Abstract
Adoptive cell therapy with tumor-infiltrating lymphocytes (TILs), an investigational cellular therapy, has demonstrated antitumor efficacy in patients with advanced solid tumors, including melanoma. Tumor-infiltrating lymphocyte cell therapy involves surgical resection of a patient's tumor, ex vivo TIL expansion under conditions that overcome immunosuppressive responses elicited by the tumor and the tumor microenvironment, administration of a lymphodepleting regimen, and infusion of the final TIL cell therapy product back into the patient followed by interleukin 2 administration to support T-cell activity. The surgeon plays a central role in patient identification and tumor selection—steps that are critical for successful outcomes of TIL cell therapy. Commercialization of TIL cell therapy and its broader access to patients will require education and collaboration among surgeons, oncologists, and cellular therapists. This review highlights the unique role that surgeons will play in the implementation of TIL cell therapy and serves as a contemporary report of best practices for patient selection and tumor resection methods.
Key Words: Best practices, surgeon, surgical considerations, TIL cell therapy, tumor-infiltrating lymphocytes, tumor resection
Adoptive cell therapy (ACT) is a form of personalized immunotherapy that leverages the body’s natural defenses against cancer. Different modalities of ACT have been explored over the years, including the use of tumor-infiltrating lymphocytes (TILs), T-cell receptor (TCR) gene modified T-cell therapy, and chimeric antigen receptor–modified T cells, each characterized by a distinct mechanism of action.1–3 Of these, TIL cell therapy represents an ideal modality for treatment in solid tumors, because of the polyclonal nature of the infusion product, which is capable of generating a response to a multitude of nonoverlapping patient-specific neoantigens. This polyclonality is expected to reduce the potential for immune escape via loss of target antigen expression by neoplastic cells.4 Tumor-infiltrating lymphocyte cell therapy involves surgical resection of a portion of a patient’s tumor, followed by ex vivo expansion of immune cells capable of recognizing patient-specific neoantigens, and reinfusion of these autologous immune cells into the patient to specifically target and eliminate tumor cells.5 The various steps involved in TIL cell therapy are outlined in Figure 1. Current TIL regimens include a preparative nonmyeloablative lymphodepletion regimen approximately 5 to 7 days preceding TIL infusion and an abbreviated course of high-dose interleukin 2 (IL-2) after TIL infusion.6,7
FIGURE 1.
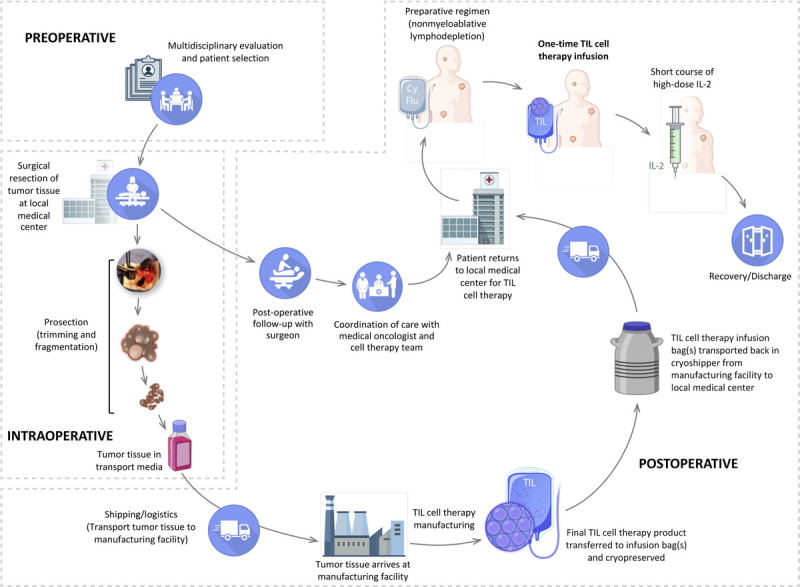
Steps involved in TIL cell therapy.
Adoptive cell therapy using investigational TIL cell therapy has demonstrated encouraging efficacy and safety in clinical trials for several patient populations with high unmet medical need, including advanced melanoma,6,8–13 non–small cell lung cancer (NSCLC),14–16 cervical cancer,12,17 and head and neck squamous cell carcinoma (HNSCC).12,18 Most of these studies included heavily pretreated patients who had failed all prior therapies and had limited treatment options. Preliminary efficacy of TIL cell therapy has also been reported in the second-line setting for human papillomavirus–associated epithelial cancers,19 epithelial ovarian cancer,20,21 colorectal cancer,22 breast cancer,23 and cholangiocarcinoma.24
Given that TIL cell therapy requires a surgical resection from which the cell therapy product is generated, the role of the surgeon in this therapy is fundamental to its success. Tumor-infiltrating lymphocyte cell therapy programs have historically been research-oriented, single-institution programs with surgeons intimately involved in the research aspects of TILs. As TIL cell therapy nears commercialization and soon may become more widely available, a broad variety of cancer surgical subspecialists, such as thoracic surgeons, head and neck surgeons, and gynecological oncologists, may be asked to procure tissue for TILs from a variety of anatomic sites. Furthermore, the success of tumor procurement for TIL manufacturing is highly dependent on active engagement among highly coordinated multidisciplinary and multifunctional teams throughout the patient’s treatment, including surgeons, medical oncologists, cell therapy teams, and laboratory personnel involved in tumor procurement. This report addresses many key aspects of the surgeon’s role in patient selection, tumor resection, and processing for TIL generation and provides surgical practice recommendations and considerations for a multidisciplinary setting, with a focus on rapid translation to clinical practice. The primary objective is not only to identify best practices that minimize patient morbidity and inconvenience during surgical resection but also optimize tumor tissue quantity and quality for TIL generation.
PATIENT SELECTION IN A MULTIDISCIPLINARY SETTING
In collaboration with the treating medical oncologists, surgeons select patients, choose appropriate lesion(s) for resection, and plan the surgical approach. As the tumor resection is not therapeutic in intent, it is crucial to design an intraoperative strategy that minimizes perioperative morbidity. Patients selected for TIL cell therapy may have received multiple prior lines of therapy and may have limited life expectancy and functional status due to advanced disease. Therefore, it is critical that these initial planning steps are performed in a timely manner to reduce time to treatment and maximize patient fitness for the full TIL treatment regimen, including administration of nonmyeloablative lymphodepletion, TIL cell therapy, and IL-2.25
Several patient and clinical characteristics have been considered when selecting patients for TIL cell therapy in clinical trials. Eligible patients typically present with Eastern Cooperative Oncology Group performance status ≤1; lack comorbidities such as irreversible cardiac dysfunction, impaired pulmonary function, or autoimmune disorders requiring immunosuppression; and are not at a high risk of bacterial infection from diseases such as biliary obstruction or indwelling catheters (i.e., palliative biliary or ureteral stents).25 Symptomatic brain metastases represent a unique point of pretherapy evaluation; lesions greater than 1 cm and those with edema, although not disqualifying, should be managed to the extent possible before TIL cell therapy infusion to minimize risk of complications, such as hemorrhage during the period that the patient has profound thrombocytopenia due to lymphodepletion. Finally, surgery and/or bridging therapy during the TIL manufacturing process may be required for patients with extensive and/or rapidly increasing tumor burden to avoid progression that would preclude receipt of TIL cell therapy.25
TUMOR/SURGICAL SITE SELECTION AND CONSIDERATIONS
Most solid tumors, especially melanoma and lung cancer, are characterized by a population of cells that exhibit phenotypic and biologic differences, leading to intratumoral and intertumoral heterogeneity.26 In addition, heterogeneity exists within the patient population in terms of tumor location, performance status, organ involvement, and extent of disease, which leads to a wide range of procedures for tumor resection.27 The tumor, as the starting material for TIL cell therapy, is essential to the final infusion product. Choosing the optimal anatomic resection site and streamlining subsequent steps are critical to optimize TIL yield and to ensure patients recover adequately before receiving the nonmyeloablative chemotherapy approximately 3 weeks after the surgical procedure and 7 days before TIL infusion.27 Therefore, surgical sites that minimize morbidity (e.g., subcutaneous nodules) and that can be performed in the outpatient setting are favored, although superficial lesions may not always yield the required amount of tumor or may need to be avoided to facilitate wound healing.25 In these instances, more complex resections may be needed to procure tumor tissue from the thorax or abdomen.
Minimally invasive options, such as laparoscopic surgery for visceral resections or robotic-assisted resection in the case of pulmonary metastasis, should always be considered to avoid delay in initiation of TIL cell therapy that might be associated with prolonged postoperative recovery. Sites that have been previously irradiated, those with ulcerated tumors, or those with high risk of bacterial growth (e.g., bowel lesions), leading to contamination of TIL cultures, should be avoided.25
Only a few published studies have reported on the choice of tumor resection site and TIL generation from a surgical perspective. Following the early encouraging responses to TIL cell therapy demonstrated by the Surgery Branch at National Cancer Institute, Klapper et al.28 reported their experience with 107 patients with metastatic melanoma undergoing surgical procedures for TIL procurement between 1998 and 2009. In this historical retrospective report, thoracotomy and video-assisted thoracoscopic surgery were the most common surgical approaches, with nonanatomic (wedge) resection and lobectomy identified as the most common surgical procedures. Although 70% of the TIL cultures exhibited antitumor activity, 83 of the 107 patients (78%) could not receive TIL cell therapy, mostly because of aggressively progressing disease or lack of TIL growth or reactivity in culture. Although this finding highlighted the need for improved patient selection, timely surgery, and refinement in TIL expansion protocol, it also demonstrated the value of thoracic metastasectomy in TIL procurement.28 In a later study conducted by Zippel et al.,27 of the 47 tumor resections from 44 patients performed for obtaining TILs, 37 were performed using general anesthesia, which included 27 open procedures (thoracotomy, laparotomy, dissection of a major lymph node basin) and 10 minimally invasive procedures (thoracoscopy or laparoscopy). Notably, 3 of the 44 patients underwent a second resection and TIL infusion. Of the 37 major surgical procedures for TIL procurement (using general anesthesia), the rate of surgical mortality and major morbidity was 0%, which the surgeons attributed to a multidisciplinary approach for careful planning and selection of resection site, simplification and streamlining of the technique, and use of “young” short-term cultured bulk TILs. Minor morbidity included only wound infections.27
In the same study by Zippel et al.,27 tumor resection was performed from a single anatomic site per patient, most commonly lymph node metastasis, followed by subcutaneous nodules, lung metastasis, and abdominal metastatic sites, including 2 liver lesions (separate patients). Each patient was infused with an average 39.4 × 109 TILs, with the lymph nodes yielding the highest number of TILs and the liver metastatic lesions yielding the lowest number of TILs. In agreement with this finding, metastatic tumor sites such as lung and lymph nodes have been shown to generate viable TILs more frequently than gastrointestinal (GI) sites, possibly due to contamination of GI tumors with gut bacteria or yeast.25,29 Although TIL clinical trials to date have been mostly limited to melanoma, TILs have been successfully resected and expanded from soft tissue sarcoma, lung, bladder, head and neck, cervical, and triple-negative breast cancer tumors, as well as pancreatic, liver, and NSCLC metastatic sites.30–34 Some cancers inherently contain more TILs than others,35 which will dictate the surgical approach and choice of resection site. Technical issues associated with each tumor resection site and tissue-specific considerations have been reviewed by Gastman et al.36
In a study by Goff et al.,29 analysis of growth and activity of TILs obtained from various resection sites showed no association with age, sex, or a specific therapy administered within 3 months of tumor harvest. Although prior chemotherapy was negatively associated with TIL growth on a per-patient basis, the effect was modest, and viable TILs could be generated from 78% of patients who received prior chemotherapy (vs. 87% who did not receive prior chemotherapy). Overall, tumors smaller in diameter, tumor digests with higher percentage of lymphocytes, and tumors resected from GI metastasis were found to be unfavorable for growth and tumor-specific activity. However, the reported analyses have not demonstrated precise cutoff values with sufficient sensitivity or specificity. Interestingly, tumor digests containing more than 60% infiltrating lymphocytes showed favorable growth, but their tumor reactivity was markedly reduced compared with tumor digests containing ≤60% infiltrating lymphocytes.29 Nevertheless, all of these studies indicate that successful growth and expansion of viable, active TILs have been possible from all resection sites and tumor types.
Contemporary ACT trials have demonstrated success with developing a TIL infusion product from multiple anatomic sites. In a multicenter phase II trial in patients with metastatic melanoma, tumors were successfully resected from multiple primary and metastatic sites that yielded an adequate number of TILs (≥1 × 109 TILs) for 1-time TIL infusion. The resection sites included skin, lung, lymph nodes, liver, spleen, lung, peritoneum, musculoskeletal sites, breast, and subcutaneous tissue.6 Of the 78 resected patients in this study, 66 (85%) received the target dose of viable TILs; 9 were not treated for patient-related factors, including progressive disease; and 3 did not have the target range of viable TILs available.6 Recently, in a multicenter phase II study of patients with advanced NSCLC, more than 50% of lesions resected were from the lung, and patients could be administered therapeutic TIL doses with minimal morbidity.16 The feasibility of TIL production from NSCLC tumors was also demonstrated in a phase I study, in which adequate tumor tissue was collected from excisional biopsy of metastases in pleural nodules or supraclavicular lymph nodes, with most patients discharged within a day following the biopsy procedure.15
USE OF MINIMALLY INVASIVE PROCEDURES FOR TUMOR RESECTION
Minimally invasive techniques are preferred to reduce postoperative morbidity, including surgical site infections, wound healing complications, anastomotic air leaks, and overall depression of the patient's performance status.36 As all patients considered for TIL cell therapy have, by definition, advanced disease, surgical resection of metastatic sites for TIL procurement is not necessarily performed with therapeutic intent and traditional oncologic margins.25
Patients without a target lesion that can be resected without minimal morbidity are not necessarily precluded from consideration for TIL cell therapy. Core needle biopsy (CNB) procedures are less invasive than open surgical biopsy and provide more substantial tissue samples than those seen with fine-needle-aspiration biopsy.37 The less invasive nature of CNB also allows a greater number of patients, including frail patients and patients with comorbidities, to benefit from TIL cell therapy. Core needle biopsy was successfully used to obtain an adequate number of TILs (>5 × 106 cells) from melanoma.38 Although the CNB sample size was small (n = 11), 4 patients (36%) achieved objective responses, including 1 complete response. The authors note that, in contrast to the discomfort, cost, and risk of complications associated with surgery, CNB is a quick, inexpensive procedure requiring only local anesthesia and can aid in streamlining TIL generation protocols across multiple centers.38 Although these data are encouraging, formal resection of a metastatic deposit should be the preferred approach for TIL procurement until refinements in TIL procurement and expansion techniques are able to demonstrate significant clinical responses from CNB.
Although a nonvisceral resection site is desired for TIL generation for ACT because of reduced morbidity, resection of liver metastasis may be considered to obtain adequate quantity of TILs in the absence of other acceptable sites. In a retrospective analysis of 22 patients who were scheduled for planned laparoscopic liver resection to generate TILs for ACT, a completely laparoscopic procedure could be performed in 91% (20 of 22) of patients, with low intraoperative blood loss and median 3 days of hospitalization. The success rate of TIL production was 82% (18 of 22 patients), demonstrating the feasibility of resection of liver metastasis using a laparoscopic approach.33
When possible, skin, soft tissue, and superficial lymph nodes are preferred owing to their feasibility in an outpatient setting with minimal risks. In the absence of these preferred sites, resection of peripheral sites and smaller nodules within visceral organs, such as the lung and liver, may be secondarily considered. However, TIL derived from secondary lymphoid organs, such as spleen or bowel, may pose limitations due to the abundance of bystander non–tumor-reactive T cells that may preferentially expand within the TIL culture.25 Metastases from the GI tract raise concerns of contamination. If possible, other sites should be attempted first. Expansion of TIL from glioma tumor tissue using a cytokine cocktail of IL-2, IL-15, and IL-21 has been reported; the authors noted that TIL expansion from glioma lesions was not possible using the standard IL-2–based expansion protocol.39 Tumor resection from central nervous system metastasis as a source of TIL generation has not been extensively studied; thus, central nervous system lesions are not an ideal resection site.
IMAGE-GUIDED APPROACH FOR LESION SELECTION
Before resection, a multidisciplinary team of physicians, including the attending surgeon, should convene to evaluate a patient’s imaging studies to determine an appropriate anatomical location for tumor resection. Image guidance for tumor procurement has been recommended for urologic tumors (e.g., renal cancer) because of the tumors being highly vascular and susceptible to hemorrhage.40 Similarly, use of interventional radiology with image-guided techniques has been suggested in the setting of recurrent disease, where surgery is not indicated.36 Image-guided CNB techniques are applied to obtain tumor tissue from complex anatomic locations, such as the spine and pelvis. As noted earlier, ultrasound-guided CNB was successfully used in patients with melanoma to obtain TILs for ACT and could be particularly safe in instances where surgery is contraindicated.38 Magnetic resonance imaging–guided percutaneous biopsy may be particularly explored for procurement of adequate tumor tissues where there is lack of tissue contrast and vascular visibility.41 Image-guided approaches coupled with minimally invasive procedures may mitigate the complications and recovery associated with open resection, potentially expanding access to TIL cell therapy to patients with significant medical comorbidities and poor performance status. The optimization of minimally invasive approaches for tumor tissue procurement for TILs requires ongoing investigation.42
Image-guided biopsy sampling for tumor characterization has been widely used for patient selection, molecular profiling, and response monitoring. Recently, a Web-based lesion-selection tool has been developed to ensure consistency in tumor biopsy site selection and to streamline the tumor sample acquisition process across a multidisciplinary team.43 Although this platform is aimed at improving the consistency in lesion selection for sequential biopsy procedures, it could potentially be applied to select appropriate tumor resection sites for TIL production and establish a uniform online workflow between the multidisciplinary team involved in patient care (medical oncologist, surgeon, and radiologist).
Examples of patient scans that can aid in lesion selection and choice of accompanying minimally invasive procedure for TIL procurement are shown in Figure 2. The procedures include minimally invasive outpatient surgery for superficial lymph nodes, laparoscopic liver resection, and video-assisted thoracoscopic surgery wedge resection for a pulmonary nodule (data on file). Additional patient case studies to explain considerations and rationale for selecting suitable tumor resection sites for TIL production can be found in a Supplementary Video by Dr. Michael E. Egger (Supplemental Digital Content 1, http://links.lww.com/PPO/A38).
FIGURE 2.
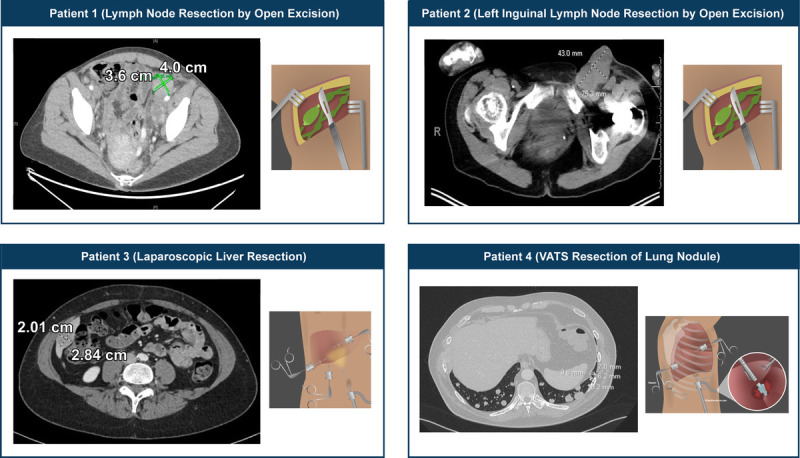
Examples of image-guided lesion selection for TIL procurement in patients with metastatic melanoma. Tumor diameter measurements are indicated on each panel. The metastatic sites were as follows: patient 1: left iliac lymph node, multiple subcutaneous sites; patient 2: bulky left inguinal lymphadenopathy (pictured), 2 hepatic metastases; patient 3: inferior right hepatic lobe, multiple smaller metastases in lymph nodes; patient 4: multiple lesions in both lungs. Additional case studies on considerations and rationale for selecting suitable tumor resection sites for production of the TIL treatment can be found in a Supplementary Video by Dr. Michael E. Egger (see Video, Supplemental Digital Content 1, http://links.lww.com/PPO/A38). All patients provided informed consent. VATS, video-assisted thoracoscopic surgery.
ADDITIONAL TUMOR TISSUE CONSIDERATIONS
The approach and conduct of the operative resection are not the only role of the surgeon in optimizing TIL cell therapy. The ex vivo prosection and preparation of the lesion before TIL manufacturing are a crucial step to ensure cell expansion success and thus should be performed by the operating surgeon (Fig. 3). During prosection, zones within the resected lesion should be selected that are more likely to be highly infiltrated with TILs; preferred sites include areas at the periphery of the tumor mass and areas adjacent to blood or lymphatic vessels, as these may be more likely to contain tertiary lymphoid structures formed from lymphocytes and antigen-presenting cells within the tumor in response to chronic inflammation.44
FIGURE 3.
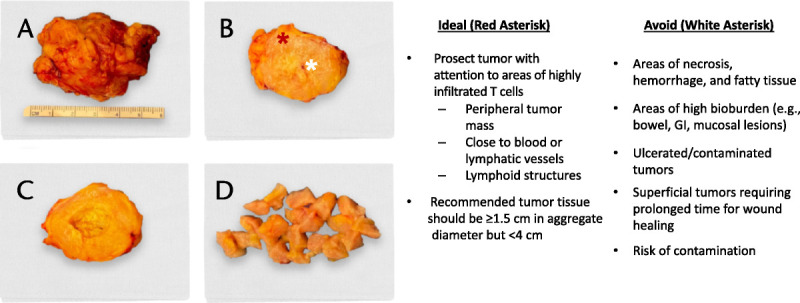
Best practices to be considered during intraoperative prosection of tumor tissue. A, Tumor tissue immediately after resection with margin of grossly normal surrounding tissue. B, Bisection of the tumor reveals areas of necrosis (white asterisk) and viable tumor (red asterisk). Necrotic portion of tumor is removed (C), and the viable portion of the tumor is fragmented in preparation for placement into transfer media (D).
The tumor must be carefully prosected by the operating surgeon in sterile fashion, with care to exclude nontumor tissue. Necrotic, hypervascular, or adipose tissue remaining in the resected tumor tissue correlates negatively with TIL growth ex vivo.45,46 The operating surgeon is best qualified to prosect and select the most viable tumor tissue for optimal TIL production. Although no formal minimal size criteria have been established for resection of viable solid tumor tissue, some studies and trials in progress recommend a postprosection aggregate tumor tissue diameter of 1 cm or greater47 or 1.5 cm or greater but less than 4 cm,6,16,48 whereas others recommend a metastatic tumor tissue of 1 cm3 or greater (>2 cm3 for lymph nodes).7 If the volume of tissue from 1 lesion is not adequate, an additional lesion can be resected to make up the size specifications, with 1.5 cm or greater in aggregate diameter after trimming to meet the minimum TIL manufacturing requirements. Some metastatic tumors, such as breast tumors, are small and may require multiple resection sites to accumulate enough tissue.35 The total volume of viable tissue resected should include sufficient samples for TIL manufacturing and separate samples for other possible testing, such as pathology review.
At the discretion of the physician, examination of an intraoperative frozen section may be performed, for example, in the case of a presumed tumor-bearing lymph node, because non–tumor-bearing lymph nodes will likely result in the expansion of non–tumor-specific lymphocytes of no therapeutic value. Material used for pathology review should be divided under sterile conditions in the operating room (OR) by the surgeon and kept separate from the tissue that will be used for TIL manufacturing. The portion of the tumor tissue designated for TIL manufacturing should be placed directly in the sterile media and sent to the cell manufacturing facility.
BEST PRACTICES TO AVOID CONTAMINATION OF THE TIL INFUSION PRODUCT
Following tumor extirpation, the risk of tumor tissue contamination can be mitigated with OR procedures akin to organ transplantation. As such, OR equipment and any instruments that may contact tumor tissue must be sterile. To ensure fidelity in the sterile processing of the tissue, recommended practice is to prepare the tumor tissue on a sterile OR back-table and to place the tumor tissue directly into the sterile transfer media after prosection. A tumor larger than 1.5 cm in diameter may be cut into smaller pieces using a sterile scalpel before placing in tumor media to allow increased surface area of tumor contact with the transfer media. The tumor tissue should leave the OR only after prosection has been completed and the tissue sealed in a sterile media transfer container. To decrease the time from resection to media placement, the prosection should be accomplished by the operating surgeon, while the first assistant attends to the closure of the surgical wound. Pathology review, if possible at the discretion of the physician, is suggested to confirm colocalization of lymphocytes and malignant cells, especially in secondary lymphoid-rich tissues where bystander T cells that lack antitumor immune specificity may expand ex vivo along with TILs.25 Any tumor material used for pathology review should be handled separately and not used for TIL generation.
Although sterile operative conditions, as noted above, are universally practiced, the inherent bioburden within the tumor poses a challenge. This is further amplified in anatomical areas that usually have a microbiome (e.g., mouth, aerodigestive tract including the GI tract, genital organs, skin lesions). Thus, tumors resected from these higher-risk areas can be expected to have a higher contamination rate, a finding that would be supported by the types of pathogens identified. As expected, tumor types that colocate in higher-risk areas, such as HNSCC and cervical carcinoma, had a higher contamination rate, whereas it can be speculated that lung cancer would be associated with a very low contamination rate. Strategies to decrease contamination for resections from higher-risk sites are being explored and could include addition of antimicrobial agents in the media that is used for tumor shipment45 or the use of closed-system tissue-processing vessels.45,49
TUMOR RESECTION SITE DOES NOT INFLUENCE TCR REPERTOIRE
Although the site of tumor procurement may influence the rate of contamination, it is not associated with expansion of TILs or quality of TILs obtained. Tumor-infiltrating lymphocytes, by definition, represent a polyclonal T-cell product with diverse antigen specificity50 and comprised a diverse patient-specific TCR repertoire.51 The TCR repertoire is defined by the presence of unique TCR β-chain complementarity-determining region 3 (CDR3) sequences in the patient's blood51,52 and can be assessed using several validated commercial assays.53,54 Each T-cell clone expresses a unique TCR, which is identifiable by the CDR3; thus, unique CDR3 sequences (uCDR3) provide a measure of clonality of the TCR repertoire within the expanded TIL product. In an analysis of pre– and post–TIL-infusion peripheral blood mononuclear cell samples from patients with metastatic melanoma enrolled in a phase II clinical trial, no significant differences in TCR diversity (Shannon Entropy Index) or clonality (Simpson Clonality Index) were observed between tumors resected from a variety of sites, such as skin, lymph node, liver, lung, peritoneum, musculoskeletal sites, breast, and other organs6 (Figs. 4A, B). Similarly, resection site did not influence the proportion of overlapping clones in tumor samples and the infused TIL cell products; nor did it influence the persistence of these clones (Figs. 4C–E).
FIGURE 4.
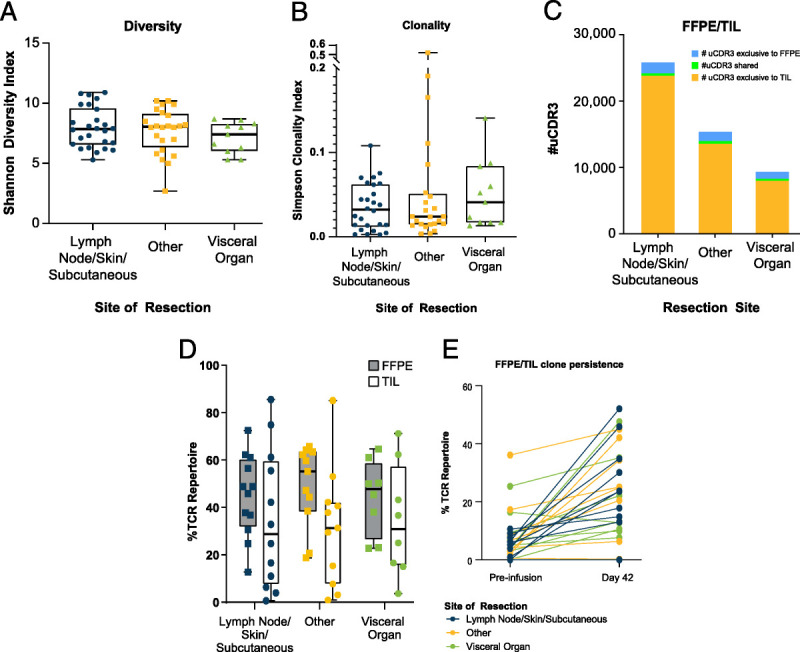
Site of tumor resection does not impact diversity, clonality, proportion of overlapping clones, or persistence of patient-specific TCR clones. The Shannon Diversity Index (A) and Simpson Clonality Index (B) are shown for the TIL products generated and indicate no significant differences between the sites of resection. A larger Shannon Entropy Index indicates a more diverse CDR3 population. Values can range from 0 (monoclonal sample) to log2(R) (evenly distributed, polyclonal sample with R unique clones). Simpson Clonality Index reflects monoclonality or polyclonality of a sample and is inversely related to diversity (Shannon Entropy Index). Values can range from 0 (evenly distributed, polyclonal sample) to 1 (monoclonal sample). Tumor samples collected at the time of resection (FFPE) were analyzed and compared with the TIL products infused and blood samples from preinfusion and postinfusion time points. Unique clonotypes identified from the FFPE tumor samples and the TIL products are shown in blue and yellow, respectively; clonotypes identified in both samples are indicated in green and reflect tumor-associated clonotypes also captured in the TIL products (C). The contribution of these shared clonotypes to the total TCR repertoire in the FFPE (gray) and the TIL (white) samples is shown in boxplots (D). These shared clonotypes were also assessed for their contribution to the total TCR repertoire in the preinfusion and postinfusion (day 42) blood (E). “Other” sites include peritoneum, musculoskeletal, breast, and other sites. FFPE indicates formalin-fixed, paraffin-embedded.
Although the patient care team may need to consider factors, such as access to the resection site, risk of contamination based on resection site, type of procedure, patient performance status, and recovery time before receipt of TILs, the above findings suggest that the site of tumor resection does not affect T-cell persistence or the clonality or diversity of TCR repertoire after TIL infusion.
Further analyses of pre– and post–TIL-infusion peripheral blood mononuclear cell samples in patients with metastatic melanoma indicated that long-term (day 42) TIL persistence,51,52 number of unique TCR clonotypes,51 and TCR diversity51 have shown no association with clinical response, but the TCR repertoire following infusion represents the only meaningful assay to describe persistence of the infusion product. Further, the in vivo fate of individual TIL clones is not dependent on their frequency in the infusion product, as minor clones within the infusion product can expand preferentially after infusion, hypothetically reflecting antigen-driven clonal proliferation.51 The TCR repertoire of TILs (LN-145) generated from NSCLC tumors of patients with advanced NSCLC demonstrated a highly polyclonal product with unique TCR clones, as indicated by the Shannon Entropy Index and Simpson Clonality Index.16 The number of unique TCR clones obtained from NSCLC tumors, as well as measures of diversity and clonality, was similar to those previously published for lifileucel for melanoma51 and LN-145 for cervical cancer.52 These results support the fact that preference of a particular resection site is unlikely to have a downstream influence on the quality of TILs as defined by the presence of unique and focused TCR clonotypes.
ADVANCES IN TIL MANUFACTURING PROCESS
Patients with advanced cancer often have limited life expectancy, leaving them unable to complete TIL cell therapy because of historically lengthy (4–6 weeks) TIL manufacturing processes.25,55 More recently, the development of a centralized, streamlined, 22-day manufacturing process has enabled faster manufacturing of investigational autologous TIL products lifileucel and LN-145 and produced a 96% manufacturing success rate in patients with melanoma.6 By comparison, viable TIL cultures have been generated in 94% to 96% of patients in previous single-center studies.10,29 Across all tumor types investigated, including melanoma, lung, cervical, and head and neck, lifileucel and LN-145 were successfully manufactured for more than 90% of patients who underwent tumor resection.10,29,56
A rapid TIL manufacturing process, with a manufacturing duration of 16 days, is being developed that will require only a small amount of starting tumor material, such as from core biopsies or tumors with poor TIL infiltration.57 Further advancements in the manufacturing timeline include addition of an IL-2/IL-15/IL-21 cytokine cocktail, which enhances TIL numbers 20% more than the use of IL-2 alone.58 Tumor-infiltrating lymphocyte expansion during manufacturing is also promoted with PD-1 blockade,31 4-1BB stimulation,31,58,59 and CD8+ T-cell enrichment via anti-OX40 antibodies,60 which are effective strategies not only to improve TIL yield but also to enhance effector cell function of TILs. Addition of an anti–PD-1 antibody to TIL cultures led to significant increase in the absolute number of TILs and also produced significantly more interferon-γ (IFN-γ) in the presence of an HLA-matched tumor line compared with TIL cultures treated with an isotype control.31 Similarly, costimulation with agonistic anti–4-1BB antibody selectively enriched the population of CD8+ cells in the TIL cultures and markedly increased IFN-γ production when compared with an HLA-mismatched tumor line.31,58,59,61 An agonistic anti-OX40 antibody added to TIL cultures promoted CD8+ TIL expansion at the expense of CD4+ T cells and significantly enhanced IFN-γ secretion compared with untreated TIL cultures, while maintaining the diverse TCR-V(β) repertoire in both CD8+ and CD4+ cell subsets.60 These preclinical data warrant further investigation into PD-1 inhibition, as well as 4-1BB and/or OX40 stimulation, as avenues to enhance the yield and function of TIL infusion products.
To date, numerous academic institutions and research centers have demonstrated the ability to resect tumors, manufacture TILs, and treat patients with TIL cell therapy. However, tumor procurement and processing protocols vary between centers, and the lack of standardization represents an opportunity to make the process more efficient and reproducible outside research protocols. As TIL cell therapy approaches commercialization, standardized protocols are required. Recent multicenter studies in advanced melanoma, HNSCC, cervical cancer, and NSCLC represent a major advance in this respect.6,12,16 These studies benefit from a centralized Good Manufacturing Practice process, which allows for timely delivery of therapy and facilitates the production of reproducible high-quality TIL cell therapy products for broader patient accessibility. Centralized manufacturing additionally drives increased capacity, and cryopreservation of the final TIL product provides flexibility in scheduling. With these manufacturing advances, TIL cell therapy enters the commercial landscape with a centralized scalable process that can generate TILs from a variety of solid tumor sources received from multiple academic centers/hospitals across the United States and/or abroad.
FUTURE DIRECTIONS AND CONCLUSIONS
Although centralization of TIL manufacturing is a significant advance, tumor resection, site selection, and procurement still require optimization and careful consideration for each patient. Excess tumor beyond what is required for routine diagnostic purposes and TIL manufacturing should be reserved for pathologic or research purposes. Streamlined protocols for rapid tumor resection, fragmentation, and processing to standardize temperature control; choice of medium, reagents, and supporting feeder cells; and sample shipping logistics will greatly enhance TIL production and potency.35
An area of concern has been the substantial proportion of patients who have their tumors resected and TILs cultured but are not able to receive TIL cell therapy because of rapid progression of disease or development of intracranial metastasis, leading to declined performance status.29 The considerably reduced centralized TIL manufacturing time represents a major step toward facilitating timely access of TIL cell therapy to high-risk patients. However, considering the aggressive nature of solid tumors, questions regarding TIL administration as an earlier line of therapy, optimal bridging therapies, and potential tumor banking must also be addressed to further expedite the time to receipt of TIL cell therapy. Implementation of core biopsies and other image-guided minimally invasive procedures for tumor resection (particularly for thoracic and abdominal tumor resections) will reduce surgical morbidity and potentially reduce time to start TIL cell therapy.27
With their expertise in immunotherapy and vital role in patient identification and tumor resection and prosection, surgeons are key contributors to the success of TIL cell therapy and its effective transition from the clinical trial setting to a standard-of-care treatment option. Surgeons, in collaboration with medical oncologists, can facilitate early patient scheduling to ensure access to TIL therapy and timely completion of surgical resection before disease progression or clinical deterioration. Finally, active educational and training opportunities and intrainstitutional and interinstitutional collaborative involvement of surgeons in conjunction with pathologists, radiologists, technicians, and cell therapists are warranted for better treatment planning algorithms, regardless of the tumor type, for wider access and benefit of TIL cell therapy.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Viktoria Gontcharova for her analysis of the TCR repertoire data. Images of image-guided lesion selection presented in Figure 2 were obtained by Dr. Sylvia Lee and Dr. Evidio Domingo-Musibay. Tumor tissue photograph presented in Figure 3 was obtained by Pilip Haurysheu, PA.
Footnotes
Conflicts of Interest and Source of Funding: The authors have disclosed that they have no significant relationships with, or financial interest in, any commercial companies pertaining to this article. Medical writing support, funded by Iovance Biotherapeutics (with specific direction and input from authors), was provided by Swati Ghatpande, PhD, and Jerylin Gan, PhD, of Second City Science (Vaniam Group LLC).
The data/data sets analyzed and interpreted in the current review article are publicly available.
Supplemental digital contents are available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.journalppo.com).
Contributor Information
Michael E. Egger, Email: michael.egger@louisville.edu.
Martin McCarter, Email: MARTIN.MCCARTER@CUANSCHUTZ.EDU.
Bradley J. Monk, Email: bmonk@gog.org.
Eric M. Toloza, Email: Eric.Toloza@moffitt.org.
Susan Brousseau, Email: Susan.Brousseau@iovance.com.
Madan Jagasia, Email: madan.jagasia@iovance.com.
Amod Sarnaik, Email: Amod.Sarnaik@moffitt.org.
REFERENCES
- 1.Kirtane K Elmariah H Chung CH, et al. Adoptive cellular therapy in solid tumor malignancies: review of the literature and challenges ahead. J Immunother Cancer. 2021;9:e002723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsimberidou AM Van Morris K Vo HH, et al. T-cell receptor–based therapy: an innovative therapeutic approach for solid tumors. J Hematol Oncol. 2021;14:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang S Sun J Chen K, et al. Perspectives of tumor-infiltrating lymphocyte treatment in solid tumors. BMC Med. 2021;19:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maeurer MJ Gollin SM Martin D, et al. Tumor escape from immune recognition: lethal recurrent melanoma in a patient associated with downregulation of the peptide transporter protein TAP-1 and loss of expression of the immunodominant MART-1/Melan-A antigen. J Clin Invest. 1996;98:1633–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rohaan MW van den Berg JH Kvistborg P, et al. Adoptive transfer of tumor-infiltrating lymphocytes in melanoma: a viable treatment option. J Immunother Cancer. 2018;6:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sarnaik AA Hamid O Khushalani NI, et al. Lifileucel, a tumor-infiltrating lymphocyte therapy, in metastatic melanoma. J Clin Oncol. 2021;39:2656–2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hawkins REJY Lorigan PC, et al. Clinical feasibility and treatment outcomes with unselected autologous tumor infiltrating lymphocyte therapy in patients with advanced cutaneous melanoma. Cancer Res. 2021;81(suppl 13):Abstract LB150. [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenberg SA Yang JC Sherry RM, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res. 2011;17:4550–4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dudley ME Wunderlich JR Yang JC, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346–2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goff SL Dudley ME Citrin DE, et al. Randomized, prospective evaluation comparing intensity of lymphodepletion before adoptive transfer of tumor-infiltrating lymphocytes for patients with metastatic melanoma. J Clin Oncol. 2016;34:2389–2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dafni U Michielin O Lluesma SM, et al. Efficacy of adoptive therapy with tumor-infiltrating lymphocytes and recombinant interleukin-2 in advanced cutaneous melanoma: a systematic review and meta-analysis. Ann Oncol. 2019;30:1902–1913. [DOI] [PubMed] [Google Scholar]
- 12.O’Malley D Lee S Psyrri A, et al. Phase 2 efficacy and safety of autologous tumor-infiltrating lymphocyte (TIL) cell therapy in combination with pembrolizumab in immune checkpoint inhibitor-naïve patients with advanced cancers. J Immunother Cancer. 2021;9:492. [Google Scholar]
- 13.Larkin J Sarnai AK Chesney JA, et al. Lifileucel (LN-144), a cryopreserved autologous tumor infiltrating lymphocyte (TIL) therapy in patients with advanced melanoma: evaluation of impact of prior anti–PD-1 therapy. J Clin Oncol. 2021;39(suppl 15):abstract 9505. [Google Scholar]
- 14.Creelan B Wang C Teer J, et al. Durable complete responses to adoptive cell transfer using tumor infiltrating lymphocytes (TIL) in non–small cell lung cancer (NSCLC): a phase I trial. Cancer Res. 2020;80:CT056. [Google Scholar]
- 15.Creelan BC Wang C Teer JK, et al. Tumor-infiltrating lymphocyte treatment for anti–PD-1–resistant metastatic lung cancer: a phase 1 trial. Nat Med. 2021;27:1410–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schoenfeld AJ, Lee S, Paz-Ares L. First phase 2 results of autologous tumor-infiltrating lymphocyte (TIL; LN-145) monotherapy in patients with advanced, immune checkpoint inhibitor-treated, non–small cell lung cancer (NSCLC). Presented at the 2021 SITC Annual Meeting; November 10–14, 2021; Washington, DC. Poster 458. [Google Scholar]
- 17.Jazaeri AA Zsiros E Amaria RN, et al. Safety and efficacy of adoptive cell transfer using autologous tumor infiltrating lymphocytes (LN-145) for treatment of recurrent, metastatic, or persistent cervical carcinoma. J Clin Oncol. 2019;37:2538. [Google Scholar]
- 18.Jimeno A Papa S Haigentz M, et al. Safety and efficacy of tumor infiltrating lymphocytes (TIL, LN-145) in combination with pembrolizumab for advanced, recurrent or metastatic HNSCC. J Immunother Cancer. 2020;8:A215–A216. [Google Scholar]
- 19.Stevanovic S Helman SR Wunderlich JR, et al. A phase II study of tumor-infiltrating lymphocyte therapy for human papillomavirus-associated epithelial cancers. Clin Cancer Res. 2019;25:1486–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pedersen M Westergaard MCW Milne K, et al. Adoptive cell therapy with tumor-infiltrating lymphocytes in patients with metastatic ovarian cancer: a pilot study. Onco Targets Ther. 2018;7:e1502905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aoki Y Takakuwa K Kodama S, et al. Use of adoptive transfer of tumor-infiltrating lymphocytes alone or in combination with cisplatin-containing chemotherapy in patients with epithelial ovarian cancer. Cancer Res. 1991;51:1934–1939. [PubMed] [Google Scholar]
- 22.Tran E Robbins PF Lu YC, et al. T-cell transfer therapy targeting mutant KRAS in cancer. N Engl J Med. 2016;375:2255–2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zacharakis N Chinnasamy H Black M, et al. Immune recognition of somatic mutations leading to complete durable regression in metastatic breast cancer. Nat Med. 2018;24:724–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kverneland AH Chamberlain CA Borch TH, et al. Adoptive cell therapy with tumor-infiltrating lymphocytes supported by checkpoint inhibition across multiple solid cancer types. J Immunother Cancer. 2021;9:e003499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crompton JG, Klemen N, Kammula US. Metastasectomy for tumor-infiltrating lymphocytes: an emerging operative indication in surgical oncology. Ann Surg Oncol. 2018;25:565–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahmed F, Haass NK. Microenvironment-driven dynamic heterogeneity and phenotypic plasticity as a mechanism of melanoma therapy resistance. Front Oncol. 2018;8:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zippel DB Besser M Shapira R, et al. Adoptive cell therapy with autologous tumor-infiltrating lymphocytes and high-dose interleukin-2 for metastatic melanoma: the surgeon’s perspective. Exp Ther Med. 2012;3:898–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klapper JA Davis JL Ripley RT, et al. Thoracic metastasectomy for adoptive immunotherapy of melanoma: a single-institution experience. J Thorac Cardiovasc Surg. 2010;140:1276–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goff SL Smith FO Klapper JA, et al. Tumor infiltrating lymphocyte therapy for metastatic melanoma: analysis of tumors resected for TIL. J Immunother. 2010;33:840–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sethuramam J Santiago L Chen JQ, et al. Successful expansion and characterization of tumor infiltrating lymphocytes (TILs) from non-melanoma tumors. Presented at the 31st Annual Meeting of the Society for ImmunoTherapy; November 11–13, 2016; National Harbor, MD. Abstract 42.
- 31.Hall M Liu H Malafa M, et al. Expansion of tumor-infiltrating lymphocytes (TIL) from human pancreatic tumors. J Immunother Cancer. 2016;4:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ripley RT Davis JL Klapper JA, et al. Liver resection for metastatic melanoma with postoperative tumor-infiltrating lymphocyte therapy. Ann Surg Oncol. 2010;17:163–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alvarez-Downing MM Inchauste SM Dudley ME, et al. Minimally invasive liver resection to obtain tumor-infiltrating lymphocytes for adoptive cell therapy in patients with metastatic melanoma. World J Surg Oncol. 2012;10:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mullinax JE Hall M Beatty M, et al. Expanded tumor-infiltrating lymphocytes from soft tissue sarcoma have tumor-specific function. J Immunother. 2021;44:63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cytiva . Improve outcomes for TIL therapies by tackling process challenges. 2020. Available at: https://www.fiercebiotech.com/sponsored/improve-outcomes-for-til-therapies-by-tackling-process-challenges. Accessed June 1, 2022.
- 36.Gastman B Agarwal PK Berger A, et al. Defining best practices for tissue procurement in immuno-oncology clinical trials: consensus statement from the Society for Immunotherapy of Cancer Surgery Committee. J Immunother Cancer. 2020;8:e001583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Joudeh AA, Shareef SQ, Al-Abbadi MA. Fine-needle aspiration followed by core-needle biopsy in the same setting: modifying our approach. Acta Cytol. 2016;60:1–13. [DOI] [PubMed] [Google Scholar]
- 38.Ullenhag GJ Sadeghi AM Carlsson B, et al. Adoptive T-cell therapy for malignant melanoma patients with TILs obtained by ultrasound-guided needle biopsy. Cancer Immunol Immunother. 2012;61:725–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu Z Meng Q Bartek J Jr., et al. Tumor-infiltrating lymphocytes (TILs) from patients with glioma. Onco Targets Ther. 2017;6:e1252894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Caoili EM, Davenport MS. Role of percutaneous needle biopsy for renal masses. Semin Intervent Radiol. 2014;31:20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weiss CR, Nour SG, Lewin JS. MR-guided biopsy: a review of current techniques and applications. J Magn Reson Imaging. 2008;27:311–325. [DOI] [PubMed] [Google Scholar]
- 42.Tam AL Lim HJ Wistuba II, et al. Image-guided biopsy in the era of personalized cancer care: proceedings from the Society of Interventional Radiology Research Consensus Panel. J Vasc Interv Radiol. 2016;27:8–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu M Tapia C Hajjar J, et al. Implementation of a novel Web-based lesion selection tool to improve acquisition of tumor biopsy specimens. J Immunother Precision Oncol. 2021;4:45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aoyama S Nakagawa R Mule JJ, et al. Inducible tertiary lymphoid structures: promise and challenges for translating a new class of immunotherapy. Front Immunol. 2021;12:675538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hopewell EL Cox C Pilon-Thomas S, et al. Tumor-infiltrating lymphocytes: streamlining a complex manufacturing process. Cytotherapy. 2019;21:307–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yugawa K Itoh S Yoshizumi T, et al. Prognostic impact of tumor microvessels in intrahepatic cholangiocarcinoma: association with tumor-infiltrating lymphocytes. Mod Pathol. 2021;34:798–807. [DOI] [PubMed] [Google Scholar]
- 47.Mullinax JE Hall M Prabhakaran S, et al. Combination of ipilimumab and adoptive cell therapy with tumor-infiltrating lymphocytes for patients with metastatic melanoma. Front Oncol. 2018;8:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Massarelli EGZ Cacovean A Yadav B, et al. A phase 2 multicenter study of autologous tumor infiltrating lymphocytes (TIL, LN-145) cell therapy in patients with metastatic non–small cell lung cancer (IOV-LUN-202). Cancer Res. 2021;81(suppl 13):Abstract CT246. [Google Scholar]
- 49.Bajgain P Mucharla R Wilson J, et al. Optimizing the production of suspension cells using the G-rex “M” series. Mol Ther Methods Clin Dev. 2014;1:14015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lu YC Yao X Crystal JS, et al. Efficient identification of mutated cancer antigens recognized by T cells associated with durable tumor regressions. Clin Cancer Res. 2014;20:3401–3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gontcharova V Suzuki S Simpson-Abelson MR, et al. Persistence of cryopreserved tumor-infiltrating lymphocyte product lifileucel (LN-144) in C-144-01 study of advanced metastatic melanoma. Cancer Res. 2019;79:LB-069. [Google Scholar]
- 52.Jazaeri AGV Blaskovich M, et al. In vivo persistence of Iovance tumour-infiltrating lymphocytes LN-145 in cervical cancer patients. Ann Oncol. 2020;31:S642. [Google Scholar]
- 53.Rosati E Dowds CM Liaskou E, et al. Overview of methodologies for T-cell receptor repertoire analysis. BMC Biotechnol. 2017;17:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang C Levy S Yang Q, et al. Evaluation of three next generation sequencing platforms for immune repertoire sequencing (58.10). J Immunol. 2012;188:58.10. [Google Scholar]
- 55.Wu R Forget MA Chacon J, et al. Adoptive T-cell therapy using autologous tumor-infiltrating lymphocytes for metastatic melanoma: current status and future outlook. Cancer J. 2012;18:160–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fardis M DiTrapani K Chartier C, et al. Current and future directions for tumor infiltrating lymphocyte therapy for the treatment of solid tumors. Cell Gene Ther Insights. 2020;6:855–863. [Google Scholar]
- 57.Wardell S Lienlaf-Moreno M Veerapathran A, et al. A 16-day generation 3 (Gen 3) tumor-infiltrating lymphocyte (TIL) manufacturing process is reproducible for low-volume tissue explants and low-infiltrate tumor indications. Abstract submitted to the 2022 Cancer Immunotherapy: Decoding the Cancer Immunity Interactome Keystone Symposium; March 20–24, 2022; Whistler, BC, Canada.
- 58.Simpson-Abelson MRMC, Frank I, Ritthipichai K, Chartier C. The T-cell growth factor cocktail IL-2/IL-15/IL-21 enhances expansion and effector function of tumor-infiltrating T cells in a novel process developed by Iovance. Presented at the SITC 2017 Annual Meeting; November 8, 2017; National Harbor, MD. Poster 357.
- 59.Chacon JA Wu RC Sukhumalchandra P, et al. Co-stimulation through 4-1BB/CD137 improves the expansion and function of CD8(+) melanoma tumor-infiltrating lymphocytes for adoptive T-cell therapy. PLoS One. 2013;8:e60031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ritthipichai K Machin M Fardis M, et al. Anti-OX40 agonistic antibody enhances ex vivo CD8+ TIL expansion with increased T-cell effector function. Cancer Res. 2018;78:LB-110-LB-10. [Google Scholar]
- 61.Sakellariou-Thompson D Forget MA Creasy C, et al. 4-1BB agonist focuses CD8(+) tumor-infiltrating T-cell growth into a distinct repertoire capable of tumor recognition in pancreatic cancer. Clin Cancer Res. 2017;23:7263–7275. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


