Abstract
Objective:
While adult cochlear implant (CI) outcomes have primarily focused on speech recognition scores, the rigorous development of a CI-specific patient-reported outcome measure provides an opportunity for a more comprehensive and ecologically valid approach to measure the real-world functional abilities of adult CI users. Here, we report for the first time normative Cochlear Implant Quality of Life (CIQOL)-35 Profile and global scores and variance for a large, multi-institutional sample of adult CI users.
Study Design:
Cross-sectional study design
Setting:
CI centers in the United States
Patients:
705 adults with bilateral moderate to profound hearing loss with at least one year of CI use.
Intervention(s):
Cochlear implantation
Main Outcome Measure(s):
CIQOL-35 Profile and CIQOL-10 Global scores
Results:
During the development of the CIQOL instruments, 1000 CI users from all regions of the United States were invited to participate in studies. Of these 705 (70.5%) completed all portions of the study and their data are reported here. Mean CIQOL domain scores were highest (indicating better function) for the emotional and social domains and lowest for listening effort. The entertainment and social domains demonstrated the widest distribution of scores and largest standard deviations, indicating greatest variability in function. Overall, there were minimal ceiling and floor effects for all domains.
Conclusions:
Normative scores from a large sample of experienced adult CI users are consistent with clinical observations, showing large differences in functional abilities and large variability. Normative CIQOL data for adult CI users has the potential to enhance pre-operative discussions with CI candidates, improve post-CI activation monitoring and establish standards for CI centers.
Introduction
Cochlear implantation is the standard of care for adults with moderate to profound hearing loss who no longer benefit from hearing aids. The vast majority of cochlear implant (CI) users demonstrate substantial improvements in speech recognition ability after implantation based on scores obtained in controlled environments, as is the current standard outcome measure in clinical practice1. However, benefits of cochlear implantation extend well beyond improvements in receptive communication abilities2,3, as indicated by absent-to-low associations between speech recognition scores (even in background noise) and patients’ reported communication and other functional abilities4–6. Thus, the reliance on speech recognition scores as the sole or primary outcome measure provides a poor surrogate for patients’ real-world communication abilities and limited knowledge of the broad impact of cochlear implantation on patients’ lives6–14.
In response to these limitations, we have developed and validated the Cochlear Implant Quality of Life-35 Profile (CIQOL-35 Profile) instrument and CIQOL-10 Global measure. The CIQOL-35 Profile provides an assessment of CI users’ functional abilities across 6 domains (communication, emotional, entertainment, environment, listening effort, and social) and the CIQOL-10 Global provides an overall measure of CI-related quality of life. Using a mixed methods research design that included stakeholder engagement and rigorous analyses, the CIQOL instruments have been shown to be more comprehensive and psychometrically sound than legacy patient-reported outcome measures (PROMs) used to assess CI outcomes3,15–17.
Here, we report for the first time mean CIQOL domain and global scores and variance for a large, multi-institutional sample of adult CI users, using data collected for the development and validation of the CIQOL insturments15–17. Although certain data for the development and validation of the CIQOL-35 Profile have been reported previously15–17, CIQOL-35 Profile scores for the entire sample of CI users were not included. These normative data, representative of typically performing experienced (≥12 months) CI users, can be compared to outcomes of individual CI users as one way of assessing their self-reported functional abilities and may be useful for counseling potential CI candidates.
Methods
IRB approval was obtained through our institution. The study sample included 705 CI users who were recruited through the 30-institution CIQOL Development Consortium15,17. The Consortium was established to recruit a large sample of CI users who were representative of the broader adult CI population. Participants (1) were between 18–89 years of age (as individuals >89 years of age are considered a special population), (2) used a CI for one year or more, (3) had post-lingual hearing loss, and (4) did not receive a CI for single sided deafness. The CIQOL-35 Profile was completed through REDCap (Research Electronic Data Capture), a secure web-based data collection platform, along with a demographic and hearing/CI history questionnaire. Participants also obtained their most recent best aided speech recognition scores from their audiologist and entered them into REDCap. These scores could include Consonant-Nucleus-Consonant (CNC) word scores and AzBio sentence scores in quiet and in noise at a +10 dB signal-to-noise ratio (SNR), as these are components of the minimum reporting standards1. Participants were not excluded if they could not obtain their speech recognition scores. Details regarding the CIQOL-35 Profile instrument (items, response options, scoring) can be found in previous publications15,16.
Participant demographics and hearing characteristics are summarized using descriptive statistics. The CIQOL-35 Profile domain and global scores are presented using descriptive and distribution statistics. To supplement previous analyses4,15, we also report Spearman correlations coefficients between CIQOL-35 Profile domain scores and duration of CI use.
Results
The CIQOL-35 Profile instrument was provided by email link to the first 1000 individuals who contacted our research team. Of these, 705 (70.5%) CI users completed all portions of the CIQOL-35 Profile and are included in the current analyses. Demographics of these participants are displayed in Table 1. Most participants were married without children living in the household. Annual household income levels were evenly split among the categories except the lowest bracket. All regions of the United States were represented with the South having the highest percentage of subjects (37.2%). Individuals from the local institution represented only 2.8% of those who completed the CIQOL-35 instrument. Overall, participants represented the full range of age, duration of CI use, speech recognition abilities, and listening modalities of the adult CI population. In addition, all three CI manufacturers’ devices were represented. (Tables 1 and 2).
Table 1:
Demographic and cochlear implant characteristics of the study sample.
| Variable | N (%) |
|---|---|
|
| |
| Sex | |
| Male | 285 (40.4) |
| Female | 420 (59.6) |
| Marital Status | |
| Married/Domestic partnership | 472 (67.0) |
| Not Married/No domestic partnership | 233 (33.0) |
| Combined Annual Household Income | |
| $0–$20,000 | 40 (5.7) |
| $20,001–$50,000 | 129 (18.3) |
| $50,001–$80,000 | 166 (23.5) |
| $80,001–$110,000 | 125 (17.7) |
| >$110,000 | 179 (25.4) |
| Unknown/Not reported | 66 (9.4) |
| Highest Level of Education | |
| Did not complete high school | 3 (0.4) |
| High school graduate or equivalent | 46 (6.5) |
| Some college/trade/technical/vocational training | 125 (17.8) |
| Associate degree | 67 (9.5) |
| Bachelor’s degree | 221 (31.3) |
| Master’s degree or higher | 243 (34.5) |
| Employment Status | |
| Employed | 311 (44.1) |
| Not employed | 90 (12.8) |
| Retired | 304 (43.1) |
| Residential Setting | |
| Urban | 167 (23.7) |
| Suburban | 408 (57.9) |
| Rural | 130 (18.4) |
| Region of US | |
| West | 176 (24.9) |
| Midwest | 158 (22.4) |
| Northeast | 96 (13.6) |
| South/Southwest | 262 (37.2) |
| Unknown/Not reported | 13 (1.8%) |
| CI Company | |
| Advanced Bionics | 138 (19.6) |
| Cochlear | 343 (48.7) |
| MED-EL | 223 (31.6) |
| Not reported | 1 (0.1) |
| Listening Modality | |
| Bilateral CI | 346 (49.1) |
| CI and Hearing Aid | 201 (28.5) |
| CI without Hearing Aid | 158 (22.4) |
| Combined electro-acoustic hearing (hybrid) | |
| No | 678 (96.3) |
| Yes | 26 (3.7) |
| No response | 1 (0.1) |
Table 2:
Participant demographic, hearing and CI history
| Variable | Mean (SD) |
|---|---|
|
| |
| Age, years | 59.5 (15.2) |
| Duration of hearing loss prior to implantation, years | 26.6 (18.1) |
| Duration of CI use, years | 7.6 (6.7) |
| CNC Word scores (%, n=371) | 68.4 (23.8) |
| AzBio Sentence scores in quiet (%, n=378) | 78.7 (24.1) |
| AzBio Sentence scores in noise at +10 dB SNR (%, n=252) | 64.4 (26.2) |
n indicates the number of participants who were able to provide speech recognition scores
Cochlear implant users’ CIQOL-35 Profile domain and the Global measure mean scores are displayed in Table 3 and the distribution of scores are displayed in Figure 1. Higher scores indicate better self-reported functional abilities. Mean scores were highest for the emotional and social domain and lowest for listening effort. In addition, the entertainment and social domains had the widest distribution of scores and the largest standard deviations (SDs). Overall, there were low ceiling (score=100) and floor (score=0) effects for all domains, as indicated by the number of CI users with the highest and lowest possible score, respectively (Table 3). The largest ceiling effects were observed for the social (n=79; 11.2%) and entertainment (n=56; 7.9%) domains and the largest floor effects were observed for the entertainment domain (n=18; 2.6%).
Table 3:
CIQOL-35 domain and global scores for study participants
| Domain | Mean (SD) | Skew | Kurtosis | Ceiling N (%) | Floor N (%) |
|---|---|---|---|---|---|
|
| |||||
| Global | 52.6 (±10.9) | 0.19 | 0.21 | 0 (0) | 0 (0) |
|
| |||||
| Communication | 51.4 (±13.3) | 0.28 | 0.89 | 4 (0.57) | 1 (0.14) |
| Emotional | 64.7 (±15.9) | −0.04 | 0.05 | 29 (4.11) | 0 (0) |
| Entertainment | 55.8 (±23.0) | −0.01 | −0.16 | 56 (7.94) | 18 (2.55) |
| Environment | 61.0 (±17.7) | 0.07 | −0.06 | 28 (3.97) | 1 (0.14) |
| Listening Effort | 41.5 (±14.8) | 0.27 | 0.20 | 0 (0) | 3 (0.43) |
| Social | 67.7 (±19.1) | −0.07 | −0.38 | 79 (11.2) | 1 (0.14) |
Figure 1:
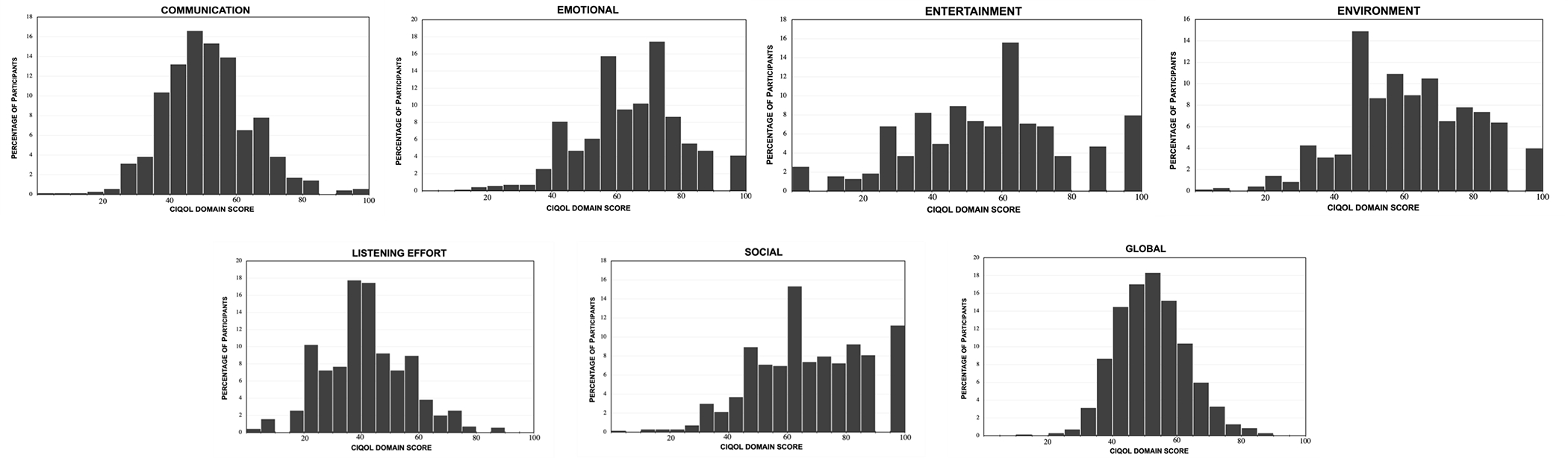
Histograms representing the distribution of CIQOL scores for the six domains, and global scores. Each dividing integer on the horizontal axis includes a CIQOL domain scores up to the previous value.
A previous study identified patient- and hearing-related factors associated with CIQOL-35 scores4. Using the normative data, we sought to determine the association between duration of CI use and CIQOL-35 domain scores. Spearman’s correlation coefficients between duration of CI use (quantified as a continuous variable) and CIQOL-35 domain scores were weak (all r < 0.20) for all domains; correlation coefficients ranged from r = 0.11 (95%CI 0.04–0.19) for the social domain to r = 0.19 (95%CI 0.11–0.26) for the communication domain. Figure 2 further illustrates that, based on these cross-sectional data, CIQOL domain and global scores did not differ based on time since cochlear implantation. The box and whisker plots also provide visual display of the differences in variability observed for each domain. While these cross-sectional results suggest that there is not, on average, a significant increase in CIQOL domain and global scores beyond one-year post-CI activation, future longitudinal studies are needed to investigate patterns of improvement for individual patients over time.
Figure 2:
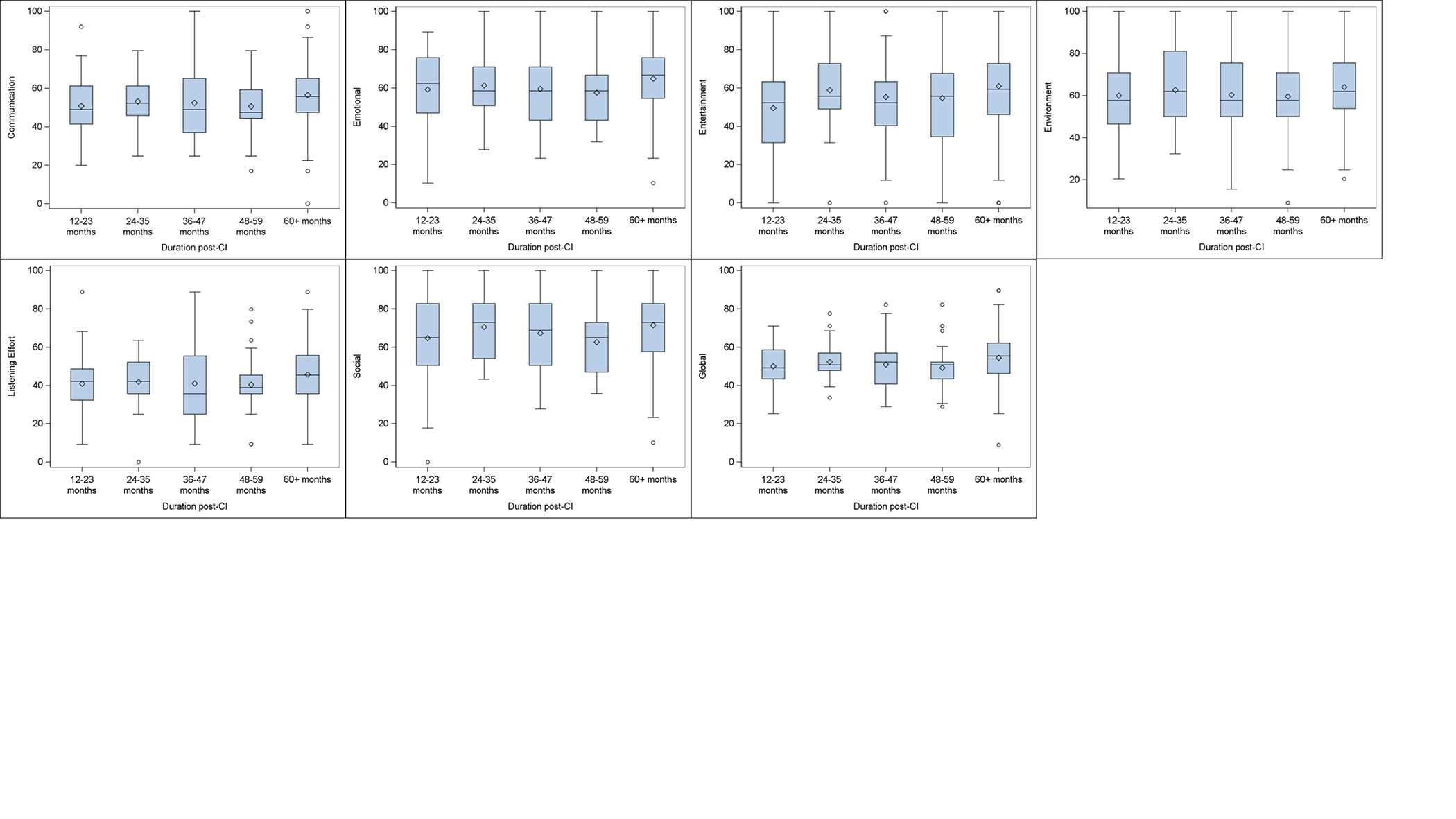
CIQOL-35 domain and global scores for five ranges of durations of CI use. Diamonds represent the mean value and circles represent outliers
Discussion
The CIQOL-35 Profile instrument and CIQOL-10 Global measure were developed to provide a more comprehensive understanding of the functional benefits of cochlear implantation that extend beyond improvements in speech recognition ability measured in control environments. As such, the CIQOL instruments allow patients to describe their functional abilities within 6 domains that have been demonstrated to be important to adult CI users. Given that this large sample of patients broadly represent experienced adult CI users across the Unites States, the means and variances of CIQOL domain and global scores serve as normative data for comparison to the functional abilities of individual adult CI users or CI candidates.
Normative CIQOL-35 scores can be helpful for numerous clinical and research applications. These data can be used during pre-operative counseling where potential CI users can compare their baseline CIQOL scores to these data to better understand the degree of potential improvement at least one year after implantation. We have summarized these data in Figure 3 in a reverse cumulative distribution plot for ease of use as a clinical tool. These distributions display the percentage of CI users that obtain each score or higher for each CIQOL domain (colors) and the global measure (black). For example, if a potential CI user’s CIQOL-communication score prior to implantation is 51.3 (x-axis), Figure 3 shows that less than 50% of patients (y-axis) achieve that score or higher at least one year after implantation. As another example, patients with domain scores on the CIQOL obtained prior to implantation that are lower than the values in Figure 3 for experienced users would anticipate some degree of improvement with time. In this way, these normative data provide the information needed for evidence-based counseling so CI candidates can have realistic expectations regarding potential functional abilities.
Figure 3:
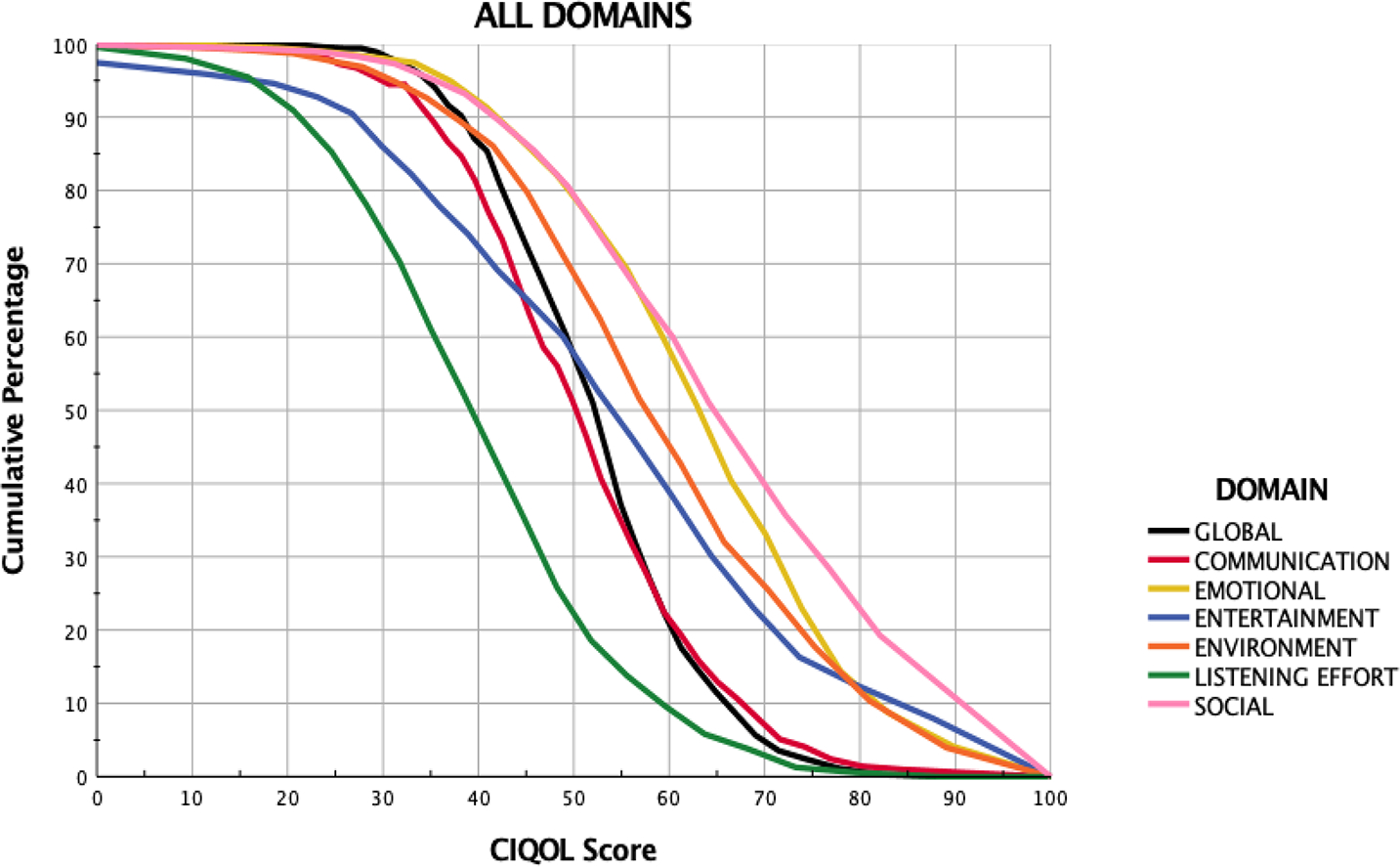
Reverse cumulative distribution curves
As a supplement to speech recognition scores, clinicians can also compare a CI user’s CIQOL scores to the normative data as a marker for success after implantation. Here, clinicians may determine whether additional domain-specific resources (e.g., alternative programming strategies, auditory rehabilitation, second CI) are needed for those experienced patients whose CIQOL scores fall well below mean values (based on Figure 2 and 3 or Table 3). Thus, CIQOL-35 Profile domain and global scores can be used to directly influence clinical decision making for individual patients, consistent with precision medicine. On a more programmatic level for quality control, these data can also be used by CI centers to provide benchmarks to ensure their patients are achieving CI outcomes that are aligned with, or within the range of, experienced CI users across the United States. Such benchmarks have never been available for previously developed PROMs and this work provides the foundation for establishing outcome standards for CI programs. Similarly, these data can be used for clinical research and clinical trials to ensure that participant outcomes are representative of typical CI users.
The large variability in CI outcomes observed in the current study are consistent with those seen for speech recognition scores8,11,12,18 and legacy PROMs5,6,19. Of interest is that the degree of variability differed based on domain, with patients’ reported abilities differing most within the entertainment and social domains. It is well established that CI users’ music appreciation and communication vary widely, but far less is known about their social abilities and listening effort20–23. One of our previous studies was designed to explain this variability by determining associations between patient-related factors and CIQOL domain scores. Using multivariable analyses, we found that higher household income, being employed, living in certain regions of United States, and using bilateral CIs were associated with higher CIQOL scores in one or more domains. However, the effect size of each was small and our regression models for each domain accounted for only a small percentage of the variance (R2= 0.08–0.17)4. Thus, additional research is needed to accurately predict potential CI user outcomes and enhance patient counseling and expectations prior to implantation.
Study Limitations
The online format of the study introduces some limitations. First, all participants were required to have access to certain devices to complete the CIQOL-35 instrument via computer/tablet/smartphone. Paper versions of the CIQOL were offered to participants but were never requested. Second, because participants were recruited through CI centers, we assumed that all participants met inclusion criteria and provided accurate information. In addition, information such as date of implantation and duration of hearing loss prior to implantation were not confirmed with physicians or audiologists. Nevertheless, the advantages of recruiting a large sample of CI users who represented the adult CI population across the United States outweigh these limitations.
Conclusions
Normative CIQOL-35 domain and global scores from a large sample of experienced adult CI users are consistent with clinical observations, showing large differences in functional abilities across six domains and large variability within each domain. The availability of normative CIQOL data for adult CI users has many potential clinical and research applications including enhancing pre-operative discussions with CI candidates, monitoring and enhancing patient outcomes following implantation, and establishing standards for CI centers. Future prospective, longitudinal cohort studies using the CIQOL-35 instruments are needed to determine patterns of post-implantation changes and factors (such as overall duration and hours of CI use) that contribute to improvements in functional abilities.
Acknowledgements:
The CIQOL instruments are available for download at: education.musc.edu/CIQOL. The Cochlear Implant Quality of Life Development Consortium consists of the following institutions (and individuals): Columbia University (Justin S. Golub, MD, MS), Duke University Hospital (Erin Blackburn, AuD; Howard Francis, MD; Amy Walker), Eastern Virginia Medical School (Stephanie Moody-Antonio, MD), Georgetown University (Michael Hoa, MD), House Ear Clinic (Eric P. Wilkinson, MD; Dawna Mills, AuD), Johns Hopkins University (John P. Carey, MD), Kaiser Permanente-Los Angeles (Nopawan Vorasubin, MD), Kaiser Permanente-San Diego (Vickie Brunk, AuD), Loyola University Medical Center (Matthew Kirchner, MD), Massachusetts Eye and Ear Infirmary (Kevin Frank, PhD; Elizabeth DesRoche, AuD), Mayo Clinic Rochester (Matthew L. Carlson, MD; Collin L. Driscoll, MD, Medical University of South Carolina (Elizabeth L. Camposeo, AuD; Paul R. Lambert, MD; Ted A. Meyer, MD PhD), New York Eye and Ear Infirmary (Maura Cosetti, MD), The Ohio State University (Aaron C. Moberly, MD), Rush University (Mike Hefferly, PhD; Mark Wiet, MD), Stanford University (Nikolas H. Blevins, MD; Jannine B. Larky, AuD), State University of New York-Downstate (Matthew Hanson, MD), Summit Medical Center (Jed Kwartler, MD), University of Arkansas for Medical Sciences (John Dornhoffer, MD), University of Cincinnati (Ravi N. Samy, MD), University of Colorado (Samuel P. Gubbels, MD), University of Maryland School of Medicine (Ronna P. Herzano, MD, PhD), University of Miami (Michael E. Hoffer, MD; Meredith A. Holcomb AuD; Sandra M. Prentiss, PhD), University of Pennsylvania (Jason Brant, MD), University of Texas Southwestern (Jacob B. Hunter, MD; Brandon Isaacson, MD; J. Walter Kutz, MD), University of Utah (Richard K. Gurgel, MD), Virginia Mason Medical Center (Daniel M. Zeitler, MD), Washington University-Saint Louis (Craig A. Buchman, MD; Jill B. Firszt, PhD); Vanderbilt University (Rene H. Gifford, PhD; David S. Haynes, MD; Robert F. Labadie, MD, PhD).
Funding and conflicts of interest:
This research was supported (in part) by a grant from the National Institutes of Health, National Institute on Deafness and Other Communication Disorders, Grant Number K23 DC016911, and a grant from the American Cochlear Implant Alliance. TRM is on the medical advisory board for Envoy Medical.
References
- 1.Adunka OF, Gantz BJ, Dunn C, Gurgel RK, Buchman CA. Minimum Reporting Standards for Adult Cochlear Implantation. Otolaryngol Head Neck Surg 2018:194599818764329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hughes SE, Hutchings HA, Rapport FL, McMahon CM, Boisvert I. Social Connectedness and Perceived Listening Effort in Adult Cochlear Implant Users: A Grounded Theory to Establish Content Validity for a New Patient-Reported Outcome Measure. Ear Hear 2018; 39:922–934. [DOI] [PubMed] [Google Scholar]
- 3.McRackan TR, Velozo CA, Holcomb MA, Camposeo EL, Hatch JL, Meyer TA, Lambert PR, Melvin CL, Dubno JR. Use of Adult Patient Focus Groups to Develop the Initial Item Bank for a Cochlear Implant Quality-of-Life Instrument. JAMA Otolaryngol Head Neck Surg 2017; 143:975–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McRackan TR, Hand BN, Velozo CA, Dubno JR. Association of Demographic and Hearing-Related Factors With Cochlear Implant-Related Quality of Life. JAMA Otolaryngol Head Neck Surg 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McRackan TR, Bauschard M, Hatch JL et al. Meta-analysis of Cochlear Implantation Outcomes Evaluated With General Health-related Patient-reported Outcome Measures. Otol Neurotol 2018; 39:29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McRackan TR, Bauschard M, Hatch JL, Franko-Tobin E, Droghini HR, Nguyen SA, Dubno JR.. Meta-analysis of quality-of-life improvement after cochlear implantation and associations with speech recognition abilities. Laryngoscope 2018; 128:982–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McRackan TR, Fabie JE, Bhenswala PN, Nguyen SA, Dubno JR. General Health Quality of Life Instruments Underestimate the Impact of Bilateral Cochlear Implantation. Otol Neurotol 2019; 40:745–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dunn C, Miller SE, Schafer EC, Silva C, Gifford RH, Grisel JJ. Benefits of a Hearing Registry: Cochlear Implant Candidacy in Quiet Versus Noise in 1,611 Patients. Am J Audiol 2020:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lenarz M, Sönmez H, Joseph G, Büchner A, Lenarz T. Long-term performance of cochlear implants in postlingually deafened adults. Otolaryngol Head Neck Surg 2012; 147:112–118. [DOI] [PubMed] [Google Scholar]
- 10.Zwolan TA, Kallogjeri D, Firszt JB, Buchman CA. Assessment of Cochlear Implants for Adult Medicare Beneficiaries Aged 65 Years or Older Who Meet Expanded Indications of Open-Set Sentence Recognition: A Multicenter Nonrandomized Clinical Trial. JAMA Otolaryngol Head Neck Surg 2020; 146:933–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holden LK, Firszt JB, Reeder RM, Uchanski RM, Dwyer NY, Holden TA. Factors Affecting Outcomes in Cochlear Implant Recipients Implanted With a Perimodiolar Electrode Array Located in Scala Tympani. Otol Neurotol 2016; 37:1662–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holden LK, Finley CC, Firszt JB et al. Factors affecting open-set word recognition in adults with cochlear implants. Ear Hear 2013; 34:342–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao EE, Dornhoffer JR, Loftus C et al. Association of Patient-Related Factors With Adult Cochlear Implant Speech Recognition Outcomes: A Meta-analysis. JAMA Otolaryngol Head Neck Surg 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dornhoffer JR, Reddy P, Meyer TA, Schvartz-Leyzac KC, Dubno JR, McRackan TR. Individual Differences in Speech Recognition Changes After Cochlear Implantation. JAMA Otolaryngol Head Neck Surg 2021; 147:280–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McRackan TR, Hand BN, Velozo CA, Dubno JR, Consortium CIQoL. Validity and reliability of the Cochlear Implant Quality of Life (CIQOL)-35 Profile and CIQOL-10 Global instruments in comparison to legacy instruments. Ear Hear 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McRackan TR, Hand BN, Velozo CA, Dubno JR, Consortium CIQoLD. Cochlear Implant Quality of Life (CIQOL): Development of a Profile Instrument (CIQOL-35 Profile) and a Global Measure (CIQOL-10 Global). J Speech Lang Hear Res 2019:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McRackan TR, Hand BN, Velozo CA, Dubno JR, Consortium CIQoLD. Development of the Cochlear Implant Quality of Life Item Bank. Ear Hear 2018. 40(4):1016–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fabie JE, Keller RG, Hatch JL et al. Evaluation of Outcome Variability Associated With Lateral Wall, Mid-scalar, and Perimodiolar Electrode Arrays When Controlling for Preoperative Patient Characteristics. Otol Neurotol 2018; 39:1122–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hinderink JB, Krabbe PF, Van Den Broek P. Development and application of a health-related quality-of-life instrument for adults with cochlear implants: the Nijmegen cochlear implant questionnaire. Otolaryngol Head Neck Surg 2000; 123:756–765. [DOI] [PubMed] [Google Scholar]
- 20.Limb CJ, Roy AT. Technological, biological, and acoustical constraints to music perception in cochlear implant users. Hear Res 2014; 308:13–26. [DOI] [PubMed] [Google Scholar]
- 21.Limb CJ, Rubinstein JT. Current research on music perception in cochlear implant users. Otolaryngol Clin North Am 2012; 45:129–140. [DOI] [PubMed] [Google Scholar]
- 22.Riley PE, Ruhl DS, Camacho M, Tolisano AM. Music Appreciation after Cochlear Implantation in Adult Patients: A Systematic Review. Otolaryngol Head Neck Surg 2018; 158:1002–1010. [DOI] [PubMed] [Google Scholar]
- 23.Hwa TP, Wen CZ, Ruckenstein MJ. Assessment of music experience after cochlear implantation: A review of current tools and their utilization. World J Otorhinolaryngol Head Neck Surg 2021; 7:116–125. [DOI] [PMC free article] [PubMed] [Google Scholar]


