Abstract
Aspergillus fumigatus produces diverse secondary metabolites whose biological functions and regulation remain to be understood. Despite the importance of the conidia for this fungus, the role of the conidia-born metabolite fumiquinazoline C (FqC) is unclear. Here, we describe a dual function of the cell-wall integrity pathway in regulating FqC biosynthesis dictated by the MAPK kinase MpkA, which phosphorylates one of the nonribosomal peptide synthetases enzymes of the cluster (FmqC), and the transcription factor RlmA, which directly regulates the expression of fmq genes. Another level of crosstalk between the FqC regulation and the cell physiology is described since the deletion of the stress-responsive transcription factor sebA provokes derepression of the fmq cluster and overproduction of FqC. Thus, we describe a mechanism by which A. fumigatus controls FqC biosynthesis orchestrated by MpkA-RlmA and SebA and hence enabling survival and adaptation to the environmental niche, given that FqC is a deterrent of ameba predation.
Keywords: cell wall, integrity pathway, fumiquinazoline C, pkcA, mpkA, rlmA, sebA, amoeba predation
Introduction
Conidia (asexual spores) are the dispersive propagules for most of the known filamentous fungi. The ability of the human pathogen Aspergillus fumigatus to produce massive amounts of such structures is a hallmark in comparison to other pathogenic molds and is recognized as a critical element that facilitates its environmental dispersion and access to the human airways (Kwon-Chung and Sugui 2013). Subsequently, conidial germination and mycelium formation in the human lungs of severely immunocompromised patients can cause the life-threatening disease invasive pulmonary aspergillosis, which is associated with high mortality rates (Brown et al. 2012; Bongomin et al. 2017; Latge and Chamilos 2019).
As an environmentally acquired pathogen, A. fumigatus spends most of its life cycle in the soil (Casadevall et al. 2019; Ferling et al. 2020). The structural and chemical elements that decorate the conidia surface, such as the secondary metabolites (SM), are considered important self-defense weapons because they can help A. fumigatus to survive in natural niches where it faces multikingdom microbial competition and predation by soil inhabitants (Hillmann et al. 2015; Ferling et al. 2020). These observations support the idea that soil ameba serves as a selection mechanism, leading to the emergence of virulence traits in different microbes via the development of strategies to counteract phagocytic uptake or intracellular passage (Erken et al. 2013; Novohradska et al. 2017; Casadevall et al. 2019).
It has been shown that the presence of SMs accumulated in the A. fumigatus aerial structures interferes in conidial uptake by mammalian phagocytes and also from soil predators such as frugivorous (Protostelium aurantium) and nonfrugivorous (Dictyostelium discoideum) amebas (Hillmann et al. 2015; Raffa and Keller 2019; Ferling et al. 2020). Among the main SMs enriched in A. fumigatus conidia, 1,8-dihydroxynaphthalene-melanin and trypacidin have received most of the attention over the past years with a focus on how these prototypical conidia-born SM representatives confer a dual-usage capability to defend against phagocytes in the environment and animal hosts (Jahn et al. 2002; Gauthier et al. 2012; Hillmann et al. 2015; Mattern et al. 2015; Hagiwara et al. 2017).
Despite the significance of the conidial structure to the biology of A. fumigatus, the entire landscape and biological activity of A. fumigatus conidia SM toolkit are still not thoroughly explored, neither their role at the infection site nor their antiphagocytic properties (Latge and Chamilos 2019; Raffa and Keller 2019). Chemical fractionation analysis indicated that five major molecules, namely tryptoquivaline F, questin, monomethylsulochrin, trypacidin, and the fumiquinazoline C (FqC) are associated with A. fumigatus conidia (Gauthier et al. 2012; Lim et al. 2014). Among them, FqC selectively accumulates in conidial tissues and its regulation is dependent on the pivotal regulator of conidiogenesis BrlA (Lim and Keller 2014; Lind et al. 2018). Interestingly, the Fq biosynthetic gene cluster (BGC) comprises five genes encoding two nonribosomal peptide synthetases (NRPS), fmqA and fmqC, a mono-oxygenase (fmqB), an oxidoreductase (fmqD), and a putative transporter (fmqE). However, the absence of a cluster-associated transcription factor (TF) suggests that global regulators may be responsible for Fq activation in A. fumigatus.
Aspergillus fumigatus is notoriously recognized for its ability to adapt to environmental changes and sustaining growth while regulating morphologic transitions to produce invasive hyphae and asexual spores depending on the growth substrate. This metabolic adjustment occurs via the activation of signaling cascades, which also influence SM production. One signaling cascade widely employed by fungi to maintain cell viability in response to different stimuli and across the A. fumigatus morphotypes transitions is the Cell Wall Integrity Pathway (CWIP; Rocha et al. 2020a). The apical kinase of the CWIP, PkcA, is central to the signaling through this cascade and leads to the activation of the mitogen-activated protein kinase (MAPK) MpkA and the downstream TF RlmA (Valiante et al. 2009; Rocha et al. 2015, 2016, 2020a, 2020b; Valiante et al. 2016). Previously, the control of SM biosynthesis by the CWIP has been described in A. fumigatus. Deletion of mpkA caused defective production of gliotoxin and increased siderophores and pyomelanin production, whereas the accumulation of the conidial pigment 1,8-dihydroxynaphthalene-melanin is perturbed in both ΔmpkA and ΔrlmA mutants (Valiante et al. 2009, 2016; Jain et al. 2011). However, the overall complexity underlying SM production and regulation is mostly unclear.
Based on our previous observations that the CWIP plays a role in the asexual differentiation of A. fumigatus (Rocha et al. 2020a), here we further probed the contribution of this signaling cascade, concerning the chemical composition of the conidia. We observed that conidial FqC production in the CWIP mutants is lower than in the wild type, especially in the ΔmpkA strain. Additionally, we proved that two general TFs, RlmA and the stress-responsive zinc finger SebA (Dinamarco et al. 2012), directly bind to the promoters of genes belonging to the Fq BGC regulating their expression. Furthermore, concomitant protein–protein interactions between MpkA and the two NRPS enzymes in the cluster are reported. We observe that MpkA is the single kinase that phosphorylates one of them (FmqC), suggesting an unprecedented role of post-translational modifications played by a fungal MAPK. Finally, we provide evidence that the FqC biological activity is to protect conidia from phagocytosis exerted by both the soil ameba D. discoideum and the mouse macrophages. We propose that the production of Fq occurs under stress conditions and that RlmA and SebA integrate signals from different pathways and induce transcriptional responses to co-ordinate the accumulation of this mycotoxin.
Materials and methods
Strains and growth media
The A. fumigatus strains used in this study are summarized in Table 1. Detailed information about the generation of mutant strains is shown in Supplementary Figures S1 and S2. All the strains were maintained in complete or minimal medium as described previously (Malavazi and Goldman 2012). To grow pyrG− auxotrophic strains, the media was supplemented with 1.2 g/L of uridine and uracil. If required, pyrithiamine or hygromycin was added to the culture medium as in reference (Valiante et al. 2009). Synchronized asexual differentiation was performed at 37°C and conducted as described previously (Rocha et al. 2020a). Samples were collected after 6, 12, and 24 h post-asexual differentiation induction, flash-frozen in liquid nitrogen, and stored at −80°C until used for RNA and protein extractions or ChIP procedures. Mycelia obtained from the submerged cultures (hyphal state) were used as control.
Table 1.
Aspergillus fumigatus strains used in this study
| Strain | Relevant genotype | Reference |
|---|---|---|
| ΔKU80 pyrG1a | ΔakuB; pyrG-MAT1-1 | da Silva Ferreira et al. (2006) |
| Afs35 | wild-type | FGSC A1159 |
| ΔrlmA | ΔrlmA::pyrG; ΔakuB | Rocha et al. (2016) |
| rlmA::GFP | rlmA::GFP::pyrG; ΔakuB | Rocha et al. (2016) |
| ΔrlmA::rlmA+ | ΔrlmA::rlmA+; ΔakuB | Rocha et al. (2016) |
| pkcA G579R | pkcA G579R::pyrG; ΔakuB | Rocha et al. (2015) |
| pkcA G579R::pkcA+ | pkcA G579R::pkcA+; ΔakuB | Rocha et al. (2015) |
| ΔmpkA | ΔmpkA::prtA; ΔakuB, PtR | Valiante et al. (2009) |
| ΔmpkA::mpkA+ | ΔmpkA::mpkA+; ΔakuB | Valiante et al. (2009) |
| ΔrlmA ΔmpkA | ΔrlmA; ΔmpkA::prtA; ΔakuB | Rocha et al. (2016) |
| ΔrlmA pkcAG579R | ΔrlmA; ΔmpkA::prtA; ΔakuB | Rocha et al. (2016) |
| ΔsebA | ΔsebA::pyrG; ΔakuB | Dinamarco et al. (2012) |
| ΔsebA::sebA+ | ΔsebA::sebA+; ΔakuB | Dinamarco et al. (2012) |
| sebA::GFP | sebA::GFP::pyrG; ΔakuB | Dinamarco et al. (2012) |
| ΔfmqA | ΔfmqA::ptrA; ΔakuB, PtR | This study |
| ΔfmqA::fmqA+ | ΔfmqA::fmqA+; ΔakuB, HygR | This study |
| TFYL48.1 | gpdA(p)::fmqA::GFP::pyrG | Lim et al. (2014) |
| TFYL50.1 | gpdA(p)::fmqC::GFP::pyrG | Lim et al. (2014) |
| TFYL34.1 | gpdA(p)::fmqD::GFP::pyrG | Lim et al. (2014) |
| fmqA::GFP mpkA::3×HA | gpdA(p)::fmqA::GFP::pyrG; mpkA::3×HA::ptrA; ΔakuB, PtR | This study |
| fmqC::GFP mpkA::3×HA | gpdA(p)::fmqC::GFP::pyrG; mpkA::3×HA::ptrA; ΔakuB, PtR | This study |
| fmqD::GFP mpkA::3×HA | gpdA(p)::fmqD::GFP::pyrG; mpkA::3×HA::ptrA; ΔakuB, PtR | This study |
| fmqC::GFP ΔmpkA | gpdA(p)::fmqC::GFP::pyrG; ΔmpkA::ptrA; ΔakuB, PtR | This study |
| ΔsakA | ΔsakA::hph; ΔakuB, HygR | Hagiwara et al. (2014) |
| ΔmpkC | ΔmpkC::prtA, PtR | Hagiwara et al. (2014) |
| ΔsakA ΔmpkC | ΔmpkC::prtA ΔsakA::hph, PtR, HygR | Bruder Nascimento et al. (2016) |
| ΔsitA | ΔsitA::pyrG; ΔakuB | Bom et al. (2015) |
| mpkA::3×HA::ptrA | mpkA::3×HA::ptrA; ΔakuB, PtR | Manfiolli et al. (2019) |
FGSC A1160 strain.
FGSC, Fungal Genetics Stock Center (http://fgsc.net); PtR, pyrithiamine resistant; HygR, hygromycin resistant.
Strains construction
The double-tagged strains containing functional mpkA::3×HA epitope and fmq genes fused with GFP (Supplementary Figure S1, A–D) were obtained by transforming the mpkA::3×HA::ptrA cassette into the recipient strains TFYL48.1, TFYL50.1, and TFYL34.1 (Lim et al. 2014), kindly provided by Dr. Nancy P. Keller. The cassette mpkA::3×HA::ptrA was amplified from the genomic DNA of the strain mpkA::3×HA::ptrA (Manfiolli et al. 2019) using the primers mpkA-5′-ORF_FW and mpkA-3′_REV. The double-tagged strains were validated by PCR using the primers mpkA-5F and linker 3×HA_REV as shown in Supplementary Figure S1, C and D. The ΔmpkA strain carrying a gpdA::fmqC::GFP fusion was generated by transforming the ΔmpkA::ptrA deletion cassette into the TFYL50.1 strain and validated by PCR as described previously (Rocha et al. 2016; Supplementary Figure S1, E and F).
For the disruption of the fmqA locus, the first 2520 bp of the open-reading frame, coding for the first adenylating domain, were deleted. In brief, 1 kb flanking regions were amplified using primer pairs fmqA1/fmqA2 and fmqA3/fmqA4 (Supplementary Figure S2A). The obtained flanking regions were recombined with the ptrA cassette, obtained from plasmid pSK275 (Szewczyk and Krappmann 2010), and amplified with primers ptrA-for2 and ptrA-rev3, in the pYES2 plasmid using yeast recombination (Malavazi and Goldman 2012). The obtained plasmid named pYES-ΔfmqA was linearized and used to transform A. fumigatus ΔKU80 pyrG1 strain (da Silva Ferreira et al. 2006). Southern blot analysis was performed by digesting the extracted genomic DNA with XbaI. Hybridizing probe was obtained by amplifying genomic DNA with primers fmqAp1/fmqAp2 (Supplementary Figure S2B). The construction of the ΔfmqA::fmqA+ strains was performed by a cotransformation strategy using a fragment of the fmqA plus the upstream flanking region spanning 5082 bp, which was amplified from the wild-type strain using the fmqAc_FW and fmqAc_REV primers (Supplementary Figure S2C), and the gpdA::hph cassette as a selection marker. The gpdA::hph (2495 bp) cassette was amplified from the genomic DNA of the ΔsakA strain (Altwasser et al. 2015) using the primers gpdA_FW_VV and higroSTOP_RE_VV, gel-purified and used in the cotransformation in a molar ratio of 10:1. The complemented strain was validated by PCR using the primers indicated in Supplementary Figure S2, C and D. The restoration of FqC production in the ΔfmqA::fmqA+ strain was evaluated by HRMS, which presented wild-type levels of accumulation (Supplementary Figure S2E). Primer sequences used in all constructions are listed in Supplementary Table S1.
HPLC-UV secondary metabolite screening
The relevant strains were grown on solid YG medium and processed according to the procedures for small-scale cultivation in solid medium and SM analysis (Sanchez and Wang 2012). Briefly, 1 × 107 conidia were spread over solid YG plates and incubated at 37°C for 5 days. Subsequently, six plugs (diameter, 6 mm) along the fungal colony’s diameter were obtained and subjected to extraction according to the micro-extraction method (Smedsgaard 1997). The extraction solvent was a mixture of methanol:dichloromethane:ethyl acetate (1:2:3 v/v/v). The extracts were filtered through 0.45-µm PTFE filters, and 10-µL samples from each fraction were injected for HPLC-UV analysis. HPLC-UV-HRMS analyses were performed on an LTQ-Orbitrap Thermo Fisher Scientific mass spectrometry system (Bremen, Germany), with the resolution set at 60 K. The HPLC was fitted with a Waters Acquity C18 (1.7 μm) column, and the samples were eluted using linear gradient elution with acetonitrile and water containing 0.1% formic acid from 25 to 90% over 30 min at a flow rate of 0.4 mL/min. The data were analyzed with Xcalibur software (Thermo Scientific).
Scale-up fermentation, isolation of Fq, and determination by HPLC-MS/MS and NMR
The culture conditions for the wild-type and mutant strains described above for SM screening were scaled up. As such, 100 Petri dishes (90 mm) were used for Fq isolation. After the fungus grew for 5 days, the agar was chopped into ∼1 cm2 pieces, collected into Erlenmeyer flasks subjected to the same extraction conditions abovementioned. The resulting extracts were dried to yield crude extract (351 mg). Initially, a pre-purification of extracts by chromatographic fractionation was performed by using silica as the stationary phase and with the following order of elution solvents: dichloromethane (100%); ethyl acetate (100%), a mixture of ethyl acetate: methanol 1:1 and methanol (100%). After being evaporated to dryness, the ethyl acetate fraction containing Fq was purified by preparative HPLC with a phenyl-hexyl Phenomenex (21.2 × 250 mm, 10 µm) column using a gradient elution of acetonitrile/H2O from 20 to 60% over 60 min at a flow rate of 10 mL/min. The yield of the vacuum-dried, high-purity FqC was 1.1 mg, and FqD was 1.0 mg. Purified FqC and FqD were further used as external standards for quantification. The 1H and 13C NMR experiments were recorded on a Bruker Avance-600 MHz spectrometer with deuteron chloroform (CDCl3) as the solvent and tetramethylsilane as the internal standard.
For FqC-D quantification, an Acquity ultra-performance LC system coupled to a Quattro Micro triple quadrupole mass spectrometer from Waters was used. The chromatographic conditions were the same as described for the SM screening. The MS was operated in ESI− in the multiple reaction monitoring (MRM) mode with the [M–H]− ions as the precursor ions. All HRMS analyses were performed at unit resolution for both quadrupoles. The MRM settings for FqC were RT (retention time) 4.9 min, precursor m/z 442.2, and fragments m/z 145.0 and m/z 240.2 (collision energy, 30 eV). For FqD, the RT was 4.7 min and the same transitions were monitored. The mass parameters were optimized as follows: source temperature, 150°C; capillary voltage, 4.5 kV; cone voltage, 15 V; extractor voltage, 3 V; desolvation temperature, 400°C; and desolvation gas flow, 600 L/h. The interchannel and interscan delays were 0.01 and 0.03 s, respectively. A calibration curve for both FqC and FqD was obtained by analyzing standard solutions using the optimized MRM method at seven concentrations (250, 125, 62.5, 31.3, 15.6, 7.8, and 3.9 µg/mL). FqC and FqD standards used were obtained after scale-up fermentation and characterized by 1H NMR.
Imaging mass spectrometry analysis
Direct mass spectrometry imaging (MSI) of agar cultures for the A. fumigatus strains employed in this study was performed as described previously (Angolini et al. 2015), with few modifications for fungal colony analyses. Briefly, 1 × 104 conidia were inoculated on a thin-layered solid YG. The inoculated medium was incubated at 37°C, and the Petri dish was sealed with parafilm to avoid premature dehydration. After the incubation period, the microscope slide was removed from the Petri dish, photographed, and put on desiccator for complete agar dehydration to obtain a completely flat agar surface. MS imaging was performed using a Prosolia DESI source (Model OS-3201) coupled to a Thermo Scientific Q Exactive Hybrid Quadrupole-Orbitrap Mass Spectrometer. DESI configuration was set in emitter height of 2.5 mm, mass spectrometer inlet height of 0.1 mm, inlet to emitter distance of 3.8 mm, 65°C sprayed angle, 5.0 kV spray voltage, inlet capillary temperature of 320°C, 100 V S-lens, 140 psi ultra-pure nitrogen nebulizing gas pressure and a sprayed solvent of methanol at a flow rate of 1.5 µL/min. Images were collected in negative mode from m/z 100–1200 with a step sized of 200 µm, a scan rate of 741 µm/s and a pixel size of 200 µm 200 µm. All collected data were converted into imaged files using Firefly data conversion software (version 2.1.05) and viewed at BioMAP software (version 3.8.04).
Quantitative real-time PCR analysis
Quantification of mRNA expression was performed using Power Sybr Green PCR Master Mix (Thermo-Fisher) and a StepOne Plus real-time PCR system (Applied Biosystems). Fungal RNA from the synchronized asexual differentiation plates was isolated using Trizol reagent (Thermo-Fisher), treated with Turbo DNAse (Ambion), and reverse transcribed into cDNA using the High Capacity cDNA Reverse Transcription kit (Thermo-Fisher). qPCR was performed as described previously (Rocha et al. 2015; Bruder Nascimento et al. 2016) using the primers listed in Supplementary Table S2. Comparative cycle threshold (Ct; ΔΔCt) analysis was used to obtain the log2 fold difference.
Identification of RlmA and SebA binding motifs at the fmq gene promoters
The analysis of promoter regions between positions −1000 and −1 of the fmq genes for the identification of the conserved poly A/T stretches (5′-TAWWWWTA-3′), which are recognized by the MADS-box RlmA TF in A. fumigatus (AFUB_040580) was performed as detailed described (Rocha et al. 2020a) based on previously reported consensus sequences obtained from different Aspergillus species (Damveld et al. 2005; Kovacs et al. 2013; Valiante et al. 2016). The search for STRE motifs in the promoters of the fmq genes was achieved by manual inspection of the conserved STRE core sequence 5′-AGGGG-3′ recognized by SebA (AFUB_066180) orthologues in Trichoderma atroviride, Saccharomyces cerevisiae, and Neurospora crassa (Peterbauer et al. 2002; Freitas and Bertolini 2004; Freitas et al. 2016).
Chromatin immunoprecipitation coupled to qPCR (ChIP-qPCR)
Mycelia from the rlmA::GFP and sebA::GFP obtained from the synchronized asexual differentiation protocol were subjected to cross-linking, sonication, and immunoprecipitation using GFP-Trap (ChromoTek) as previously described (Ries et al. 2016; Rocha et al. 2020a). Primers flanking the predicted RlmA or SebA binding motifs were designed to amplify the promoter region of the target genes (Supplementary Table S3). Relative signal abundance in regions of interest in sample DNA was measured by qPCR as in reference (Rocha et al. 2020a). Cross-linked and sonicated samples (nonimmunoprecipitated) were used as positive controls (input). Relative signal enrichment was calculated and normalized according to the percent input method (Ries et al. 2016). Statistical differences were analyzed by a one-tailed paired t-test by comparing binding in the tagged strain versus the nontagged (wild-type) strain.
Co-immunoprecipitation assays and immunoblotting
The double-tagged strains containing both in-frame GFP fusions with either fmqA, fmqC, or fmqD and 3×HA with mpkA were used. Co-immunoprecipitation (Co-IP) experiments using ChromoTek GFP-Trap were performed by using 5 mg of protein, which were extracted and processed for IP as described by Ries et al. (2016) and Fabri et al. (2018). For reciprocal Co-IP using α-HA affinity gel (Sigma), samples were extracted and 5 mg of protein was used as input as described previously (Manfiolli et al. 2017; Rocha et al. 2020a). Proteins were released from the resins by boiling in 1× Laemmli buffer and run on an 8% SDS-PAGE gel. Proteins were electroblotted onto PVDF membranes for Western blot assay and detected with α-HA or α-GFP antibodies as described previously (Rocha et al. 2020a). α-Pgk1 was used as input control and assayed as described by Rocha et al. (2018). To assess if MpkA phosphorylates FmqC during conidiation, proteins obtained from the strains expressing gpdA(p)::fmqC::GFP and gpdA(p)::fmqC::GFP ΔmpkA strains were extracted as described by Caster et al. (2016) and Rocha et al. (2020a) and resolved on 100-μM Phos-tag (AAL-107; FujiFilm Wako Chemicals) acrylamide gels containing 100 μM MnCl2. To verify phosphate-specific signals, 100 µg of protein samples were treated with 100 U of CIP (NEB, #M0290) for 40 min (37°C), followed by boiling in 1× Laemmli sample buffer. Protein samples separated on either 6% Phos-tag or regular SDS-PAGE gels were electroblotted onto a PVDF membrane and processed for Western blots as abovementioned. Chemiluminescent detection for each antibody was achieved using an ECL Prime Western Blot Detection Kit (GE HealthCare). Images were generated by exposing the membranes to the ChemiDoc XRS gel imaging system (BioRad).
Confrontation of D. discoideum with A. fumigatus conidia
Cells of D. discoideum were initially grown to confluence as described previously (Hillmann et al. 2015). Cells were grown in Petri dishes containing HL5 (Axenic) medium supplemented with streptomycin sulfate (300 µg/mL) during 16 h at 22°C and harvested from the plates using fresh HL5 medium. The ameba cell number was obtained by counting in a Neubauer chamber and adjusted to 2 × 106 cells/mL with HL5. Conidia and ameba co-incubation experiments were done at a multiplicity of infection (MOI) of 10:1. In a 6-well plate, 1 × 105 of D. discoideum was mixed with 1 × 106 conidia of each relevant strain in 500 µL of HL5 medium and coincubated for 6 h at 22°C. Subsequently, 50 ameba cells were inspected at the microscope to assess the phagocytosis index. For the phagocytosis experiments in the presence of exogenous FqC, the same MOI was employed and purified FqC dissolved in DMSO (100 µg/mL) was added directly to the wells. DMSO was used as vehicle control in these assays. To evaluate the kinetics of conidia internalization, cells of D. discoideum were counted hourly up to 5 h.
Viability of D. discoideum coincubated with conidia or exposed to purified FqC
The relevant strains were grown in MM at 37°C for 5 days. Conidia were harvested and adjusted to a concentration of 2 × 107 conidia/mL. Cells of D. discoideum were grown and harvested as described above. The procedures were those described previously (Mattern et al. 2015) with slight modifications. In brief, ameba numbers were determined by counting in a hemocytometer and adjusted to a final concentration of 2 × 106 ameba/mL. Ameba coincubated with conidia at an MOI of 10:1 or exposed to 50 or 100 µg/mL of FqC were incubated in 1 mL of HL5 medium at 22°C for 24 h in 6-well plates. After incubation, the number of D. discoideum was determined using a hemocytometer.
Killing of A. fumigatus conidia after predation by D. discoideum
Conidia killing was expressed as the decrease in the total number of CFUs during coincubation with D. discoideum, following the methodology described by Hillmann et al. (2015). Coincubations were carried out at 22°C for 5 h at an MOI of 10:1. Aliquots of the recovered conidia (100 µL) were plated on YG agar plates and incubated for 36–48 h at 37°C. The total number of fungal colonies was determined, and death was expressed as the percentage of the number of CFUs from coincubations versus ameba-free controls.
Confrontation of bone marrow-derived macrophages with conidia
Bone marrow-derived macrophages (BMDMs) from C57BL/6 mice were prepared as previously described by culturing bone marrow cells from the femurs of adult mice for 6 days in RPMI 1640 supplemented with 20% fetal bovine serum and 30% L-929 cell-conditioned media (Marim et al. 2010). A phagocytic assay was performed at an MOI of 5:1 for 2 h, following the description reported by Mech et al. (2011 and Rocha et al. (2015).
Statistical analysis
All statistical analyses were performed in GraphPad Prism 8, using the comparison tests described for each experiment in the legend of figures. Error bars indicate standard deviation (SD) of the mean. Individual data points in the graphs indicate independent biological samples. α was set at 5%.
Results
The CWIP mutants are deficient in Fumiquinazoline C production
We have previously shown that the CWIP plays a crucial role in the conidiation cascade in A. fumigatus and that rlmA is required for the onset of conidiation by directly regulating key components of the asexual developmental pathway (Rocha et al. 2020a). The vegetative and conidiation defects displayed by the ΔrlmA mutant prompted us to investigate the relevance of the CWIP in the SM diversity of this fungus. Therefore, we performed a high-throughput SM screening based on a small-scale cultivation approach in solid medium, followed by chemical identification by HPLC-HRMS (High-Resolution Mass Spectrometry) analyses (Sanchez and Wang 2012). A comparison of the HPLC-UV chromatographic profiles between the wild-type and the CWIP mutants indicated a substantial decrease in the detection of an SM with a retention time of 13.3 min (Figure 1A). HRMS analysis and 1H NMR structure elucidation assigned the peak as the conidia-born SMs FqC (m/z = 444.1657) and fumiquinazoline D (FqD, m/z = 444.1664; Figure 1, B and C and Supplementary File S1). As previously reported, FqD is a congener and a direct product of FqC formed through a nonenzymatic spontaneous reaction in aqueous solution, as a result of the intramolecular cyclization of FqC, originating a bridging eight-membered ring (Lim et al. 2014; Resende et al. 2019; Figure 1D). As an alternative metabolomic protocol, we used ambient MSI on agar cultures to assess the spatial distribution of FqC directly in the colonies of the CWIP mutants. The MSI results confirmed the SM production differences in the mutant strains, showing that FqC highly accumulates in the wild-type colony, whereas it is poorly detected in the ΔrlmA mutant and almost absent in the ΔmpkA strain (Figure 2A). Quantitative analytic measurement confirmed significant decreases in the FqC levels in the pkcAG579R, a hypomorphic mutation in pkcA (Rocha et al. 2015), and ΔrlmA strains, also in the double-mutant ΔrlmA pkcAG579R in comparison to the wild-type (24.7, 27.9, and 61.3% reduction, respectively; P ≤ 0.0031). The FqC concentrations were 10.5- and 20.6-fold lower in the ΔmpkA and ΔrlmA ΔmpkA mutant strains, respectively (P < 0.0001). The FqC content in the ΔmpkA strain was also significantly reduced compared to pkcAG579R, ΔrlmA, and ΔrlmA pkcAG579R strains (P < 0.0001). Overall, FqD accumulation was 5.6-fold lower in comparison to FqC in the wild-type strain (Figure 2, B and C). We observed a decrease in the FqD concentration by 6.1-, 4.1-, and 2.4-fold in the pkcAG579R, ΔrlmA, and ΔmpkA, respectively, compared to the wild-type strain (Figure 2C). These data collectively suggest that the accumulation of FqC relies on the PkcA-MpkA-RlmA circuitry, with MpkA playing a major regulatory role.
Figure 1.
Identification of fumiquinazoline C and D in the CWIP mutants. (A) HPLC-UV FqC profiles for the wild-type and CWIP single mutants (254 nm). (B, C) The high-resolution mass spectrum of fumiquinazolines (C24H21N5O4). FqC and FqD were detected as the m/z 442.1657 and m/z 444.1664, respectively. (D) The chemical structures of FqC and its spontaneous conversion to FqD in aqueous solution.
Figure 2.
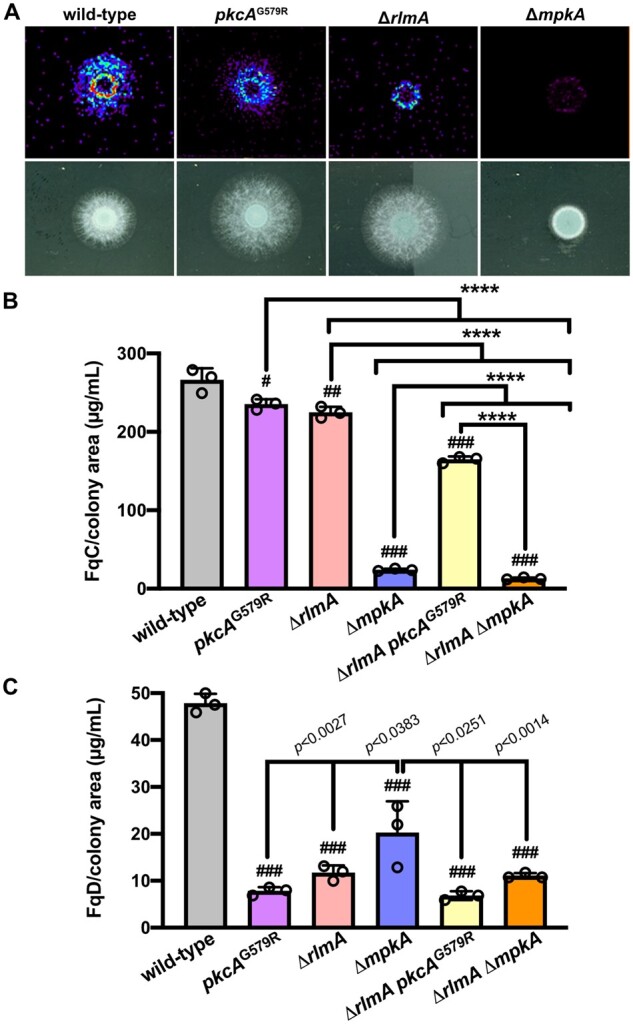
The CWIP mutants accumulate lower amounts of FqC and FqD in their conidial tissue. (A) FqC spatial distribution visualized by mass spectrometry imaging on agar. In brief, 1 × 104 conidia of each strain were incubated on solid YG slides and grown for 24 h at 37°C. Slides were dried in a desiccator and analyzed. A representative experiment (n = 3) is shown. (B, C) FqC and FqD were quantified in the mutant strains by HPLC-MS/MS. Purified and 1H NMR validated FqC and FqD were used as standards. Significant differences were observed by using one-way ANOVA followed by Tukey’s post hoc test. #P = 0.0031; ##P = 0.0002, and ###P < 0.0001 indicate significant differences from comparisons to the wild-type strain (n = 3 ± SD). All other comparisons are indicated by the bars. ****P < 0.0001.
MpkA and RlmA positively modulate fmq cluster during conidiogenesis
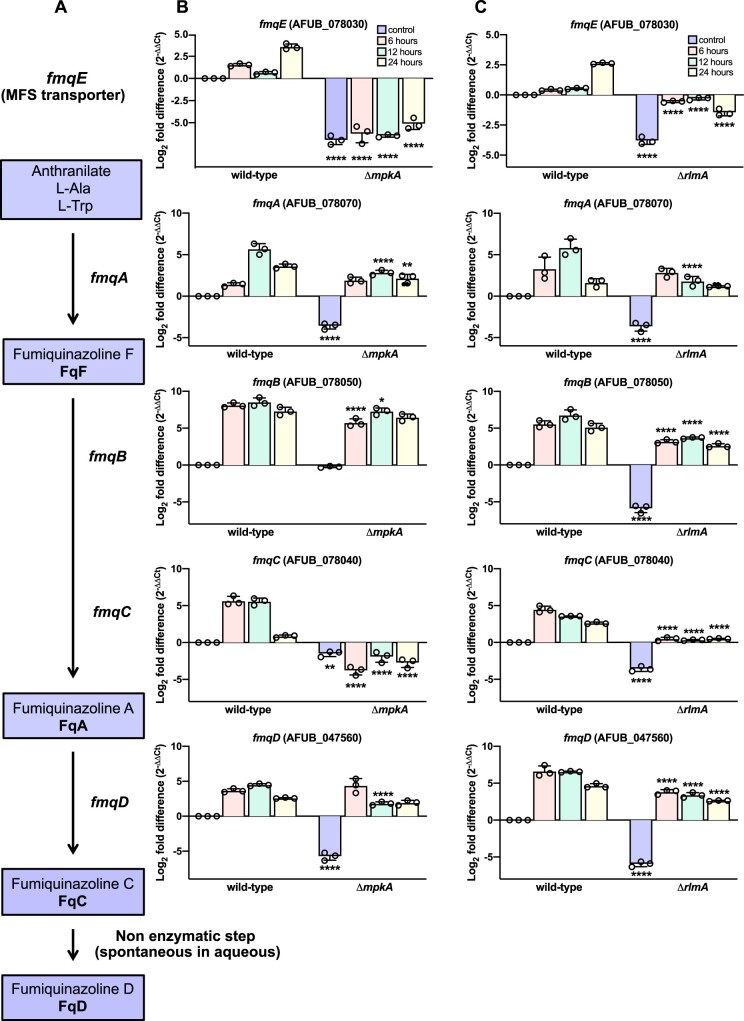
Considering that FqC production is influenced by the CWIP, we next investigated the contribution of mpkA and rlmA to the fmq cluster activation. The collection of Fq molecules, i.e., FqF, FqA, FqC, and FqD are produced in this sequence in A. fumigatus using anthranilate as a key nonproteinogenic amino acid building block (Ames and Walsh 2010; Ames et al. 2010, 2011; Lim et al. 2014). In brief, the trimodular NRPS enzyme fmqA is required to produce all the Fq metabolites because it condenses antranilic acid, l-tryptophan, and l-alanine in the presence of ATP to form FqF (Figure 3A). Consequently, the fmqA null mutant lacks all the Fq congeners (Lim et al. 2014). fmqB encodes a FAD-dependent mono-oxygenase responsible for the oxidization of FqF, which is then transformed to FqA by the action of fmqC gene product, encoding the second NRPS in the BGC. The fmqD gene encodes a FAD-dependent oxidoreductase, required for the last enzymatic step in the pathway that converts FqA to FqC. FqD, in turn, is generated spontaneously (Lim et al. 2014).
Figure 3.
The expression of fmq cluster genes during conidiation is lower in the ΔmpkA and ΔrlmA strains. (A) Biosynthetic pathway of Fq production in A. fumigatus. fmqA, a trimodular NRPS, condenses alanine, tryptophan, and anthranilic acid to form FqF. The tandem action of a flavoprotein (fmqB) and a monomodular NRPS (fmqC) converts FqF to FqA. Finally, fmqD, a FAD-dependent oxidoreductase converts FqA to the heptacyclic FqC. Gene expression measured by RT-qPCR in the ΔmpkA (B) and ΔrlmA (C) strains subjected to synchronized asexual differentiation at 37°C during the indicated time points. Control indicates the hyphal state (submerged culture). n = 3±SD. One-way ANOVA with Sidak’s multiple comparison test relative to wild type at the same time point of differentiation was performed. ****P < 0.0001, **P < 0.001, and *P < 0.01.
Given that FqC selectively accumulates in conidia and this accumulation requires the conidiation-specific TF BrlA (Lim et al. 2014), we hypothesized that changes in the mRNA abundance of the fmq genes during the asexual differentiation prepare the cells to generate mature FqC-enriched conidia. We used RT-qPCR to evaluate the expression of the fmq cluster genes in the wild-type and the CWIP mutants ΔmpkA and ΔrlmA. We observed that throughout the asexual differentiation, the mRNA abundance of all fmq genes was highly induced in the wild-type strain (Figure 3, B and C), in line with the high accumulation of FqC (Figure 2). For instance, the mRNA levels of fmqA increased by approximately 3.2–5.8 log2 fold, whereas the mRNA abundance of fmqB increased the most among all the fmq genes, reaching about an 8.5 log2-fold increase after 12 h post-asexual developmental induction. The levels of fmqD also peaked after 12 h of conidiation (5 log2-fold increase). In contrast, we observed marked decreases in the mRNA levels of all fmq genes in both ΔmpkA and ΔrlmA mutants (Figure 3, B and C), suggesting a regulatory role of those proteins in positively controlling fmq expression.
RlmA binds to conserved motifs in the fmq promoters
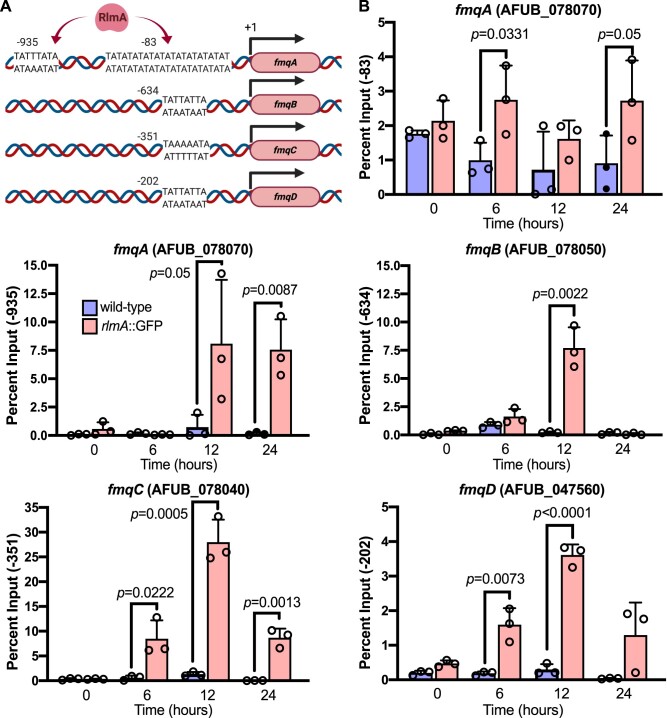
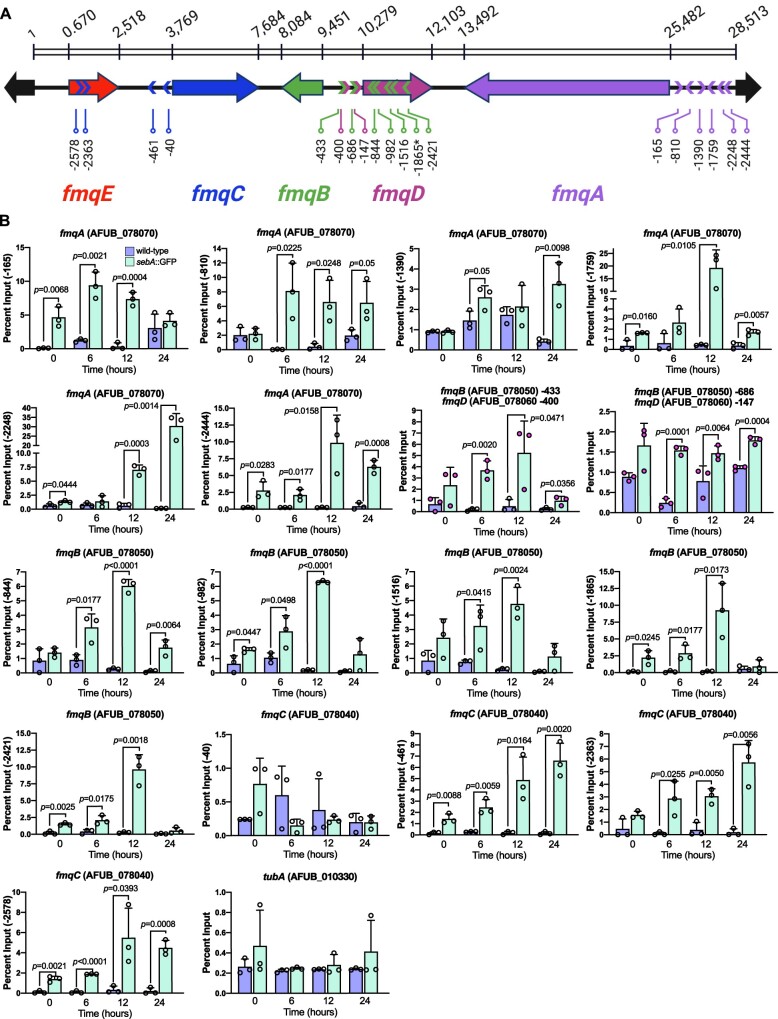
As aforementioned, the fmq BGC in A. fumigatus lacks associated TFs (Lim et al. 2014), suggesting that Fq biosynthesis relies on the function of unknown conidiation-responsive TFs. The results of gene expression analyses prompted us to test whether RlmA directly binds to the fmq promoters. To address this hypothesis, we searched the fmqA-E promoters for potential RlmA binding sites (5′-TAWWWWTA-3′), according to a previous definition and genome-wide identification of RlmA-binding motifs (Rocha et al. 2020a). Excepting the fmqE, we observed that the fmqA-D gene promoters presented putative DNA core sequences that could be recognized by RlmA (Figure 4A). We subsequently performed chromatin immunoprecipitation followed by quantitative PCR (ChIP-qPCR) in wild-type and rlmA::GFP strains submitted to synchronized asexual differentiation to analyze in vivo RlmA binding to the fmq genes. We observed significant binding of RlmA to the fmqA-B-C-D promoters compared to the negative (non-GFP tagged) control strain (Figure 4B). The fmqA, fmqB, and fmqC promoters were the most enriched in our analysis, with input above 7.5%, suggesting the importance of RlmA in the transcriptional activation of the BGC. Although we observed a tandem repeat of three T/A stretches located at the fmqA promoter at the position -83 (Figure 4A), the binding values to this region were much lower than those for the position -935. Taken together, these results indicate that RlmA specifically associates with the target fmq promoters during conidiation, activating the Fq biosynthesis pathway to supplement mature conidia with FqC.
Figure 4.
RlmA binds to the promoters of fmq biosynthetic gene cluster. (A) Location of RlmA binding motifs (5′-TAWWWWTA-3′; W = A or T) at the promoters of the fmq genes. The DNA motif positioned at −83 bp of fmqA comprises a repeat region containing three overlapped core sequences. (B) ChIP-qPCR analysis of RlmA occupancy on the fmqA-D promoters at the indicated time points after induction of asexual differentiation. Quantification of enrichment was represented as percent fold-enrichment over the non-GFP tagged wild-type strain. A two-tailed unpaired Student’s t test was used for statistical analysis (n = 3 ± SD). Created with BioRender.com.
MpkA interacts with FmqA and FmqC during conidiogenesis and phosphorylates FmqC
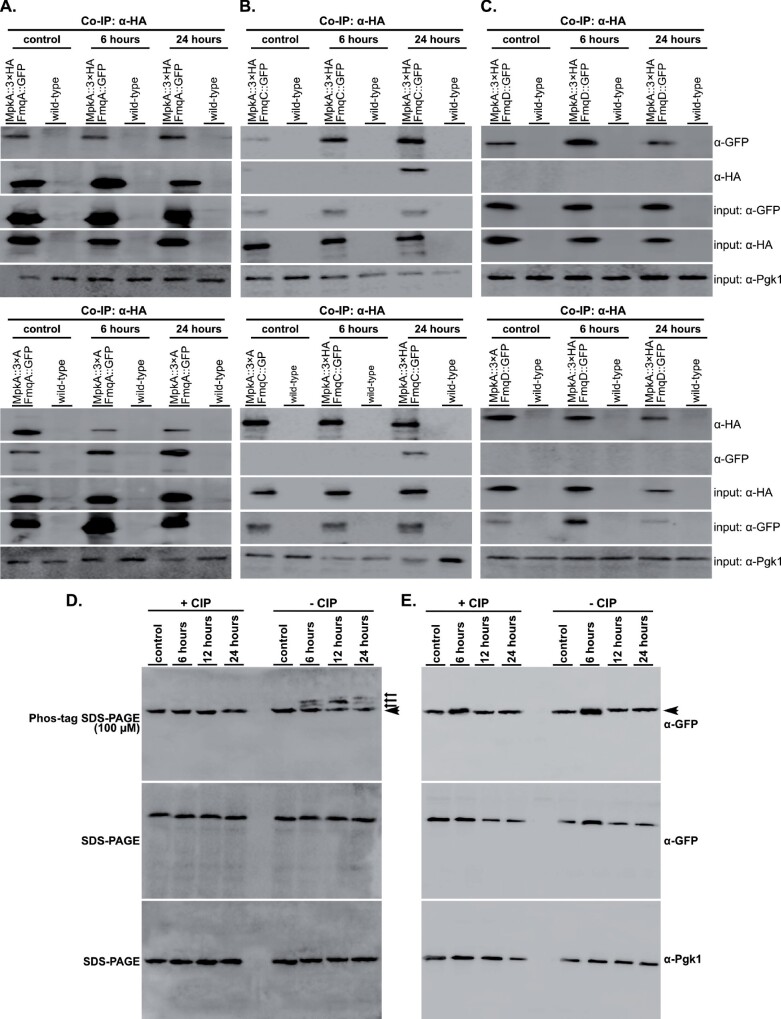
Among the CWIP regulators, conidia of ΔmpkA and ΔmpkA ΔrlmA strains are notably FqC depleted, supporting an epistatic interaction between these two genes during the FqC synthesis (Figure 2), and confirming the direct role of RlmA in the transcriptional activation of fmq genes (Figure 4). Since deletion of mpkA does not completely repress fmqA expression, but strongly affects FqC production, we investigated if MpkA could be responsible for potential post-transcriptional modifications of Fq biosynthetic enzymes. To test this hypothesis, we initially undertook Co-IP experiments using crude protein extracts from wild-type and double-tagged functional strains expressing MpkA::3×HA and FmqA::GFP, FmqC::GFP or FmqD::GFP obtained after 6 and 24 h post-asexual differentiation. The mutants expressing the tagged proteins did not show any growth defects when compared to wild type (Supplementary Figure S1, A and B). We observed that both NRPS enzymes, FmqA and FmqC, interacted with MpkA. Interestingly, the FmqA and MpkA association was detected throughout the asexual development, including the hyphal state (control), whereas the FmqC interaction was detected after 24 h post-asexual differentiation (Figure 5, A and B, top panels). Despite the fact that mpkA deletion appeared to affect mRNA accumulation of fmqD (Figure 3B), no physical interaction was recorded for FmqD::GFP and MpkA::3×HA (Figure 5C). To further confirm the specificity of the detected interactions, we performed reciprocal Co-IP assays, in which MpkA::3×HA was pulled-down first and then probed with α-HA or α-GFP antibodies (Figure 5, lower panels). To determine if MpkA phosphorylates both enzymes, we investigated their phosphorylation state in ΔmpkA mutants expressing the GFP-tagged proteins (Supplementary Figure S1, C–F). Phosphorylated FmqC was observed 6–24 h postinduction of conidiation, whereas the unphosphorylated form of the protein predominated in the hyphal state (Figure 5D). Consistently, the bands indicating slower migrating forms of FmqC disappeared when protein extracts were treated with calf intestinal alkaline phosphatase. In contrast, the phosphorylation signal was completely absent in the ΔmpkA strain (Figure 5E). We were not able to analyze the FmqA phosphorylation, most likely owing to its high molecular weight (437.8 kDa). Altogether, these results suggest that multiple phosphorylation sites are present in FmqC and that MpkA is a kinase acting on this enzyme, leading to the activation of the FqC production.
Figure 5.
MpkA physically interacts with the NRPS enzymes FmqA and FmqC during conidiation. Co-IP was performed with total protein extracts from the wild type and the relevant strains expressing the GFP and 3×HA tags subjected to synchronized asexual differentiation. GFP-Trap resin was used to immunoprecipitate FmqA::GFP (A), FmqC::GFP (B), and FmqD::GFP (C) upper panels. EzView α-HA resin was used to immunoprecipitate MpkA::3×HA in a reciprocal experiment (bottom panels). Co-immunoprecipitated proteins were investigated by Western blot analysis using α-HA and α-GFP antibodies. α-Pgk1 was used as input control. Western blot of protein extracts from the strains expressing gpdA::fmqC::GFP (D) and gpdA::fmqC::GFP ΔmpkA (E) treated (+CIP) or not (−CIP) with Calf Intestinal Alkaline Phosphatase. FmqC::GFP was probed with α-GFP antibody onto membranes obtained from 6% Phos-tag or regular SDS-PAGE. The arrows point to the phosphorylated FmqC and the arrowhead points to the unphosphorylated protein. Predicted protein sizes on blot: MpkA (48.5 kDa), FmqA::GFP (464.6 kDa), FmqC::GFP (155.5 kDa), and FmqD::GFP (82.0 kDa).
FqC is overproduced in the ΔsebA mutant
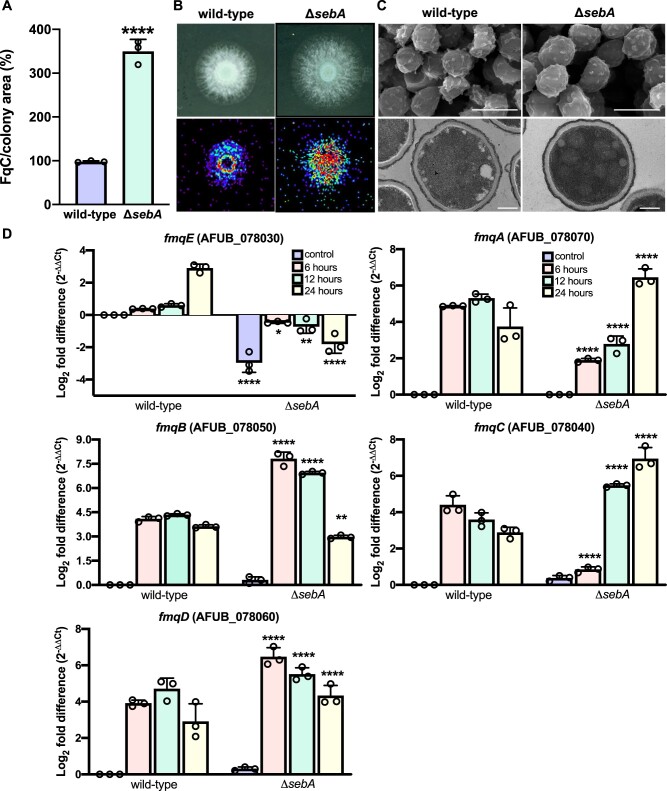
We demonstrated that FqC production is reduced when the CWIP is not functional; however, this phenotype does not provide information regarding the reason why A. fumigatus produces such a massive amount of FqC even under standard laboratory growth conditions. To further understand the regulation of FqC synthesis, we screened an arbitrary collection of selected mutant strains deleted on genes responsive to general stressing conditions and involved in different signaling pathways, including kinases and phosphatases. Interestingly, we did not observe a decrease in the accumulation of FqC in the mutants of the High Osmolarity Glycerol pathway MAP kinases (ΔsakA, ΔmpkC, and the double-mutant strain), further supporting the prominent role of the CWIP in the accumulation of this SM (Supplementary Figure S3A). The mutant collection included the ΔsebA strain, a zinc finger TF previously described as involved in oxidative stress response, heat shock, and virulence in A. fumigatus (Dinamarco et al. 2012). Surprisingly, we observed that the ΔsebA strain accumulated 3.6-fold more FqC than the wild type (Figure 6A), suggesting that in the absence of this TF, the production of Fq is derepressed. Further quantification of the FqC accumulation and MSI analysis on agar cultures confirmed this finding and showed an intense distribution of FqC across the ΔsebA colony (Figure 6B). We observed no difference in the ultrastructure of ΔsebA conidia analyzed by transmission and scanning electron microscopy compared to the wild type, indicating that the cell-wall structure and the hydrophobic out layer of the conidia are similar to wild type (Figure 6C). To test whether the enhanced FqC production in this mutant correlates to increased gene expression of the Fq BGC, additional RT-qPCR experiments were performed to analyze the expression of the Fq cluster genes in the ΔsebA strain. Excepting the putative transporter encoding gene (fmqE), which was repressed in the mutant strain, the mRNA abundance of all other genes was significantly increased by at least two log2-fold. It includes the genes fmqB and fmqD, in which mRNA abundance was higher than the other fmq genes (Figure 6D). To exclude the possibility that this is an indirect effect owing to undisclosed roles of sebA during the asexual differentiation in A. fumigatus, we additionally evaluated the expression of sebA under these conditions. We observed that sebA mRNA levels were highly increased (34-fold) during the asexual differentiation (Supplementary Figure S3B). Given that no conidiation defects were observed in the ΔsebA strain (Supplementary Figure S3C), we suggest that high levels of this TF are required during conidiation without playing a direct role in the activation of the conidiation cascade. Since the sporulation-specific TF BrlA is required for FqC biosynthesis (Lim et al. 2014), we interrogated if brlA mRNA levels are misregulated in the ΔsebA mutant, what could account for the increased FqC accumulation observed in this mutant. The results showed normal levels of brlA expression in the ΔsebA strain since the differences in the mRNA abundance are lower than 1.2-fold (Supplementary Figure S3D).
Figure 6.
Expression profile of fmq cluster genes during conidiation in the ΔsebA strain. (A) Quantification of FqC in the ΔsebA strain by HPLC-MS/MS. Purified and 1H NMR-validated FqC was used as standard. A two-tailed unpaired Student’s t test was used for statistical analysis (n = 3 ± SD). (B) FqC spatial distribution visualized by mass spectrometry imaging on agar. In brief, 1 × 104 conidia of each strain were incubated on solid YG slides and grown for 24 h at 37°C. Slides were dried in a desiccator and analyzed. A representative experiment (n = 3) is shown. (C) Scanning (upper panels; bars 3 µm) and Transmission (lower panels; bars, 500 nm) electron microscopy comparing the wild-type and ΔsebA strains. (D) Gene expression measured by RT-qPCR in the wild-type and ΔsebA strains subjected to synchronized asexual differentiation during the indicated time points (hours). Control indicates the hyphal state (submerged culture). n = 3 ± SD. One-way ANOVA with Sidak’s multiple comparison test relative to wild type at the same time point of differentiation was performed. ****P < 0.0001, **P ≤ 0.0020, and *P ≤ 0.0337.
Although sebA was initially described as essential to withstand different stressing conditions (Dinamarco et al. 2012), deletion of this TF caused a derepression of FqC production, suggesting that other unidentified TFs are intertwined to regulate stress adaptation concomitantly with FqC overaccumulation. To probe further into the potential mechanism underlying the increased production of FqC in the ΔsebA mutant, we searched for STRE motifs at the promoters of the Fq BCG. In other filamentous fungi, such as T. atroviride and N. crassa, the SebA orthologues recognize the STRE motifs (5′-AGGGG-3′; Peterbauer et al. 2002; Freitas et al. 2016). We used this core DNA sequence to search for STRE in the fmq promoters. Interestingly, we found several STRE motifs in the promoters of all fmq genes, excepting fmqE. The fmqA gene has the most extended intergenic region (3031 bp), and six STRE motifs were identified in this region (Figure 7A, purple lines). Besides, according to the organization of the BGC, fmqB and fmqD share a bidirectional promoter (828 bp). Since the mRNA levels of fmqB were substantially increased in the ΔsebA strain in comparison to other fmq genes (Figure 6D), we sought to determine if additional STRE is present inside the fmqD coding region (green < symbols). We identified five STRE inside the fmqD, one of them (−1865 bp) located into an intronic region. Our ChIP-qPCR analyses indicated that SebA bound to all STRE motifs in the fmqA promoter during the asexual differentiation, having those located at positions −2248 and −1759 presented the highest values (Figure 7B). Interestingly, for the fmqB gene, the binding of SebA to STRE was more evident for the motifs located into the fmqD open-reading frame, such as those at the positions −1865, −982, and −844. The STRE motifs located at the fmqB (−686)/fmqD (−147) bidirectional promoter (Figure 7B, pink dots) were bound with lower affinity by SebA. SebA also bound to STRE motifs located upstream the fmqC gene (including two of them inside the fmqE ORF), except the STRE located at −40 of fmqC promoter. Together, these results suggest that SebA binds to fmq promoters repressing the expression of such genes under basal conditions during the conidiation. The absence of sebA, however, is sufficient to propagate cell compensatory effects causing transcriptional activation of fmqA-B-C-D by specific TFs that gain access to the fmq promoters, such as RlmA, thus causing over-accumulation of FqC (Figure 6).
Figure 7.
SebA binds to multiple stress response elements (STRE) distributed on the fmq biosynthetic gene cluster promoters. (A) Graphical representation of Fq gene cluster. The numbers above the scheme show the length of the BGC. The colored symbols (<) or (>) show the approximate location of the STRE for a given Fq cluster gene and indicate the orientation of the motif (sense or anti-sense direction) regarding its associated gene. The asterisk (*) indicates an STRE for the fmqB gene (−1865) located inside an intron of the fmqD gene. (B) ChIP-qPCR analysis of SebA occupancy on the fmqA-D promoters at the indicated times (hours) after induction of asexual differentiation. The y-axis indicates the position of the STRE tested for each gene. Quantification of enrichment was represented as percent fold-enrichment over the non-GFP-tagged wild-type strain. A two-tailed unpaired Student’s t-test was used for statistical analysis (n = 3 ± SD). An exonic region of tubA was used as a negative binding control for the STRE motif. Created with BioRender.com.
FqC is an antiphagocytic molecule
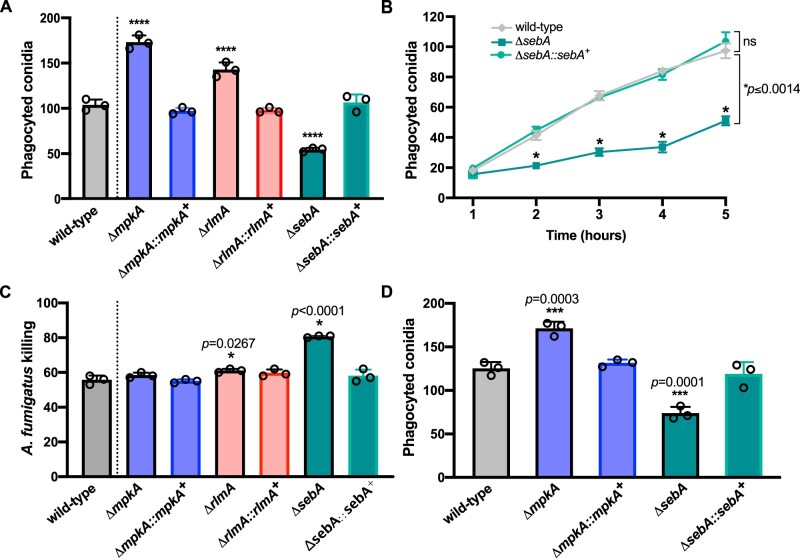
It has been shown that fungi face ameba predation in their environmental niches and constant competition for nutrients. This scenario may evolve grazing defenses that potentially result in enhanced fitness and pathogenicity of fungal organisms (for review see Erken et al. 2013; Novohradska et al. 2017). We then used D. discoideum as a model to initially investigate if there is a correlation between the FqC content accumulated in the conidia of the mutant strains with the ability of the ameba to phagocytize these structures. Confrontation assays were then conducted between conidia of the CWIP mutants and the ΔsebA strain, which accumulate lower and higher amounts of FqC, respectively. Conidia of the CWIP mutants were efficiently taken up by D. discoideum, yielding a higher number of ingested conidia by the cells (P < 0.0001). In contrast, the ingestion was significantly lower when ΔsebA FqC-enriched conidia were coincubated with the ameba (P < 0.0001; Figure 8A). Besides, the phagocytosis of ΔsebA conidia was monitored over time, and we observed that it is decreased since the initial times of coincubation, suggesting that it is not an effect caused by the conidial swelling or subsequent germination (P < 0.001; Figure 8B). Regardless the genetic background of the conidia used in the confrontation assays, no differences in the proliferation of the ameba were observed after 24 h of coincubation (Supplementary Figure S4A), suggesting that at an MOI of 10, the FqC accumulated in the ΔsebA conidia did not compromise the phagocyte viability.
Figure 8.
Phagocytosis of conidia of the CWIP and ΔsebA mutants by soil ameba and macrophages. (A) Deletion of mpkA, rlmA, and sebA results in increased and decreased phagocytosis, respectively. The phagocytic index was scored by assessing the number of ingested conidia by D. discoideum after 6 h of coincubation at an MOI of 10 (n = 3 ± SD). One-way ANOVA with Dunnett’s multiple comparison test was performed. ****P < 0.0001 indicates comparison to the wild-type control condition. (B) Phagocytosis of ΔsebA conidia over time. The phagocytic index was scored by assessing the number of ingested conidia by the D. discoideum hourly up to 5 h of co-incubation at an MOI of 10 (n = 3 ± SD). Two-way ANOVA with Holm–Sidak’s multiple comparisons test was performed, as indicated by the bars. (C) Percentages of conidia killed by D. discoideum (killing) in vitro. Wild-type, ΔmpkA, ΔrlmA, and ΔsebA conidia were coincubated with the ameba at an MOI of 10 (n = 3 ± SD). After 5 h, samples were removed to determine the total number of survivors. Values are expressed as the percentage of CFUs in ameba-free controls at the same time point. One-way ANOVA with Dunnett’s multiple comparison test was performed in comparison to the wild type. (D) Phagocytosis of ΔmpkA and ΔsebA by BMDM is increased and decreased, respectively. The phagocytic index was scored by assessing the number of ingested conidia by the BMDM after 2 h of coincubation (MOI = 5:1). One-way ANOVA with Dunnett’s multiple comparison test was performed. ****P < 0.0001 indicates comparison to the wild-type control condition (n = 3 ± SD).
To further explore the role of FqC in the phagocytosis by D. discoideum, we next examined the ameba’s ability to kill A. fumigatus phagocyted conidia carrying different amounts of FqC in their surfaces. We observed no difference in the killing of the ΔmpkA strain, whereas a significant subtle increase was observed for the ΔrlmA strain. Despite the lower phagocytosis number, the ΔsebA strain conidia were more efficiently killed by the ameba (P < 0.0001; Figure 8C), as previously reported for alveolar macrophage killing (Dinamarco et al. 2012). To test whether the lower phagocytosis index for the ΔsebA conidia is conserved in other phagocytes or is a specific phenomenon in confrontation with soil ameba, we examined the percentage of phagocytized conidia by BMDM. Consistently, phagocytosis was lower in the ΔsebA and increased in the ΔmpkA strain in the BMDM model (Figure 8D). A similar increase in the phagocytosis index under the same experimental conditions was previously reported for the ΔrlmA and pkcAG579R mutants (Rocha et al. 2015, 2016).
Together, these results emphasize that SebA is crucial for the stress response in A. fumigatus and suggests that the accumulated FqC in the conidial surface of ΔsebA correlates with strain-perturbed phagocytosis, whereas it does not protect the cells from killing.
Our data point out that the FqC levels in the A. fumigatus conidia are potentially associated with differential phagocytosis by D. discoideum, suggesting that this phagocyte can discriminate the chemical composition of the conidia or respond to the chemical milieu where the phagocytosis is occurring. Therefore, we hypothesized that FqC is an important deterrent to ameba predation in natural niches, which can also have undescribed consequences to A. fumigatus persistence inside the human host during the initiation of infection. To further validate this hypothesis, we investigated if phagocytosis is impacted when purified exogenous FqC was added to A. fumigatus and D. discoideum confrontation assays. First, we generated a deletion mutant for the fmqA gene (Supplementary Figure S2, A–D), which rendered cells devoided of all Fq congeners, but especially FqC, which is mostly enriched in the conidia compared to FqA and FqF (Lim et al. 2014). Mass spectrometry analyses confirmed no production of FqC in the ΔfmqA mutant and phenotypic analysis indicated that this mutant has wild-type levels of susceptibility to cell-wall damaging agents (Supplementary Figure S2, E and F). Consistent with our hypothesis that FqC is an antiphagocytic SM, the phagocytosis index of the ΔfmqA strain was increased twice (P < 0.0001), thus recapitulating the phenotype of the lower FqC accumulation strains ΔmpkA and ΔrlmA (Figure 9A). We next observed that the addition of purified FqC to the confrontation experiments did not significantly alter the phagocytosis index of the wild-type conidia by the ameba at an MOI of 10:1 (Figure 9B). Also, the ameba’s viability under the concentration of FqC used in our experiments (100 µg/mL) was not significantly affected (Figure 9C). In contrast, in the FqC devoided conidia from the ΔfmqA strain, the higher phagocytic index was reverted when exogenous FqC was added to the media (P = 0.0028; Figure 9B). A similar outcome was observed for the CWIP mutants for which the naturally high basal levels of phagocytosis were reduced to values close to the wild-type strain after the addition of purified FqC (P ≤ 0.0053). To assure that FqC can diffuse from the conidia to the aqueous environment, such as the culture medium, we quantified the partition of FqC when 2 × 108 conidia of each relevant strain were inoculated in 20 mL of culture medium. This experiment was planned as a proxy to understand if soil predators in the environment can face a concentration gradient of naturally occurring FqC. The results showed that FqC is transferred to the culture medium and the concentration of the recovered FqC coincides with the accumulated molecule in each one of the strains (Supplementary Figure S4B). Taken together, these results strongly indicate that conidia harboring a high content of FqC were protected from general recognition mechanisms of D. discoideum and that a chemically modified ambient enriched with FqC similarly culminated in a different rate of phagocytosis of the CWIP mutants although the ameba and BMDM macrophages more easily recognized these conidia.
Figure 9.
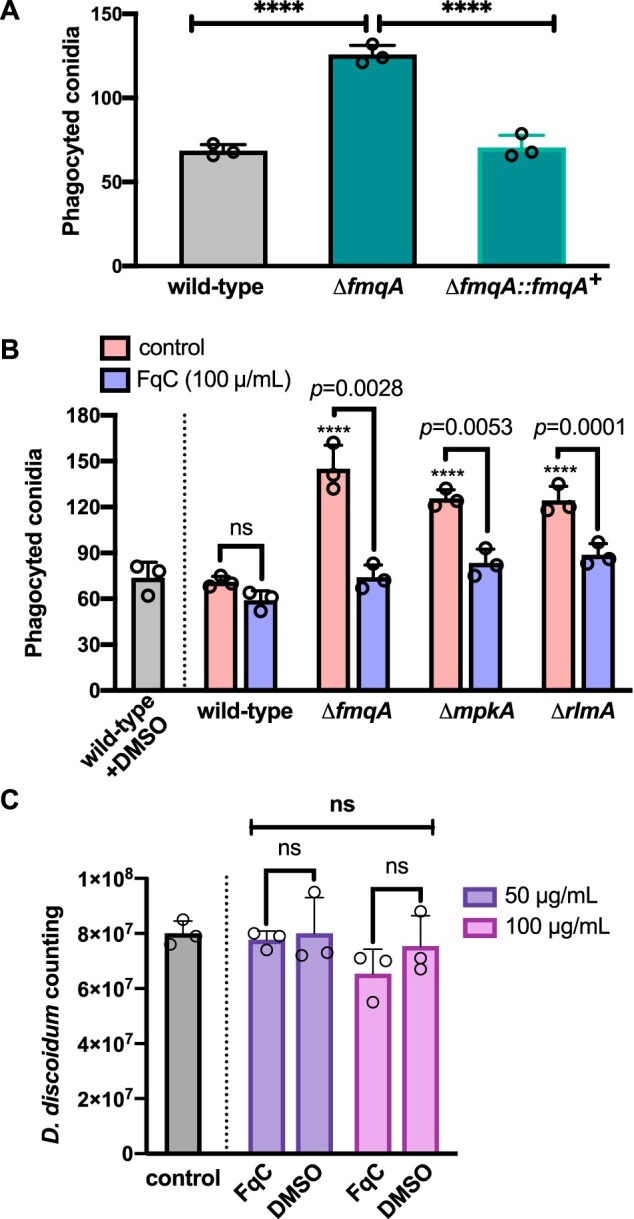
Exogenous FqC modulates phagocytosis of A. fumigatus conidia by soil ameba. (A) Deletion of fmqA results in increased phagocytosis. Phagocytic index of wild-type, ΔfmqA and complemented strain were scored by assessing the number of ingested conidia by D. discoideum after 6 h of coincubation (MOI = 10:1). One-way ANOVA with Dunnett’s multiple comparison test was performed. ****P < 0.0001 indicates comparison to the wild-type control condition (n = 3 ± SD). (B) Effect of FqC on the phagocytosis of conidia by soil ameba. Phagocytic index of wild-type, ΔfmqA, ΔmpkA, and ΔrlmA strains was scored by assessing the number of ingested conidia by D. discoideum after 6 h of coincubation at an MOI of 10:1 (n = 3 ± SD). Two-way ANOVA with Holm–Sidak’s multiple comparison test was performed. ****P < 0.0001 indicates comparison to the wild-type control condition. For all other comparisons indicated by the bars, P values are shown. (C) Viability of D. discoideum is not affected upon incubation to 100 µg/mL of pure FqC isolated from A. fumigatus conidia. Ameba were exposed to FqC or to the vehicle (DMSO) during 24 h at 22°C. Viable ameba cells were counted using a hemocytometer and plotted in the graph. Two-way ANOVA with Holm–Sidak’s multiple comparison test was performed. n = 3 ± SD; ns: non-significant.
Discussion
FqC is one of the five most abundant SM accumulated in the conidia of A. fumigatus (Gauthier et al. 2012; Lim et al. 2014; Lim and Keller 2014); however, it remains a significant gap in knowledge how the Fq biosynthesis is co-ordinated and more important, what molecular mechanisms contribute to the regulation of the fmq BGC. Herein, we demonstrate that the CWIP is a key signaling cascade acting as a positive regulator of Fq biosynthesis. In addition, we show that the loss of the global stress-responsive TF SebA (Dinamarco et al. 2012; Freitas et al. 2016) leads to derepression of the fmq cluster and increased amounts of FqC in the conidial tissue, which impacts the phagocytosis ability of the conidia by soil ameba and macrophages (Figure 10).
Figure 10.
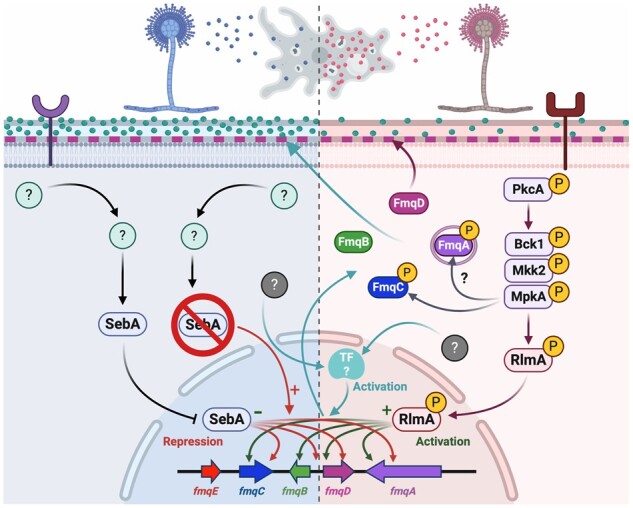
Proposed regulation for FqC accumulation during conidiogenesis. The CWIP comprising the apical kinase PkcA, the MAP kinase module (Bck1-Mkk2-MpkA), and the associated TF RlmA plays a direct role in the activation of the FqC BGC. The activation of MpkA causes the subsequent activation of RlmA, which in turn binds to the RlmA box motifs located at the promoters of fmqA-B-C-D genes (green arrows), activating their expression (+). Concomitantly, MpkA phosphorylates FmqC, suggesting that this event is crucial to the FqC production since ΔmpkA mutant is highly deficient in FqC. MpkA also associates with FmqA but phosphorylation activity remains to be determined. FmqA, FmqB, and FmqC reside in the cytosol, whereas FmqA is localized in vesicles, the other enzymes are evenly distributed in the cytoplasm. FmqD is located at the cell wall (pink dashed lines). Together, these enzymes process the substrates to produce FqC (greenish spheres), accumulating at the conidia surface by unknown mechanisms. The loss of PkcA-MpkA-RlmA signal leads to a low accumulation of FqC (right-hand side). The stress-responsive TF SebA (left-hand side) is activated by unknown mechanisms (green question marks) and is highly expressed during the asexual differentiation. SebA binds to the STRE motifs located at the fmqA-B-C-D promoters (black arrows) and represses (−) fmq expression under physiological conditions. This event contributes to the bona fide accumulation of FqC. Upon deletion of SebA, the recognition of STRE at the Fq cluster genes promoter is lost (red arrow), and compensatory mechanisms activate additional TFs (ciano box and arrows) or hyperactivate the CWIP to enhance FqC production, which causes over-accumulation of the molecule at the ΔsebA conidia. The overproduction of FqC is a result of the perturbation of endogenous stressing response mechanisms elicited by SebA and concerted actions of MpkA and RlmA. However, how cells integrate information from multiple pathways do produce FqC remains undescribed (gray question marks). The soil ameba D. discoideum selectively discriminates conidia harboring high or low FqC content, leading to decreased (left) or increased (right) phagocytosis. This diagram is based on the data from this article and from references Lim et al. (2014) and Rocha et al. (2020a). Created with BioRender.com.
Overall, the production of SM is one of the fungal physiological responses to abiotic and biotic stresses or congruent with fungal development (Macheleidt et al. 2016; Keller 2019). Earlier studies on the FqC biosynthesis and regulation have demonstrated that the accumulation of Fq is dependent on BrlA as the deletion of this conidiation-specific TF halts the accumulation of FqA or FqC in A. fumigatus (Lim et al. 2014; Lind et al. 2018). Recently, it has been shown that hbxA, which has a conidiation defect, regulates the expression of genes involved in the production of fumiquinazoline, and the regulation of the genes involved in the synthesis of these compounds is indirect and mediated, at least in part, by its effect on brlA (Satterlee et al. 2020). This regulatory event links conidiogenesis to accumulation of FqC; however, no information is available regarding the signaling pathways contributing to the biosynthesis of this SM. By comparing the SM profile of the wild-type and ΔrlmA mutant, we found that FqC production was significantly reduced in ΔrlmA (Figure 2). Previously, we demonstrated that rlmA is required for the onset of conidiation as this TF binds to the promoter of brlA and abaA, regulating their expression (Rocha et al. 2020a). Here, we observed that the same regulatory events of RlmA are extended to the fmqA-B-C-D, given that these genes are direct RlmA transcriptional targets presenting conserved RLM-binding motifs at their promoters (Figure 4). Supporting this idea, RlmA is highly phosphorylated during the conidiation (Rocha et al. 2020a), suggesting that the maturation of conidia and associated FqC enrichment relies partially on this TF and it is not strictly dependent on BrlA. Our initial analysis of the cell responses of the ΔrlmA strain through quantitation of the fmqA-E mRNA levels during conidiation confirms that RlmA is not the single TF activating the Fq BGC (Figure 3). Although RlmA binds to fmqA-D promoters with different affinities (Figure 4), the mRNA abundance of such genes is not completely absent. Interestingly, an RLM box domain was not identified in the fmqE promoter although it was the most repressed gene of the BGC in both ΔmpkA and ΔrlmA strains. Consistently, the deletion of fmqE causes no impact on Fq production as reported previously (Lim et al. 2014).
Although all the CWIP mutants accumulated less FqC during the conidiation, the decrease of FqC content in the ΔmpkA strain was more severe in comparison to ΔrlmA and pkcAG579R strains, indicating that MpkA and RlmA/PkcA play major and minor roles, respectively, in the FqC production (Figure 2). MpkA is known to play a role in the production of several SM in A. fumigatus (Valiante et al. 2009; Jain et al. 2011; Valiante et al. 2016; Valiante 2017), thus supporting our hypothesis that the CWIP is connected to the asexual development and as such, participates in the biosynthesis of FqC. Both FmqB and FmqC act in tandem to complete a single biosynthetic step: the conversion of FqF to FqA, which in turn generates FqC via the activity of fmqD (Lim and Keller 2014). Given that our expression analyses indicated that fmqC was one of the most downregulated genes, both in ΔmpkA and in ΔrlmA strains, we propose that the CWIP critically regulate this gene. In line with this idea, the values for RlmA binding to the fmqC promoter were very high after 12 h postasexual differentiation. Here, we also showed that the two NRPS enzymes in the fmq cluster (FmqA and FmqC) physically associate with MpkA, suggesting a direct and undescribed role of this MAPK in the regulation of secondary metabolism in A. fumigatus via post-translational modification by phosphorylation. Consistently, MpkA is the single kinase that phosphorylates FmqC during conidiogenesis (Figure 5). Interestingly, we did not observe physical interaction between MpkA and FmqD, which is the last enzyme of the BGC-generating FqC in the conidia surface. This is not surprising as FmqD is the only enzyme of the pathway located at the cell wall, in contrast to FmqA and FmqC, which are both cytosolic (Lim et al. 2014; Figure 10). Our data suggest that the phosphorylation of the NRPS enzymes of the fmq cluster by MpkA sustains the Fq production, which coincides with the remarkable reduction of FqC observed in the ΔmpkA strain. We have previously demonstrated that MpkA activation is enhanced during conidiogenesis (Rocha et al. 2020a), and this again coincides with the cytosolic actions of MpkA in the phosphorylation of FmqC. The cell-wall localization of FmqD is an actin-dependent event that requires ER–Golgi transport and it is mandatory for the selective accumulation of FqC to the conidia (Lim et al. 2014). However, how this protein is attached to the cell wall and is activated is an open question. To this end, the definition of the mechanism(s) of how MpkA directly mediates the FqC production, the identification of the specific residues that undergo phosphorylation in FmqA and FmqC and the impact of these events on Fq pathway would be a significant advance to understanding the underlying basis of the contribution of a MAP kinase in the secondary metabolism in fungi.
It has been demonstrated that there is a substantial genetic diversity in the Fq BGC in different isolates, which consequently accumulate different amounts of FqC (Frisvad et al. 2009; Knox et al. 2016; Lind et al. 2017), suggesting that a myriad of environmental stresses may influence the accumulation of this metabolite (Knox et al. 2016; Keller 2019). Recent evidence in this direction indicated that FqC protects conidia from UV-C radiation (Blachowicz et al. 2020), further highlighting the environmental importance of this SM. An RNA-seq experiment designed to detect the expression values of genes belonging to different BGC under different temperatures revealed that lower temperature increased the mRNA abundance of trypacidin BGC, another member of the top five SM enriched in the conidia (Hagiwara et al. 2017). Accordingly, trypacidin was more abundant in the conidia grown at 25°C. By analyzing the same data set, we noticed that a similar behavior was observed for the NRPS genes fmqA and fmqC. Although differences in the accumulation of FqC were investigated here only at 37°C, it should be interesting to determine if FqC would also be more abundant at 25°C.
The regulation of SM production is complex and one of the regulatory layers of these co-ordinated events relies on signaling pathways that funnel through a downstream TF. Approximately, up to 50% of fungal BGCs contain a cluster-specific TF (Lim and Keller 2014); however, fmq BGC is one exception. We provide evidence here that FqC accumulation occurs in an MpkA-RlmA-dependent manner, which is exacerbated when sebA is absent (Figure 10). We propose that multiple TF integrates the signals emerging from MpkA and elicits overlapping and conserved transcriptional responses to shape the levels of FqC in the conidia. This hypothesis is consistent with the substantial increase in the fmqA-D mRNA levels in the ΔsebA strain and the associated increase in FqC accumulation (Figure 6). As SebA binds to the STRE motifs at the fmqA-D promoters and negatively control gene expression under physiological conditions, we conclude that the basal concentration of FqC derives from the concerted actions of different TFs, including RlmA, acting as a positive regulator, and possibly BrlA. However, it is currently unknown if BrlA directly binds to the fmq promoters. Recent reports have indicated that other TFs also influence the accumulation of FqC. For instance, the deletion of cbfA, a TF identified as necessary for the caspofungin paradoxical effect, also caused the derepression of FqC production (Valero et al. 2020). The lessons learned from the FqC depression in the ΔsebA strain suggest a similar scenario for ΔcbfA or other regulators. However, how these events connect with the CWIP or whether the overproduction of FqC results in additional fitness benefits beyond protecting against phagocytosis awaits further experimentation. In addition, given the broad spectrum of regulation exerted by sebA (Dinamarco et al. 2012), it is possible that this TF regulates the synthesis of other SM. Additional ChIP seq experiments will be helpful to further elucidate the contribution of sebA in SM biosynthesis control.
Currently, it is not known whether the different amounts of FqC in the conidia of an invading A. fumigatus strain would have a significant effect in the persistence in the lungs or effectively interfere in the phagocytosis during the initial steps of infection (for a review see Keller 2019; Latge and Chamilos 2019). However, at the saprophytic niche, where a gradient of FqC release is conceivable, it can support our hypothesis that FqC is originally important for the dispersal and permanence of conidia in the environmental niche upon competition with soil predators (Figures 8 and 9). Our data indicate that FqC is easily transferred from the conidia surface to the aqueous solution (Supplementary Figure S4B). Consequently, the idea of an FqC gradient in the soil, together with other conidium-bound SMs such as trypacidin, which also presents antiphagocytic properties (Mattern et al. 2015), is consistent with the differential phagocytosis of the conidia from the CWIP mutants, ΔsebA and ΔfmqA strains by D. discoideum.
Conclusion
In conclusion, this is the first comprehensive study to demonstrate a connection between the A. fumigatus CWIP, the accumulation of Fqs, and the impact of FqC during interkingdom encounters with phagocytes. Finally, we argue that exploiting the effect of FqC overproduction in different genetic backgrounds in A. fumigatus can provide an opportunity to increase the purification efficiency of FqC and to seek for medical and biotechnological applications for this specific A. fumigatus molecule in other cellular systems such as bacteria or virus.
Data availability
Strains and plasmids are available upon request. The authors affirm that all data necessary for confirming the conclusions of the article are present within the article, figures, and tables. Supplementary material is available at GENETICS online with Supplementary material is available at figshare: https://doi.org/10.25386/genetics.13650392.
Acknowledgments
The authors thank Magda R. Ometto Patricio (FAPESP 2019/00967-5) and thank Daniela Hildebrandt (HKI) for excellent technical assistance. The authors are also indebted to Dr. Nancy P. Keller for providing the fmqA-E GFP fusion strains and to Dr. Robert A. Cramer for critical reading of the manuscript.
Funding
This study was funded by FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo; 2015/17541-0, 2016/07870-9 and 2017/19694-3) and CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico—301058/2019-9, 404735/2018-5, and 462383/2014-8).
Conflicts of interest
None declared.
Literature cited
- Altwasser R, Baldin C, Weber J, Guthke R, Kniemeyer O, et al. 2015. Network modeling reveals crosstalk of MAP kinases during adaptation to caspofungin stress in Aspergillus fumigatus. PLoS One. 10:e0136932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames BD, Haynes SW, Gao X, Evans BS, Kelleher NL, et al. 2011. Complexity generation in fungal peptidyl alkaloid biosynthesis: oxidation of fumiquinazoline A to the heptacyclic hemiaminal fumiquinazoline C by the flavoenzyme Af12070 from Aspergillus fumigatus. Biochemistry. 50:8756–8769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames BD, Liu X, Walsh CT.. 2010. Enzymatic processing of fumiquinazoline F: a tandem oxidative-acylation strategy for the generation of multicyclic scaffolds in fungal indole alkaloid biosynthesis. Biochemistry. 49:8564–8576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames BD, Walsh CT.. 2010. Anthranilate-activating modules from fungal nonribosomal peptide assembly lines. Biochemistry. 49:3351–3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angolini CF, Vendramini PH, Araujo FD, Araujo WL, Augusti R, et al. 2015. Direct protocol for ambient mass spectrometry imaging on agar culture. Anal Chem. 87:6925–6930. [DOI] [PubMed] [Google Scholar]
- Blachowicz A, Raffa N, Bok JW, Choera T, Knox B, et al. 2020. Contributions of spore secondary metabolites to UV-C protection and virulence vary in different Aspergillus fumigatus strains. mBio. 11:e03415-19. 10.1128/mBio .03415-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bom VL, de Castro PA, Winkelstroter LK, Marine M, Hori JI, et al. 2015. The Aspergillus fumigatus sitA phosphatase homologue is important for adhesion, cell wall integrity, biofilm formation, and virulence. Eukaryot Cell. 14:728–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bongomin F, Gago S, Oladele RO, Denning DW.. 2017. Global and multi-national prevalence of fungal diseases-estimate precision. J Fungi. 3: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, et al. 2012. Hidden killers: human fungal infections. Sci Transl Med. 4:165rv113. [DOI] [PubMed] [Google Scholar]
- Bruder Nascimento AC, Dos Reis TF, de Castro PA, Hori JI, Bom VL, et al. 2016. Mitogen activated protein kinases SakA(HOG1) and MpkC collaborate for Aspergillus fumigatus virulence. Mol Microbiol. 100:841–859. [DOI] [PubMed] [Google Scholar]
- Casadevall A, Fu MS, Guimaraes AJ, Albuquerque P.. 2019. The ‘amoeboid predator-fungal animal virulence’ hypothesis. J Fungi. 5: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caster SZ, Castillo K, Sachs MS, Bell-Pedersen D.. 2016. Circadian clock regulation of mRNA translation through eukaryotic elongation factor eEF-2. Proc Natl Acad Sci USA. 113:9605–9610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva Ferreira ME, Kress MR, Savoldi M, Goldman MH, Hartl A, et al. 2006. The akuB(KU80) mutant deficient for nonhomologous end joining is a powerful tool for analyzing pathogenicity in Aspergillus fumigatus. Eukaryot Cell. 5:207–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damveld RA, Arentshorst M, Franken A, vanKuyk PA, Klis FM, et al. 2005. The Aspergillus niger MADS-box transcription factor RlmA is required for cell wall reinforcement in response to cell wall stress. Mol Microbiol. 58:305–319. [DOI] [PubMed] [Google Scholar]
- Dinamarco TM, Almeida RS, de Castro PA, Brown NA, dos Reis TF, et al. 2012. Molecular characterization of the putative transcription factor SebA involved in virulence in Aspergillus fumigatus. Eukaryot Cell. 11:518–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erken M, Lutz C, McDougald D.. 2013. The rise of pathogens: predation as a factor driving the evolution of human pathogens in the environment. Microb Ecol. 65:860–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabri J, Godoy NL, Rocha MC, Munshi M, Cocio TA, et al. 2018. The AGC kinase YpkA regulates sphingolipids biosynthesis and physically interacts with SakA MAP kinase in Aspergillus fumigatus. Front Microbiol. 9:3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferling I, Dunn JD, Ferling A, Soldati T, Hillmann F.. 2020. Conidial melanin of the human-pathogenic fungus Aspergillus fumigatus disrupts cell autonomous defenses in amoebae. mBio. 11:e00862-20. 10.1128/mBio.00862-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas FZ, Bertolini MC.. 2004. Genomic organization of the Neurospora crassa gsn gene: possible involvement of the STRE and HSE elements in the modulation of transcription during heat shock. Mol Genet Genom. 272:550–561. [DOI] [PubMed] [Google Scholar]
- Freitas FZ, Virgilio S, Cupertino FB, Kowbel DJ, Fioramonte M, et al. 2016. The SEB-1 transcription factor binds to the STRE motif in Neurospora crassa and regulates a variety of cellular processes including the stress response and reserve carbohydrate metabolism. G3. 6:1327–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisvad JC, Rank C, Nielsen KF, Larsen TO.. 2009. Metabolomics of Aspergillus fumigatus. Med Mycol. 47:S53–71. [DOI] [PubMed] [Google Scholar]
- Gauthier T, Wang X, Sifuentes Dos Santos J, Fysikopoulos A, Tadrist S, et al. 2012. Trypacidin, a spore-borne toxin from Aspergillus fumigatus, is cytotoxic to lung cells. PLoS One. 7:e29906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara D, Sakai K, Suzuki S, Umemura M, Nogawa T, et al. 2017. Temperature during conidiation affects stress tolerance, pigmentation, and trypacidin accumulation in the conidia of the airborne pathogen Aspergillus fumigatus. PLoS One. 12:e0177050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara D, Suzuki S, Kamei K, Gonoi T, Kawamoto S.. 2014. The role of AtfA and HOG MAPK pathway in stress tolerance in conidia of Aspergillus fumigatus. Fungal Genet Biol. 73:138–149. [DOI] [PubMed] [Google Scholar]
- Hillmann F, Novohradska S, Mattern DJ, Forberger T, Heinekamp T, et al. 2015. Virulence determinants of the human pathogenic fungus Aspergillus fumigatus protect against soil amoeba predation. Environ Microbiol. 17:2858–2869. [DOI] [PubMed] [Google Scholar]
- Jahn B, Langfelder K, Schneider U, Schindel C, Brakhage AA.. 2002. PKSP-dependent reduction of phagolysosome fusion and intracellular kill of Aspergillus fumigatus conidia by human monocyte-derived macrophages. Cell Microbiol. 4:793–803. [DOI] [PubMed] [Google Scholar]
- Jain R, Valiante V, Remme N, Docimo T, Heinekamp T, et al. 2011. The MAP kinase MpkA controls cell wall integrity, oxidative stress response, gliotoxin production and iron adaptation in Aspergillus fumigatus. Mol Microbiol. 82:39–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller NP. 2019. Fungal secondary metabolism: regulation, function and drug discovery. Nat Rev Microbiol. 17:167–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox BP, Blachowicz A, Palmer JM, Romsdahl J, Huttenlocher A, et al. 2016. Characterization of Aspergillus fumigatus isolates from air and surfaces of the international space station. mSphere. 1: :e00227-16. doi:10.1128/mSphere.00227-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs Z, Szarka M, Kovacs S, Boczonadi I, Emri T, et al. 2013. Effect of cell wall integrity stress and RlmA transcription factor on asexual development and autolysis in Aspergillus nidulans. Fungal Genet Biol. 54:1–14. [DOI] [PubMed] [Google Scholar]
- Kwon-Chung KJ, Sugui JA.. 2013. Aspergillus fumigatus—what makes the species a ubiquitous human fungal pathogen? PLoS Pathog. 9:e1003743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latge JP, Chamilos G.. 2019. Aspergillus fumigatus and Aspergillosis in 2019. Clin Microbiol Rev. 33: e00140-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim FY, Ames B, Walsh CT, Keller NP.. 2014. Co-ordination between BrlA regulation and secretion of the oxidoreductase FmqD directs selective accumulation of fumiquinazoline C to conidial tissues in Aspergillus fumigatus. Cell Microbiol. 16:1267–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim FY, Keller NP.. 2014. Spatial and temporal control of fungal natural product synthesis. Nat Prod Rep. 31:1277–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind AL, Lim FY, Soukup AA, Keller NP, Rokas A.. 2018. An LaeA- and BrlA-dependent cellular network governs tissue-specific secondary metabolism in the human pathogen Aspergillus fumigatus. mSphere. 3: e00050-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind AL, Wisecaver JH, Lameiras C, Wiemann P, Palmer JM, et al. 2017. Drivers of genetic diversity in secondary metabolic gene clusters within a fungal species. PLoS Biol. 15:e2003583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macheleidt J, Mattern DJ, Fischer J, Netzker T, Weber J, et al. 2016. Regulation and role of fungal secondary metabolites. Annu Rev Genet. 50:371–392. [DOI] [PubMed] [Google Scholar]
- Malavazi I, Goldman GH.. 2012. Gene disruption in Aspergillus fumigatus using a PCR-based strategy and in vivo recombination in yeast. Methods Mol Biol. 845:99–118. [DOI] [PubMed] [Google Scholar]
- Manfiolli AO, de Castro PA, Dos Reis TF, Dolan S, Doyle S, et al. 2017. Aspergillus fumigatus protein phosphatase PpzA is involved in iron assimilation, secondary metabolite production, and virulence. Cell Microbiol. 19: e12770. [DOI] [PubMed] [Google Scholar]
- Manfiolli AO, Siqueira FS, Dos Reis TF, Van Dijck P, Schrevens S, et al. 2019. Mitogen-Activated Protein Kinase Cross-Talk Interaction Modulates the Production of Melanins in Aspergillus fumigatus. mBio. 10: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marim FM, Silveira TN, Lima DS Jr., Zamboni DS.. 2010. A method for generation of bone marrow-derived macrophages from cryopreserved mouse bone marrow cells. PLoS One. 5:e15263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattern DJ, Schoeler H, Weber J, Novohradska S, Kraibooj K, et al. 2015. Identification of the antiphagocytic trypacidin gene cluster in the human-pathogenic fungus Aspergillus fumigatus. Appl Microbiol Biotechnol. 99:10151–10161. [DOI] [PubMed] [Google Scholar]
- Mech F, Thywissen A, Guthke R, Brakhage AA, Figge MT.. 2011. Automated image analysis of the host-pathogen interaction between phagocytes and Aspergillus fumigatus. PLoS One. 6:e19591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novohradska S, Ferling I, Hillmann F.. 2017. Exploring virulence determinants of filamentous fungal pathogens through interactions with soil amoebae. Front Cell Infect Microbiol. 7:497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterbauer CK, Litscher D, Kubicek CP.. 2002. The Trichoderma atroviride seb1 (stress response element binding) gene encodes an AGGGG-binding protein which is involved in the response to high osmolarity stress. Mol Genet Genomics. 268:223–231. [DOI] [PubMed] [Google Scholar]
- Raffa N, Keller NP.. 2019. A call to arms: mustering secondary metabolites for success and survival of an opportunistic pathogen. PLoS Pathog. 15:e1007606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resende D, Boonpothong P, Sousa E, Kijjoa A, Pinto MMM.. 2019. Chemistry of the fumiquinazolines and structurally related alkaloids. Nat Prod Rep. 36:7–34. [DOI] [PubMed] [Google Scholar]
- Ries LN, Beattie SR, Espeso EA, Cramer RA, Goldman GH.. 2016. Diverse regulation of the CreA carbon catabolite repressor in Aspergillus nidulans. Genetics. 203:335–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha MC, de Godoy KF, Bannitz-Fernandes R, Fabri J, Barbosa MMF, et al. 2018. Analyses of the three 1-Cys peroxiredoxins from Aspergillus fumigatus reveal that cytosolic Prx1 is central to H2O2 metabolism and virulence. Sci Rep. 8:12314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha MC, Fabri J, Simões IT, Silva-Rocha R, Hagiwara D, et al. 2020a. The cell wall integrity pathway contributes to the early stages of Aspergillus fumigatus asexual development. Appl Environ Microbiol. 86:e02347-19. 10.1128/AEM.02347-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha MC, Fabri JH, Franco de Godoy K, Alves de Castro P, Hori JI, et al. 2016. Aspergillus fumigatus MADS-box transcription factor rlmA is required for regulation of the cell wall integrity and virulence. G3. 6:2983–3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha MC, Godoy KF, de Castro PA, Hori JI, Bom VL, et al. 2015. The Aspergillus fumigatus pkcAG579R mutant is defective in the activation of the cell wall integrity pathway but is dispensable for virulence in a neutropenic mouse infection model. PLoS One. 10:e0135195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha MC, Minari K, Fabri J, Kerkaert JD, Gava LM, et al. 2020b. Aspergillus fumigatus Hsp90 interacts with the main components of the cell wall integrity pathway and cooperates in heat shock and cell wall stress adaptation. Cell Microbiol. 23:e13273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez JF, Wang CC.. 2012. The chemical identification and analysis of Aspergillus nidulans secondary metabolites. Methods Mol Biol. 944:97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterlee T, Nepal B, Lorber S, Puel O, Calvo AM.. 2020. The transcriptional regulator HbxA governs development, secondary metabolism, and virulence in Aspergillus fumigatus. Appl Environ Microbiol. 86: e01779-19. 10.1128/AEM.01779-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smedsgaard J. 1997. Micro-scale extraction procedure for standardized screening of fungal metabolite production in cultures. J Chromatogr A. 760:264–270. [DOI] [PubMed] [Google Scholar]
- Szewczyk E, Krappmann S.. 2010. Conserved regulators of mating are essential for Aspergillus fumigatus cleistothecium formation. Eukaryot Cell. 9:774–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valero C, Colabardini AC, Chiaratto J, Pardeshi L, de Castro PA, et al. 2020. Aspergillus fumigatus transcription factors involved in the caspofungin paradoxical effect. mBio. 11:e00816-20. 10.1128/mBio.00816-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valiante V. 2017. The cell wall integrity signaling pathway and its involvement in secondary metabolite production. J Fungi. 3:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valiante V, Baldin C, Hortschansky P, Jain R, Thywißen A, et al. 2016. The Aspergillus fumigatus conidial melanin production is regulated by the bifunctional bHLH DevR and MADS-box RlmA transcription factors. Mol Microbiol. 102:321–335. [DOI] [PubMed] [Google Scholar]
- Valiante V, Jain R, Heinekamp T, Brakhage AA.. 2009. The MpkA MAP kinase module regulates cell wall integrity signaling and pyomelanin formation in Aspergillus fumigatus. Fungal Genet Biol. 46:909–918. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Strains and plasmids are available upon request. The authors affirm that all data necessary for confirming the conclusions of the article are present within the article, figures, and tables. Supplementary material is available at GENETICS online with Supplementary material is available at figshare: https://doi.org/10.25386/genetics.13650392.