production of preparative amounts of xylulose. Enzyme Microb Technol. 1994;16:388–394. [Google Scholar]
- 21.Richard P, Toivari M H, Penttilä M. Evidence that the gene YLR070c of Saccharomyces cerevisiae encodes a xylitol dehydrogenase. FEBS Lett. 1999;457:135–138. doi: 10.1016/s0014-5793(99)01016-9. [DOI] [PubMed] [Google Scholar]
- 22.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 23.Sarthy A V, McConaughy B L, Lobo Z, Sundstrom J A, Furlong C E, Hall B D. Expression of the Escherichia coli xylose isomerase gene in Saccharomyces cerevisiae. Appl Environ Microbiol. 1987;53:1996–2000. doi: 10.1128/aem.53.9.1996-2000.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schrunder J, Gunge N. Extranuclear expression of the bacterial xylose isomerase (xylA) and the UDP-glucose dehydrogenase (hasB) genes in yeast with Kluyveromyces lactis linear killer plasmids as vectors. Curr Microbiol. 1996;33:323–330. doi: 10.1007/s002849900122. [DOI] [PubMed] [Google Scholar]
- 25.Shamanna D K, Sanderson K E. Uptake and catabolism of d-xylose in Salmonella typhimurium LT2. J Bacteriol. 1979;139:64–70. doi: 10.1128/jb.139.1.64-70.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sherman F, Fink G, Hicks J B. Methods in yeast genetics: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1983. [Google Scholar]
- 27.Skoog K, Hahn-Hägerdal B. Intermediary metabolite concentrations in xylose fermenting Candida tropicalis at varying oxygen limitations. Biotechnol Tech. 1989;3:1–6. [Google Scholar]
- 28.Skoog K, Hahn-Hägerdal B. Effect of oxygenation on xylose fermentation by Pichia stipitis. Appl Environ Microbiol. 1990;56:3389–3394. doi: 10.1128/aem.56.11.3389-3394.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Southern E M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975;98:503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- 30.Teusink B, Walsh M C, van Dam K, Westerhoff H V. The danger of metabolic pathways with turbo design. Trends Biochem Sci. 1998;23:162–169. doi: 10.1016/s0968-0004(98)01205-5. [DOI] [PubMed] [Google Scholar]
- 31.Thestrup H N, Hahn-Hägerdal B. Xylitol formation and reduction equivalent generation during anaerobic xylose conversion with glucose as cosubstrate in recombinant Saccharomyces cerevisiae expressing the xyl1 gene. Appl Environ Microbiol. 1995;61:2043–2045. doi: 10.1128/aem.61.5.2043-2045.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Toivari M H, Aristidou A, Ruohonen L, Penttilä M. Conversion of xylose to ethanol by recombinant Saccharomyces cerevisiae: importance of xylulokinase (XKS1) and oxygen availability. Metab Eng. 2001;3:236–249. doi: 10.1006/mben.2000.0191. [DOI] [PubMed] [Google Scholar]
- 33.van Zyl W H, Eliasson A, Hobley T, Hahn-Hägerdal B. Xylose utilisation by recombinant strains of Saccharomyces cerevisiae on different carbon sources. Appl Microbiol Biotechnol. 1999;52:829–833. doi: 10.1007/s002530051599. [DOI] [PubMed] [Google Scholar]
- 34.Verduyn C, Postma E, Scheffers W A, Van Dijken J P. Effect of benzoic acid on metabolic fluxes in yeasts: a continuous- culture study on the regulation of respiration and alcoholic fermentation. Yeast. 1992;8:501–517. doi: 10.1002/yea.320080703. [DOI] [PubMed] [Google Scholar]
- 35.von Sivers M, Zacchi G. A techno-economical comparison of three processes for the production of ethanol from pine. Bioresour Technol. 1995;51:43–52. [Google Scholar]
- 36.Wahlbom C F, Eliasson A, Hahn-Hägerdal B. Intracellular fluxes in a recombinant xylose-utilizing Saccharomyces cerevisiae cultivated anaerobically at different dilution rates and feed concentrations. Biotechnol Bioeng. 2001;72:289–296. doi: 10.1002/1097-0290(20010205)72:3<289::aid-bit5>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 37.Walfridsson M, Bao X, Anderlund M, Lilius G, Bülow L, Hahn-Hägerdal B. Ethanolic fermentation of xylose with Saccharomyces cerevisiae harboring the Thermus thermophilus xylA gene, which expresses an active xylose (glucose) isomerase. Appl Environ Microbiol. 1996;62:4648–4651. doi: 10.1128/aem.62.12.4648-4651.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Winston F, Chaleff D T, Valent B, Fink G R. Mutations affecting Ty-mediated expression of the HIS4 gene of Saccharomyces cerevisiae. Genetics. 1984;107:179–197. doi: 10.1093/genetics/107.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamanaka K. Inhibition of D-xylose isomerase by pentitols and D-lyxose. Arch Biochem Biophys. 1969;131:502–506. doi: 10.1016/0003-9861(69)90422-6. [DOI] [PubMed] [Google Scholar]
- 40.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]