Abstract
Estrogen regulation of gonadotropin-releasing hormone (GnRH) neuronal activity plays a crucial role in homeostatic regulation of the hypothalamic-pituitary-gonadal axis. Estrogen also coordinates a complex series of physiological changes culminating with a surge of gonadotropin secretion that triggers ovulation of a developed follicle from the ovary. The coordinated functions of estrogen ensure that the female will elaborate appropriate reproductive behaviors ultimately designed to deliver sperm to the oocyte and to provide a receptive uterine environment for the fertilized embryo. Although the effects of estrogen on GnRH neuronal function have long been proposed to be indirect due to the presumed lack of estrogen receptors in GnRH neurons, the identification of alternative estrogen signaling pathways, including estrogen receptor (ER)β and membrane ERs such as GPR30, has put the focus back on estrogen’s effect at the level of the GnRH neuron itself. One candidate to mediate the effects of estrogen is the β isoform of the estrogen receptor. We review the evidence for a role for ERβ-mediated regulation of GnRH neuronal function.
Keywords: GnRH, estrogen receptor-β, HPG axis
Organization of the Hypothalamic-Pituitary-Gonadal Axis
Reproductive function is regulated by the secretions of gonadotrophs from the pituitary and the steroid hormones from the gonads, which regulate secondary sex traits, cellular function, and behavior. Hypothalamic gonadotropin-releasing hormone (GnRH) neurons secrete the GnRH decapeptide to the surface of the median eminence, which then travels via the portal vasculature to the anterior pituitary. There GnRH stimulates the synthesis and secretion of the pituitary gonadotropins, luteinizing hormone (LH) and follicle-stimulating hormone (FSH), from the gonadotrophs of the anterior pituitary. Circulating LH and FSH, in turn, stimulate the development and maturation of the gonads and direct the synthesis and secretion of the gonadal steroid hormones; testosterone from testes and estradiol (E2) and progesterone from ovaries. The linear organization of the axis allows for complex regulatory events to be mediated at the level of the hypothalamus, the pituitary, or the gonads themselves.
GnRH neurons, with cell bodies located in the basal hypothalamus, represent the final common pathway for neuronally derived signals to the pituitary. As such, they serve as integrators of a myriad of signals, including sensory inputs mediating information about circadian, seasonal, behavioral, pheromonal, and emotional cues. Additionally, information about peripheral physiological function may also be included in the integrative signal to the GnRH neuron. These signals may communicate information about metabolic status, disease, or infection.
Gonadal steroid hormones also arguably exert the most important effects on GnRH neuronal function. In both males and females, the gonadal steroid hormones exert negative feedback regulation on axis activity at both the level of the pituitary and the hypothalamus. These negative feedback loops regulate homeostasis of steroid hormone levels. At the level of the GnRH neuron, estrogen-negative feedback has been shown to reduce GnRH pulse frequency and amplitude in rats,1 sheep,2 and primates.3 Interestingly, recent work in the mouse has demonstrated that high sensitivity to negative feedback contributes to restrained axis activity during the prepubertal period and supports a model in which a reduction in sensitivity to estrogen-negative feedback contributes to the activation of the GnRH neuron during puberty.4 However, these effects were shown to be afferent to the GnRH neuron, at the level of the kisspeptin (kiss1) neuron, and mediated by the estrogen receptor (ER)α isoform.
In females, a cyclic reversal of estrogen feedback produces a positive feedback loop at both the hypothalamic and pituitary levels. Central positive feedback results in a dramatic increase in GnRH secretion.5–8 This is coupled with an increase in pituitary sensitivity to GnRH,9,10 which produces the massive surge in secretion of LH that triggers ovulation.
Kiss1 neurons have emerged as an important locus for steroid feedback effects. There are two predominant populations of kiss1 neurons in the rodent brain: one located in the periventricular preoptic area (also called the anteroventral periventricular nucleus [AVPV]), the other in the arcuate nucleus (Arc).11–13 AVPV kiss1 is upregulated in females under conditions of elevated estrogen levels and suppressed by removal of estrogen, thereby providing a neural circuitry potentially controlling surge generation by E2 (Fig. 1A). In contrast, removal of E2 by gonadectomy upregulates kiss1 expression in the arcuate nucleus and replacement with exogenous E2 downregulates kiss1 expression providing a neuronal circuit that could regulate estrogen-negative feedback (Fig. 1A). Both kiss1 neuron populations possess ERα and require the receptor for regulation of gene expression.14 Strong evidence for an essential role for ERα in the elaboration of both positive and negative feedback comes from studies of mice with a neuron-specific conditional knockout of the ERα gene that showed a loss of both positive and negative steroid feedback.15 In primates, the infundibular nucleus (analogous to the Arc) is thought to contain most kiss1 neurons and may mediate both positive and negative feedback possibly through separate populations of kiss1 neurons within this nucleus.16,17 Although recent work in the rhesus monkey suggests that Arc kiss1 neurons can be upregulated during the late follicular phase when estrogen levels are high, a population of kiss1 neurons in the preoptic area (POA) are also upregulated at this time.18 This is analogous to what is observed in the sheep, where kiss1 in the rostral portion of the Arc is down-regulated by E2 while kiss1 in the caudal portion of the Arc is upregulated at the same time that kiss1 neurons in the POA are robustly upregulated19 (Fig. 1B). The relative contribution of Arc and POA kiss1 signaling to the generation of the preovulatory surge is not yet known.
Figure 1.
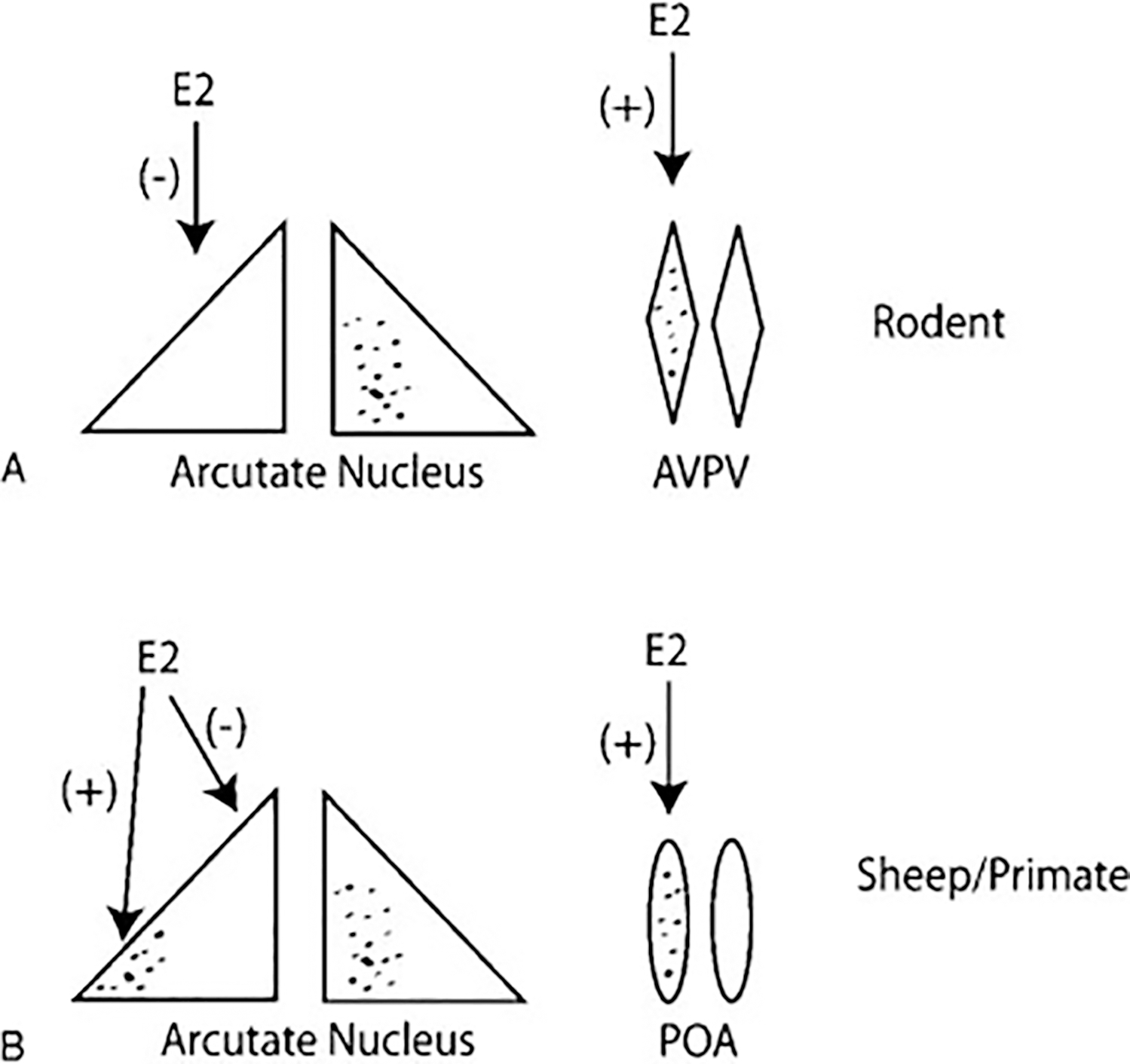
(A) Model of estradiol (E2) regulation of different kiss1 neuronal populations in rodents. Shaded regions represent neurons expressing kiss1. E2 negatively regulates arcuate kiss1 expression and positively regulates anteroventral periventricular nucleus (AVPV) kiss1 expression. (B) Model of E2 regulation of different kiss1 neuronal populations in sheep or primates. E2 negatively regulates kiss1 expression in the rostral arcuate but positively regulates kiss1 in the caudal arcuate. E2 also positively regulates kiss1 expression in the preoptic area (POA).
Besides an essential role in mediating estrogen-positive and -negative feedback, kiss1 had also been proposed to mediate metabolic and stress signals to the GnRH neurons20,21 and to play an essential role in mediating pubertal onset.22,23 However, the role of the kiss1 system in some of these processes has come into question. Recent reports have indicated that there may be kiss1-independent activation of GnRH neuronal activity at puberty24 and beyond.25 Additionally, estrogen-induced LH surges, albeit blunted, were able to be elicited in kiss1 receptor knockout (KO) mice,13 although this finding has been disputed by others.26 Although alternative afferent systems could act in parallel with the kisspeptinergic system to mediate estrogen induction of the LH surge, a role for estrogen signaling directly at the level of the GnRH neuron via estrogen receptors (i.e., ERα, ERβ, GPR30) could also contribute to the physiological responses of the GnRH neuron to E2.
Estrogen Receptors
ERα is a member of the nuclear hormone family of receptors that share five evolutionarily conserved structural and functional domains: an N-terminal domain (A/B), a DNA binding domain (DBD), a hinge region, a ligand-binding domain (LBD), and a C terminal region (F).27 In 1996, a second ER isoform was identified, ERβ,28,29 that contains significant homology to ERα in the DBD (96%) and the LBD (58%) but little homology in the remaining domains.29 ERβ is coded by eight exons and located on chromosome 14 in humans and chromosome12 in mouse. It emerged from a duplication event along with ERα >450 million years ago.30 Several ER splice variants have been identified in mouse and human (Fig. 2). In humans, two protein encoding isoforms of ERβ exist: hERβ1 and hERβ2, the latter an alternatively spliced species in which an alternative exon 8 is included producing a protein shorter than hERβ1 by 35 amino acids. Only hERβ1 exhibits estrogen-binding capacity.31 In mice, Chung et al32 identified a splice variant of ERβ called mERβ2 that could serve as a dominant negative partner when heterodimerized with mERβ1 or mERα; mERβ2 has an insertion of 18 amino acids in the LBD that results in reduced ligand binding affinity. Note that the hERβ2 and the mERβ2 are not named as such because of structural similarities but rather because of the order of discovery. To avoid this confusion, some investigators refer to hERβ2 as hERβcx (cx refers to the alternatively spliced exon 8) and mERβ2 as mERβins.33 mERβ2 had been shown to bind to E2 with lower affinity and to exhibit dominant negative function when heterodimerized with mERα or mERβ and to possess transactivating capacity at high levels of E2. However, mERβ1 and mERα are the primary estrogen-responsive members of the mouse ER family and exhibit overlapping but distinct expression patterns. Both ERα and ERβ are expressed in the hypothalamus where GnRH neurons are located.34
Figure 2.
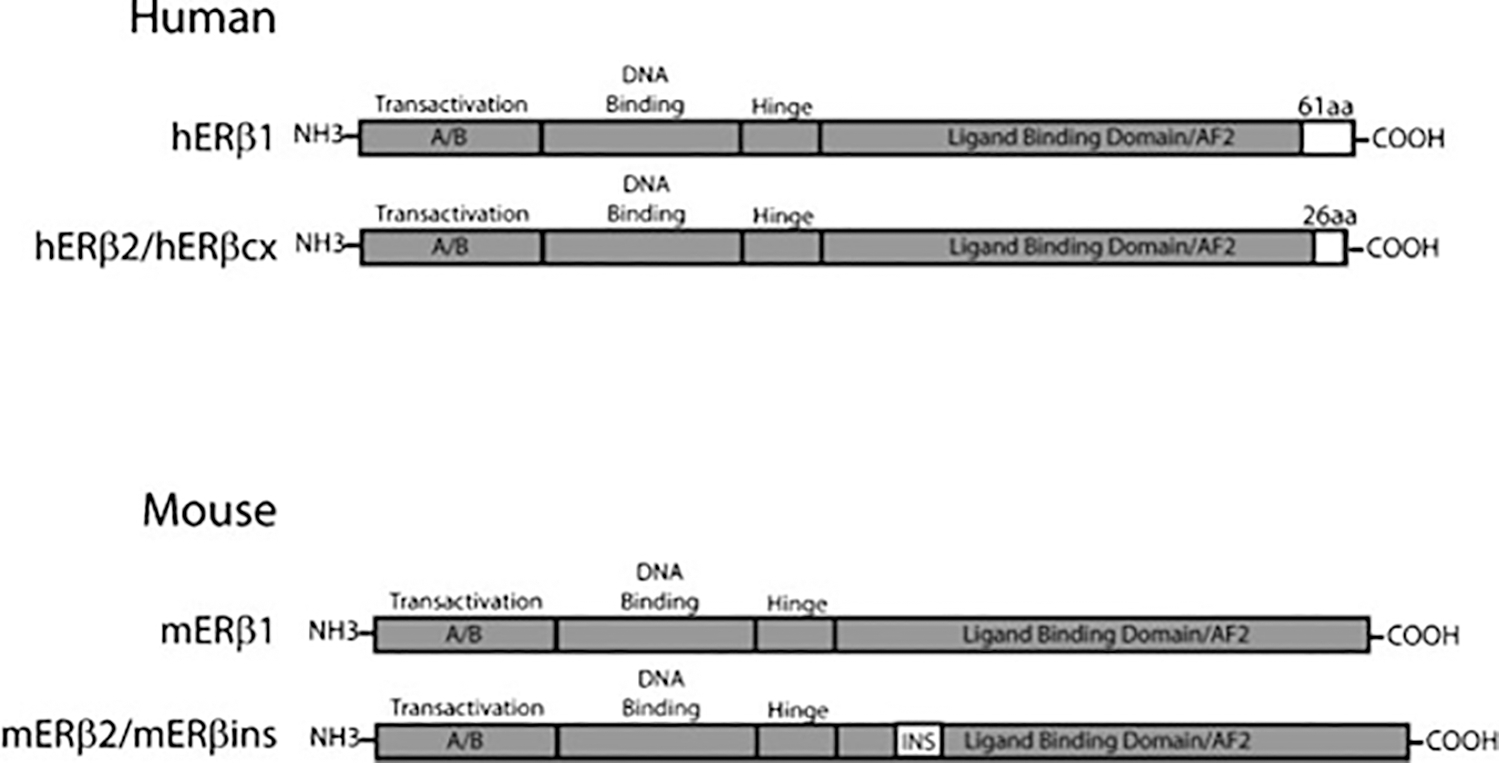
Human and mouse estrogen receptor (ER)β splice variants. Human (h)ERβ1 is encoded by eight exons; the splice variant hERβ2 is encoded by the alternative cx exon. The cx exon produces a truncated AF2 domain (26 amino acids versus 61 amino acids). The full-length mERβ1 is also encoded by eight exons, and mERβ2 is produced by splice insertion of an 18aa fragment in the ligand binding domain (INS).
Estrogen binding to ER results in disassociation from heat shock proteins and recruitment of coregulator proteins. Subsequent binding to estrogen response elements (EREs) on gene promoters results in regulated gene transcription. E2 is known to regulate gene transcription by binding to high-affinity receptor dimers and by facilitating dimer formation between ERs.35 In fact, ERα and ERβ can form heterodimers in tissues where they are coexpressed36,37 and the DBD was sufficient to allow for heterodimerization.37 The hsp90 chaperone was also found to be required for the ERα and ERβ heterodimer to form.36
Agonists for the two ER isoforms have been developed; propyl pyrazole triol (PPT) is a specific agonist for the ERα receptor and binds with a 410-fold higher affinity to ERα than ERβ,38 whereas diarylpropionitrile (DPN) is a specific agonist for the ERβ receptor that binds with 70-fold higher affinity and is 170-fold more potent in transcriptional assays with ERβ than with ERα.39 These receptor specific agonists have been used to tease apart the relative contribution of each receptor isoform to cellular or physiological function and have specifically been used to address these questions regarding the regulation of hypothalamic-pituitary-gonadal (HPG) axis function.40–42
ER has also been shown to transregulate genes in the absence of classic EREs in the promoter. ERs can interact with other transcription factors such as AP-1 or SP-1 at response elements specific for those transcription factors. This mechanism allows for signaling cross-talk between the reproductive hormone axis and growth factor signaling pathways (reviewed in Heldring et al43). Interestingly, it has been proposed that ERα and ERβ may exert opposite effects on promoter activity via this mechanism, with ERα activating gene expression and ERβ repressing gene expression.44,45
Studies from the Catt laboratory have suggested that ERα and ERβ located in the plasma membrane can interact with Gi and GS subunits, respectively, to regulate adenylyl cyclase activity.46 These results were observed in both cultured hypothalamic GnRH neurons and in GT1–7 cells (described later) and represent an alternative estrogen signaling pathway that nevertheless includes the ERα and ERβ proteins.
A membrane estrogen receptor that is distinct from either ERα or ERβ was recently identified. The GPR30 orphan receptor, a member of the G-protein coupled family of receptors, has been identified as a putative estrogen receptor. GPR30 located in the endoplasmic reticulum, Golgi apparatus, and nuclear membranes has been shown to be activated by E2 and other estrogen agonists (reviewed in Prossnitz et al47). GPR30 has been found in primate and mouse GnRH neurons48,49 and is proposed to be located at the plasma membrane as membrane-impermeable bovine serum albumin–conjugated E2 can exert rapid effects on GnRH function.48
Studies of the hypothalamic-pituitary axis in ERα KO mice provide evidence that genetic disruption of ERα impairs feedback regulation by E2 both at the level of the hypothalamus and the pituitary.50,51 The presence of ERs in the gonadotroph is well accepted, and it is thought that E2 action on the gonadotroph is direct. Studies in a pituitary ERα conditional KO mouse have shown that both positive as well as negative E2 feedback was disrupted.52 In contrast, the role of ERβ in the HPG axis, and specifically in the GnRH neurons, is less clear and the focus of this review.
Anatomical Evidence that Estrogen Receptor-β Is expressed in Gonadotropin-Releasing Hormone Neurons
Initial studies exploring the ERβ KO mice failed to identify a significant reproductive phenotype. However, it was noted later that ER KO mice produced by insertion of an ER allele containing a neomycin resistance gene could produce a chimeric protein that is partially estrogen responsive53–55 Complete ERβ KO mice demonstrate subfertility,55–57 although gonadotropin levels appear to be unchanged compared with control mice. These data suggest that ERβ may not be important for central E2 negative feedback of the axis. However, gonadotropin levels are less elevated in ERα KO versus combined ERαβ KO mice, indicating that ERβ may have a negative feedback role in the axis.58
Although E2 action on the pituitary is direct, the mechanism by which E2 regulates GnRH neurons is not well understood. The prevailing doctrine is that E2 action on GnRH neurons is indirect and conveyed through presynaptic afferents from kiss1 neurons that express ER (as discussed earlier). An accumulating body of in vitro and in vivo evidence, however, documents the presence of functional ERs in GnRH neurons. Although some authors have found ERα transcripts in GnRH neurons in the preoptic area,59,60 the predominant ER found in GnRH neurons is believed to be ERβ. The first report of colocalization came in 2000 in rats using in situ hybridization to localize ERβ mRNA and immunohistochemistry for GnRH.61 This study also used in vivo binding studies to localize estrogen binding sites to the GnRH neuron. Using a sensitive ERβ antibody, this same group was able to demonstrate that ~80% of GnRH neurons were colocalized with ERβ immunoreactivity.62 Nearly the same percentage of GnRH neurons were found to colocalize ERβ by Legan and Tsai,63 who further noted that the proportion did not change after short- or long-term E2 administration. A slightly lower percentage (52 to 63%) of GnRH neurons were found to contain ERβ in a gonadectomized rat model,64 and in contrast to the study by Legan and Tsai, Kalló et al observed a significant decline in ERβ protein in GnRH neurons in ovariectomized (OVX) rats in response to treatment with E2.64 ERβ protein has also been shown to be located in GnRH neurons in sheep65 and humans.66
Although the role of the splice variants of the ER has been frequently studied in cancer biology,67–69 recent analysis of the mERβ2 isoform suggests a role in GnRH neurons. mERβ2 has been shown to bind to E2 with lower affinity than to ERα or mERβ1, and to exhibit dominant negative function when heterodimerized with mERα or mERβ1 or to possess transactivating capacity at high levels of E2. Because mERβ2 was found to be expressed in the GnRH neurons along with mERβ1, it was proposed to mediate estrogen effects at high circulating E2 levels such as seen preceding the preovulatory LH surge.32
The study of GnRH neurons has been greatly aided by the development of GnRH-expressing cell lines such as the GT1–7,70 GN11,71,72 and SN5673 lines. In addition to their histological analysis of the brain, Kalló et al also explored ER expression in the GT1–7 cell line and found that they expressed ERβ,64 which several other laboratories confirmed.46,74–76 Studies in SN56 cells, another cell model of the GnRH neuron, have also identified ERβ expression,73,77 which suggests that mouse GnRH neurons express ERβ as well. This is supported by several electrophysiological studies described later and studies using the isoform-specific agonists PPT and DPN in GN11 and GT1–7 cells.75
Studies by Sharifi et al used GnRH neurons obtained from 11.5-day mouse embryos and maintained in culture for up to 28 days to determined ER expression patterns.78 They found mERβ but not mERα to be expressed in the cells at all time points studied; however, the number of cells expressing ERβ decreased over time. Adding to the controversy, a study by Hu et al showed that rat fetal and adult GnRH neurons express both mERα and mERβ, and GT1–7 cells also express both forms of the ER.79 Interestingly, this study showed that expression of ER varies with the estrous cycle and that high E2 levels, both in vivo during proestrus and in vitro are associated with decreasing ER expression, which correlates with results obtained by Ng et al.75
It is not clear why there are such discrepancies in results obtained from different laboratories. In vivo studies could differ based on different developmental stages assessed, species differences, or technical variability. Differing results obtained in GT1–7 cells could reflect variations in culture conditions or technical considerations.
Estrogen Receptor-β Regulation of Channel Properties
Much of the work looking at the role of ERβ on the biophysical properties of the GnRH neuron has been performed in slice studies using the GnRH-GFP mouse.80,81 This model has the advantages of the GnRH neurons maintaining their intercellular connections with many regulatory afferent neurons. Neuronal function can be assessed following physiological manipulations in the intact animal or following acute application of drugs or hormones directly to the cultured GnRH neurons.
Experiments performed in the Moenter laboratory using whole cell recordings of GnRH neurons from OVX mice found that application of high levels of E2 to GnRH neurons, in which intercellular communication was blocked, increased GnRH neuronal activity in an ERβ-specific manner that depended on protein kinase A. Application of low levels of E2 did not affect intrinsic firing, but if intercellular communication was not blocked, E2 reduced firing of GnRH neurons. This suggested high E2 directly stimulated GnRH neurons and that lower levels of E2 exerted effects via estrogen-responsive interneurons. They further proposed that GABA neurons served as the interneuron mediating the effects of low E2 and that these effects were via ERα. Despite commonly serving as an inhibitory neurotransmitter, gamma-aminobutyric acid (GABA) has been shown to activate GnRH neuronal activity. Corresponding to this modality of response to GABA, they showed that PPT (ERα agonist) inhibited and DPN (ERβ agonist) stimulated GABAergic transmission.82
In a subsequent study, Sun et al49 also explored the circadian effects of E2 on GnRH neurons. Their studies suggest that E2 modulates HVA currents in GnRH neurons in a diurnal manner with activating effects in the evening (when positive feedback occurs) and inactivating effects in the morning (during negative feedback). Acute bath application of E2 rapidly potentiated calcium currents from both morning and evening neurons mediated by both ERβ and by GPR30. This suggests that the circadian differences observed from systemic E2 administration are mediated indirectly. These studies were in line with the results obtained from other laboratories on E2 regulation of firing activity and the frequency of oscillations in intracellular calcium communication.83–86 Yet there is not universal agreement that it is mediated by ERβ, with some proposing that the circadian effects of E2 on GnRH neurons are mediated by ERα.84
Intrinsic regulation by both ERα and ERβ was demonstrated by Hu et al in rat fetal and adult GnRH neurons, as well as in GT1–7 cells. They propose a cell-autonomous unifying model in which low levels of E2 activate the ERα receptor and inhibit action potential frequency and reduces cyclic adenosine monophosphate (cAMP) production. The effects of higher levels of E2 are mediated by ERβ and cause increased action potential firing frequency and accumulation of cAMP.79 By this mechanism, low circulating levels of E2 that in the female mouse would be exerting negative feedback, and high circulating E2 levels such as those that occur preceding the preovulatory GnRH surge, would in part exert its effects directly at the level of the GnRH neuron.
Additional studies in GT1–7 cells also found that E2 induced an ERβ-mediated increase in calcium currents, perhaps by increasing expression of the α- and β4 subunits of large-conductance Ca2+-activated K+(BK) channel subunits.87 GT1–7 cells were also used as a model by Morales et al88 to explore catecholamine sensitivity. They found that norepinephrine signaling was modulated by rapid ERα signaling but used imaging to show ERα and ERβ at the cell membrane.
Estrogen Receptor-β Regulation of Gene Expression in the Gonadotropin-Releasing Hormone Neurons
Classically, steroid receptors exert their effects by serving as ligand-activated transcription factors that interact with response elements in the promoters of genes to modify gene expression. Although E2 has been shown to rapidly regulate the electrical properties of the GnRH neurons, they also have been shown to regulate transcription of genes, including the GnRH gene.
Roy et al74 identified both ERα and ERβ transcript and protein in GT1–7 cells. They found that long-term treatment with E2 (10–48 hours) attenuated GnRH mRNA levels mediated by ERα, although these studies were done in advance of the development of the receptor-specific agonists DPN and PPT. However, this result was confirmed by Ng et al in both GN11 and GT1–7 cells. These studies identified ERβ in both GN11 and GT1–7 cells by polymerase chain reaction but ERα only in GN11 cells. They further assessed the receptor isoform mediating these effects and observed that both DPN and PPT negatively regulated GnRH mRNA levels in GN11 cells but only DPN negatively regulated GnRH mRNA levels in GT1–7 cells (Fig. 3). These results correlate with the ER isoform expression in these cells.
Figure 3.
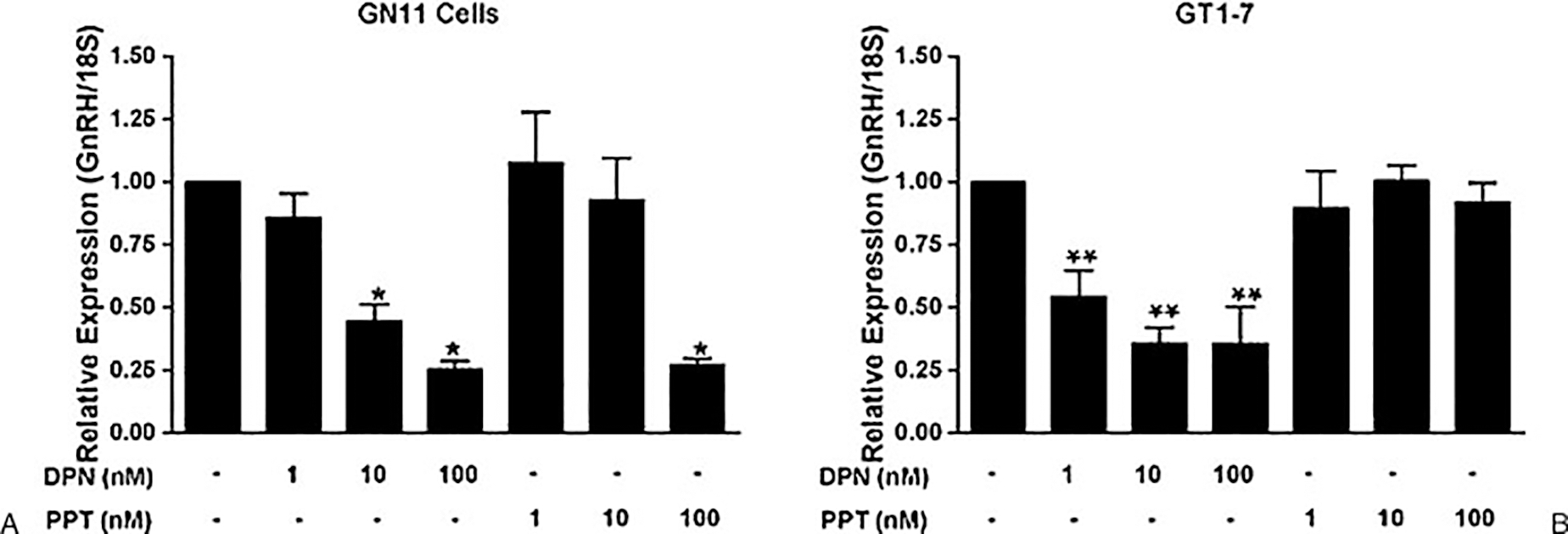
Gonadotropin-releasing hormone (GnRH) gene expression in GN11 and GT1–7 cells treated with estrogen receptor (ER) agonists. (A) GN11 and (B) GT1–7 neuronal cells were treated with 1 nM, 10 nM, or 100 nM of an estrogen receptor (ER)β agonist (diarylpropionitrile [DPN]) or ER-α agonist (propyl pyrazole triol [PPT]) for 16 hours. Total RNA was extracted, and quantitative real-time polymerase chain reaction was performed with primers specifically designed to amplify mGnRH. GN11 data are expressed as the mean plus or minus the standard error of mean (SEM) (n = 6). GT1–7 data are expressed as the mean plus or minus SEM (n = 5). *p < 0.001 versus control. **p < 0.05 versus control.
Ng et al also explored estrogen regulation of the gonadal steroid hormone receptors, both ER isoforms and the progesterone receptor (PR).75 These studies demonstrated that E2 negatively regulated expression of ERα and ERβ in GN and GT cells. As they had observed for GnRH gene expression, PPT and DPN both negatively regulated ERα and ERβ in GN11 cells, but only the ERβ-specific ligand (DPN) negatively regulated expression in the GT1–7 cells. Estrogen has been shown to positively regulate PR expression in several tissues including the pituitary and the uterus,41,89 and regulation occurs by interactions with defined promoter regions,90 although defined EREs have not been identified in the human PR gene.91 Therefore, Ng et al explored whether E2 regulated PR expression in GN11 and GT1–7 cells. These studies demonstrated that E2 positively regulated PR expression in both GN11 and GT1–7 cells and suggest that GN11 cells might be a good model to concurrently explore both positive and negative gene regulation by ERα and ERβ.
Pak et al92 used transfection studies in GT1–7 cells to explore the role of mERβ1 and mERβ2 in the transcriptional regulation of the GnRH gene. By cotransfecting ERβ expression vectors with GnRH promoter reporter constructs, they identified a ligand-independent activation of GnRH promoter activity mediated by both mERβ1 and mERβ2. E2 was found to eliminate the effect, demonstrating a potential mechanism for E2-mediated negative regulation of GnRH gene expression. These effects were mapped to the proximal promoter using truncated promoter reporter constructs, and no ERE was identified in the estrogen-responsive region, indicating a novel ERβ ERE is essential for function.
An alternative mechanism for negative regulation by E2 was proposed by Abrahám et al,93 who explored the effects of E2 on cAMP response element binding (CREB) protein phosphorylation in mice. E2 treatment of OVX female mice stimulated CREB phosphorylation at serine 133. These effects were maintained in ERα KO mice but not in ERβ KO mice, indicating an indispensable role for ERβ in this process. They further suggest that CREB activation may be contributing to negative feedback regulation in GnRH neurons. To date, no role has been ascribed to CREB in regulating GnRH neuronal function, although this work was the first to demonstrate an ERβ-mediated effect in GnRH neurons.
Estrogen Receptor-β Regulation of Gonadotropin-Stimulating Hormone Secretion
The effects of ERβ on GnRH secretion has not been directly assessed, although the analysis of GnRH neuronal activity described earlier suggests that the ERβ is mediating positive feedback effects of E2 via increased neuronal activity, which is in contrast to many of the studies exploring the regulation of gene expression where ERβ-mediated negative regulation is frequently observed. To explore the role of ERβ in vivo, mice with a conditional deletion of ERβ in GnRH neurons need to be developed to conclusively understand the role of estrogen signaling at the level of the GnRH neuron via ERβ.
Although results in vivo are lacking, several studies have assessed the effects of E2 on GnRH secretion from GnRH neuronal cell lines. For example, Ng et al75 used a static culture system to evaluate secretion of GnRH in response to E2 treatment and found a significant reduction in both GN11 and GT1–7 cells, although the receptor subtype was not explored. Hu et al also used a perfusion system to measure pulsatile secretion of GnRH from GT1–7 cells and concluded that ERα mediated a decrease in GnRH pulsatile secretion in response to low levels of E2 and that ERβ mediated an increase in pulse amplitude of secretion in response to elevated levels of E2,79 demonstrating that the intrinsic biphasic nature of the response of GnRH neurons is manifest at the level of regulating action potential frequency and the pulsatile secretion of GnRH.
On the whole, in vivo studies suggest that intrinsic E2 signaling is mediated by ERβ, whereas analysis of GnRH expressing cell lines such as the GT1–7 or GN11 cells has often identified signaling via ERα. Perhaps this is a phenotypic difference between transformed cell lines and native GnRH neurons, or it could reflect a developmental expression of ERα that is captured as a “snapshot” of the GnRH neurons when they become transformed.
Conclusion
Estrogen feedback regulation of the GnRH neuron represents a crucial physiological process that underlies pubertal development, homeostasis, and fertility in females. There is convincing evidence that both negative and positive estrogen feedback regulation of GnRH neuronal function are, in part, exerted indirectly via estrogen-responsive afferent neurons, likely via ERα. However, estrogen regulation directly at the level of the GnRH neuron likely plays an important role in regulating neuronal activity, gene expression, and pulsatile secretion of GnRH, and ERβ may contribute in each of these processes (Fig. 4).
Figure 4.
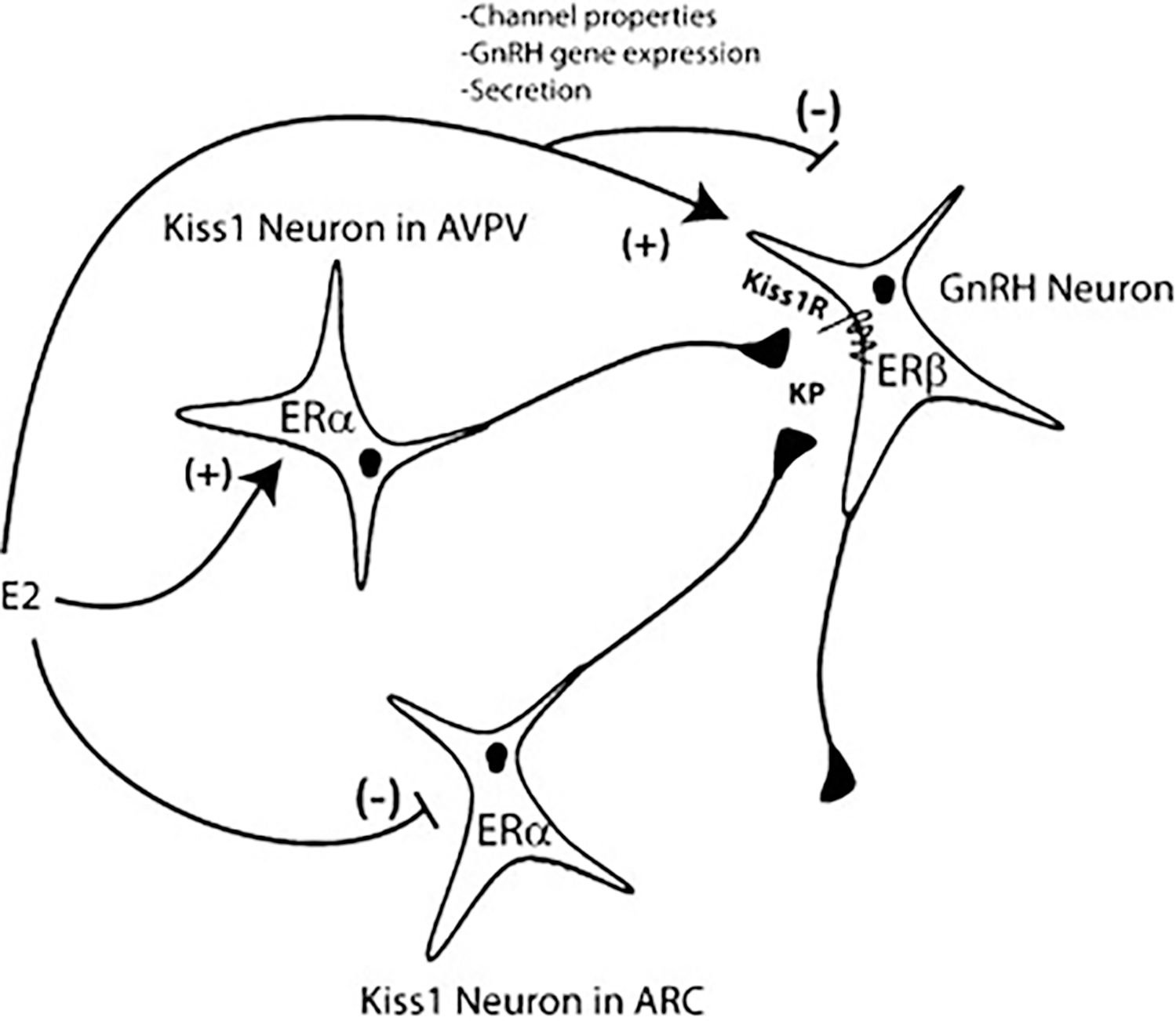
Model for estradiol (E2) regulation of gonadotropin-releasing hormone (GnRH) neuronal function. Kiss1 neurons in the Arc and anteroventral periventricular nucleus (AVPV) nuclei mediate negative and positive feedback regulation of the GnRH neuron, respectively. Estrogen receptor (ER)β expressed in the GnRH neuron allows for cell autonomous E2 regulation. E2 regulates various functional properties in GnRH neurons including biophysical function, GnRH gene expression, and GnRH secretion.
Acknowledgments
The authors are grateful to Dr. Sally Radovick and Dan Diaczok for their critical evaluation of the manuscript. The authors also thank Dr. Gloria Hoffman for assistance with the figures.
References
- 1.Duffy KR, Pardridge WM. Blood-brain barrier transcytosis of insulin in developing rabbits. Brain Res 1987;420(1):32–38 [DOI] [PubMed] [Google Scholar]
- 2.Karsch FJ, Cummins JT, Thomas GB, Clarke IJ. Steroid feedback inhibition of pulsatile secretion of gonadotropin-releasing hormone in the ewe. Biol Reprod 1987;36(5):1207–1218 [DOI] [PubMed] [Google Scholar]
- 3.Plant TM, Dubey AK. Evidence from the rhesus monkey (Macaca mulatta) for the view that negative feedback control of luteinizing hormone secretion by the testis is mediated by a deceleration of hypothalamic gonadotropin-releasing hormone pulse frequency. Endocrinology 1984;115(6):2145–2153 [DOI] [PubMed] [Google Scholar]
- 4.Mayer C, Acosta-Martinez M, Dubois SL, et al. Timing and completion of puberty in female mice depend on estrogen receptor alpha-signaling in kisspeptin neurons. Proc Natl Acad Sci U S A 2010;107 (52):22693–22698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sisk CL, Richardson HN, Chappell PE, Levine JE. In vivo gonadotropin-releasing hormone secretion in female rats during peripubertal development and on proestrus. Endocrinology 2001;142 (7):2929–2936 [DOI] [PubMed] [Google Scholar]
- 6.Clarke IJ. Variable patterns of gonadotropin-releasing hormone secretion during the estrogen-induced luteinizing hormone surge in ovariectomized ewes. Endocrinology 1993;133(4): 1624–1632 [DOI] [PubMed] [Google Scholar]
- 7.Moenter SM, Brand RC, Karsch FJ. Dynamics of gonadotropin-releasing hormone (GnRH) secretion during the GnRH surge: insights into the mechanism of GnRH surge induction. Endocrinology 1992;130(5):2978–2984 [DOI] [PubMed] [Google Scholar]
- 8.Xia L, Van Vugt D, Alston EJ, Luckhaus J, Ferin M. A surge of gonadotropin-releasing hormone accompanies the estradiol-induced gonadotropin surge in the rhesus monkey. Endocrinology 1992;131(6):2812–2820 [DOI] [PubMed] [Google Scholar]
- 9.Turzillo AM, DiGregorio GB, Nett TM. Messenger ribonucleic acid for gonadotropin-releasing hormone receptor and numbers of gonadotropin-releasing hormone receptors in ovariectomized ewes after hypothalamic-pituitary disconnection and treatment with estradiol. J Anim Sci 1995;73(6):1784–1788 [DOI] [PubMed] [Google Scholar]
- 10.Savoy-Moore RT, Schwartz NB, Duncan JA, Marshall JC. Pituitary gonadotropin-releasing hormone receptors during the rat estrous cycle. Science 1980;209(4459):942–944 [DOI] [PubMed] [Google Scholar]
- 11.Kauffman AS, Gottsch ML, Roa J, et al. Sexual differentiation of Kiss1 gene expression in the brain of the rat. Endocrinology 2007;148(4):1774–1783 [DOI] [PubMed] [Google Scholar]
- 12.Smith JT, Clifton DK, Steiner RA. Regulation of the neuroendocrine reproductive axis by kisspeptin-GPR54 signaling. Reproduction 2006;131(4):623–630 [DOI] [PubMed] [Google Scholar]
- 13.Dungan HM, Gottsch ML, Zeng H, et al. The role of kisspeptin-GPR54 signaling in the tonic regulation and surge release of gonadotropin-releasing hormone/luteinizing hormone. J Neurosci 2007;27(44):12088–12095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology 2005;146(9):3686–3692 [DOI] [PubMed] [Google Scholar]
- 15.Wintermantel TM, Campbell RE, Porteous R, et al. Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron 2006;52(2):271–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Plant TM, Ramaswamy S. Kisspeptin and the regulation of the hypothalamic-pituitary-gonadal axis in the rhesus monkey (Macaca mulatta). Peptides 2009;30(1):67–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rometo AM, Krajewski SJ, Voytko ML, Rance NE. Hypertrophy and increased kisspeptin gene expression in the hypothalamic infundibular nucleus of postmenopausal women and ovariectomized monkeys. J Clin Endocrinol Metab 2007;92(7):2744–2750 [DOI] [PubMed] [Google Scholar]
- 18.Smith JT, Shahab M, Pereira A, Pau KY, Clarke IJ. Hypothalamic expression of KISS1 and gonadotropin inhibitory hormone genes during the menstrual cycle of a non-human primate. Biol Reprod 2010;83(4):568–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caraty A, Franceschini I, Hoffman GE. Kisspeptin and the preovulatory gonadotrophin-releasing hormone/luteinising hormone surge in the ewe: basic aspects and potential applications in the control of ovulation. J Neuroendocrinol 2010;22(7):710–715 [DOI] [PubMed] [Google Scholar]
- 20.Castellano JM, Bentsen AH, Mikkelsen JD, Tena-Sempere M. Kisspeptins: bridging energy homeostasis and reproduction. Brain Res 2010;1364;129–138 [DOI] [PubMed] [Google Scholar]
- 21.Castellano JM, Bentsen AH, Romero M, et al. Acute inflammation reduces kisspeptin immunoreactivity at the arcuate nucleus and decreases responsiveness to kisspeptin independently of its anorectic effects. Am J Physiol Endocrinol Metab 2010;299(1): E54–E61 [DOI] [PubMed] [Google Scholar]
- 22.Seminara SB, Messager S, Chatzidaki EE, et al. The GPR54 gene as a regulator of puberty. N Engl J Med 2003;349(17):1614–1627 [DOI] [PubMed] [Google Scholar]
- 23.Shahab M, Mastronardi C, Seminara SB, Crowley WF, Ojeda SR, Plant TM. Increased hypothalamic GPR54 signaling: a potential mechanism for initiation of puberty in primates. Proc Natl Acad Sci U S A 2005;102(6):2129–2134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mayer C, Boehm U. Female reproductive maturation in the absence of kisspeptin/GPR54 signaling. Nat Neurosci 2011;14(6):704–710 [DOI] [PubMed] [Google Scholar]
- 25.Chan YM, Broder-Fingert S, Wong KM, Seminara SB. Kisspeptin/Gpr54-independent gonadotrophin-releasing hormone activity in Kiss1 and Gpr54 mutant mice. J Neuroendocrinol 2009;21 (12):1015–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clarkson J, d’Anglemont de Tassigny X, Moreno AS, Colledge WH, Herbison AE. Kisspeptin-GPR54 signaling is essential for preovulatory gonadotropin-releasing hormone neuron activation and the luteinizing hormone surge. J Neurosci 2008;28(35):8691–8697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mangelsdorf DJ, Thummel C, Beato M, et al. The nuclear receptor superfamily: the second decade. Cell 1995;83(6):835–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci U S A 1996;93(12):5925–5930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mosselman S, Polman J, Dijkema R. ER beta: identification and characterization of a novel human estrogen receptor. FEBS Lett 1996;392(1):49–53 [DOI] [PubMed] [Google Scholar]
- 30.Kelley ST, Thackray VG. Phylogenetic analyses reveal ancient duplication of estrogen receptor isoforms. J Mol Evol 1999;49 (5):609–614 [DOI] [PubMed] [Google Scholar]
- 31.Zhao C, Dahlman-Wright K, Gustafsson JA. Estrogen signaling via estrogen receptor beta. J Biol Chem 2010;285(51):39575–39579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chung WC, Pak TR, Suzuki S, Pouliot WA, Andersen ME, Handa RJ. Detection and localization of an estrogen receptor beta splice variant protein (ERbeta2) in the adult female rat forebrain and midbrain regions. J Comp Neurol 2007;505(3):249–267 [DOI] [PubMed] [Google Scholar]
- 33.Palmieri C, Saji S, Sakaguchi H, et al. The expression of oestrogen receptor (ER)-beta and its variants, but not ERalpha, in adult human mammary fibroblasts. J Mol Endocrinol 2004;33(1):35–50 [DOI] [PubMed] [Google Scholar]
- 34.Kuiper GG, Shughrue PJ, Merchenthaler I, Gustafsson JA. The estrogen receptor beta subtype: a novel mediator of estrogen action in neuroendocrine systems. Front Neuroendocrinol 1998; 19(4):253–286 [DOI] [PubMed] [Google Scholar]
- 35.Katzenellenbogen BS, Bhardwaj B, Fang H, et al. Hormone binding and transcription activation by estrogen receptors: analyses using mammalian and yeast systems. J Steroid Biochem Mol Biol 1993;47(1–6):39–48 [DOI] [PubMed] [Google Scholar]
- 36.Powell E, Wang Y, Shapiro DJ, Xu W. Differential requirements of Hsp90 and DNA for the formation of estrogen receptor homodimers and heterodimers. J Biol Chem 2010;285(21): 16125–16134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pace P, Taylor J, Suntharalingam S, Coombes RC, Ali S. Human estrogen receptor beta binds DNA in a manner similar to and dimerizes with estrogen receptor alpha. J Biol Chem 1997;272 (41):25832–25838 [DOI] [PubMed] [Google Scholar]
- 38.Stauffer SR, Coletta CJ, Tedesco R, et al. Pyrazole ligands: structure-affinity/activity relationships and estrogen receptor-alpha-selective agonists. J Med Chem 2000;43(26):4934–4947 [DOI] [PubMed] [Google Scholar]
- 39.Meyers MJ, Sun J, Carlson KE, Marriner GA, Katzenellenbogen BS, Katzenellenbogen JA. Estrogen receptor-beta potency-selective ligands: structure-activity relationship studies of diarylpropionitriles and their acetylene and polar analogues. J Med Chem 2001;44(24):4230–4251 [DOI] [PubMed] [Google Scholar]
- 40.Arreguin-Arevalo JA, Davis TL, Nett TM. Differential modulation of gonadotropin secretion by selective estrogen receptor 1 and estrogen receptor 2 agonists in ovariectomized ewes. Biol Reprod 2007;77(2):320–328 [DOI] [PubMed] [Google Scholar]
- 41.Sánchez-Criado JE, Martín De Las Mulas J, Bellido C, Tena-Sempere M, Aguilar R, Blanco A. Biological role of pituitary estrogen receptors ERalpha and ERbeta on progesterone receptor expression and action and on gonadotropin and prolactin secretion in the rat. Neuroendocrinology 2004;79(5):247–258 [DOI] [PubMed] [Google Scholar]
- 42.Sánchez-Criado JE, de Las Mulas JM, Bellido C, et al. Gonadotropin-secreting cells in ovariectomized rats treated with different oestrogen receptor ligands: a modulatory role for ERbeta in the gonadotrope? J Endocrinol 2006;188(2):167–177 [DOI] [PubMed] [Google Scholar]
- 43.Heldring N, Pike A, Andersson S, et al. Estrogen receptors: how do they signal and what are their targets. Physiol Rev 2007;87(3): 905–931 [DOI] [PubMed] [Google Scholar]
- 44.Saville B, Wormke M, Wang F, et al. Ligand-, cell-, and estrogen receptor subtype (alpha/beta)-dependent activation at GC-rich (Sp1) promoter elements. J Biol Chem 2000;275(8):5379–5387 [DOI] [PubMed] [Google Scholar]
- 45.Paech K, Webb P, Kuiper GG, et al. Differential ligand activation of estrogen receptors ERalpha and ERbeta at AP1 sites. Science 1997;277(5331):1508–1510 [DOI] [PubMed] [Google Scholar]
- 46.Navarro CE, Abdul Saeed S, Murdock C, et al. Regulation of cyclic adenosine 3′,5′-monophosphate signaling and pulsatile neurosecretion by Gi-coupled plasma membrane estrogen receptors in immortalized gonadotropin-releasing hormone neurons. Mol Endocrinol 2003;17(9):1792–1804 [DOI] [PubMed] [Google Scholar]
- 47.Prossnitz ER, Arterburn JB, Smith HO, Oprea TI, Sklar LA, Hathaway HJ. Estrogen signaling through the transmembrane G protein-coupled receptor GPR30. Annu Rev Physiol 2008;70;165–190 [DOI] [PubMed] [Google Scholar]
- 48.Noel SD, Keen KL, Baumann DI, Filardo EJ, Terasawa E. Involvement of G protein-coupled receptor 30 (GPR30) in rapid action of estrogen in primate LHRH neurons. Mol Endocrinol 2009;23 (3):349–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun J, Chu Z, Moenter SM. Diurnal in vivo and rapid in vitro effects of estradiol on voltage-gated calcium channels in gonadotropin-releasing hormone neurons. J Neurosci 2010;30(11):3912–3923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Scully KM, Gleiberman AS, Lindzey J, Lubahn DB, Korach KS, Rosenfeld MG. Role of estrogen receptor-alpha in the anterior pituitary gland. Mol Endocrinol 1997;11(6):674–681 [DOI] [PubMed] [Google Scholar]
- 51.Lindzey J, Wetsel WC, Couse JF, Stoker T, Cooper R, Korach KS. Effects of castration and chronic steroid treatments on hypothalamic gonadotropin-releasing hormone content and pituitary gonadotropins in male wild-type and estrogen receptor-alpha knockout mice. Endocrinology 1998;139(10):4092–4101 [DOI] [PubMed] [Google Scholar]
- 52.Singh SP, Wolfe A, Ng Y, et al. Impaired estrogen feedback and infertility in female mice with pituitary-specific deletion of estrogen receptor alpha (ESR1). Biol Reprod 2009;81(3):488–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen M, Wolfe A, Wang X, Chang C, Yeh S, Radovick S. Generation and characterization of a complete null estrogen receptor alpha mouse using Cre/LoxP technology. Mol Cell Biochem 2009;321; (1–2):145–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Couse JF, Curtis SW, Washburn TF, et al. Analysis of transcription and estrogen insensitivity in the female mouse after targeted disruption of the estrogen receptor gene. Mol Endocrinol 1995;9 (11):1441–1454 [DOI] [PubMed] [Google Scholar]
- 55.Antal MC, Krust A, Chambon P, Mark M. Sterility and absence of histopathological defects in nonreproductive organs of a mouse ERbeta-null mutant. Proc Natl Acad Sci U S A 2008;105(7): 2433–2438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Krege JH, Hodgin JB, Couse JF, et al. Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proc Natl Acad Sci U S A 1998;95(26):15677–15682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, Mark M. Effect of single and compound knockouts of estrogen receptors alpha (ERalpha) and beta (ERbeta) on mouse reproductive phenotypes. Development 2000;127(19):4277–4291 [DOI] [PubMed] [Google Scholar]
- 58.Couse JF, Curtis Hewitt S, Korach KS. Receptor null mice reveal contrasting roles for estrogen receptor alpha and beta in reproductive tissues. J Steroid Biochem Mol Biol 2000;74(5):287–296 [DOI] [PubMed] [Google Scholar]
- 59.Leng G Might oestrogen act directly on GnRH neurones? J Neuroendocrinol 1999;11(5):323–324 [DOI] [PubMed] [Google Scholar]
- 60.Butler JA, Sjöberg M, Coen CW. Evidence for oestrogen receptor alpha-immunoreactivity in gonadotrophin-releasing hormone-expressing neurones. J Neuroendocrinol 1999;11(5):331–335 [DOI] [PubMed] [Google Scholar]
- 61.Hrabovszky E, Shughrue PJ, Merchenthaler I, et al. Detection of estrogen receptor-beta messenger ribonucleic acid and 125I-estrogen binding sites in luteinizing hormone-releasing hormone neurons of the rat brain. Endocrinology 2000;141(9):3506–3509 [DOI] [PubMed] [Google Scholar]
- 62.Hrabovszky E, Steinhauser A, Barabás K, et al. Estrogen receptor-beta immunoreactivity in luteinizing hormone-releasing hormone neurons of the rat brain. Endocrinology 2001;142(7): 3261–3264 [DOI] [PubMed] [Google Scholar]
- 63.Legan SJ, Tsai HW. Oestrogen receptor-alpha and -beta immunoreactivity in gonadotropin-releasing hormone neurones after ovariectomy and chronic exposure to oestradiol. J Neuroendocrinol 2003;15(12):1164–1170 [DOI] [PubMed] [Google Scholar]
- 64.Kalló I, Butler JA, Barkovics-Kalló M, Goubillon ML, Coen CW. Oestrogen receptor beta-immunoreactivity in gonadotropin releasing hormone-expressing neurones: regulation by oestrogen. J Neuroendocrinol 2001;13(9):741–748 [DOI] [PubMed] [Google Scholar]
- 65.Skinner DC, Dufourny L. Oestrogen receptor beta-immunoreactive neurones in the ovine hypothalamus: distribution and colocalisation with gonadotropin-releasing hormone. J Neuroendocrinol 2005;17(1):29–39 [DOI] [PubMed] [Google Scholar]
- 66.Hrabovszky E, Kalló I, Szlávik N, Keller E, Merchenthaler I, Liposits Z. Gonadotropin-releasing hormone neurons express estrogen receptor-beta. J Clin Endocrinol Metab 2007;92(7):2827–2830 [DOI] [PubMed] [Google Scholar]
- 67.Davies MP, O’Neill PA, Innes H, et al. Correlation of mRNA for oestrogen receptor beta splice variants ERbeta1, ERbeta2/ERbetacx and ERbeta5 with outcome in endocrine-treated breast cancer. J Mol Endocrinol 2004;33(3):773–782 [DOI] [PubMed] [Google Scholar]
- 68.Mitter D, Ortmann O, Treeck O. Estrogen receptor Beta isoforms — functions and clinical relevance in breast cancer. [in German]. Zentralbl Gynakol 2005;127(4):228–234 [DOI] [PubMed] [Google Scholar]
- 69.Taylor SE, Martin-Hirsch PL, Martin FL. Oestrogen receptor splice variants in the pathogenesis of disease. Cancer Lett 2010;288 (2):133–148 [DOI] [PubMed] [Google Scholar]
- 70.Mellon PL, Windle JJ, Goldsmith PC, Padula CA, Roberts JL, Weiner RI. Immortalization of hypothalamic GnRH neurons by genetically targeted tumorigenesis. Neuron 1990;5(1):1–10 [DOI] [PubMed] [Google Scholar]
- 71.Radovick S, Wray S, Lee E, et al. Migratory arrest of gonadotropin-releasing hormone neurons in transgenic mice. Proc Natl Acad Sci U S A 1991;88(8):3402–3406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zakaria M, Dunn IC, Zhen S, et al. Phorbol ester regulation of the gonadotropin-releasing hormone (GnRH) gene in GnRH-secreting cell lines: a molecular basis for species differences. Mol Endocrinol 1996;10(10):1282–1291 [DOI] [PubMed] [Google Scholar]
- 73.Martínez-Morales JR, López-Coviella I, Hernández-Jiménez JG, et al. Sex steroids modulate luteinizing hormone-releasing hormone secretion in a cholinergic cell line from the basal forebrain. Neuroscience 2001;103(4):1025–1031 [DOI] [PubMed] [Google Scholar]
- 74.Roy D, Angelini NL, Belsham DD. Estrogen directly represses gonadotropin-releasing hormone (GnRH) gene expression in estrogen receptor-alpha (ERalpha)- and ERbeta-expressing GT1–7 GnRH neurons. Endocrinology 1999;140(11):5045–5053 [DOI] [PubMed] [Google Scholar]
- 75.Ng Y, Wolfe A, Novaira HJ, Radovick S. Estrogen regulation of gene expression in GnRH neurons. Mol Cell Endocrinol 2009;303; (1–2):25–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Otani H, Otsuka F, Takeda M, et al. Regulation of GNRH production by estrogen and bone morphogenetic proteins in GT1–7 hypothalamic cells. J Endocrinol 2009;203(1):87–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Martínez-Morales JR, Morales A, Marín R, et al. Estrogen modulates norepinephrine-induced accumulation of adenosine cyclic monophosphate in a subpopulation of immortalized luteinizing hormone-releasing hormone secreting neurons from the mouse hypothalamus. Neurosci Lett 2001;298(1):61–64 [DOI] [PubMed] [Google Scholar]
- 78.Sharifi N, Reuss AE, Wray S. Prenatal LHRH neurons in nasal explant cultures express estrogen receptor beta transcript. Endocrinology 2002;143(7):2503–2507 [DOI] [PubMed] [Google Scholar]
- 79.Hu L, Gustofson RL, Feng H, et al. Converse regulatory functions of estrogen receptor-alpha and -beta subtypes expressed in hypothalamic gonadotropin-releasing hormone neurons. Mol Endocrinol 2008;22(10):2250–2259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Spergel DJ, Krüth U, Hanley DF, Sprengel R, Seeburg PH. GABA- and glutamate-activated channels in green fluorescent protein-tagged gonadotropin-releasing hormone neurons in transgenic mice. J Neurosci 1999;19(6):2037–2050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Suter KJ, Song WJ, Sampson TL, et al. Genetic targeting of green fluorescent protein to gonadotropin-releasing hormone neurons: characterization of whole-cell electrophysiological properties and morphology. Endocrinology 2000;141(1):412–419 [DOI] [PubMed] [Google Scholar]
- 82.Chu Z, Andrade J, Shupnik MA, Moenter SM. Differential regulation of gonadotropin-releasing hormone neuron activity and membrane properties by acutely applied estradiol: dependence on dose and estrogen receptor subtype. J Neurosci 2009;29(17):5616–5627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Temple JL, Laing E, Sunder A, Wray S. Direct action of estradiol on gonadotropin-releasing hormone-1 neuronal activity via a transcription-dependent mechanism. J Neurosci 2004;24(28): 6326–6333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Romanò N, Lee K, Abrahám IM, Jasoni CL, Herbison AE. Nonclassical estrogen modulation of presynaptic GABA terminals modulates calcium dynamics in gonadotropin-releasing hormone neurons. Endocrinology 2008;149(11):5335–5344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Abe H, Keen KL, Terasawa E. Rapid action of estrogens on intracellular calcium oscillations in primate luteinizing hormone-releasing hormone-1 neurons. Endocrinology 2008;149(3):1155–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Abe H, Terasawa E. Firing pattern and rapid modulation of activity by estrogen in primate luteinizing hormone releasing hormone-1 neurons. Endocrinology 2005;146(10):4312–4320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nishimura I, Ui-Tei K, Saigo K, Ishii H, Sakuma Y, Kato M. 17beta-estradiol at physiological concentrations augments Ca(2þ) -activated K+ currents via estrogen receptor beta in the gonadotropin-releasing hormone neuronal cell line GT1–7. Endocrinology 2008;149(2):774–782 [DOI] [PubMed] [Google Scholar]
- 88.Morales A, Gonzalez M, Marin R, Diaz M, Alonso R. Estrogen inhibition of norepinephrine responsiveness is initiated at the plasma membrane of GnRH-producing GT1–7 cells. J Endocrinol 2007;194(1):193–200 [DOI] [PubMed] [Google Scholar]
- 89.Kurita T, Lee K, Saunders PT, et al. Regulation of progesterone receptors and decidualization in uterine stroma of the estrogen receptor-alpha knockout mouse. Biol Reprod 2001;64(1):272–283 [DOI] [PubMed] [Google Scholar]
- 90.Kraus WL, Montano MM, Katzenellenbogen BS. Cloning of the rat progesterone receptor gene 5′-region and identification of two functionally distinct promoters. Mol Endocrinol 1993;7(12): 1603–1616 [DOI] [PubMed] [Google Scholar]
- 91.Kastner P, Krust A, Turcotte B, et al. Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. EMBO J 1990;9(5):1603–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pak TR, Chung WC, Roberts JL, Handa RJ. Ligand-independent effects of estrogen receptor beta on mouse gonadotropin-releasing hormone promoter activity. Endocrinology 2006;147(4):1924–1931 [DOI] [PubMed] [Google Scholar]
- 93.Abrahám IM, Han SK, Todman MG, Korach KS, Herbison AE. Estrogen receptor beta mediates rapid estrogen actions on gonadotropin-releasing hormone neurons in vivo. J Neurosci 2003;23 (13):5771–5777 [DOI] [PMC free article] [PubMed] [Google Scholar]


