Abstract
Background
The outbreak of Lumpy skin disease (LSD) in cattle caused by LSD virus (LSDV) was first reported in August 2019 in China. Since then, several LSD outbreaks have been reported in seven different provinces of China. Until now, several Lumpy skin disease virus (LSDV) strains from China have been reported and sequenced including LSDV/Xinjiang/2019 (MN598005.1), China/GD01/2020 (MW355944.1), and LSDV/Hongkong/2021 (MW732649.1). In October 2020, more than 1,700 cattle imported from Chile arrived in Xilingol, Inner Mongolia, and were diagnosed with LSD. Currently, limited data on the origin of the virus is available.
Methods
Nucleotide sequences of the ORF11, ORF36, ORF74, ORF117, ORF126 genes and the complete genome of LSDV strains and isolates were downloaded from NCBI database. MEGA7.0 was used to perform phylogenetic analysis with Neighbor-Joining (NJ). DNASTAR software is used to analyze homologous comparison analysis with related genes of reference strains included in Genbank.
Results
Compared with other strains isolated from China, the results of full genome sequence analysis showed the LSDV/NMG/2020 strain belonged to the recombinant strains. The LSDV/NMG/2020 strain is different from the current LSDV field isolates in Africa, the Middle East, Europe, and the newly emerged LSDV Russia variants. Based on the identities of P32, RPO30, EEV, GPCR and LSDV117 genes (99.8%, 99%, 99.8%, 99% and 98.7%), the sub-cluster recombinant containing LSDV/NMG/2020 strain is phylogenetically closer to the Russia strain (Saratov/2017).
Conclusions
In this study, we reported a new isolated LSDV strain named LSDV/NMG/2020. The results of genomic characterization and phylogenetic analysis demonstrated that the LSDV/NMG/2020 isolate was a vaccine-like recombinant strain.
Keywords: Lumpy skin disease virus, Molecular characterization, Recombination
Background
Lumpy skin disease (LSD) is a transboundary, viral disease of cattle with severe economic impact and is listed as a notifiable disease by the OIE[1]. LSD is caused by lumpy skin disease virus (LSDV), a pathogen of the genus Capripoxvirus of the family Poxviridae, the severity of clinical signs of LSD varies from subclinical to fatal depending on the virulence of the strains and the cattle breed’s susceptibility [2, 3]. Morbidity can range from 1% to almost 100%, with mortality most often between 1% and 3% [4]. It is mainly transmitted through insects and does not infect humans, but could spread to cattle, water buffalo and certain antelopes[5]. LSD was first discovered in Zambia in 1929. In 1988, the first report in Egypt from where it spread in 1989 to the Middle East. Intercontinental transmission occurred again in 2015 [6, 7], and now it was expanded into Greece, eastern Europe, Russia and India [8–10]. But lumpy skin disease virus was eradicated in Greece and eastern Europe using vaccination. LSD was reported in China and neighbouring countries of Indian and Vietnam during 2019–2020 [11, 12].
A live-attenuated vaccine, based on the Neethling-type field strain was developed by multiple passages in lamb kidney cells and chorioallantoic membranes of embryonated eggs in the 1950s in South Africa [13]. Live attenuated LSD vaccines with the strain of the “Neethling” as a prototype has been used in Africa for over 50 years and are now widely used in most affected countries [14, 15]. In 2017, a vaccine-like strain has been discovered linked to circulating to LSD outbreaks in Russia [16]. A novel LSDV strain, known as Saratov/2017, from diseased cattle in southern Russia in 2017 revealed similarities with the Neethling and KSGPO-like vaccine strains [17]. This is the first report demonstrating that an attenuated LSD vaccine strain has been identified in Russian cattle given the ban on the use of live attenuated vaccines against LSDV[18]. After the introduction of vaccines in Kazakhstan in 2017, vaccine-like isolates underwent multiple dispersal events across a wide area toward the East along the Russian border, resulting in a new epidemiological wave of genetically different LSDV[8, 19, 20].
In 2019, LSD was introduced to the Xinjiang region of China, which then swept through a vast geographic area in China [21]. In 2020, seven provinces and cities in Xinjiang, Fujian, Jiangxi, Guangdong, Anhui, Zhejiang and Taiwan have all experienced epidemics [11]. In October 2020, more than 1,700 cattle imported from Chile arrived in Xilingol, Inner Mongolia, and were diagnosed with LSD. At the same time, cattle destined for the Baotou city, Ningxia province and Shandong province were also affected. Currently, limited data on the origin of the virus is available. Here, we reported phylogenetic analysis of the genome of LSDV LSDV/NMG/2020 strain reported by China. LSDV/NMG/2020(ON616408), LSDV/Xinjiang/2019(MN598005.1), China/GD01/2020 (MW732649.1), 20L43Ly-Quoc/VITM/20(MZ577074.1), and LSDV/Hongkong/2020 (MW732649.1) belong to the same branch. These strains are different from all previously reported LSDV strains, but the cluster is a new lineage.
Materials and methods
Clinical histories and collections of samples
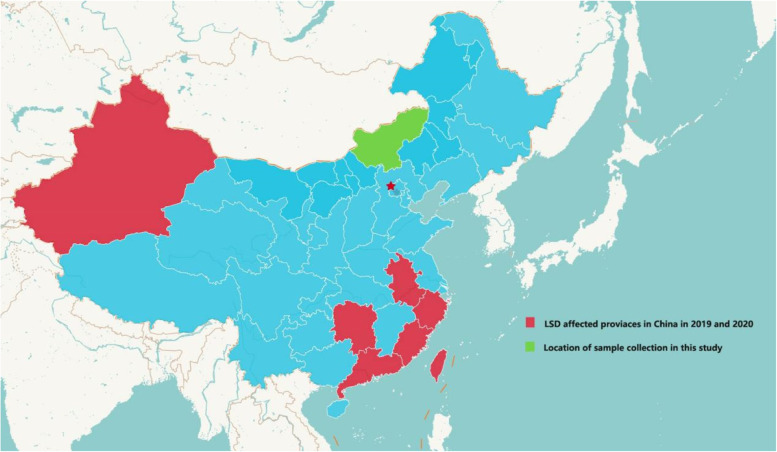
Samples of cattle (n = 3) collected in Xilingol City (Fig. 1) were collected aseptically in a sterile tube containing a virus transport medium and stored at 4 °C. These samples were then transported to the third-level biological safety protection laboratory (BSL3) of Jinyu Paulin Biopharmaceutical Co., Ltd on ice.
Fig. 1.
Location of study area. Districts where outbreaks occurred are shown in green range (Xilingol, Inner Mongolia)
Sample processing
The samples were processed by cutting up the scab tissue with a sterile scalpel and tweezers, pulverizing with sterile sand using a sterile mortar and pestle, and preparing a suspension in DMEM containing antibiotics. The tissue homogenate was submitted to two freeze-thaw cycles to release the intracellular virus followed by centrifugation at 5,000 rpm for 10 min at 4 °C to remove any coarse particles. Collect the supernatant of the tissue homogenate in a separate sterile tube for detection of LSDV by PCR and virus isolation. The sample processing process is carried out in the third -level biological safety protection laboratory (BSL3) of Jinyu Paulin Biopharmaceutical Co., Ltd.
Agents identification
Total DNA was extracted from 600 µl of clinical specimens using a QIAamp DNA Mini Kit following the manufacturer’s instructions. Extracted DNA was quantified using NanoDrop One. This was subjected to the P32 gene targeting PCR to identify and discriminate LSDV using the primers and protocol designed by OIE: F-5’-TTT CCT GAT TTT TCT TAC TAT-3’; R-5’-AAA TTA TAT ACG TAAATAAC-3’. Amplification was performed using the following conditions: initial denaturation cycle at 95℃ for 5 min, 34 cycles (denaturation at 95℃ for 45 s, annealing at 50℃ for 30 s, and extension at 72 ℃ for 1 min), followed by a final extension cycle at 72℃ for 5 min. The PCR assay was performed in 25 µL volume, including 12.5 µL Dream Taq green PCR master mix (2×) (Thermo Scientific, Germany), 1 µL of each primer (10 pmol/µl), 8.5 µL deionized water, and 2 µL of DNA template.
Nucleotide sequencing
In order to further confirm the identity of the virus of LSDV/NMG/2020, duplicate samples were submitted for complete genome sequencing and only high-quality sequences were submitted to Genbank database with Accession Numbers of DN616408 (Lumpy skin disease virus strain LSDV/NMG/2020, complete genome), OL977078 (GPCR), OL977075 (EEV), OL977074 (P32), OL977077 (LSDV117) and OL977076 (RPO30) .
Phylogenetic analysis
Nucleotide sequences of the ORF11, ORF36, ORF74, ORF117, ORF126 genes and the full-length genes of LSDV strains and isolates were downloaded from NCBI database. Multiple alignments of these sequences were performed by MEGA 7.0 with ClustalW method. MEGA7.0 was used to perform phylogenetic analysis with Neighbor-Joining (NJ). The bootstrap values were determined from 1000 replicates of the original data. DNASTAR software is used to analyze homologous comparison analysis with related genes of reference strains included in Genbank.
Results and discussion
The earliest recorded LSD outbreak occurred in several epidemics in seven provinces were confirmed and caused considerable economic losses to the cattle industry in China. Currently, the sequences of three strains, LSDV/Xinjiang/2020 (MN598006.1), China/GD01/2020 (MW355944.1), LSDV/Hongkong/2020 (MW732649.1) have been reported in China. This study investigated the molecular characterization of the new isolated LSDV/NMG/2020 strain has been analyzed and compared with vaccine and wild-type strains of China, Russia, Indian, Vietnam, and several other countries.
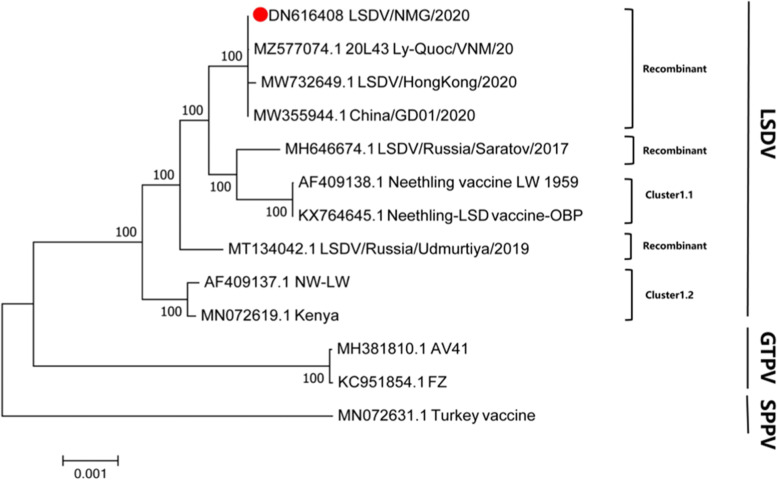
The previous studies have demonstrated that the LSDV virus strains could be divided into two major subgroups[22]. In this study, the full-length genomic analysis showed that all strains isolated from China have been clustered into the same lineages (Fig. 2). Significantly, these strains in the same lineages were assembled into a new cluster, which is different from the vaccine and the current wild-type strains. According to the full-length sequence, LSDV/NMG/2020 belongs to the same sub-cluster as 20L43Ly-Quoc/VNM/20(MZ577074.1), China/GD01/2020(MW732649.1) and LSDV/HongKong/2020 (MW732649.1). Although they both belong to recombinant strains, LSDV/NMG/2020, LSDV/Russia/Udmurtiya/2019 and LSDV/Russia/Saratov/2017 belong to different branches. It is worth noting that the sequences based GPCR, LSDV117 is highly similarity with 99%, 99.6% with field-type, and less similar with 98.9%, 98.7% compared with the Neethling/LW-1959/vaccine strains. Base on the RPO30, P32, and EEV genes, it was more similar with 99.7%, 99.8%, and 99.8% compared with the Neethling/LW-1959/vaccine and less similar with 99.3%, 99.3%, 94.1% compared to wildtype strains (Table 1). Interestingly, the LSDV/NMG/2020 strain has the characteristics of the Neethling/LW-1959/vaccine and KSGPO-like strains in the virus genome. It was identified as having the P32, RPO30 and EEV genes derived from the vaccine strains and the genes of GPCR and LSDV117 derived from field isolates (Figs. 3 and 4). The phenomenon of recombination within the family Poxviridae has been reported [23]. Two novel recombinant LSDV strains generated from NI-2490/KSGPO-like and Neethling/LW-1959/vaccine isolates named Saratov/2017 and Russia/Udmurtiya/2019 were isolated in Russia and clustered into the different branches according to the virus genes. The LSDV RUSSIA/Saratov/2017 strain is more closely genetically related to the Neethling/LW-1959/vaccines compared (> 99.3%), than the field strains (> 98.64%) [17]. The 2019-2020 outbreak of the LSDV strain in China was not identical to the outbreak in India (Table 1). The origin of the LSDV in these outbreaks is still unclear.
Fig. 2.
Phylogenetic tree with Neighbor-Joining (NJ) shows the relationship between LSDV full-length genomic sequences from LSDV/NMG/2020, marked with red round, with other Capripoxvirus gene sequences from GenBank
Table 1.
The nucleotide homology between LSDV/NMG/2020 and other Capripoxvirus gene sequences
| Genbank ID | Abbreviation name | virus type | Year | Origin | Wildtype or vaccine | Identity to Inner Mongolia | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| GPCR | RPO30 | P32 | EEV | LSDV117 | |||||||
| 1 | KX764645.1 | Neethling-LSD_vaccine-OBP | LSDV | U | South Africa | vaccine | 98.9 | 99.7 | 99.8 | 99.8 | 98.7 |
| 2 | KX764644.1 | Neethling-Herbivac_vaccine | LSDV | U | South Africa | vaccine | 98.9 | 99.7 | 99.8 | 99.8 | 98.7 |
| 3 | KX764643.1 | SIS-Lumpyvax_vaccine | LSDV | U | South Africa | vaccine | 98.9 | 99.7 | 99.8 | 99.8 | 98.7 |
| 4 | AF409138.1 | Neethling_vaccine_LW_1959 | LSDV | U | South Africa | vaccine | 98.9 | 99.7 | 99.8 | 99.8 | 98.7 |
| 5 | MG972412.1 | Cro2016 | LSDV | U | Croatia | vaccine | 98.9 | 99.7 | 99.8 | 99.8 | 98.7 |
| 6 | MN072619.1 | Kenya | LSDV | 1958 | Kenya | Wild type | 99.1 | 99.2 | 99.3 | 94.1 | 100 |
| 7 | AF325528.1 | NI-2490 | LSDV | 1958 | Kenya | Wild type | 99.1 | 99.2 | 99.3 | 94.1 | 100 |
| 8 | KX683219.1 | KSGP_0240 | LSDV | 1974 | Kenya | vaccine | 99.1 | 99.2 | 99.3 | 94.1 | 100 |
| 9 | AF409137.1 | NW-LW | LSDV | 1999 | South Africa | Wild type | 98.1 | 99.3 | 99.3 | 94.3 | 99.6 |
| 10 | KX894508.1 | 155,920/2012 | LSDV | 2012 | Israel | Wild type | 98.1 | 99.3 | 99.3 | 94.1 | 99.6 |
| 11 | MN995838.1 | pendik | LSDV | 2014 | Turkey | Wild type | 98.1 | 99.3 | 99.3 | 94.1 | 99.6 |
| 12 | MH893760.2 | LSDV/Russia/Dagestan/2015 | LSDV | 2015 | Russia | Wild type | 98.1 | 99.3 | 99.3 | 94.1 | 99.6 |
| 13 | KY829023.3 | Evros/GR/15 | LSDV | 2015 | Greece | Wild type | 98.1 | 99.3 | 99.3 | 94 | 99.6 |
| 14 | KY702007.1 | SERBIA/Bujanovac/2016 | LSDV | 2016 | Serbia | Wild type | 98.1 | 99.3 | 99.3 | 94 | 99.6 |
| 15 | MH646674.1 | LSDV/Russia/Saratov/2017 | LSDV | 2017 | Russia | Wild type | 99 | 99 | 99.8 | 99.8 | 98.7 |
| 16 | MT134042.1 | LSDV/Russia/Udmurtiya/2019 | LSDV | 2019 | Russia: Udmurtiya | Wild type | 98.9 | 99.2 | 99.1 | 99.8 | 99.8 |
| 17 | MW452642 | LSDV/IND/ODI/30PR-LT/2019 | LSDV | 2019 | India | Wild type | 99 | / | / | / | / |
| 18 | MW452630 | LSDV/IND/ODI/30PR-LT/2019 | LSDV | 2019 | India | Wild type | / | 99.2 | / | / | / |
| 19 | MW452618 | LSDV/IND/ODI/30PR-LT/2019 | LSDV | 2019 | India | Wild type | / | / | 99.3 | / | / |
| 20 | MW452654 | LSDV/IND/ODI/30PR-LT/2019 | LSDV | 2019 | India | Wild type | / | / | / | 94.1 | / |
| 21 | MW452648 | LSDV/IND/WB/GS2-LT/2019 | LSDV | 2019 | India | Wild type | 98.9 | / | / | / | / |
| 22 | MW452636 | LSDV/IND/WB/GS2-LT/2019 | LSDV | 2019 | India | Wild type | / | 99.2 | / | / | / |
| 23 | MW452624 | LSDV/IND/WB/GS2-LT/2019 | LSDV | 2019 | India | Wild type | / | / | 99.3 | / | / |
| 24 | MW452660 | LSDV/IND/WB/GS2-LT/2019 | LSDV | 2019 | India | Wild type | / | / | / | 94.1 | / |
| 25 | MW452650 | LSDV/IND/WB/JS10-LT/2019 | LSDV | 2019 | India | Wild type | 99.1 | / | / | / | / |
| 26 | MW452638 | LSDV/IND/WB/JS10-LT/2019 | LSDV | 2019 | India | Wild type | / | 99.2 | / | / | / |
| 27 | MW452626 | LSDV/IND/WB/JS10-LT/2019 | LSDV | 2019 | India | Wild type | / | / | 99.3 | / | / |
| 28 | MN452662 | LSDV/IND/WB/JS10-LT/2019 | LSDV | 2019 | India | Wild type | / | / | / | 94.1 | / |
| 29 | MN598006.1 | LSDV/Xinjiang/2019 | LSDV | 2019 | China | Wild type | 99.9 | / | / | / | / |
| 30 | MN598007.1 | LSDV/Xinjiang/2019 | LSDV | 2019 | China | Wild type | / | 100 | / | / | / |
| 31 | MN598005.1 | LSDV/Xinjiang/2019 | LSDV | 2019 | China | Wild type | / | / | 100 | / | / |
| 32 | MW355944.1 | China/GD01/2020 | LSDV | 2020 | China | Wild type | 100 | 100 | 100 | 100 | 100 |
| 33 | MW732649.1 | LSDV/HongKong/2020 | LSDV | 2020 | China | Wild type | 100 | 100 | 100 | 99.6 | 100 |
| 34 | MZ577074.1 | 20L43_Ly-Quoc/VNM/20 | LSDV | 2020 | VietNam | Wild type | 100 | 100 | 100 | 100 | 100 |
| 35 | MN072631.1 | SPPV-Turkey vaccine | SPPV | U | Turkey | vaccine | 92.5 | 94.7 | 98 | 97.1 | 94.2 |
| 36 | KT438551.1 | SPPV-GL | SPPV | 2013 | China | Wild type | 93.4 | 94.9 | 98 | 97.1 | 94.2 |
| 37 | KT438550.1 | SPPV-GH | SPPV | 2013 | China | Wild type | 93.4 | 94.9 | 98 | 97.1 | 94.2 |
| 38 | AY077835.1 | GTPV-Pellor | GTPV | 2000 | Kazakhstan | Wild type | 96.3 | 98.5 | 98.3 | 97.1 | 97.1 |
| 39 | KC951854.1 | GTPV-FZ | GTPV | 2012 | China | Wild type | 94.6 | 98.5 | 98 | 97.3 | 96.6 |
| 40 | MH381810.1 | GTPV-AV41 | GTPV | 2018 | China | vaccine | 94.7 | 98.5 | 98.1 | 97.3 | 96.6 |
U unknow; /:no data for this cell
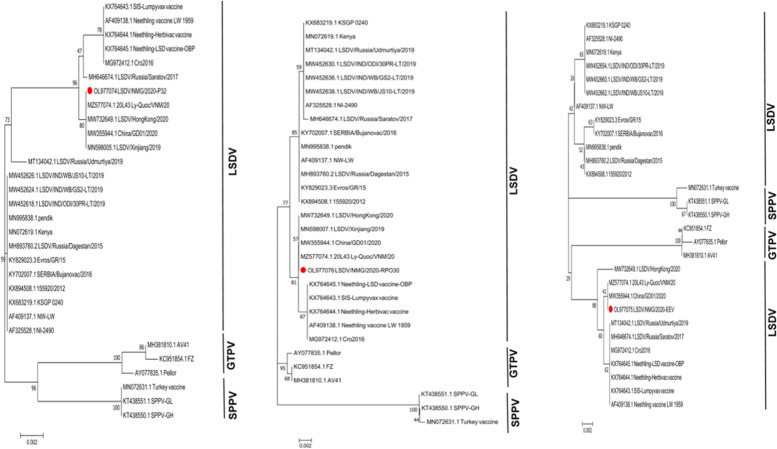
Fig. 3.
Phylogenetic trees with Neighbor-Joining (NJ) show the relationship between LSDV P32, RPO30 and EEV genomic sequences from LSDV/NMG/2020, marked with red round, with other Capripoxvirus gene sequences from GenBank
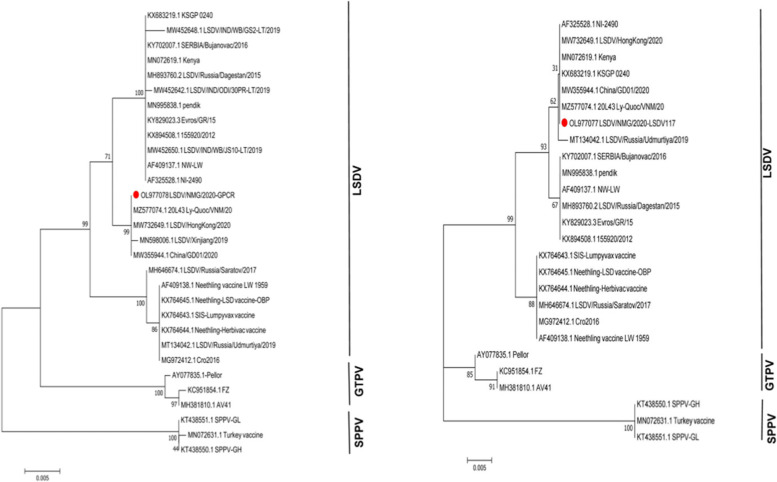
Fig. 4.
Phylogenetic trees with Neighbor-Joining (NJ) show the relationship between LSDV GPCR and LSDV117 genomic sequences from LSDV/NMG/2020, marked with red round, with other Capripoxvirus gene sequences from GenBank
Overall, the similarities to the full genome, two subgroups were grouped according to the P32 gene, an envelope protein of the virus, which is homologous to the P35 protein encoded by the Vaccinia virus (Vaccinia virus VV) H3L gene. The gene is located on the envelope surface of the mature virus particle. According to the nucleotide sequence of the P32 gene of LSDV, the homology of the LSDV/NMG/2020 strain with the SPPV strains is 98%, the homology with the GTPV strains is 98-98.3%, and the homology with the LSDV strain is 99.1 − 100%. The nucleotide sequence homology between LSDV/NMG/2020 and LSDV vaccine strains of South Africa was 99.8%. The homology with Russia vaccine-like recombinant LSDV/Russia/Udmurtiya/2019 (MT134042.1) strain is 99.1%. The homology with the LSDV/Xinjiang/2019 (MN598006.1) strain is 100%. LSDV/NMG/2020 strain and all other strains isolated in China along with the 20L43Ly-Quoc/VITM/20(MZ577074.1) strain belonged to the same subgenotype by full-genome analysis.
The RPO30 gene is a nucleotide sequence homologous gene of the vaccinia virus E4L gene, which encodes a DNA-dependent RNA polymerase subunit and plays a role in virus replication [24]. Comparisons between the LSDV strains generated in China with the LSDV vaccine strains obtained in South Africa, the homology of the RPO30 gene sequence was 99.7%. By analysis of the EEV gene of 27 bp insertions/deletion, all strains obtained in China were grouped into the same group. With reference to the nucleotide sequence of the LSDV EEV gene, the homology of the LSDV/NMG/2020 strain and the SPPV strain is 97.1%, the GTPV strain is 97.1–97.3% and the LSDV strain is 94.1-100%. The homology of the nucleotide sequence is 99.8% between LSDV/NMG/2020 and LSDV vaccine strains from South Africa. The homology with the Russia vaccine-like recombinant virus LSDV/Russia/Udmurtiya/2019 (MT134042.1) is 99.8%. The homology with the China/GD01/2020 strain (MW355944.1) is 100%.
Capripoxvirus G-protein-coupled chemokine receptor (GPCR) was supposed as a suitable gene for capripoxvirus discrimination as the GPCR of field type strains has a deletion of about 12 bp compared with the vaccine strain [25]. The LSDV/NMG/2020 strain has a 12-bp insertion similar to the vaccine strain. In this study, the field strains were clustered into distinct lineages by GPCR analysis. Our results suggested that the highest homology of the LSDV strains isolated in China was China/GD01/2020 (MW355944.1), LSDV/Hongkong/2020 (MW732649.1), and 20L43_Ly-Quoc/VNM/20 (MZ577074.1). We analyzed the LSDV117 gene, which was rarely reported previously, according to the nucleotide sequence of the LSDV117 gene of LSDV, the LSDV/NMG/2020 shares significant homology with Russia vaccine-like recombinant virus LSDV/Russia/Udmurtiya/2019 (MT134042.1) is 99.8%, and different lineage with the Neethling strain and LSDV/Saratov/2017.
We conclude that the LSDV/NMG/2020 isolate belongs to a new emerging subgenotype of the LSDV in the genus Capripoxvirus. Further study to evaluate the biological and epidemiological features of the strain can establish effective control programs for LSD outbreaks.
Acknowledgements
This study is supported by the Science and Technology Major Project of Inner Mongolia Autonomous Region of China (2020ZD0006), JiebangGuashuai Project of Inner Mongolia (2022JBGS0004), and the Science and Technology Major Project of Inner Mongolia Autonomous Region of China to the State Key Laboratory of Reproductive Regulation and Breeding of Grassland Livestock(2019ZD031).
Authors' contributions
Wei Wang designed and initiated the study. Xiaohui Zan and Dongdong Di participated in the design and conducted most of the experiments in the study and drafted the manuscript. Yu Guo, Haibi Huang, Cun Fu, Shirong Wang, Youzhi Wu, Jialei Wang, Yan Wang, Yanhua Ma, Rui Su, Qingqing Song and Chunxia Chai participated in the molecular genetic analysis. All authors read and approved the final manuscript.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author upon reasonable request. The datasets generated and/or analysed during the current study are available in the NCBI GenBank database repository, [OL977078 (GPCR), OL977075 (EEV), OL977074 (P32), OL977077 (LSDV117), OL977076 (RPO30), DN616408 (Lumpy skin disease virus strain LSDV/NMG/2020, complete genome)].
Declarations
Ethics approval and consent to participate
The authors confirm that the ethical policies of the journal, as noted on the journal’s author guidelines page, have been adhered to. This study was conducted in strict accordance with the Animal Management Regulations of the People’s Republic of China. All experiments were approved and supervised by the Research Ethics Committee of Inner Mongolia University. This study was conducted in accordance with ARRIVE guidelines.
Competing interest
The authors have agreed that there is no conflict of interest between them.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xiaohui Zan, Haibi Huang, Yu Guo and Dongdong Di contributed equally to this work.
Contributor Information
Qingqing Song, Email: 280413215@qq.com.
Wei Wang, Email: wangwei@imu.edu.cn.
References
- 1.OIE. (2017). Chapter 2.4.13. Lumpy skin disease OIE Terrestrial Manual 2017. Available online: https://www.oie.int/fileadmin/home/eng/healt.
- 2.Liu F, Zhang H, Liu W. Construction of recombinant capripoxviruses as vaccine vectors for delivering foreign antigens: Methodology and application. Comp Immunol Microbiol Infect Dis. 2019;65:181–8. doi: 10.1016/j.cimid.2019.05.013. [DOI] [PubMed] [Google Scholar]
- 3.Tageldin MH, Wallace DB, Gerdes GH, et al. Lumpy skin disease of cattle: an emerging problem in the Sultanate of Oman. Trop Anim Health Prod. 2014;46(1):241–6. doi: 10.1007/s11250-013-0483-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coetzer JAW. Lumpy skin disease. In: Coetzer JAW, Tustin RC, editors. Infectious diseases of livestock. 2. Oxford, UK: University Press Southern Africa; 2004. pp. 1268–1276. [Google Scholar]
- 5.Shen YJ, Shephard E, Douglass N, et al. A novel candidate HIV vaccine vector based on the replication deficient Capripoxvirus, Lumpy skin disease virus (LSDV) Virol J. 2011;8:265. doi: 10.1186/1743-422X-8-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tasioudi KE, Antoniou SE, Iliadou P, et al. Emergence of Lumpy Skin Disease in Greece, 2015. Transbound Emerg Dis. 2016;63(3):260–5. doi: 10.1111/tbed.12497. [DOI] [PubMed] [Google Scholar]
- 7.Agianniotaki EI, Tasioudi KE, Chaintoutis SC, et al. Lumpy skin disease outbreaks in Greece during 2015-16, implementation of emergency immunization and genetic differentiation between field isolates and vaccine virus strains. Vet Microbiol. 2017;201:78–84. doi: 10.1016/j.vetmic.2016.12.037. [DOI] [PubMed] [Google Scholar]
- 8.Saltykov YV, Kolosova AA, Filonova NN, Chichkin AN, Feodorova VA. Genetic Evidence of Multiple Introductions of Lumpy Skin Disease Virus into Saratov Region, Russia. Pathogens. 2021;10(6):716. [DOI] [PMC free article] [PubMed]
- 9.Sudhakar SB, Mishra N, Kalaiyarasu S, Jhade SK, Singh VP. Genetic and phylogenetic analysis of lumpy skin disease viruses (LSDV) isolated from the first and subsequent field outbreaks in India during 2019 reveals close proximity with unique signatures of historical Kenyan NI-2490/Kenya/KSGP-like field strains. Transbound Emerg Dis. 2021;69,e451–e462. [DOI] [PubMed]
- 10.Tran H, Truong AD, Dang AK, et al. Lumpy skin disease outbreaks in vietnam, 2020. Transbound Emerg Dis. 2021;68(3):,977–80. doi: 10.1111/tbed.14022. [DOI] [PubMed] [Google Scholar]
- 11.Lu G, Xie J, Luo J, Shao R, Jia K, Li S. Lumpy skin disease outbreaks in China, since 3 August 2019. Transbound Emerg Dis. 2021;68(2):216–9. doi: 10.1111/tbed.13898. [DOI] [PubMed] [Google Scholar]
- 12.Mathijs E, Vandenbussche F, Nguyen L, et al. Coding-Complete Sequences of Recombinant Lumpy Skin Disease Viruses Collected in 2020 from Four Outbreaks in Northern Vietnam. Microbiol Resour Announc. 2021;10(48):,e89721. doi: 10.1128/MRA.00897-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Rooyen PJ, Munz EK, Weiss KE. The optimal conditions for the multiplication of Neethling-type lumpy skin disease virus in embryonated eggs. Onderstepoort J Vet Res. 1969;36(2):165–74. [PubMed] [Google Scholar]
- 14.Hunter P, Wallace D. Lumpy skin disease in southern Africa: a review of the disease and aspects of control. J S Afr Vet Assoc. 2001;72(2):68–71. doi: 10.4102/jsava.v72i2.619. [DOI] [PubMed] [Google Scholar]
- 15.Calistri P, DeClercq K, Gubbins S, et al. Lumpy skin disease: III. Data collection and analysis. EFSA J. 2019;17(3):e5638. doi: 10.2903/j.efsa.2019.5638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kononov A, Byadovskaya O, Kononova S, et al. Detection of vaccine-like strains of lumpy skin disease virus in outbreaks in Russia in 2017. Arch Virol. 2019;164(6):1575–85. doi: 10.1007/s00705-019-04229-6. [DOI] [PubMed] [Google Scholar]
- 17.Sprygin A, Babin Y, Pestova Y, et al. Analysis and insights into recombination signals in lumpy skin disease virus recovered in the field. Plos One. 2018;13(12):,e207480. doi: 10.1371/journal.pone.0207480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sprygin A, Pestova Y, Prutnikov P, Kononov A. Detection of vaccine-like lumpy skin disease virus in cattle and Musca domestica L. flies in an outbreak of lumpy skin disease in Russia in 2017. Transbound Emerg Dis. 2018;65(5):1137–44. doi: 10.1111/tbed.12897. [DOI] [PubMed] [Google Scholar]
- 19.Haegeman A, De Leeuw I, Saduakassova M, Van Campe W, Aerts L, Philips W, Sultanov A, Mostin L, De Clercq K. The Importance of Quality Control of LSDV Live Attenuated Vaccines for Its Safe Application in the Field. Vaccines (Basel). 2021;9(9):1019. doi: 10.3390/vaccines9091019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sprygin A, Pestova Y, et al. "Evidence of recombination of vaccine strains of lumpy skin disease virus with field strains, causing disease. PLoS One. 2020;15(5):e0232584. doi: 10.1371/journal.pone.0232584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y, Zhao L, Yang J, et al. Analysis of vaccine-like lumpy skin disease virus from flies near the western border of China. Transbound Emerg Dis. 2021;69:1813–23. [DOI] [PubMed]
- 22.Biswas S, Noyce RS, Babiuk LA, et al. Extended sequencing of vaccine and wild-type capripoxvirus isolates provides insights into genes modulating virulence and host range. Transbound Emerg Dis. 2020;67(1):80–97. doi: 10.1111/tbed.13322. [DOI] [PubMed] [Google Scholar]
- 23.Aleksandr K, Olga B, David WB, et al. Non-vector-borne transmission of lumpy skin disease virus. Sci Rep. 2020;10(1):7436. doi: 10.1038/s41598-020-64029-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lamien CE, Le GC, Silber R, et al. Use of the Capripoxvirus homologue of Vaccinia virus 30kDa RNA polymerase subunit (RPO30) gene as a novel diagnostic and genotyping target: development of a classical PCR method to differentiate Goat poxvirus from Sheep poxvirus. Vet Microbiol. 2011;149(1–2):30–9. doi: 10.1016/j.vetmic.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 25.Le GC, Lamien CE, Fakhfakh E, et al. Capripoxvirus G-protein-coupled chemokine receptor: a host-range gene suitable for virus animal origin discrimination. J Gen Virol. 2009;90(Pt 8):1967–77. doi: 10.1099/vir.0.010686-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. The datasets generated and/or analysed during the current study are available in the NCBI GenBank database repository, [OL977078 (GPCR), OL977075 (EEV), OL977074 (P32), OL977077 (LSDV117), OL977076 (RPO30), DN616408 (Lumpy skin disease virus strain LSDV/NMG/2020, complete genome)].