Abstract
To-be-attended information can be specified either with positive cues (I’ll be wearing a blue shirt) or with negative cues (I won’t be wearing a red shirt). Numerous experiments have found that positive cues help search more than negative cues. Given that negative cues produce smaller benefits compared to positive cues, it stands to reason that searchers may choose to use positive templates instead of negative templates if given the opportunity. Here, we evaluate this possibility with behavioral measures as well as by directly measuring the formation of positive and negative templates with event-related potentials. Analysis of the contralateral delay activity (CDA) elicited by cues revealed that positive and negative templates relied on working memory to the same extent, even when negative working memory templates could have been circumvented by relying on long-term memories of target colors. Whereas the CDA did not discriminate positive and negative templates, a CNV-like potential did, suggesting cognitive differences between positive and negative templates beyond visual working memory. However, when both positive and negative information were presented in each cue, participants preferred to make use of the positive cues, as indicated by a CDA contralateral to the positive color in negative cue blocks, and a lack of search benefits for positive- and negative-color cues relative to positive-color cues alone. Our results show that searchers elect to selectively encode only positive information into visual working memory when both positive and negative information are available.
Keywords: Visual search, attention, working memory, event-related potentials
1. Introduction
Our visual system provides us with a wealth of potentially useful information, but a key to successful behavior is selecting just the information that is useful in a given moment. This selection has been variously explained as prioritization of information we want to attend to (e.g., Wolfe, Cave, & Franzel, 1989; Wolfe & Gray, 2007) and suppression of information we do not want to attend (Treisman & Sato, 1990). In principle, foreknowledge of relevant and irrelevant information should be equally helpful in selecting desired information, but research in visual search shows that in fact there is an asymmetry: cues telling you what to attend to (positive cues) are more helpful that cues telling you what not to attend to (negative cues; Arita, Carlisle, & Woodman, 2012; Beck & Hollingworth, 2015; Becker, Hemsteger, & Peltier, 2015; Beck, Luck, & Hollingworth, 2018). Because negative cues provide smaller benefits, it stands to reason that searchers would employ positive templates instead of negative templates when the opportunity presents itself. In the present study, we used a combination of behavior and event-related potentials elicited by positive and negative cues to directly measure which cues participants use.
The question of how we process information about what not to do, think, or believe has a long history in experimental psychology (Clark & Chase, 1972; Logan, Schachar, & Tannock, 1997; Verbruggen & Logan, 2008; Wason, 1959; Wegner, 1994). Across many tasks, receiving negative information presents cognitive challenges compared to positive information. That is, information about what is not true, or what will not occur is more difficult to represent or use than information about what is true, or what will occur. For example, Clark and Chase (1972) found that the time it takes to verify that a sentence accurately describes a picture is slower overall when the sentence includes a negation (e.g., the star is not above the plus). This was attributed to an additional cognitive step of reversing judgments when the subjects of the statement otherwise matched the picture.
More recently, research on visual search has addressed the question of how negative information is used to control attention. These studies have presented cues that tell participants what color, for example, a target will not be before presenting a search array (Arita et al., 2012; Moher & Egeth, 2012). Two general findings are worth emphasizing. First, positive cues generally lead to better search performance than do negative cues. Second, negative cues can provide benefits relative to conditions where no cues are provided (Arita, et al., 2012, Carlisle & Nitka, 2019, Reeder, Olivers, & Pollman, 2017, Reeder, Olivers, & Pollman, 2018), but some studies fail to find a negative cue benefit (see Beck & Hollingworth, 2015; Becker, Hemsteger, & Peltier, 2016), and sometimes negative cues instead leads to costs (Moher & Egeth, 2012, Beck & Hollingworth, 2018).
Currently there is no consensus on how negative cues are used (Geng, Won, & Carlisle, in press). One position is that negative templates cannot be directly used, but that searchers must first attend to irrelevant information before they can exclude it (Moher & Egeth, 2012) and subsequently attend to the remaining, relevant information using either spatial (Beck & Hollingworth, 2015) or feature-based (Becker, Hemsteger, & Peltier, 2016) recoding strategies. An alternative position is that negative templates can be used to directly suppress irrelevant information, but that attentional weights for ignored information are not set to zero (Arita, et al., 2012, Carlisle & Nitka, 2019), which would account for the relatively lower benefits of negative cues. While the former positions holds that using negative information involves two cognitive steps, and the latter position holds that negative information can be used in a single cognitive step, all sides agree that negative cues do not provide the same performance advantages that positive cues do.
While the debate regarding negative templates has largely focused on what searchers are capable of, a complete account of how we implement control over attention requires an understanding of what searchers choose to do when multiple strategies are available (Irons & Leber, 2016; Pauszek & Gibson, 2018; Rajsic, Wilson, & Pratt, 2015). Accounting for strategies and processing preferences can reveal a capacity for cognitive control over seemingly automatic processes that would otherwise go unnoticed (Bacon & Egeth, 1994; Carlisle & Woodman, 2011; Kiyonaga, Enger, & Soto, 2012; Leber & Egeth, 2006; Woodman & Luck, 2007). There is growing evidence that choice or strategy can determine the pattern of results obtained in visual search tasks. For example, spatially mixing relevant and irrelevant items in search discourages searchers from relying on negative templates (Beck & Hollingworth, 2015). In contrast, when the same non-effective spatially mixed arrays from Beck & Hollingworth (2015) were randomly mixed into a block where the majority of trials contained spatially separated arrays where negative cues are effective, a negative cue benefit was found for both the spatially mixed and spatially separated arrays (Carlisle and Nitka, 2019). Similarly, Conci, Deichsel, Müller, and Töllner (2019) have shown that negative color cues do not lead to benefits during a search task which can easily be performed based on target shape, but that benefits emerge when the task cannot be completed based on simple shape features. This suggests that searchers will only utilize negative cues when the task becomes extremely demanding or impossible to complete without using the cues, even though they are helpful in principle. This is consistent with the idea that they are more difficult to use than positive cues (see also Beck & Hollingworth, 2015).
In the present study, we sought to address the question of whether positive information is preferred to negative information in the guidance of attention by directly measuring the maintenance of both positive and negative templates in working memory using electrophysiology and examining the behavioral impact of template choice. We reasoned that if negative templates are less useful than positive templates, then opportunities to instead use positive templates should lead to a reduction the frequency with which negative cues are encoded into working memory as a search template. Although we are interested in the nature of attentional dynamics during actual searching, our experiments here focus on preparatory processes. That is, we measured the formation and maintenance of templates based on cue displays in advance of search. Following Carlisle, et al., (2011; see also Woodman, Carlisle, & Reinhart, 2013; Reinhart & Woodman, 2015), we measured an event-related potential (ERP) known as the contralateral delay activity (CDA) to cues that either showed colors that needed to be later attended (positive cues) or ignored (negative cues). The CDA is a negative slow wave measured at posterior electrodes contralateral to stimuli that are being maintained in working memory. Previous experiments have established that this component tracks the maintenance of positive search templates (Woodman & Arita, 2011), decreases in amplitude when working memory templates can be replaced by long-term memory templates (Carlisle, et al., 2011; Woodman, Carlisle, & Reinhart, 2013), and increases when emphasis is placed on search performance in an upcoming trial (Reinhart, McClenahan, & Woodman, 2016; Reinhart & Woodman, 2014). This demonstrates that the CDA is sensitive to the use cues to form positive search templates. As a result, we expected reliance on negative templates would be captured by changes in amplitude of the CDA.
Here, we outline the purpose of each experiment and preview the results. In Experiment 1, we compared ERPs of working memory storage elicited when participants were shown what to attend (positive cues) to those elicited from cues showing what to ignore (negative cues). In this experiment, no opportunities were given for recoding of negative cues into positive templates prior to the onset of the search array. With any given negative cue, participants could not predict what color they would eventually attend, as it was selected at random from the remaining set of colors. We found similar amplitude CDA effects for positive and negative search templates. Experiment 1, then, establishes a baseline for how negative cues are stored in working memory in comparison to positive cues. In Experiment 2, we added an opportunity for participants to rely on their memory for target features rather than negative templates: within short runs of trials, as long as a given negative cue color repeated, so did the corresponding target color for those searches. If guiding attention using knowledge of previous target features is preferable to relying on negative cues, the CDA in the negative cue condition should drop below that of the positive condition as cues repeat. However, we found that participants still represented the negative templates in working memory. This suggested that participants were still choosing to use negative cues, even when positive templates could have been used instead. In Experiment 3, we analyzed the CDA when both a positive and negative color cue were available prior to the search array. Specifically, the two colors presented in each lateralized cue array were the two colors that appeared in that trial’s search, with pre-cues and instructions specifying the cued color as positive or negative in a given block. When given both cues in this manner, we found a CDA contralateral to the cue indicating the target’s color, regardless of instructions. This suggests that while participants can prepare a negative cue in working memory, when given the choice between using a negative and positive cue, they have a strong tendency to use the positive cue information to guide attention to search targets rather than negative cue information. Finally, to confirm that the results of Experiment 3 reflect the use of the positive cue when both types of cues are available, Experiment 4 compared the behavioral impact of receiving positive, negative, and both cues compared to a neutral cue condition. By measuring the size of response time benefits in the both cue condition to the positive cue only condition, we could see whether adding negative cues produced any extra search gains. The results showed that providing both positive and negative colors in a cue produced no additional benefit when compared to the positive cue alone, suggesting participants were largely choosing to use the positive information alone even when a negative cue provides additional information, confirming our interpretation of the CDA results in Experiment 3.
2. Experiment 1
In Experiment 1, we used a simple conjunction search task that could be completed with either positive or negative search templates. Subjects searched for Landolt C’s with a gap on their left or right side. Across different blocks of trials the subjects were instructed that the cued object (i.e., to the left in Figure 1a) indicated the color in which the distractors would appear on negative-cue condition. In the positive-template condition the cued object indicated the color that the target would appear in. Following previous studies (Carlisle, et al., 2011; Woodman, Carlisle, & Reinhart, 2013; Reinhart & Woodman, 2015), we expected to see a CDA emerge for positive and negative cues, reflecting the creation of positive and negative templates, respectively. Importantly, a horizontal Landolt-C of both the cued color and another color was presented in each search, ensuring that it was not possible to correctly report the target without knowing the cue’s color (Becker, Hemsteger, & Peltier, 2015). Without this addition, participants could have ignored the cues entirely and simply looked for a horizontal Landolt-C.
Figure 1.
A. Depiction of the task structure used in Experiments 1, 2, and 3. Stimuli are not drawn to scale but drawn to maximize stimulus discriminability. Stimuli on search displays were positioned at twice the eccentricity from fixation of the cues. Examples in lower panels all provide two possible search displays in a run of negative-cue repetitions, given a green cue (as pictured in the upper panels).
2.1. Methods
2.1.1. Participants.
Thirty-one volunteers from the Vanderbilt community participated in Experiment 1. Our goal for each ERP experiment was to collect at least 20 participants, whose data passed inclusion criteria, to be consistent with the sample sizes of previous studies measuring the CDA to cues in a visual search task (typically 15-20 participants: Carlisle, Arita, Pardo, & Woodman, 2011; Grubert, Carlisle, & Eimer, 2016; Reinhart & Woodman, 2013; Servant, Cassey, Woodman, & Logan, 2018). Participants’ data were included for analysis if they met the following criteria: fewer than 25% of trials lost to ocular artifacts in either the cue epoch or the search epoch (mean of 10.9% trials rejected across remaining subjects), an average error rate of less than 15% (mean of 93.5% correct across remaining subjects), and less than 3.2μv of residual HEOG towards cues after rejecting ocular artifacts. Blocking artifacts (Luck, 2005) were excluded on a trial and electrode-wise basis. One additional participant was excluded for excessive blocking artifacts. Twenty-one participants remained after these criteria were applied. All participants provided informed consent and were paid for their time. Experimental procedures were approved by the Vanderbilt University Institutional Review Board.
2.1.2. Apparatus.
Stimuli were presented on a CRT monitor in a soundproof, electrically shielded booth. Participants viewed stimuli from approximately 150 cm. Stimuli were generated with Matlab using the Psychophysics toolbox (Kleiner et al., 2007), and responses were collected using a Logitech gamepad. Subjects’ EEG was recorded using an SA instrumentation isolated bioelectric amplifier from tin electrodes embedded in a elastic cap (Electro-cap International Inc., Eaton, OH) using the following locations from the International 10/20 system: F3, F4, Fz, C3, C4, Cz, T3, T4, T5, T6, P3, P4, Pz, PO3, PO4, OL (PO7), OR (PO8), O1, O2, along with bipolar HEOG (electrodes placed 2 cm from the outer canthi of both eyes) and bipolar VEOG (electrodes placed 1cm below the lower right eyelid and 1cm above the right eyebrow). All electrodes were kept at 4kΩ or lower. The voltages were amplified 20,000 times, digitally sampled at 250Hz, using the right mastoid as an online-reference and re-referenced offline to the average of the left and right mastoids.
2.1.3. Stimuli and procedure.
Stimuli presented on each trial consisted of five displays, all with a uniform gray background (27 cd/m2). The first display indicated which of the two upcoming, lateralized stimuli would be the trial’s cue color. This was indicated using two arrowheads facing left (“<<”) or right (“>>”), centered on the screen, 0.5° width and 0.15° height, lasting a variable interval between 1000ms and 1400ms. Following the offset of this screen, a fixation display was presented for 1000ms containing a central “+” symbol, 0.15° width and height. The cue display appeared next for 100ms, which showed two line-drawn circles centered 1.5° to the left and right of fixation. The color of these circles was randomly selected from four colors: green (x = .282, y = .586, Y = 44 cm/m2), red, (x = .631, y = .328, Y = 17 cm/m2), cyan (x = .209, y = .310, Y = 41 cm/m2), and yellow (x = .400, y = .500, Y = 44 cm/m2), with the constraint that the two circles could never be the same color. They had a diameter of 0.63° and a thickness of 0.1°. On positive search blocks, participants were instructed that the target in the search display would be the cued color. On negative search blocks, participants were instructed that the target in the search display would be whichever color in the search display was not the cued color. Following the cue display a fixation display was again presented for 900ms. Lastly, participants were shown a search display. Search displays were made up of four Landolt C stimuli, the same dimensions as the cues, presented 3° to the left, right, top, and bottom of fixation, with gaps of 0.2°. Two of these Landolt C’s had vertical gaps (distractors) and two of the Landolt C’s had horizontal gaps (potential targets). One of each of these Landolt stimuli appeared in two possible colors: the cued color and a non-cued color, which could vary between all of the three non-cued colors. This meant that participants needed to know the cued color in order to provide a correct response. In this way, we ensured that any differences between positive and negative search performance would not be due to a difference in the strategic use of templates (Becker, Hemsteger, & Peltier, 2016; Carlisle & Nitka, 2019; Conci, et al, 2019), that is, the choice to simply look for a sole target (left or right facing Landolt-C) irrespective of its color. Search displays were presented for 2000ms or until a response was collected. Subjects responded by pressing one of the two response buttons to signal their decision (the leftmost and rightmost buttons on a Logitech gamepad, indicating left target gap and right target gap, respectively). The next trial began immediately after the search trial offset from the previous trial. Participants were instructed to maintain fixation at the fixation cross at all times, and to blink only in the period between their response and the onset of the following cue display.
Participants each completed six blocks of 360 trials within an experimental session, which lasted approximately three hours, not including EEG setup. An experimental session consisted of three positive cue (attend) blocks and three negative cue (ignore) blocks, which were completed in an alternating fashion. Half of participants completed a positive cue block first, and half completed a negative cue block first. Following the design of Carlisle, Arita, Pardo, & Woodman (2011)’s third experiment, trials were structured so, within a block, that the same cue color would repeat for three, five, or seven trials before changing. For each cue-repetition trial, the non-cued color could change on every trial, and matched the non-cued search set at chance levels (33%, given that there were always three potential non-target colors). Likewise, the non-cued search color (i.e., the non-target color on positive cue blocks and the target color on negative cue blocks) could change on any given trial. Participants were instructed verbally with a visual aid depicting sample trials for each block type. Before beginning their first recorded block, participants practiced trials of whichever block they were to do first until they were comfortable with the task and were able to maintain fixation and control their blinks, as indicated by experimenter observation of the EOG during practice trials and by participant self-report. During this time, verbal feedback on eye control was given by the experimenter as deemed necessary to encourage fixation and proper blink timing (between trials). Once eye control and trial completion became satisfactory, the participant was invited to begin the first block, or to continue practicing. Experimental blocks began when participants elected to start.
2.1.4. EEG analysis.
Continuous EEG data for each participant were sorted into epochs locked to the onset of the cue on each trial, beginning 200ms before the onset of cue displays until 1000ms following the onset of the cue display. EEG was baseline corrected by subtracting the mean of 200ms period before each stimulus onset. Artifacts were identified and rejected using a two-step procedure based on Woodman and Luck (2003). Time windows with differences exceeding threshold values were rejected (mean thresholds across subjects were 71 μv for blinks and 25μv for saccades, with thresholds set individually for each subject) as were individual electrodes on trials with amplifier saturation or whose voltage exceeded +/−75μv. Averaging across participants and CDA electrode, the resulting number of trials remaining after exclusions was 178, 180, 303, and 242 for the 4 repetition bins (1, 2, 3:4, 5:7, respectively) in the positive cue condition, and 167, 171, 286, and 228 for the 4 repetition bins in the negative cue condition. Finally, EEG data were algebraically re-referenced to the average of the left and right mastoids (Luck, 2005). Filtered ERPs were also calculated from the overall EEG time series, low-pass filtered at 30hz, and we used these data to plot results. Mean amplitude measurements were calculated using unfiltered data.
Our analysis focused on the contralateral delay activity, or CDA (Vogel & Machizawa, 2004), elicited by the cue to measure the use of visual working memory in representing the cue as a template. The CDA was measured as the mean amplitude between 300 and 1000ms after cue onset (Vogel & Machizawa, 2004) at O1/O2, PO3/PO4, OL/OR, and T5/T6 (Carlisle, et al., 2011). ERPs were calculated only for trials where a correct response was given, and on trials with no identified saccades or blinks.
2.2. Results
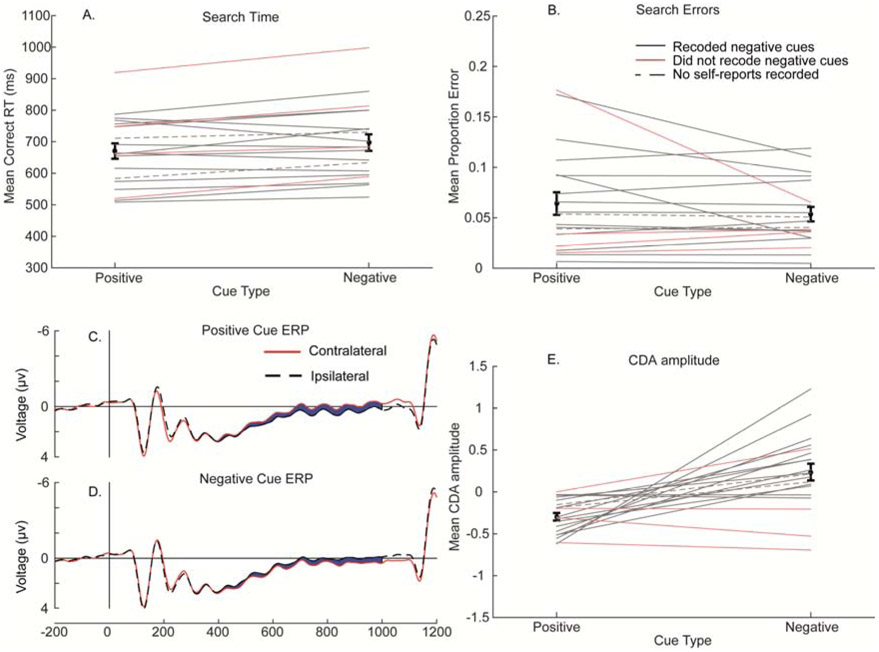
Consistent with previous reports, mean reaction time (RT, see Figure 2, panel A) was faster following positive cues than negative cues, F(1, 20) = 378.82, p < .001, η2p = 0.95. Response times declined over cue repetitions, F(6, 120) = 3.54, p = .003, η2p = .15. Cue type and repetition did not interact, F(3.72, 74.4)1 = 2.18, p = .08, η2p = .10. The same was true of error rate, with fewer errors for positive than negative cues, F(1, 20) = 45.37, p < .001, η2p = 0.69, and a decline in error rate over cue repetition, F(3.46, 69.12) = 3.91, p = .009, η2p = .16. Cue type and repetition did not interact, F(3.69, 73.74) = 0.40, p = .80, η2p = .02. As can be seen in Figure 2, however, the reduction in RT was modest.
Figure 2.
Results from Experiment 1. Panels A and B depict behavioral data (search time and error rate, respectively; error bars show one SEM; lines are individual participants), Panels C and D show contralateral and ipsilateral grand-average ERPs to the cues for positive and negative cues, respectively (differences in the CDA epoch filled in blue), and panel E depicts averaged CDA amplitude (error bars show one SEM; lines are individual participants).
Having verified that negative cues indeed led to poorer search performance in our task, we asked whether both positive and negative cues were stored in working memory in the same way. To assess whether participants prepare for search differently when given a positive versus a negative cue, we analyzed cue-locked CDAs. For both positive and negative cue trials, we observed a cue-locked CDA, F(1, 20) = 25.38, p < .001, η2p = 0.56, with no differences in amplitude due to cue type, F(1, 20) = 0.92, p = .35, η2p = 0.04. This shows that participants simply stored the color of the cue in working memory regardless of cue type (see Figure 2, panels C and D). We did not find a systematic change in the CDA over cue repetitions, F(3, 60) = 0.95, p = .42, η2p = 0.05, suggesting that participants tended to rely on working memory-based templates across repetitions. Considering the type of cue (positive or negative) in this interaction did not provide support for an effect of repetition on the CDA either, F(2, 60) = 2.14, p = .11, η2p = .10. Thus, the results suggest that the cued object is held in visual working memory regardless of whether the cue indicates an item to-be-attended, or to-be-ignored.
2.3. Discussion
Experiment 1 demonstrated that both positive and negative cues were held in working memory, as measured by the CDA. Although participants could have recoded the negatively cued color into the remaining three colors, or even suppressed the cued color (i.e., creating an inhibitory tag for the cued feature, manifesting as a Pd; Sawaki, Geng, & Luck, 2012), their strategy was to simply remember the single color they would either attend or ignore later.
Before we push the apparent tendency of participants to remember the items they were supposed to ignore, we wanted to address some additional analyses that we performed on the data from Experiment 1. Specifically, informed readers may be aware of previous work suggesting that when the searched for target remains the same across trials, that people exhibit faster RTs when performing search, their CDA component appears to disappear, and frontal components indexing long-term memory appear to systematically change (e.g., Reinhart & Woodman, 2015). Above we showed that in Experiment 1, we observed a significant speeding in RTs across target repetitions, but did not see the CDA component decrease in amplitude across these repetitions. The anterior P1 (or P170) showed the same pattern as the CDA, in that it was insensitive to the repetition of targets (Fz, 180ms – 220ms post-stimulus measurement window), F(3, 60) = 1.69, p = .18, η2p = .08. And to preview our subsequent experiments, we did not find significant effects of repetition on the anterior P1 in Experiment 2, F(3, 66) = 0.18, p = .91, η2p = .008, or Experiment 3, F(3, 57) = 1.55, p = .21, η2p = .08, either. Although it is a tangent to the current question of how negative and positive information is handled to guide attention, these learning-related findings suggest that subjects may have control over whether they use long-term memory or working memory to guide attention during visual search (Reinhart, McClenahan, & Woodman, 2016).
3. Experiment 2
In Experiment 2, we pursued the question of whether participants would ignore negative cues if the target color was largely predictable. In Experiment 1, only the target color (in the positive cue condition) and the distractor color (in the negative cue condition) would repeat for a short run of trials within each block. In Experiment 2, each run of trials involved repetition of both the target and distractor colors in every search display for both cue conditions. This meant that participants could potentially learn to ignore negative cues and instead use their memory of the previous trial’s target color as a positive template once they had completed the first trial of a given run. If participants elect to ignore negative cues when they can predict a target’s color, then we should observe equivalent search performance and ERPs in the two cue conditions on later trials in a run, and a large drop in the CDA in the negative cue condition over repetitions of cue colors, as participants opt not to represent the to-be-ignored color in working memory. Instead, if participants rely on negative cues instead of memory for the previous trial’s color, Experiment 2 should replicate the results of Experiment 1.
Experiment 2 was identical to Experiment 1 with one exception. In the negative-cue condition, runs of trials where the cue color repeated also involved repetitions of whatever target color was used (e.g., if the cue signaled a non-red target and the target was blue on that trial, the same was true for the two, four, or six cue repetition trials that followed). This is invited the potential for subjects to not waste working memory capacity on representing the negative cues and rely on target-color memory instead for search guidance. All other aspects of the experiment were the same.
3.1. Method
3.1.1. Participants.
Thirty-one volunteers from the Vanderbilt participant pool participated in Experiment 2. None of these participants had been in Experiment 1. Eight participants’ data were excluded, for the same reasons laid out in Experiment 1 (mean of 8.8% trials excluded for ocular artifacts and a mean of 94% accuracy in remaining participants). All participants provided informed consent before participating.
3.1.2. Apparatus, stimuli, procedure, and EEG analysis.
All methods were identical to Experiment 1 save for one difference. For each trial in a run of repeated cues, both the target color and the non-target color repeated in search displays. This ensured that the color of the object that participants ultimately selected and responded to on each trial in a run repeated in both the positive and negative search conditions, and allowed the target’s color to be largely predictable in negative-cue blocks. The mean VEOG threshold across subjects was 62μv and mean HEOG threshold was 27μv. Averaging across participant and CDA electrode, the average number of trials remaining after exclusions was 179, 182, 304, and 242 for each repetition bin (1, 2, 3:4, 5:7, respectively) in the positive cue condition, and 172, 174, 290, and 233 for each repetition bin in the negative cue condition.
3.2. Results
Despite the opportunity for recoding during the runs in Experiment 2’s design, participants still performed better in the positive-cue condition (Figure 3, panel A). Mean correct RTs were faster in the positive-cue condition, F(1, 20) = 149.03, p < .001, η2p = .87, and declined over cue repetitions, F(3.85, 84.76) = 9.44, p < .001, η2p = .30. The decline was more pronounced for the negative-cue condition, as indicated by an interaction between cue type and repetition, F(6, 132) = 2.47, p = .027, η2p = .10. Errors were also lower in the positive than negative-cue condition, F(1, 20) = 52.13, p < .001, η2p = .70, but did not decline significantly with cue repetition, F(4.11, 90.48) = 1.77, p = .14, η2p = .075. To behaviorally test whether participants benefitted from target-color repetitions in the negative cue condition, we compared negative-cue performance between Experiments 1 and 2. Neither RT, F(4.50, 188.86) = 1.27, p = .28, η2p = .03, nor error-rate, F(4.13, 173.30) = 1.04, p = .39, η2p = .02, provided any evidence for a benefit of target-color predictability.
Figure 3.
Results from Experiment 2. Panels A and B depict behavioral data (search time and error rate, respectively; error bars show one SEM; lines are individual participants), Panels C and D show contralateral and ipsilateral grand-average ERPs to the cues for positive and negative cues, respectively (differences in the CDA epoch filled in blue), and panel E depicts CDA amplitude in the four cue repetition bins (error bars show one SEM; lines are individual participants).
As in Experiment 1, we again found a CDA, F(1, 22) = 18.27, p < .001, η2p = 0.45, that did not interact with cue type F(1, 22) = 0.37, p = .55, η2p = 0.017. This provides strong evidence that even when the target color was predictable, negative cue colors were simply maintained in working memory like positive cue colors. While the CDA overall did not reduce as a function of repetitions, F(3, 66) = 0.39, p = .76, η2p = .017, the cue-repetition effect on the CDA marginally differed as a function of cue type, F(3, 66) = 2.63, p = .057, η2p = .11. As can be seen in Figure 3, this interaction is driven by the smaller CDA in the positive cue condition than the negative condition on the first cue repetition. While this could be taken to indicate greater reliance on visual working memory for new, negative templates, it instead appears that it is the positive-cue CDA that is unusually small early on. Separate repeated-measures ANOVAs for positive and negative-cue conditions that included only the early (repetition 1) and late (repetitions 5-7) bins substantiated this impression: for the negative-cue CDA, the CDA was larger early than late, F(1, 22) = 4.55, p = .044, η2p = .17, but the positive-cue CDA was smaller early than late, F(1, 22) = 5.42, p = .029, η2p = .20. While an unusual pattern, it is important to emphasize that it is entirely inconsistent with the prediction that repeating target colors would allow strategic avoidance of visual-working-memory-based negative templates later in a run of searches. In sum, neither the CDA nor response times provided evidence that being able to predict the target’s color in the negative condition led participants to rely less on visual-working-memory-based negative templates. Instead, both positive and negative cue colors remained in working memory.
3.3. Discussion
The results of Experiment 2 showed that even when the target color could be predicted on a majority of trials, participants held colors that they needed to ignore in working memory, as evidenced by the CDA in the negative cue condition. This predictability of target colors did not improve performance following negative cues, consistent with a lack of relying on their memory of target features. Although recoding was possible in Experiment 2, it would have relied on an internal representation of the target’s color, as well as recognition of the cue repetition. Given that positive cues are more effective than negative cues, it was surprising that participants did not adopt a strategy of relying on their memory for recent target features. In Experiment 3, we provided participants both negative and positive cues in advance of each search to test whether participants would rely on positive and negative cues equally, or whether they would choose to only rely on positive cues.
4. Experiment 3
In Experiment 3, we used the same task and instructions as Experiments 1 and 2, but provided both the target and distractor colors in each cue display. That is, we fully equated visual presentation sequence of the positive-cue and negative-cue conditions by using the same colors for all cue and search displays within a given run of trials. We did this by reliably pairing target and non-target colors in the search displays (as in Experiment 2) and in the cue displays as well. In other words, the non-cued color in each negative cue display reliably predicted the target color in that subsequent search display, and the non-cued color in each positive display predicted the distractor color. This allowed us to test whether participants preferentially form positive templates when both types of information are available. Although we instructed participants that the cued color would show them the to-be-ignored color on negative blocks, we anticipated that they could learn that the non-cued color was always the target color. If it is the case that positive search is a cognitively simpler process than negative search (Carlisle & Nitka, 2018; Clark & Chase, 1972; Rajsic, Wilson, & Pratt, 2015) then participants might instead encode the non-cued color in the negative-cue condition, which would reverse the CDA’s polarity. On the other hand, if participants simply encode the cue they are informed about, Experiment 3’s results should look just like Experiments 1 and 2. Alternatively, if participants encoded both positive and negative colors, the two CDAs would cancel out and we would observe no CDA.
4.1. Method
4.1.1. Participants.
Twenty-five volunteers from the Vanderbilt community participated in Experiment 3. Three of the participants had participated in Experiment 1, but at least two months elapsed between sessions, and participants did not recall the details of the earlier session when asked. Five participants were excluded for exceeding artifact criteria described in Experiment 1 (mean of 91% trials remaining after rejecting ocular artifacts for included participants, mean of 94% accuracy in included participants).
4.1.2. Apparatus, stimuli, procedure, and EEG analysis.
All apparatus, stimuli, procedure, and analysis were identical to Experiment 2 except as follows. On each trial, in both positive and negative cue blocks, search displays were constrained to include the same two colors shown in the cue display for that trial. Specifically, on positive cue trials, the cued color would be the target color on that trial and the uncued color (in the hemifield the central arrows pointed away from) would be the distractor color. The opposite was true on negative trials. The cued color was used for the distractor objects, and the uncued color was used for the target objects. The mean VEOG threshold across participants was 65μv and the mean HEOG threshold was 26μv. Averaging across participant and CDA electrode, the number of trials remaining after exclusions was 175, 178, 297, and 240 for each repetition bin (1, 2, 3:4, 5:7, respectively) in the positive cue condition and 180, 184, 302, and 243 for each repetition bin in the negative cue condition.
4.2. Results
Showing both the target and non-target color almost completely equated the positive and negative cue conditions. Mean response time (see Figure 4A) for the positive cue condition were still faster, than for the negative cue condition, F(1, 19) = 7.85, p = .01, η2p =.29. While different, the magnitude of the difference is considerably smaller than Experiment 1 and 2, as shown by an experiment X cue type interaction, F(2, 61) = 50.23, p < .001, η2p = .62. For perspective, the negative cue condition was 155ms slower than the positive cue condition in Experiment 1, 157ms slower than the positive cue condition in Experiment 2, but only 26ms slower than the positive cue condition in Experiment 3. Response time did not reduce as a function of cue repetitions, F(6, 114) = 0.46, p = .83, η2p = 0.02. No difference in error rate was found between the positive cue and negative cue conditions, F(1, 19) = 1.54, p = .23, η2p = 0.08, but error rate did reduce with cue repetition, F(3.28, 62.22) = 7.23, p < .001, η2p = .28.
Figure 4.
Results from Experiment 3. Panels A and B depict behavioral data (search time and error rate, respectively; error bars show one SEM; lines are individual participants), Panels C. and D. show contralateral and ipsilateral grand-average ERPs to the cues for positive and negative cues, respectively (differences in the CDA epoch filled in blue), and panel E depicts averaged CDA amplitude (error bars show one SEM; lines are individual participants). For panels A, B, and E, participant data are visually coded according to their reported strategy (see legend in panel B).
Most dramatically, the polarity of the CDA reversed in the negative cue condition, such that we observed an interaction between cue condition and laterality, F(1, 19) = 21.07, p < .001, η2p = 0.53, with no main effect of laterality, F(1, 19) = 0.56, p = .46, η2p = 0.029. To be sure, the positive cue CDA was different from zero, F(1, 19) = 47.61, p < .001, η2p = 0.72, and so was the polarity-reversed, negative cue CDA, F(1, 19) = 5.02, p = .037, η2p = 0.21. To check whether the positive cue and negative cue CDAs were of similar amplitudes, we multiplied the negative-cue amplitudes by −1 and checked for an interaction with laterality. No such interaction was present, F(1, 19) = 0.56, p = .46, η2p = 0.03, suggesting that participants nearly fully relied on the non-cued color in the negative cue condition. Consistent with response times, the CDA amplitude did not change as a function of cue repetitions, F(3, 57) = 1.35, p = .27, η2p = .07. The CDA was largest at OL/OR, F(1.72, 32.66) = 3.25 p = .028, η2p = .15, but was larger at T5/T6 for negative compared to positive cues, F(2.15, 40.93) = 7.79, p = .001, η2p = .29.
The CDA reversal (Figures 4C, D, E) shows that when participants were always shown what they would later attend opposite what they were cued to ignore, they preferred to instead encode what color they would later attend into working memory. Informal conversations following the experiment confirmed this result, with the clear majority of those participants asked about strategy (14 out of 18) verbally reporting that they chose to remember the uncued color in negative cue blocks. As can be seen in Figure 4E, the CDA for the participants who reported no strategic selection of positive cues on negative cue blocks (plotted in red) tended to be negative in the negative-cue condition, supporting this distinction. Indeed, when only the participants reporting recoding are included in the analysis, the small difference between positive and negative cues in RT is no longer evident, F(1, 13) = 1.69, p = .22, η2p = 0.12.
4.3. Discussion
In Experiment 3, we found that when participants were given access to both target and distractor color information prior to search, most chose to encode only the positive cue information, even when they were told to use the negative cue in negative cue blocks. This suggests that, when equally available, searchers prefer to rely only on positive information instead of negative information, or both kinds of information. However, since Experiment 3 did not have a condition in which only positive or negative cues were provided, it is not possible to be sure that negative information was not also used, but to a lesser extent than positive information.
5. Midline ERPs discriminate positive from negative cues
As a brief summary, Experiments 1 and 2 showed that our ERP measure of visual working memory storage (the CDA) did not discriminate between positive and negative cues despite the rather large difference evident in response times and error rates. Only when participants were given the opportunity to selectively encode cues (Experiment 3) did we observe a difference in how visual working memory was used to store these cues. Although we designed our experiment to look at this established marker of template preparation (Carlisle, et al., 2011), the experimental design also provided an opportunity to look for other possible electrophysiological markers of the negative-cue disadvantage (or positive-cue advantage).
Previous investigations have found that midline ERPs can distinguish between how different search tasks employ identical cues. Gunseli, Olivers, & Meeter (2014; Gunseli, Meeter, & Olivers, 2014) have found that more difficult target discriminations lead to more positive, sustained voltage shifts over central and parietal electrodes (the LPC), which they have interpreted as the amount of effort devoted to maintaining a representation in visual working memory. More closely related to the present experiment, Kawashima and Matsumoto (2016) found that the P3b elicited by a to-be-remembered cue was larger when it reliably predicted the colour of the search target in an intervening search.
To see whether these components might provide a clue as to whether differences in positive and negative search might be partially explained by differences in cue processing, we computed midline ERPs time-locked to the cue for Experiment 1 – 3. Based on previous reports we computed average amplitude for Fz, Cz, and Pz in the 275-375ms time range (P3: Kawashima and Matsumoto, 2016) and in the 475-700ms time range (LPC: Gunseli, Olivers, & Meeter, 2014; Gunseli, Meeter, and Olivers, 2014). In both Experiments 1 and 2, where negative information needed to be stored on negative blocks, a sustained midline ERP can be seen (see Figure 5). In Experiment 1, there were no cue-related effects in the P3 range, Fs < 2.06, ps > .11, η2p s < .10, but a marginally different LPC, F(1, 20) = 3.26, p = .09, η2p = .14. In Experiment 2, both the P3, F(1, 22) = 6.73, p = .017, η2p = .23, and the LPC , F(1, 22) = 12.39, p = .002, η2p = .36, were more positive in the negative cue condition overall2. Importantly, in Experiment 3, when the CDA results suggested that only positive information was stored, these ERP differences vanished. Fs(1, 57) < 0.10, ps > .76, η2ps ≤ 0.005.
Figure 5.
Grand average, midline ERPs for Experiments 1 – 3, time-locked to the appearance of the cue.
While we observed a LPC difference between the positive and negative cue conditions, it is not yet clear what cognitive process is indexed by this ERP. Gunseli and colleagues (2014) have tended to interpret the LPC as a marker of the amount of effort invested in maintaining a template, given the differences they observed when search difficulty was varied. Intuitively, our findings would fit this explanation. Given that participants chose to use positive cues over negative ones, one could infer that negative templates are more effortful, and therefore aversive (Kool et al., 2010). However, this side steps the question of what cognitive process is marked by the LPC. It may also be that this ERP reflects a change in the contingent negative variation (CNV), which reflects preparatory processes in the period leading up to a target. Indeed, spatial cuing studies have found a similar sustained, central potential that is more negative when cues are spatially informative (Talsma, Slagter, Nieuwenhuis, Hage, & Kok, 2005; Wright, Geffen, & Geffen, 1995) and more negative for spatial cues when targets must be identified rather than localized (Eimer, 1993).
Given the breadth of potential interpretations of this component, it is premature to draw conclusions about what it may tell us about positive and negative templates. Nonetheless, it does provide evidence that the cognitive representation of positive and negative templates does differ beyond visual working memory, as measured by the CDA. As such, it may not be simply the case that the difference between positive and negative search can be solely explained as the result of memory-driven attention (see the General Discussion).
6. Experiment 4
The electrophysiological results of Experiment 3 suggest that participants largely chose to use positive cues instead of negative cues when both positive and negative information were provided before search. While the CDA results imply that the positive, but not the negative, colors were encoded, it is difficult to rule out the possibility that negative colors were encoded, but to a lesser extent. To do so, we would need to compare search with both positive and negative cues to search with only positive cues, to see if the additional negative information produces any extra benefits. Fortunately, N.B.C had independently conducted a behavioral experiment with this pair of conditions. If cues with both positive and negative information improve search time compared to cues with only positive information, then negative information is clearly being incorporated into the template, but if they do not, then one can conclude that only positive information is stored as a template.
6.1. Method
6.1.1. Participants.
Twenty-five participants were recruited from Lehigh University’s Participant pool. Five participants were replaced for search accuracy in one or more conditions that was 2 standard deviations below the mean. The mean age of the final sample was 19, and there were 12 females in the sample. All participants gave informed consent, and the procedures were approved by Lehigh University’s IRB.
6.1.2. Stimuli.
Stimuli were presented using Matlab (Kleiner et al., 2007) and viewed from approximately 105 cm. Trials began with a central fixation dot (0.3°) on a gray background. After 500 ms, a color cue (1.2°) was presented via a filled circle for 150ms. Positive cues indicated the color of the upcoming target and were presented 1.2° below the fixation dot. Negative cues indicated the color of the upcoming distractors and were presented 1.2° above the fixation dot. Neutral cues were presented surrounding the fixation dot. A 500ms fixation screen was presented before the 12- item visual search array of Landolt-Cs (1.2°) was presented on an imaginary circle (5.2° radius) centered on the fixation dot (see Figure 1). On each trial, two colors were randomly selected for the search array from red, green, blue, magenta, orange, and cyan. All items in one hemifield shared one color. The target Landolt-C had a gap (.2°) opening facing the top (0°) or bottom (180°). Each distractor had a gap facing 45°, 90°, 135°, 225°, 270°, or 315°. The search array remained on the screen until response (or for a maximum of 3500 ms).
6.1.3. Procedure.
All participants completed four blocks of trials, where the meaning of the cue was held the same throughout a block. In positive cue blocks, the cue indicated the color of the upcoming target. In negative cue blocks, the cue indicated the color of the upcoming distractor. In neutral cue blocks, the cued color would not appear in the search array. Finally, in both cue blocks, participants received both a positive and a negative cue. They were instructed to use both cues to aid in performance in finding the target. For each condition, participants received verbal and visual instructions and performed a practice block of 8 trials. Participants could repeat the practice trials if they were not comfortable with the task. Then participants completed the experimental block of 72 trials with breaks including feedback on performance every 18 trials. The instructions, practice and experimental blocks were then repeated for the other conditions. An illustration of a sample trial is presented in Figure 6.
Figure 6.
A sample trial from Experiment 4 (not drawn to scale). The bottom panels depict the potential cues that could have been shown in the sample trial, depending on block.
6.2. Results
As can be seen in Figure 7, providing positive and negative information in cues before search noticeably improved search performance (RT: F(3, 72) = 72.34, p < .001, η2p = 0.75; accuracy: F(3, 72) = 22.22, p < .001, η2p = .48). Importantly, although search was again faster, t(24) = 6.26, p < .001, and more accurate, t(24) = 3.71, p = .001, for positive than negative cues, it was no faster or more accurate (ps > .61) with both cues compared to positive cues. This suggests that participants only rely on positive cues when both positive and negative information are presented.
Figure 7.

Response time (panel A) and error rate (panel B) for each cue type in Experiment 4. Error bars depict one standard error of the mean, individual lines depict participant means.
6.3. Discussion
The results of Experiment 4 provide a direct comparison of RT benefits for positive cues, negative cues, and both cues. We replicated the pattern of faster RTs for positive than negative cues shown in our previous experiments, and additionally found that the RT benefits and accuracy benefits for the both condition were not significantly different than the benefits for the positive cue condition. This demonstrates that when both positive and negative information are available, participants prefer to guide their search using only the positive information, and substantiates our interpretation of the CDA results in Experiment 3.
One explanation for the lack of an extra benefit of both cues over positive cues alone in Experiment 4 is that searchers try to minimize working memory load. Several studies have provided evidence suggesting that attention can only be controlled by a single representation at a time (Houtkamp & Roelfsema, 2009; van Mooreselaar, Theeuwes, & Olivers, 2014; but see Bahle, Beck, & Hollingworth, 2018; Beck, Hollingworth, & Luck, 2012). That is to say, participants here may have relied on the positive cues because they were incapable of using both types of information at once3, or may simply be attempting to minimize cognitive load. Be that as it may, it is still the case that participants reliably chose to rely on the positive cue when both positive and negative cues were available in Experiments 3 and 4. Clearly there is a preference for how searchers allocate their limited cognitive resources, and that preference is towards positive information.
7. General Discussion
In four experiments, we used subjects’ electrophysiology and behavior to ask how we prepare templates to guide attention when we are given positive or negative information. In Experiment 1, participants were provided cues signaling a color that they needed to attend (the positive search condition) and cues signaling a color that they needed to ignore (the negative search condition). Following these cues, participants searched arrays with pairs of colored Landolt C’s, two possessing the target color (the cued color in the positive search condition) and two possessing another color (the cued color in the negative search condition). Participants were markedly slower at reporting a target in the negative search condition than in the positive search condition (Arita, et al., 2012; Beck & Hollingworth, 2015; Becker, Hemsteger, & Peltier, 2015). Scalp potentials showed that in both cases participants stored the cued colors in working memory, as indicated by a cue-locked CDA. This occurred as well in Experiment 2, where the target’s color was predictable over short runs of trials and participants could have relied on memory for the previous target’s color to create a positive template. However, when cue displays presented both the to-be-attended and to-be-ignored color, participants preferred to rely on the positive cue information in Experiments 3 and 4. Moreover, subjects’ brain activity suggests that they elect to encode the to-be-attended color, as demonstrated by a reversal in the CDA’s polarity in Experiment 3. This is despite the fact that instructions only communicated to participants that, in negative cue blocks, the cued color would not be the target’s color. Clearly, the relationship between the non-cued color and the target’s color was learned and strategically exploited by most participants. Thus, our final experiments demonstrate that participants will choose to use the more potent positive cue than the negative cue when given the opportunity.
By providing a direct measure of template formation, our experiments demonstrate that the contents of working memory for positive and negative templates are simply the color shown in the cue. Although selection and inhibition in ERPs of visual attention have been associated with different polarities (Luck & Hillyard, 1994; Sawaki, Geng, & Luck, 2012), the CDA clearly does not code the attentional valence (attend versus ignore) of the information being stored. Recently, de Vries, Savran, van Driel, & Olivers (2019) found that lateralized alpha oscillations likewise do not differentiate between positive and negative templates, implying similar activations of the to-be-attended and to-be-ignored features. Thus, it is simplest to assume that when shown cues that predict either the target or the non-target color, participants simply remember this color and some other process uses information this to compute the attentional valence of the color. The notion that attentional templates consist of separate representations for features and task rules is consistent with broader accounts of working memory that propose separate systems for declarative and procedural aspects of cognitive components of actions (Oberauer, 2002; Oberauer, Souza, Druey, & Gade, 2013; Myers, Stokes, & Nobre, 2017). However, it is nonetheless possible that positive and negative templates rely on distinct populations of neurons within the same cortical areas (e.g., Wallis, Anderson, & Miller, 2001, Reeder, et al., 2017, 2018). Our findings also cast doubt on the possibility that negative templates are less helpful because participants re-code them into the remaining positive set (Becker, Hemsteger, & Peltier, 2016; Beck, Luck, & Hollingworth, 2018). For example, when told not to look for red, one could opt to instead prepare to look for a green, blue, or yellow target. This sort of search would be less efficient due to the multiplicity of potential target colors (Stroud, Menneer, Cave, & Donnelly, 2012). Insofar as the CDA tracks the number of active representations used to guide attention (Carlisle, et al., 2011; Grubert, Carlisle, & Eimer, 2016), our results do not support this possibility. Either the positive, recoded representations rely on a different format than visual working memory, or no such recoding of negative templates occurs. However, this is not to say that recoding could not occur following the onset of the search array, rather than in advance of it (Becker, Hemsteger, & Peltier, 2016). Currently evidence for this possibility is mixed, with ERP findings failing to support a biphasic, seek-and-destroy process (Carlisle, & Nitka, 2019), but eye-tracking findings suggesting early selection of negatively cued features followed by later suppression (Beck, Luck, & Hollingworth, 2018).
Because negative templates involve active maintenance of the non-target color, a simple explanation of their reduced benefit is memory-driven attentional capture. Previous research has found that while memory-driven capture is reduced or prevented when the contents of memory reliably match distracting information (Carlisle & Woodman, 2011a; Carlisle & Woodman, 2011b; Kiyonaga, Egner, & Soto, 2012), some attentional capture may still occur (Carlisle & Woodman, 2013; Carlisle & Woodman, in press; van Loon, Olmos-Solis, & Olivers, 2017) So while there is more to attentional guidance than the contents of working memory (Downing & Dodds, 2004; Olivers, Peters, Houtkamp, & Roelfsema, 2011; Dube & Al-Aidroos, 2019), having recently stored a feature makes it more difficult to ignore that feature in the future. This bias would help performance for positive cues, but hurt it for negative cues, providing a simple explanation of the positive cue benefit.
Another factor that may contribute to the positive cue benefit is that, in our task, the negative cue must be actively maintained, because the correct response cannot be given without knowing which color to avoid. In many other negative search studies, negative cues are provided as hints, but nothing about the tasks prevented participants from finding the search target without using the cue (Beck & Hollingworth, 2015; Becker, Hemsteger, & Peltier, 2016), as the target has another unique feature (albeit one that is usually less salient, such as Landolt rotation or letter identity). As noted in the introduction, recent research has demonstrated that negative cues are more likely to be used in search tasks where they are strategically beneficial (Carlisle & Nikta, 2019) or that are difficult to complete without guidance from the negative cue (Conci et al. 2019). The same argument holds for memory-driven capture experiments; since search targets are necessarily defined by some other unique feature than the remembered feature, differences in the magnitude of memory-driven capture could be due to strategic changes in whether or not the remembered feature is maintained as part of the search task set. Thus, tasks where the negative cue is necessary for discriminating targets from non-targets may measure a different kind of positive template advantage (or negative template cost) that reflects the cognitive demand of needing to monitor for the presence of a feature that should be avoided (Wegner, 1994; Moher & Egeth, 2012; Huffman, Rajsic, & Pratt, 2016). Directly comparing search performance between tasks that require negative templates and tasks that simply provide negative cues would provide a good test of this hypothesis. Indeed, comparing the CDA findings between these conditions may be telling as to how, and when, negative cues are used.
A reader might object that in our experiments we only used two colors in the search displays and that this may have encouraged strategic recoding that allowed search to be more efficient, especially in light of the fact that participants did choose to encode only positive cues in Experiments 3 and 4 (Beck & Hollingworth, 2015; Becker, Hemsteger, & Peltier, 2016). While this is a possibility, the alternative choice of coloring non-target stimuli heterogeneously is unattractive for different reasons. In some studies of negative search, the cued (and therefore irrelevant) set is drawn in a homogenous color, and the uncued set (and therefore relevant) is then drawn in multiple colors (Kugler, Marius, ‘t Hart, Kohlbecher, Einhäuser, & Schneider, 2015; Kawashima & Matsumoto, 2018; Beck, Luck, & Hollingworth, 2018). While this discourages strategic recoding, it necessarily confounds the positive and negative stimulus subsets with visual heterogeneity. As a consequence, it is inherently unclear whether differences in search efficiency between positive and negative search conditions in such designs reflect difficulties in grouping heterogeneous stimuli (Duncan & Humphreys, 1989), or difficulties in suppressing irrelevant information using top-down control. One possible solution is to cue multiple colors in both negative and positive cue conditions, and present displays with an equal number of cued and uncued colors (see Experiment 2 of Kugler et al., 2015). This ensures that both stimulus subsets (relevant and irrelevant) are heterogeneous, which may discourage recoding.
In Experiment 3, we found that participants overwhelmingly chose to rely on positive information in both positive and negative blocks. Because the non-cued color always ended up being the target color for negative-cue blocks, participants were clearly able to realize that they could form a template by reversing the arrow-cue. Although this is not a complex strategy to learn, it is noteworthy that we did not allude to it being available when instructing participants, and that participants were explicitly aware of the shift (that is, it was not an implicit bias). Most strikingly, the CDA was able to track this strategy switch, providing a neural correlate of these subjective templates. We interpret this result as an attempt by participants to choose the task strategy that minimized the number of cognitive operations required on each trial (Kool et al., 2010; Pauszek & Gibson, 2018). Across various tasks, negative information is seen to involve additional cognitive steps (Becker, Hemsteger, & Peltier, 2016; Clark & Chase, 1972; Moher & Egeth, 2012), and so choosing to rely on positive cues is a cognitive path of least resistance. Cognitive neuroscience is beginning to develop a better understanding of how cognitive effort is computed and minimized (Shenav et al., 2017), and our results suggest that the CDA provides a viable neural correlate of the information that participants choose to rely on during tasks. A preference for cues that allow for a visual matching strategy fits with related arguments suggesting the concept of sameness is somehow cognitively fundamental (Hochmann, Mody, & Carey; 2016; Zentall, Andrews, & Case, 2018). Relatedly, it is surprising that we did not observe a decline in the CDA as expected when cues repeated (Carlisle, et al., 2011). It is not clear why this is the case, though the present experiments differed from previous experiments in several ways. We used highly discriminable colors as cues (compared to Landolt Cs and photographs of objects: Carlisle et al., 2011; Reinhart & Woodman, 2015; Servant, Cassey, Woodman, & Logan, 2017), and the cues displayed a feature that needed to be attended, but not reported, which could exhibit less learning (Olivers & Meeter, 2006).
Finally, it is worth considering whether the advantage for positive cues is merely a consequence of visual priming (Awh, Belopolsky, & Theeuwes, 2012). As we discussed earlier, our results are consistent with a strategic, memory-driven capture account. That is, participants choose to do the task in such a way that they can take advantage of their memory for the cue color. However, on the basis of these results alone we cannot determine whether the critical component of the positive template advantage is in knowing the target’s color or actually having that color stored in visual working memory. While we do not yet have an answer to this question, the literature on memory-driven capture may provide some indication. Kawashima and Matsumoto (2017) compared the magnitude of memory-driven capture when then contents of working memory were either a visual code (i.e., remember a particular colored square) or a verbal code (i.e., remember the word “red”). The authors varied the probability that either a target or distractor would match the feature held in memory. They found that both remembered codes led to memory-driven capture (see also Soto & Humphreys, 2007; Beck, Luck, & Hollingworth, 2018), and that memory driven capture was weaker for both when distractors were more likely to match memory than the target. While this could reflect strategic changes in the state of working memory during search, it nonetheless is consistent with the possibility that knowing a target’s features may be part of the positive template advantage over and above having the target’s features stored in visual working memory.
Overall, our results demonstrate that both positive and negative cues lead to working-memory based templates, as indicated by participants’ brain activity. When participants were provided with both positive and negative cues prior to search both explicitly and implicitly, they preferred to rely solely on the positive cues to perform the visual search task. This provides evidence that positive cues may be easier to implement as templates than negative cues, but both types of cues are used and stored as templates in visual working memory when they are all that is available.
Search is better with positive (find red) than negative (find non-red) templates
We tested whether people avoid storing negative templates in working memory
Neural measures of working memory were consistently found for both templates
Participants selectively stored positive templates when both cues were given
Acknowledgements
We thank Chong Zhao and Sarah Whitaker for helping with data collection. We also thank Sisi Wang, Christopher Sundby, David Sutterer, and Mathieu Servant for technical help and useful discussions. Findings from this paper were presented at the 2019 Vision Sciences Society Meeting.
Funding
Grants support was provided by the National Institutes of Health to GFW (R01-EY025275, R01-EY019882, R01-MH110378, and P30-EY08126).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Greenhouse-Geisser corrections are reported throughout where sphericity assumptions were violated.
There were also sporadic interactions, but we are wary to over-interpret them. While we use the time-windows from previous studies for consistency, it is important to note that the midline ERP difference is both spatially and temporally broad in both our ERPs and in previously published ERPs. Indeed, computing mean amplitude over a broader, 400-1000ms time window supported the simple, consistent finding of a main effect of cue type and electrode, but nothing else, for Experiments 1 and 2.
We thank an anonymous reviewer for this suggestion.
References
- Arita JT, Carlisle NB, & Woodman GF (2012). Templates for rejection: configuring attention to ignore task-irrelevant features. Journal of experimental psychology: human perception and performance, 38(3), 580–5884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awh E, Belopolsky AV, & Theeuwes J (2012). Top-down versus bottom-up attentional control: A failed theoretical dichotomy. Trends in cognitive sciences, 16(8), 437–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacon WF & Egeth HE (1994). Overriding stimulus-driven capture. Perception & Psychophysics, 55(5), 485–496. [DOI] [PubMed] [Google Scholar]
- Bahle B, Beck VM, & Hollingworth A (2018). The architecture of interaction between visual working memory and visual attention. Journal of Experimental Psychology: Human Perception and Performance, 44(7), 992–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck VM, & Hollingworth A (2015). Evidence for negative feature guidance in visual search is explained by spatial recoding. Journal of Experimental Psychology: Human Perception and Performance, 41(5), 1190–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck VM, Hollingworth A, & Luck SJ (2012). Simultaneous control of attention by multiple working memory representations. Psychological Science, 23(8), 887–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck VM, Luck SJ, & Hollingworth A (2018). Whatever you do, don’t look at the…: Evaluating guidance by an exclusionary attentional template. Journal of Experimental Psychology: Human Perception and Performance, 44(4), 645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker SI (2010). The role of target–distractor relationships in guiding attention and the eyes in visual search. Journal of Experimental Psychology: General, 139(2), 247–265. [DOI] [PubMed] [Google Scholar]
- Becker MW, Hemsteger S, & Peltier C (2015). No templates for rejection: A failure to configure attention to ignore task-irrelevant features. Visual Cognition, 23(9-10), 1150–1167. [Google Scholar]
- Bundesen C (1990). A theory of visual attention. Psychological Review, 97(4), 523–547. [DOI] [PubMed] [Google Scholar]
- Carlisle NB, Arita JT, Pardo D, & Woodman GF (2011). Attentional templates in visual working memory. Journal of Neuroscience, 31(25), 9315–9322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlisle NB (2019). Flexibility in Attentional Control: Multiple Sources and Suppression. The Yale Journal of Biology and Medicine, 92(1), 103. [PMC free article] [PubMed] [Google Scholar]
- Carlisle NB & Nitka AW (2019). Location-based explanations do not account for active attentional suppression. Visual Cognition. [Google Scholar]
- Carlisle NB, & Woodman GF (2011a). Automatic and strategic effects in the guidance of attention by working memory representations. Acta Psychologica, 137(2), 217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlisle NB & Woodman GF (2011b). When memory is not enough: Electrophysiological evidence for goal-dependent use of working memory representations in guiding visual attention. Journal of Cognitive Neuroscience, 23(10), 2650–2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlisle NB & Woodman GF (2013). Reconcling conflicting electrophysiological findings on the guidance of attention by working memory. Attention, Perception, & Psychophysics, 75(7), 1330–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlisle NB, & Woodman GF (in press). Quantifying the attentional impact of working memory matching targets and distractors. Visual Cognition. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark HH, & Chase WG (1972). On the process of comparing sentences against pictures. Cognitive psychology, 3(3), 472–517. [Google Scholar]
- Conci M, Deischel C, Müller HJ, & Töllner T (2019). Feature guidance by negative attentional templates depends on search difficulty. Visual Cognition. doi: 10.1080/13506285.2019.1581316 [DOI] [Google Scholar]
- de Vries IEJ, Savran E, van Driel J, & Olivers CNL (2019). Oscillatory mechanisms of preparing for visual distraction. Journal of Cognitive Neuroscience. doi: 10.1162/jocn_a_01460 [DOI] [PubMed] [Google Scholar]
- Downing PE (2000). Interactions between visual working memory and selective attention. Psychological science, 11(6), 467–473. [DOI] [PubMed] [Google Scholar]
- Dube B & Al-Aidroos N (2019). Distinct prioritization of visual working memory representations for search and for recall. Attention, Perception, & Psychophysics. [DOI] [PubMed] [Google Scholar]
- Duncan J, & Humphreys GW (1989). Visual search and stimulus similarity. Psychological review, 96(3), 433–458. [DOI] [PubMed] [Google Scholar]
- Eimer M (1993). Spatial cueing, sensory gating and selective response preparation: an ERP study on visuo-spatial orienting. Electroencephalography and clinical Neurophysiology, 88, 408–420. [DOI] [PubMed] [Google Scholar]
- Geng JJ, Won BY, & Carlisle NB (in press). Distractor ignoring: strategies, learning, and passive filtering. Current Directions in Psychological Science. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubert A, Carlisle NB, & Eimer M (2016). The control of single-color and multiple-color visual search by attentional templates in working memory and in long-term memory. Journal of Cognitive Neuroscience, 28(12), 1947–1963. [DOI] [PubMed] [Google Scholar]
- Gunseli E, Meeter M, & Olivers CNL (2014). Is a search template an ordinary working memory? Comparing electrophysiological markers of working memory maintenance for visual search and recognition. Neuropsychologia, 60, 29–38. [DOI] [PubMed] [Google Scholar]
- Gunseli E, Olivers CNL, & Meeter M (2014). Effects of search difficulty on the selection, maintenance, and learning of attentional templates. Journal of Cognitive Neuroscience, 26(9), 2042–2054. [DOI] [PubMed] [Google Scholar]
- Hochmann JR, Mody S, & Carey S (2016). Infants’ representations of same and different in match-and non-match-to-sample. Cognitive psychology, 86, 87–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houtkamp R & Roelfsema PR (2009). Matching of visual input to only one item at any one time. Psychological Research, 73(3), 317–326. [DOI] [PubMed] [Google Scholar]
- Huffman G, Rajsic J, & Pratt J (2019). Ironic capture: top-down expectations exacerbate distraction in visual search. Psychological Research, 83(5), 1070–1082. [DOI] [PubMed] [Google Scholar]
- Irons JL, & Leber AB (2016). Choosing attentional control settings in a dynamically changing environment. Attention, Perception, & Psychophysics, 78(7), 2031–2048. [DOI] [PubMed] [Google Scholar]
- Kawashima T, & Matsumoto E (2016). Electrophysiological evidence that top–down knowledge controls working memory processing for subsequent visual search. NeuroReport, 27(5), 345–349. [DOI] [PubMed] [Google Scholar]
- Kawashima T & Matsumoto E (2017). Cognitive control of attentional guidance by visual and verbal working memory representations. Japanese Psychological Research, 59(1), 49–57. [Google Scholar]
- Kawashima T, & Matsumoto E (2018). Negative cues lead to more inefficient search than positive cues even at later stages of visual search. Acta Psychologica, 190, 85–94. [DOI] [PubMed] [Google Scholar]
- Kiyonaga A, Egner T, & Soto D (2012). Cognitive control over working memory biases of selection. Psychonomic Bulletin & Review, 19(4), 639–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiner M, Brainard DH, & Pelli DG (2007). What is new in Psychophysics Toolbox. Perception, 36. [Google Scholar]
- Kool W, McGuire JT, Rosen ZB, & Botvinick MM (2010). Decision making and the avoidance of cognitive demand. Journal of Experimental Psychology: General, 139(4), 665–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugler G, Marius‘t Hart B, Kohlbecher S, Einhäuser W, & Schneider E (2015). Gaze in visual search is guided more efficiently by positive cues than by negative cues. PloS one, 10(12), e0145910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leber AB & Egeth HE (1994). It’s under control: Top-down search strategies can override attentional capture. Psychonomic Bulletin & Review, 13(1), 132–138. [DOI] [PubMed] [Google Scholar]
- Logan GD, Schachar RJ, & Tannock R (1997). Impulsivity and inhibitory control. Psychological Science, 8(1), 60–64. [Google Scholar]
- Luck SJ (2005). An introduction to the event-related potential technique. [Google Scholar]
- Luck SJ, & Hillyard SA (1994). Spatial filtering during visual search: evidence from human electrophysiology. Journal of Experimental Psychology: Human Perception and Performance, 20(5), 1000–1014. [DOI] [PubMed] [Google Scholar]
- Moher J, & Egeth HE (2012). The ignoring paradox: Cueing distractor features leads first to selection, then to inhibition of to-be-ignored items. Attention, Perception, & Psychophysics, 74(8), 1590–1605. [DOI] [PubMed] [Google Scholar]
- Olivers CN, & Eimer M (2011). On the difference between working memory and attentional set. Neuropsychologia, 49(6), 1553–1558. [DOI] [PubMed] [Google Scholar]
- Olivers CN, & Meeter M (2006). On the dissociation between compound and present/absent tasks in visual search: Intertrial priming is ambiguity driven. Visual Cognition, 13(1), 1–28. [Google Scholar]
- Olivers CN, Meijer F, & Theeuwes J (2006). Feature-based memory-driven attentional capture: visual working memory content affects visual attention. Journal of Experimental Psychology: Human Perception and Performance, 32(5), 1243. [DOI] [PubMed] [Google Scholar]
- Olivers CN, Peters J, Houtkamp R, & Roelfsema PR (2011). Different states in visual working memory: When it guides attention and when it does not. Trends in cognitive sciences, 15(7), 327–334. [DOI] [PubMed] [Google Scholar]
- Pauszek JR, & Gibson BS (2018). The Least Costs Hypothesis: A rational analysis approach to the voluntary symbolic control of attention. Journal of experimental psychology: human perception and performance, 44(8), 1199. [DOI] [PubMed] [Google Scholar]
- Rajsic J, Wilson DE, & Pratt J (2015). Confirmation bias in visual search. Journal of experimental psychology: human perception and performance, 41(5), 1353–1364. [DOI] [PubMed] [Google Scholar]
- Reeder RR, Olivers CN, & Pollmann S (2017). Cortical evidence for negative search templates. Visual Cognition, 25(1-3), 278–290. [Google Scholar]
- Reeder RR, Olivers CN, Hanke M, & Pollmann S (2018). No evidence for enhanced distractor template representation in early visual cortex. Cortex, 108, 279–282. [DOI] [PubMed] [Google Scholar]
- Reinart RMG, McClenahan LJ, & Woodman GF (2016). Attention’s accelerator. Psychological Science, 27(6), 790–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhart RMG & Woodman GF (2013). High stakes trigger the use of multiple memories to enhance the control of attention. Cerebral Cortex, 24(8), 2022–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhart RMG & Woodman GF (2015). Enhancing long-term memory with stimulation tunes visual attention in just one trial. Proceedings of the National Academy of Sciences, 112(2), 625–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawaki R, Geng JJ, & Luck SJ (2012). A common mechanism for preventing and terminating the allocation of attention. Journal of Neuroscience, 32(31), 10725–10736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servant M, Cassey P, Woodman GF, & Logan GD (2018). Neural bases of automaticity. Journal of Experimental Psychology; Learning, Memory, and Cognition, 44(3), 440–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenhav A, Musslick S, Lieder F, Kool W, Griffiths TL, Cohen JD, & Botvinick MM (2017). Toward a rational and mechanistic account of mental effort. Annual review of neuroscience, 40, 99–124. [DOI] [PubMed] [Google Scholar]
- Soto D, & Humphreys GW (2007). Automatic guidance of visual attention from verbal working memory. Journal of Experimental Psychology: Human Perception and Performance, 33(3), 730–737. [DOI] [PubMed] [Google Scholar]
- Stroud MJ, Menneer T, Cave KR, & Donnelly N Using the Dual-Target Cost to Explore the Nature of Search Target Representations. Journal of Experimental Psychology: Human Perception and Performance, 38(1), 113–122. [DOI] [PubMed] [Google Scholar]
- Talsma D, Slagter HA, Nieuwenhuis S, Hage J, & Kok A (2005). The orienting of visuospatial attention: An event-related brain potential study. Cognitive Brain Research, 25(1), 117–129. [DOI] [PubMed] [Google Scholar]
- Treisman A, & Sato S (1990). Conjunction search revisited. Journal of Experimental Psychology: Human Perception and Performance, 16(3), 459–478. [DOI] [PubMed] [Google Scholar]
- van Moorselaar D, Theeuwes J, & Olivers CNL (2014). In competition for the attentional template: Can multiple items within visual working memory guide attention? Journal of Experimental Psychology: Human Perception and Performance, 40(4), 1450–1464. [DOI] [PubMed] [Google Scholar]
- van Loon AM, Olmos-Solis K, & Olivers CN (2017). Subtle eye movement metrics reveal task-relevant representations prior to visual search. Journal of Vision, 17(6), 1–15. [DOI] [PubMed] [Google Scholar]
- Vatterott DB, & Vecera SP (2012). Experience-dependent attentional tuning of distractor rejection. Psychonomic bulletin & review, 19(5), 871–878. [DOI] [PubMed] [Google Scholar]
- Verbruggen F, & Logan GD (2008). Response inhibition in the stop-signal paradigm. Trends in cognitive sciences, 12(11), 418–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel EK, & Machizawa MG (2004). Neural activity predicts individual differences in visual working memory capacity. Nature, 428(6984), 748–751. [DOI] [PubMed] [Google Scholar]
- Wallis JD, Anderson KC, & Miller EK (2001). Single neurons in prefrontal cortex encode abstract rules, Nature, 411, 953–956. [DOI] [PubMed] [Google Scholar]
- Wason PC (1959). The processing of positive and negative information. Quarterly Journal of Experimental Psychology, 11(2), 92–107. [Google Scholar]
- Wegner DM (1994). Ironic processes of mental control. Psychological review, 101(1), 34. [DOI] [PubMed] [Google Scholar]
- Wolfe JM, & Gray W (2007). Guided search 4.0. Integrated models of cognitive systems, 99–119. [Google Scholar]
- Wolfe JM, Cave KR, & Franzel SL (1989). Guided search: an alternative to the feature integration model for visual search. Journal of Experimental Psychology: Human perception and performance, 15(3), 419. [DOI] [PubMed] [Google Scholar]
- Woodman GF & Arita JT (2011). Direct electrophysiological measurement of attentional templates in visual working memory. Psychological Science, 22(2), 212–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodman GF, & Luck SJ (2007). Do the contents of visual working memory automatically influence attentional selection during visual search?. Journal of Experimental Psychology: Human Perception and Performance, 33(2), 363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright MJ, Geffen GM, Geffen LB (1995). Event related potentials during covert orientation of visual attention: Effects of cue validity and directionality. Biological Psychology, 41, 183–202. [DOI] [PubMed] [Google Scholar]
- Zentall TR, Andrews DM, & Case JP (2018). Sameness May Be a Natural Concept That Does Not Require Learning. Psychological science, 0956797618758669. [DOI] [PubMed] [Google Scholar]