Summary
Traumatic brain injury (TBI) can have lifelong and dynamic effects on health and wellbeing. Research on the long-term consequences emphasises that, for many patients, TBI should be conceptualized as a chronic health condition. Evidence suggests that functional outcomes after TBI can show improvement or deterioration up to two decades after injury, and rates of all-cause mortality remain elevated for many years. Furthermore, TBI represents a risk factor for a variety of neurological illnesses, including epilepsy, stroke and neurodegenerative disease. With respect to neurodegeneration after TBI, post-mortem studies on the long-term neuropathology after injury have identified complex persisting and evolving abnormalities best described as polypathology which includes chronic traumatic encephalopathy. Despite growing awareness of the life-long consequences of TBI, substantial gaps in research exist. Improvements are therefore needed in understanding chronic pathologies and their implications for survivors of TBI, which could inform long-term health management in this sizeable patient population.
Introduction
Evidence accumulated in the past decades has led to recognition that for many patients traumatic brain injury (TBI) does not cease to evolve after the acute period and initial recovery. This injury is not a time-bound event, but a chronic health condition1 with lifelong effects on both morbidity and mortality. Estimates based on patients with TBI treated in hospital indicate that 1.1% of the US population have lifelong disabilities as a consequence of this injury,2 whereas if the substantial number of people who do not seek hospital treatment for injury are included, the percentage might be more than three times greater.3 Furthermore, outcomes in TBI are not fixed long term, and there can be improvement and deterioration many years after injury.4,5
The long-term consequences of TBI are a matter of substantial concern for affected individuals and their families, and for society owing to the substantial economic burden; moreover, the costs are greater in the elderly and therefore set to increase in an ageing population.6 Multiple negative effects on lifelong health have been associated with TBI (panel), and there is growing evidence that many people in the chronic phase are living with under-recognised and poorly managed sequelae of injury.29,30 Understanding of the long-term consequences of TBI is important because this knowledge will allow identification of risk factors for poor outcomes and appropriate targeting of health care resources and interventions.
One area that has received recent attention is the long-term effects of repetitive concussion in sports.31,32 The potential effects of concussion should be understood in the broader context of TBI as a risk factor for long-term neurological disease. Studies of cohorts with TBI of all severities and causes can help to elucidate the neurological consequences of brain injury, and neuroimaging and pathological investigations in the past decade have enabled better characterisation of late neurodegenerative features associated with TBI.
Other reviews have provided detailed accounts of long-term pathology,33,34 imaging,35 disease,1,10,36 functioning,4 emotional adjustment,26 and cognition.12,37 In this Series paper, we aim to bring together key evidence concerning the chronic consequences of TBI in adults, with an emphasis on neurological changes and how they evolve over time, focusing on studies that have included follow-up of 5 years or more after injury.
We begin by examining studies of late changes in global functional outcome and long-term mortality. We then evaluate the specific contribution of TBI-related neurodegenerative and other neurological diseases to morbidity and mortality, and consider characterization of underlying neurodegeneration. Finally, we emphasise the gaps in knowledge and highlight areas of research needed to improve understanding and awareness of the long-term consequences of TBI.
Dynamic long-term functional outcomes of TBI
A range of problems can persist after TBI, including post-concussion symptoms, emotional difficulties, cognitive impairment, and functional limitations.38 However, until recently relatively little has been known about changes in outcome many years after injury. Understanding of global outcome changes has been enhanced by information from longitudinal studies involving two cohorts,28,39–42 both with follow-up of functional status of more than 10 years (table 1). In the USA the TBI Model Systems (TBIMS) collaboration has assembled an invaluable national database of long-term outcomes of patients who have received rehabilitation after brain injury, and in the UK, a representative cohort of patients admitted to hospital with TBI originally identified by Thornhill and colleagues49 in Glasgow, UK, has been successively followed up.
Table 1.
Key studies of long-term functional outcome and mortality after traumatic brain injury.
| Study | Study design | Sample | Follow-up* | Outcomes | Main findings | Risk factors |
|---|---|---|---|---|---|---|
| Whitnall et al (2006)39 Glasgow, UK | Prospective cohort study | 475 adults who were admitted to hospital for TBI (all severities) and were alive 1 year after injury; 58% aged ≤ 40 years at injury | 5–7 years | Mortality, functional outcome, cognition, emotional adjustment, health status, alcohol and drug use, and social deprivation | 24% of patients had died by 5–7 years; 53% of survivors were disabled, 29% had improved and 25% deteriorated by follow-up at 5–7 years. | Disability was more strongly related to emotional adjustment and self-esteem than to injury severity or cognitive impairment. |
| McMillan et al (2012)40 Glasgow, UK | Prospective cohort study | 219 adults who were admitted to hospital for TBI (all severities) and were alive at 5–7 years after injury; 65% aged ≤ 40 years at injury | 12–14 years | Mortality, functional outcome, cognition, emotional adjustment, health status, alcohol use, and social deprivation | 16% of patients had died by 12–14 years; 51% of survivors were disabled, 23% had improved, and 32% had deteriorated by follow-up at 12–14 years | Disability was associated with older age at injury, premorbid brain illness or physical disability and current self-esteem and stress. |
| McMillan et al (2014)28 Glasgow, UK | Prospective case-controlled, record linkage study | 2,428 adults who were admitted to hospital for mild TBI; median age 39 years at injury; 2428 individuals without TBI in the community, matched for age, sex, and social deprivation | 15 years | Mortality | 37% of patients had died by follow up at 15 years; death rate was 24.5 per 1,000 patients versus 13.3 per 1000 community controls (ratio=1.84). | Age was a risk factor for mortality; younger adults with TBI had a 4.2 times greater risk of death than community controls; in addition to age, independent risk factors at time of injury included habitual alcohol excess, number of previous admissions to hospital with TBI, preinjury physical limitations, and social deprivation. |
| Corrigan et al (2014)43 TBI Model Systems national database, USA | Prospective cohort study | 4064 adults who received inpatient rehabilitation for TBI; 53% aged <60 years at injury | 5 years | Mortality, functional outcome, societal participation, emotional adjustment, and alcohol and drug use | Estimated that for the US acute inpatient rehabilitation population 21% had died by 5 years; 57% of survivors were disabled and 39% had deteriorated since 1–2 years after injury | Poorer functional outcome was associated with older age, whereas younger groups had poorer mental health and emotional outcomes. |
| Pretz et al (2013)41 and Dams-O’Connor et al (2015)42 TBI Model Systems national database, USA | Prospective cohort study | 3,870 adults who received inpatient rehabilitation for TBI; mean age 36 at injury years at injury | 1–20 years | Mortality, functional outcome | Group mean outcome ratings were in the moderately disabled range at all time points; functional outcome improved initially, reached a peak at about 10 years after injury, then subsequently declined. | Growth curves were influenced by age, race, disability at admission, and length of rehabilitation stay; trajectories for those who died at least 5 years after injury began with lower functional status and declined more rapidly than trajectories for those who survived. |
| Harrison-Felix et al (2012)27,44 TBI Model Systems national database, USA | Prospective cohort study | 8,573 adults who received inpatient rehabilitation for TBI; mean age at injury 39 years | 1–20 years | Mortality, life expectancy, cause of death | Patients with TBI were 2.25 times more likely to die than the general population (adjusted for age, sex, and race or ethnicity). SMR was elevated in all subgroups (age, gender, race, and injury severity), and remained higher 10 years after injury; SMR was raised for all causes of death, particularly seizures (33.38), aspiration pneumonia (13.35), sepsis (10.37), accidental poisonings (9.54), and falls (9.87) | Independent risk factors for death included: older age, being a man, non-Hispanic ethnicity, being unemployed or unmarried at injury, preinjury drug use, and greater disability at discharge; risk factors for mortality varied by age group. Increased deaths in younger age groups were mainly due to external causes and accidents; life expectancy in the youngest men was decreased by 16 years. |
| Baguley et al (2012)45 and Nott et al (2012)46 NSW, Australia | Prospective cohort, record linkage study. | 2,545 adults who received inpatient rehabilitation for severe TBI; mean age 35 years at injury | 2–20 years | Mortality | Overall mortality was 10% and patients with TBI were 3·19 times more likely to die than the general population (adjusted for age and sex); risk of death was increased for 8 years or more after discharge; SMR was raised for causes of death related to abnormal clinical and laboratory findings (14.1), respiratory system (10.2), nervous system (6.4), digestive system (5.2), mental and behavioural disorders (5.4), and external causes (5.2). | Independent risk factors included disability at discharge, older age at injury, being a man, preinjury drug and alcohol misuse, preinjury epilepsy, and discharge to an aged care facility; crude mortality rates increased with age, but younger adults had the highest risk of death compared with population norms. |
| Flaada et al (2007)47 Olmsted County, MN, USA | Retrospective population-based cohort, record linkage study | 1,433 patients of all ages who sought any help from medical services for TBI (89% had mild TBI, 11% had moderate or severe TBI); mean age 28 years at injury; | 6 months and 10 years | Mortality | Observed survival at 10 years after injury for 1303 patients with TBI who were alive at 6 months (93·1%) was not significantly different to expected survival (92·8%) based on population norms | Mortality increased with age, did not differ from population norms after survival to six months. |
| Dams-O’Connor et al (2013)48 Adult Changes in Thought study, Seattle, WA, USA | Prospective population-based cohort study | 4,225 individuals without dementia, of whom 606 reported a lifetime history of TBI with loss of consciousness; aged ≥ 65 years at enrolment;. | 0-to ≥ 40 years | Mortality, recurrent TBI, and dementia | Lifetime history of TBI was not associated with increased risk of mortality or dementia in individuals who were alive and did not have dementia at enrolment | A history of TBI was associated with elevated risk of further brain trauma during follow-up. |
Abbreviations: SMR = standardized mortality ratio.
Interval between exposure to TBI and study observation; in the case of lifetime reported TBI in some studies, this follow-up period can comprise long intervals and some studies do not always report the exact range, because of uncertainty around the timing of the TBI.
Interval between exposure to TBI and study observation; in the case of lifetime reported TBI, this follow-up period can comprise long intervals and some studies do not always report the exact range, because of uncertainty around the timing of the TBI.
The TBIMS programme was established in 1987 to collect longitudinal data that could be used to improve outcomes of TBI,50 and there are currently 16 funded civilian centres. Individuals enrolled in the project are aged 16 years and older receiving inpatient rehabilitation for a primary diagnosis of TBI, and they are followed up at 1 year, 2 years, and 5 years after injury, and every 5 years thereafter. The 15,000th person was enrolled in the TBIMS national database in 2016, with early participants now followed up for 25 years.
As might be expected, older age at the time of injury tends to be associated with poorer outcomes and a faster rate of decline on functional outcomes.51–53 Studies have taken advantage of the longitudinal nature of the TBIMS national database to examine trajectories of change over time. One study43 weighted this database to provide estimates for the US population receiving acute inpatient rehabilitation: by 5 years after injury, approximately one in five had died, 12% of survivors were living in institutional settings, and 50% had been readmitted to hospital at least once. Of those who survived, the majority were moderately or severely disabled and more than a third had deteriorated from a previously achieved level after injury. Deterioration in functional outcome was evident across all age groups, implying that decline is not simply age-related.
Another study41 used individual growth curve analysis to evaluate functional status over time as measured by the Glasgow Outcome Scale-Extended (GOSE), and found that for individuals in the TBIMS national database as a whole, the typical trajectory was to improve gradually over time for about 10 years, plateau, and then decline. This method allowed covariates to be entered into the model to explain variability in rates and levels of improvement and decline, and factors such as age, race, injury severity and length of rehabilitation hospital stay were found to influence functional trajectories. Another study42 used a similar approach to compare functional outcome trajectories (as defined by the GOSE and Disability Rating Scale) of those who survived with those who died more than 5 years after injury. This study found that those who died had a worse functional status at the time of discharge from inpatient rehabilitation, and that they also had more rapid functional decline over time (figure 1).42 These findings suggest that there might be readily detectable, although commonly missed, opportunities to identify patients at risk for poor outcomes and to deploy interventions to improve their health and quality of life.
Figure 1. Longitudinal trajectories, based on data modelling, for functional outcome after inpatient rehabilitation for traumatic brain injury in surviving versus deceased patients.
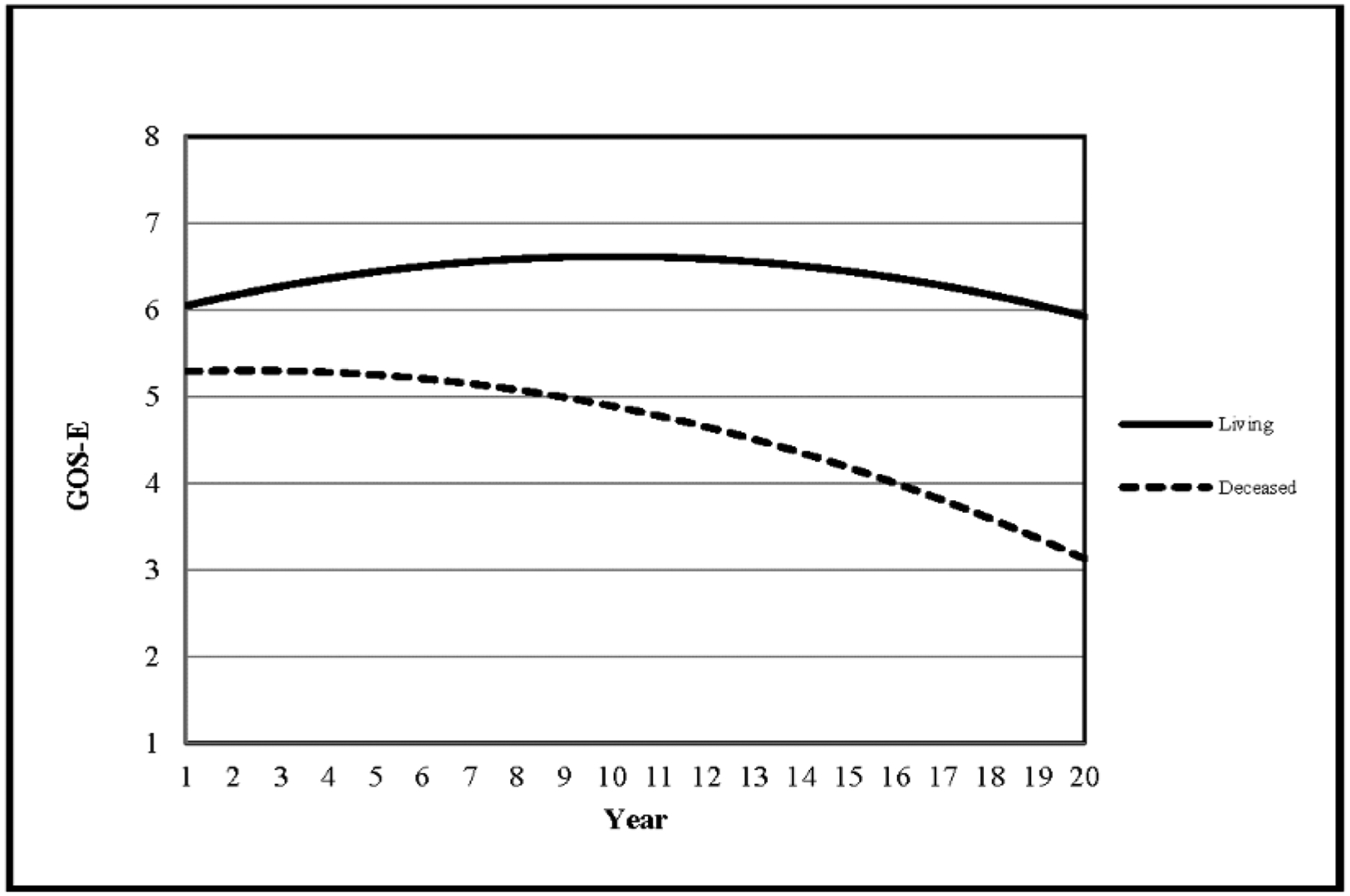
Trajectories are fitted curves showing expected change in functioning of patients with TBI as measured with the GOSE for survivors compared with those who were deceased (died >5 years after injury). These sample trajectories were generated with a modelling approach known as individual growth curve analysis with use of longitudinal data from the TBI Model Systems national database from patients aged ≥ 16 years who had completed at least three study visits (ie, having survived for a minimum of 2 years); the curves shown represent individuals, who were white, aged 26 years when their first GOSE score was recorded, had 30 days of rehabilitation, and had the same level of disability on admission to rehabilitation (cognitive FIM score of 11 and motor FIM score of 33). Although profiles vary substantially between individuals, it is evident from these examples that the trajectory for those who were deceased is markedly different from those who survived: those who were deceased started out with more disability initially–a GOSE score nearly a point below those who survived–and their trajectories suggest a near constant decline in function. By contrast, the trajectory for surviving patients shows slight improvement in outcomes, which could take many years to reach a peak, followed by a delayed decline from about 10 years after injury. Reproduced from Dams-O’Connor and colleagues,42 by permission of Wolters Kluwer Health. TBI= traumatic brain injury. GOSE = Glasgow Outcome Scale–extended. FIM=Functional Independence Measure.
Similar findings have been reported from long-term follow-up of patients with TBI in the cohort admitted to hospital in Glasgow.49 At five to seven years after injury 70% of those originally assessed at 1 year were re-evaluated.39 At this timepoint nearly a quarter of those alive at 1 year had died. Over half of survivors were still disabled on the GOSE, and, although 29% had improved, 25% had deteriorated between 1 year and 5–7 years after injury. Both late improvement and late deterioration were strongly related to self-rating of depression, anxiety, stress and self-esteem, rather than injury severity and cognitive impairment. Similar associations were apparent at a further follow-up 12 – 14 years after injury40 Strikingly, despite the fact that the majority of the sample had injuries considered to be mild on the basis of Glasgow Coma Scale criteria, more than half of the individuals were disabled at each follow-up timepoint.
The findings from long-term longitudinal studies suggest that changes in functional outcome are common even many years after TBI, and both improvement and decline can be observed. Most studies described in this section had no control groups; therefore, the extent to which reported disability is accounted for by normal aging processes is not clear. However, it appears that age is not solely responsible for decline,43 and impaired cognition does not seem to be the primary cause.39 The long-term effects of mild TBI are particularly concerning, and further research is needed on this issue.
The evidence from studies of adults is in accord with reports of poor long-term outcomes following childhood TBI.54,55 A recent population-based study56 in Sweden found that injury in childhood increased the risk of later disability, psychiatric illness, and premature death. Whether injury in children generally results in poorer outcomes than in adults is a complex issue57 that is beyond the scope of this Series paper.
Late mortality after TBI
Many studies have examined mortality within the first 6 months after brain injury,58 but far fewer studies have assessed mortality risks in the long term (table 1).28,39–48 There is consensus that moderate-to-severe injuries have an enhanced risk of mortality that might persist for many years, but a puzzling observation is that high mortality rates might be observed even after mild TBI.28
Long-term mortality after moderate to severe TBI
The TBIMS national database was used to examine survival status of 8,573 TBI patients who had received inpatient rehabilitation.27,44 This work addressed the issue of a control group by comparing mortality related to TBI with mortality rates of the general population, adjusted for age, sex and race or ethnicity. Patients with TBI were more than twice as likely to die than individuals in the general population (standardized mortality rate [SMR] 2.25; 95% CI 2.10 – 2.40); life expectancy was on average 7 years shorter. Mortality rates were increased for a variety of causes (table 1). Older age increased the absolute risk of death, but as found in a previous study,59 the greatest relative risk of death after TBI occurred in younger age groups. In those aged 15 –19 years at injury the mortality rate was nearly five times that of matched individuals. Younger people more commonly died secondary to accidental injuries, suggesting that dysexecutive symptoms might have been causal in mortality risk, whereas older people more frequently died for reasons suggesting chronic medical conditions or comorbidities.44 Multivariate predictors of mortality included disability at discharge and preinjury drug or alcohol abuse,27 and were found to vary by age at injury.44
Similar results were reported in a study from New South Wales, Australia, of 2,545 patients discharged from rehabilitation.45,46 An enhanced risk of death in this population was observed (SMR 3.19; 95% CI 2.80 – 3.60), particularly within the first year after injury, and this risk remained above population norms for at least 8 years. Predictors of mortality included functional dependence, age, preinjury drug and alcohol misuse, and preinjury epilepsy. Older patients were at the highest absolute risk of death, but the relative risk was greatest for younger adults (aged <50 years) with severe TBI. This group had a four-to-six times increase in risk of mortality compared with a control group matched for age and sex.
Therefore, studies of well-characterized cohorts of rehabilitation patients show that moderate-to-severe TBI carries an enhanced risk of late mortality (table 1); deaths occur from a wide variety of causes, some of which might be preventable. When considering estimates of risk, it should be noted that these estimates are based on the use of population comparison groups, with matching limited to a small set of demographic variables.
Long-term mortality after mild TBI
Substantially elevated long-term death rates after mild TBI have been reported in the cohort admitted to hospital in Glasgow.28 Medical records of 2,428 patients who had mild head injury 15 years previously were traced and compared with individuals without head injury in the community. Overall, more than a third of patients had died despite the relatively young age at injury (median 39 years). As expected, there were more deaths in older age groups, but again when standardized against those without head injury the highest rates were in the younger age groups. Apart from age, independent predictors of mortality included preinjury factors such as habitual alcohol excess or drug use, previous admissions with head injury or neurological illness, previous physical limitation, and social deprivation. Over the follow-up period patients with TBI had a rate of subsequent head injury 19 times greater than those in the control group.
The results from the Glasgow cohort contrast with those of a US population-based study47 of 1,433 patients with predominantly mild TBI (89%) in Olmsted County, Minnesota, the geographical area served by the Mayo Clinic. For patients who were alive at 6 months the death rate by 10 years was not significantly different than expected. The findings of the Glasgow cohort28 also contrast with results of the Adult Changes in Thought (ACT) cohort study,48 which identified 606 participants aged 65 years or older with a history of head injury and loss of consciousness at any time in their lives, and included follow up assessments every 2 years after enrolment for an average of 7 years. In this study no association was found between history of TBI and subsequent death.48 However, a relationship was found between a history of TBI reported at baseline and risk of subsequent injury during follow up, particularly in those aged 55 years or older at the time of first injury (hazard ratio [HR] 3.8; 95% CI 1.89 – 7.62). Furthermore, recent TBI (i.e. injury since the previous follow-up) was associated with increased risk of death (adjusted HR 2.0; 95% CI 1.51 – 2.58).
In studies of retired professional American football players, increases in overall risk of mortality were not observed—eg, Guskiewicz and colleagues60 studied 2,552 former American National Football League (NFL) players and found no evidence for elevated mortality despite the fact that about 60% would be expected to have a history of mild TBI.60 In another study of 3,439 former NFL players,18 all-cause mortality was about half that of the national population, indicating long-term health benefits of participation.
The association between mild head injury and long-term mortality appears to be sample-dependent: in some cohorts being admitted to hospital with mild TBI serves as a marker that the person belongs to a high risk group,28 whereas in other cohorts this association is not apparent.42–44 Results from the Glasgow study of mild head injury28 suggest that pre-existing status and lifestyle factors, such as socio-economic status and risk-taking behaviour, are major determinants of mortality in this particular population.
TBI as a risk factor for later neurological disease
The possibility that TBI is associated with neurological diseases, and neurodegenerative disease in particular, has had substantial attention over the years, and recent studies have provided new evidence concerning a link. TBI disproportionally affects young adults and, despite lengthy follow-up periods, most of the studies described earlier have involved substantial proportions of people with TBI who are still at a relatively young age. Any increased risk for neurodegenerative illness attributable to TBI might not become fully apparent until late life: therefore studies focusing on older age groups or that have very long term follow-up, or both, are important (table 2).
Table 2.
Key studies of long-term neurodegenerative and other neurological diseases after traumatic brain injury
| Study | Study design | Sample | Follow-up* | Outcomes | Covariates | Main findings |
|---|---|---|---|---|---|---|
| Crane et al (2016)16 Adult Changes in Thought study, Religious Orders Study, and Memory and Aging Project, USA | Pooled data from three prospective cohort studies | 7,130 participants of whom 865 reported a lifetime history of TBI with loss of consciousness; aged ≥65 years at enrolment. | 0 – to ≥ 40 years | Diagnosis of MCI, dementia, AD, and PD; abnormalities on neuropathology. | Age at enrolment, sex, education level, and cohort | No association between TBI and dementia or AD was observed; TBI was associated with PD in the three datasets (HR 3·56 for patients with LOC >1 h in Adult Changes in Thought study; pooled OR 1·65 for patients with LOC >1 h and 2·23 for patients with LOC >1 h in the Religious Orders Study and Memory and Aging Project) after adjustment. TBI was associated with dementia with Lewy bodies (pooled RR 1.59–5.73) and microinfarcts (pooled RR 1.58–2.12). |
| Nordstrom et al (2014)61 Military conscript study, Sweden | Retrospective population-based cohort, record linkage study | 81,1622 men of whom 45,249 had at least one diagnosis of TBI over the follow-up interval; mean age 18 years at enrolment | 0 – 43 years | ICD-8, ICD-9, and ICD-10 codes for TBI, AD, dementia, and selected other diagnoses. | Age, place and year of conscription, cognitive function at conscription, alcohol intoxication, weight, height, knee strength, TBI or dementia in parents, income, education level, blood pressure, drug intoxication, depression, and cerebrovascular disease | TBI was not associated with risk of AD, but was associated with risk of other types of dementia (adjusted HR 1.7 for a single mild TBI; 1.7 for at least two occurrences of mild TBI; 2.6 for a single severe TBI). |
| Gardner et al (2014)62 California state databases, USA | Retrospective population-based case-controlled, record linkage study | 51,799 patients admitted to hospital for TBI (all severities); 112,862 patients admitted to hospital for non-TBI trauma (eg fractures); aged ≥ 55 years at injury without baseline dementia | 5–7 years | ICD-9 diagnosis of dementia ≥1 year after TBI | Age, sex, race or ethnicity, income, comorbidities, healthcare use, and trauma severity | TBI was associated with a diagnosis of dementia (8.4% of patients with TBI vs 5.9% without TBI adjusted HR 1.26). Moderate or severe TBI was significantly associated with dementia in all age groups (HR 1.72 for 55–64 years; 1.46 for 65–74 years), whereas mild TBI was associated with an elevated risk (1.25) only in those aged ≥65 years. |
| Gardner et al (2015)63 California state databases, USA | Retrospective population-based case-controlled, record linkage study | 52,393 patients admitted to hospital for TBI (all severities); 113 406 patients admitted to hospital for non-TBI trauma (fractures); aged ≥ 55 years at injury without baseline dementia or PD. trauma. | 5–7 years | ICD-9 diagnosis of Parkinson’s Disease one year or more after TBI | Age, sex, race or ethnicity, income, comorbidities, healthcare use, and trauma severity | TBI was associated with diagnosis of PD (1·7% of patients with TBI vs 1·1% without TBI; adjusted HR 1.44); There was a dose-response relationship with both severity (HR 1.24 for mild TBI; 1.50 for moderate/severe TBI) and frequency of TBI (1.45 for single TBI; 1.87 for more than one TBI) |
| Wang et al (2012)64 Longitudinal Health Insurance Database, Taiwan | Retrospective population-based case-controlled, record linkage study | 44,925 patients who received outpatient or hospital care for TBI; 224,625 patients who received outpatient or hospital care without TBI, matched for sex, age, and year of index use of health care; mean age 41 years at injury | 5 years | ICD-9 diagnosis of dementia | Region and selected comorbidities (stroke, diabetes, hypertension, hyperlipidaemia, and heart disease) | TBI was associated with an increased risk of dementia at 5 years (adjusted HR 1.68). |
| Chen et al (2011)22 Longitudinal Health Insurance Database, Taiwan | Retrospective population-based case-control, record linkage study | 23,199 patients who received outpatient or hospital care for TBI; 69,597 patients who received outpatient or hospital care without TBI, matched for sex, age, and year of index use of health care; mean age 42 years at injury | 3 months to 5 years | ICD-9 diagnosis of stroke | Income, region, and selected comorbidities (stroke, diabetes, hypertension, hyperlipidaemia, and heart disease) | TBI was associated with an increased risk of stroke at 5 years (adjusted HR 2.32). |
| Lehman et al (2012)18 National Football League pension fund database, USA | Retrospective cohort, record linkage study | 3,439 retired male American football players; 62% of those alive were aged < 60 years at date last observed | 19- to ≥ 48 years | Mortality and neurodegenerative causes of death. | Age, race, and calendar year | Overall mortality in players was lower than that of the general US population (adjusted SMR 0.53); overall neurodegenerative deaths were increased (SMR 2.83–3.26), and were elevated for AD (3.86), and ALS (4.31) |
| Guskiewizc et al (2005)60 study of National Football League players, USA | Retrospective cohort study | 2,552 retired male American football players, of whom 61% reported at least one concussion, and 24% reported three or more concussions; mean age 54 years at follow-up. | Up to ≥ 60 years | Diagnosis of MCI, AD, memory complaints, and health-related quality of life. | Unadjusted comparison between groups | In 758 retired players, aged ≥50 years and who had completed memory questionnaires, recurrent concussion was significantly associated with MCI (p=0.02), self-reported memory impairments (p=0.001), and spouse or relative-reported memory impairments (p=0.04); in participants with three or more concussions there was a five-time increase in MCI diagnosis, and a three-time increase in reported memory problems compared to players who had not been concussed. |
| Chio et al (2005)65 study of Italian professional soccer players | Retrospective cohort, record linkage study | 7,325 professional soccer players; aged 18 – 69 years at last date of follow-up. | 0 – 31 years | Diagnosis of ALS | Age and sex | Five cases of ALS were identified, with a mean age of onset of 43.4 years; overall adjusted SMR was 6.5 and there was a dose-response relationship with length of career (adjusted SMR 15.2 for > 5 years = 15.2; 3.5 for ≤5 years). |
| McMillan et al (2016)66 study of Scottish Rugby Union players, UK | Retrospective case-controlled study | 52 retired male international rugby players with a median of 7 concussions; 29 individuals who had not had concussion matched for sex, age, and social deprivation; mean age 54 at follow-up.. | 1 – 48 years | Cognitive assessment, reported concussion symptoms, emotional adjustment, functional status, health-related quality of life, alcohol use, chronic stress biomarkers. | Unadjusted comparison between groups. | Players had lower scores on tests of verbal learning and fine motor co-ordination than did the control group; persisting symptoms were more common in players with more than nine concussions. No other group differences were significant. |
Abbreviations: ACT=Adult Changes in Thought, AD = Alzheimer’s disease, ALS = amyotrophic lateral sclerosis, HR = hazard ratio, MAP= Memory and Aging Project, MCI = mild cognitive impairment, NFL = National Football League, OR = odds ratio, PD = Parkinson’s Disease, ROS = Religious Orders Study, RR= relative risk, SMR = standardized mortality ratio.
Interval between exposure to TBI and study observation; in the case of lifetime reported TBI, this follow-up period can comprise long intervals and some studies do not always report the exact range, because of uncertainty around the timing of the TBI.
Neurodegenerative disease after single TBI
Acceptance that TBI is a risk factor for dementia is growing.33 Investigators of a meta-analysis67 of studies done up to 2001 with an overall sample of 4,639 patients found that a history of TBI was associated with a two-to-four times increased risk of Alzheimer’s disease, with a dose-response effect- ie, risk was highest in patients with severe injury.67 Similarly, the MIRAGE study68 showed an increased risk of Alzheimer’s disease in patients with a history of TBI, with greater risk in patients reporting injury with loss of consciousness than in those without loss of consciousness. These and other early studies largely used case-controlled designs, and were subject to limitations including recall bias, reverse causation, differing definitions of TBI, potential misclassification of neurodegenerative disease, and inadequate control for covariates. Studies that include a greater number of health and lifestyle variables in the models tend to find no relationship or a weak relationship between TBI and dementia.69
Addressing some of the key limitations of study design in earlier work, Gardner and colleagues62 used hospital databases from California, USA, to compare the incidence of all-cause dementia in almost 52,000 patients with TBI with that of a control population of more than 112,000 patients exposed to trauma without brain injury. An increased risk of dementia after a single moderate-to-severe TBI up to 7 years later was observed; the HR adjusted for covariates was 1.26 (95% CI 1.21–1.32). Furthermore, even a single, mild TBI increased the risk of dementia in patients aged 65 years or older at the time of injury (HR 1.25; 95% CI 1.20–1.31). A similar population based study from Taiwan compared about 45,000 patients with TBI with around 225,000 patients without TBI and reported that TBI was associated with an elevated risk of dementia (HR 1.68; 95% CI 1.57 – 1.80) after adjusting for demographics and comorbidities.64
By contrast, the Adult Changes in Thought cohort study48 found no association between TBI and dementia or probable Alzheimer’s disease in participants aged 65 years or older reporting a history of head injury with loss of consciousness and no evidence of dementia at enrolment. Building on this work, Crane and colleagues16 pooled data from this study and two additional prospective cohorts,70,71 yielding a total sample of 7,130 participants, and found no apparent association between TBI of different severities (loss of consciousness >1 h or < 1 h) and clinically diagnosed all-cause dementia or Alzheimer’s disease.16 Limitations of these studies include the use of interview to ascertain TBI severity, and exclusion of patients with young-onset dementia (occurring before age 65 years).
The risk of developing young-onset dementia after TBI was examined in a landmark study61 that followed up about 800,000 Swedish military recruits for up to three decades, around 45,000 of whom had sustained a TBI. TBI was not associated with risk of Alzheimer’s disease. After correcting for covariates (including cognitive function in young adulthood and alcohol intoxication), the authors reported a HR for other young-onset dementia (non-Alzheimer’s disease) types of 1·7 (95% CI 1·2–2·3) for mild TBI and 2·6 (1·6–4·1) for severe TBI. However, the absolute risk of illness was very low: only 0.07% of the cohort developed dementia.
Recent studies have provided evidence for an association between TBI and Parkinson’s disease. A comprehensive meta-analysis by Jafari and colleagues15 of 22 studies reported a pooled odds ratio (OR) of 1.57 (95% CI 1.35–1.83) for risk of Parkinson’s disease after TBI. In this meta-analysis, 19 of the 22 studies reported an OR greater than 1.0. A well-controlled study using the California cohort63 found that TBI sustained after 55 years of age is associated with a 44% increased risk of developing Parkinson’s disease within the subsequent 5–7 years. Furthermore, in their pooled analysis Crane and colleagues16 identified a dose-response relationship between TBI and Parkinson’s disease, with patients with more severe TBI having the greatest risk. These associations were present even when injury had occurred before the age of 25 years, suggesting processes with a protracted time course. However, these results are limited by the relatively small numbers of patients who developed Parkinson’s disease (1.6% of the sample).16
In conclusion, the evidence favours a link between single TBI and neurodegenerative disease. The evidence is perhaps strongest for Parkinson’s disease,15,16 whereas an association specifically with Alzheimer’s disease is less certain. Recent research has involved stronger study designs than earlier work, but they still have considerable shortcomings, with characterization of injury severity a weakness in all of the studies discussed. A further limitation is the absence of prospective neuropathological reviews of clinical diagnoses informed by current understanding of the complex pathology of neurodegeneration after TBI. There is a pressing need for suitably designed and powered studies of the association between single incident TBI and neurodegenerative illness.
Neurodegenerative disease after repetitive mild TBI
Exposure to repetitive mild TBI from boxing has long provided the best evidence for the association between such injury and risk of neurodegenerative disease.72 More recently, attention has turned to other groups exposed to repetitive mild TBI (table 2). In the report of 3,439 former NFL players,18 about two thirds of whom were younger than 60 years of age, deaths due to neurodegenerative illness were three times higher than in the general population; in particular deaths due to amyotrophic lateral sclerosis and Alzheimer’s disease were increased. However, the absolute risk was low: in total only 17 deaths involved neurodegenerative illness, and in only ten individuals was this the principal cause of death. Mortality overall is lower in this group, and, arguably, as noted above, positive effects of participation in sports on long term health73 might be at the cost of a small increased risk of dementia for some sports.
Supporting a modest increase in risk of dementia, the study by Guskiewicz and colleagues60 of 2,552 former NFL players showed a significant association between concussion and both clinically diagnosed mild cognitive impairment and reported memory impairment. There was a higher prevalence of Alzheimer’s disease (1.37; 95% CI 0.98–1.56) in the study sample than in the general population, with the difference particularly apparent in the youngest group (aged ≤69 years), but these findings were not statistically robust. Again, the overall rate of Alzheimer’s disease was low: only 1.3% had this diagnosis.
Studies in former rugby players66,74,75 have reported detectable, although clinically insignificant, neurocognitive deficits. In one study,66 formal neuropsychological assessment findings in a cohort of 52 retired male international rugby players who had a median of seven mild TBIs were compared with assessment results of matched controls with no history of repeated concussion. Rugby players had poorer verbal learning and fine motor co-ordination than did the control group.
In soccer, prevalence of amyotrophic lateral sclerosis might be increased. Investigators of a study of 7,325 former Italian professional soccer players65 found that the risk of amyotrophic lateral sclerosis was approximately six times higher than expected for the population. The authors argue that this effect is specific to soccer and not simply related to exercise: increased risk of amyotrophic lateral sclerosis was absent in parallel cohorts of cyclists and basketball players.76 This finding might be a consequence of exposure to repetitive concussive or sub-concussive impacts, such as in heading the ball in soccer (ie, hitting the ball with their heads). However, no data have been presented in support of functional consequences of heading beyond mild, short lived and reversible brain impairment.77
Adding to historical studies of boxers, evidence of an association between repetitive TBIs and neurodegenerative illness in groups of athletes has now been reported,18,60 although degenerative illness apparently has a low prevalence in the relatively young cohorts that have been studied. Again, studies to date are subject to considerable limitations to study designs, including retrospective designs and little control for covariates.78 Moreover, progress in understanding sports brain injury has been hampered by inconsistent classification of TBI and by disparity in outcome assessments. In particular, the common division of “concussion” and “mild TBI” has been unhelpful79 and fails to acknowledge that TBI spans a spectrum from mild TBI or concussion to severe TBI, which is reflected in commonality in underlying pathology33,34,80 and in lifelong health consequences.3 Little can be offered by way of evidence-based advice to people who have had multiple mild TBIs. Longitudinal studies of athletes that address gaps in evidence are a high priority.
Stroke and other neurological disorders after TBI
Although recent attention has focused on the association between exposure to TBI and the potential increased risk for various neurodegenerative diseases, such injury might also affect later risk for other neurological disorders.
Results of a large scale population study22 showed a link between TBI and increased risk of stroke in the first 5 years after injury. At 5 years patients with TBI had 2.3 times the risk of stroke after adjusting for socio-demographics and comorbidities (Table 2). A similar finding has been reported for a cohort of 1,173,000 patients with trauma in the USA,23 of whom 37% had TBI. After adjusting for confounding covariates the HR was 1.31 (95% CI 1.25–1.36), potentially placing TBI as a more significant stroke risk factor than hypertension. An association between TBI and stroke early after injury is not surprising, but the mechanism underlying a persisting link has not been established.81
Various other diseases associated with TBI have a significant influence on long-term outcomes including epilepsy,20,21 neuroendocrine disorders,24 and neuropsychiatric illness, particularly depression.10 A detailed discussion of these conditions is beyond the scope of this Series paper.
Characterisation of neurodegeneration after TBI
Late neuropathology of TBI
Early observations on the brains of boxers formed the basis of Corsellis and colleagues’72 landmark publication in 1973 describing dementia pugilistica. However, only recently has due attention been paid to the significance of neurodegenerative pathology after TBI, which has now been documented in a growing number of serendipitously observed brains from individuals exposed to such injury in a range of contexts, including participants in boxing, American football, ice hockey, soccer, and rugby;19,33,72,82–85 military personnel;86 and survivors of a single moderate or severe TBI.34,87 Reflecting that it is exposure to TBI that is associated with risk of late neurodegeneration, and not the sport or environment or, solely, injury severity and frequency, this pathology is now termed chronic traumatic encephalopathy (CTE).34
Notwithstanding this recent increased awareness of and attention to CTE, reports on neuropathology after TBI account for just more than 300 cases in the scientific literature, with many studies being retrospective examinations on donated brains. These studies are therefore subject to inevitable case selection biases and limitations in clinical evaluation. Furthermore, few pathological studies include adequate numbers and appropriate samples of non-injured control brains in their assessments to allow observations to be placed in the context of potentially confounding pathologies, particularly ageing associated pathology.33 Nevertheless, evidence is emerging of a distinctive neurodegenerative pathology88 that, within acknowledged limitations of retrospective studies thus far, is almost exclusive to circumstances in which there has been previous exposure to TBI.89 Although many reports focus on aspects of tau neuropathology in CTE,88 the pathology after TBI is complex and, in addition to tau, features a range of abnormalities including amyloid beta and TDP-43 deposition, neuroinflammation, axonal degeneration, white matter degradation, neuronal loss and blood-brain barrier disruption (figure 2).33,34,90–92
Figure 2: Neuropathology after traumatic brain injury.
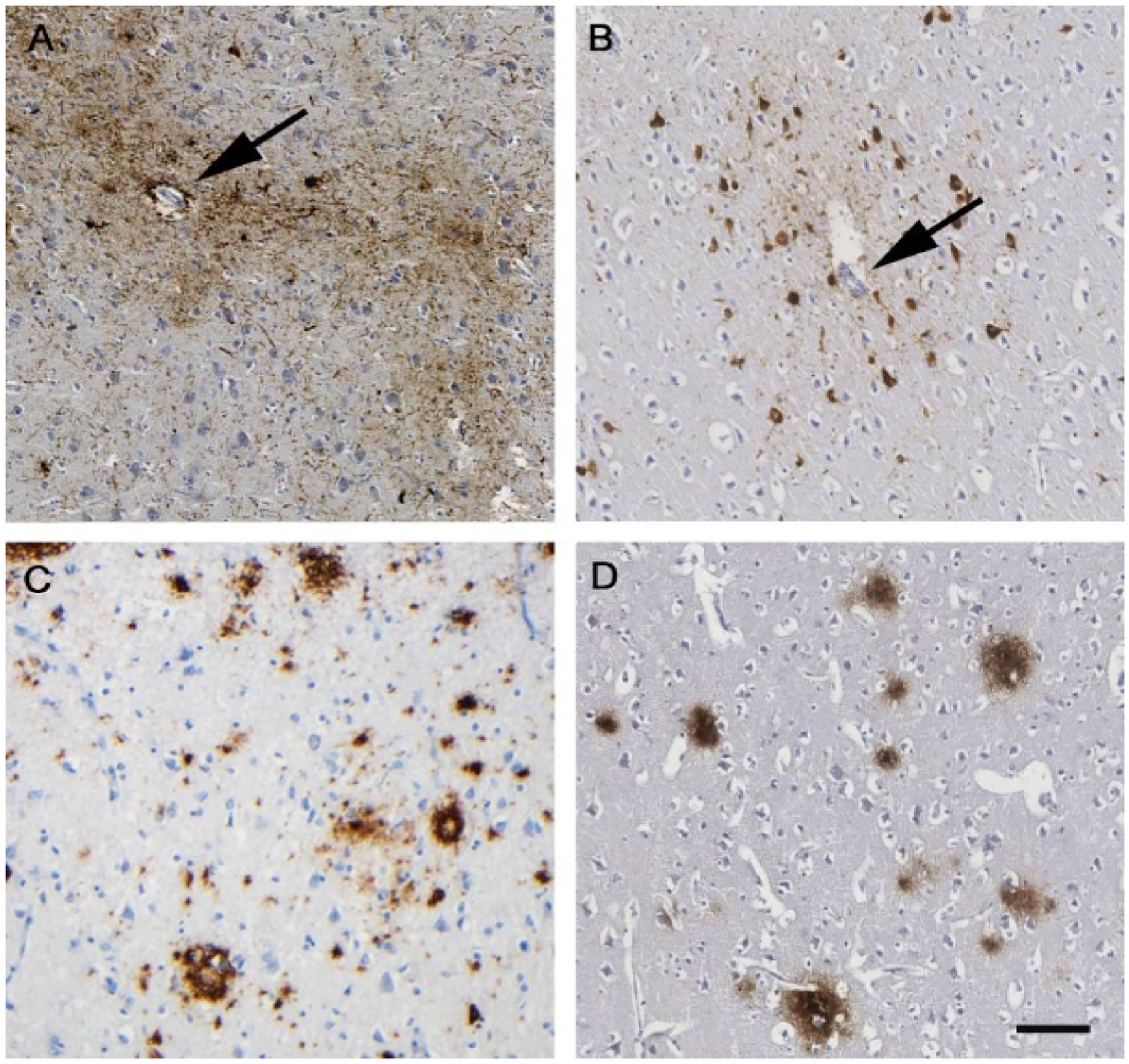
Appearance of hyperphosphorylated tau aggregates within neurons and astrocytes (brown staining in panels A and B) clustered around small cortical vessels (arrows), characteristically in a patchy distribution towards the depths of cortical sulci, is emerging as the distinctive pathology of chronic traumatic encephalopathy. This pathology appears to be virtually exclusive to circumstances in which there has been exposure to brain injury in life, whether as repetitive mild TBI as shown in a 61-year-old male former boxer (A) or single moderate or severe TBI as shown in a 48-year-old man with 3 years of survival after single severe TBI (B). In addition to this distinctive tau pathology, neurodegeneration after TBI is increasingly recognised as a complex pathology, including abnormal amyloid plaque deposition (brown staining in panels C and D). As with tau, aspects of these pathologies can be recognised in case material from patients exposed to either repetitive mild TBI as shown in a 59-year-old male former soccer player (C) or single moderate or severe TBI (D; same patient as in panel B). Phosphorylated tau using antibody CP13 (A) or PHF-1 (B). Amyloid β was stained with antibody 6F3D (C and D). TBI= traumatic brain injury. Scale bar 100 microns for all images.
Despite heterogeneity in mechanisms of closed head injury, there is increasing acknowledgment of common diffuse pathologies across TBI, such as diffuse axonal injury80 and blood-brain barrier disruption,92 which are also reflected in the neuropathology after injury, perhaps offering potential candidates for development of targeted therapeutic interventions.
Although recognition of CTE beyond former boxers has increased, no operational criteria exist for its clinical diagnosis; currently diagnosis requires autopsy confirmation. Perhaps as a consequence, and because of the low numbers of autopsies in patients with neurodegenerative disease, CTE remains an infrequent diagnosis. Therefore, current reporting provides no insight into the prevalence of CTE.
It is an unfortunate omission in research on outcomes from TBI that few studies include prospective neuropathology assessment. Furthermore, studies that do include neuropathology findings in patients with dementia after TBI, rely on archival reporting and assessments, which precede current understanding of the complex pathology in these patients.16 In this context, it is worth noting that dementia in survivors of TBI has a clinical syndrome that appears distinct from that of typical Alzheimer’s disease in the absence of a history of TBI, and is perhaps more in keeping with CTE.93,94
Imaging endophenotypes of late neurodegeneration after TBI
Neuroimaging can provide epidemiological, conceptual, and practical advances, and resolve uncertainties concerning the presence, extent and type of neurodegeneration in survivors of TBI. Pathological characterisation is confounded by several variables. In particular the clinical relevance of specific neurodegenerative pathologies (such as amyloid deposition) is difficult to quantify in patients with TBI, because the clinical picture might be modulated by several other factors. These factors include preinjury reserve,61 loss of cognitive reserve as a direct consequence of injury, neuroinflammation, and activation of processes that are shared with neurodegenerative diseases. In view of the difficulties in relating neurodegenerative processes to clinical outcome, imaging offers two key approaches to identify endophenotypes of late cognitive decline.
The first approach is use of either late, or preferably serial, MRI to characterise global or regional volume reduction or cortical thinning, or white matter loss measured with use of diffusion tensor MRI. Although most studies have used MRI in the acute (days to weeks) and subacute (weeks to months) phases after TBI, some studies have acquired MRI data later,95–97 and in a few cases, several years after injury.98–100 These studies have shown that a minority of survivors of TBI display late or ongoing cortical or white matter loss, or both, years after injury.
The second approach is use of molecular imaging (e.g. PET) to identify neuropathological processes that might be activated following TBI, and could hence provide signatures for the cognate neurodegenerative diseases. Key processes that have started to be characterised in this context include amyloid deposition,101–105 tau deposition,104,106 and neuroinflammation.107–109 Although the location and progress of pathology on MRI can provide useful clues to underlying neuropathology, the data from molecular imaging techniques provide insights that cannot be achieved even with advanced MRI.
These combined imaging advances are useful for several reasons. In an appropriate population with TBI, they can provide data on the prevalence of molecular neurodegenerative processes, and (on serial MRI) its effect on neural loss, both of which can also be tied to late neurocognitive outcome and its progression. Serial MRI also enables careful exploration of the interaction between exposure to injury and recognised genetic drivers of neurodegeneration in contributing to late and progressive cortical loss.99 These data are also important conceptually, because they allow parcellation of different molecular processes that underlie late cognitive decline, such as tau deposition, amyloid deposition, or neuroinflammation, with the promise of precision-medicine approaches to choosing specific therapies, if these become available. Finally, the literature on Alzheimer’s disease suggests that imaging tools can provide very early characterisation of disease, at a stage at which clinical deterioration is undetectable.110 Given the increased risk of late neurodegeneration described in this Series paper, survivors of TBI represent a population that is enriched for patients at risk of neurodegenerative disease. Consequently, imaging approaches in this population could be particularly rewarding in terms of stratifying risk of late cognitive decline and identifying the molecular mechanisms involved at a stage when related clinical characteristics are not yet evident. These tools can therefore be used in the population of survivors of TBI to select patients for clinical trials, provide intermediate endpoints for such trials, and where successful allow selection of patients for effective treatments to prevent late neurodegeneration.
Conclusions and future directions
Multiple lines of evidence suggest that, for many patients, TBI is a chronic, evolving, and perhaps lifelong disorder. This disorder manifests as altered risk not only for various neurological pathologies but also systemic pathologies, with associated increased morbidity and mortality, extending for many decades in survivors of TBI. These negative outcomes are a major concern. However, research to identify and quantify late outcomes from TBI has been remarkably scarce, with few studies including longitudinal follow-up of more than 5 years after injury. Therefore, there is an urgent need for further research to address the limitations of available studies, which are confounded by inconsistencies in definitions of injury severity and recording of outcomes and follow-up procedures, and by largely retrospective methods. Furthermore, heterogeneity of TBI is an important issue that needs to be addressed in future work: there is well recognized heterogeneity in mechanisms of injury and brain abnormalities, and poorly understood variability in host factors.
In studies that have documented late outcomes after TBI, poor long-term outcomes, late deterioration in functional status, and high rates of mortality have been reported across the age range. Therefore, age-related processes do not seem to explain these findings, and, particularly in younger age groups, seem likely to play a limited role. Associations between TBI and higher rates of mortality have been identified, with predictors for both mild28 and moderate/severe injuries27,44,45 including pre injury factors such as alcohol and drug abuse and previous neurological illness, although severity of injury appears not to have substantial influence. These associations suggest that lifestyle factors play a role in poor outcome after injury, and such factors might be amenable to change with appropriate interventions.
Patients discharged with moderate or severe disability are a readily identifiable group, who potentially have a chronic disease. Individuals living with moderate-to-severe TBI might benefit from a long-term health management approach with enhanced medical monitoring and supported proactive health-maintenance interventions. Positive long-term outcomes from injury have been reported where co-ordinated health care is available.111 There is a need for comparative effectiveness research on long-term TBI outcomes within different systems to establish optimal health care and interventions that parallels current assessments of acute and sub-acute care.112
Neuropathological evidence suggests the occurrence of complex pathological changes after TBI that might best be described as a polypathology.17,33,91 This pathology might be superimposed on normal ageing or accelerate existing age-related changes.33 Many factors will influence the point at which clinical threshold is reached including preinjury cognitive reserve.61 By some estimates, it has been calculated that TBI might currently contribute to between 5% to 15% of all incident cases of dementia.13 As such, TBI represents not just a major contribution to neurodegenerative illness, which might be preventable, but also a substantial economic burden in healthcare.
Unquestionably, the link between TBI and neurodegenerative illness warrants further study. Long-term longitudinal studies of patients with TBI would be particularly informative. Objective imaging and molecular biomarkers of neurodegeneration related to TBI are needed, because experience shows that such biomarkers are essential for accurate diagnosis and for targeting therapy.113 It would be helpful if projects assembling large prospective cohorts of patients with TBI could include steps to facilitate future long-term follow-up of patients. Such cohorts could provide systematic selection of patients for imaging studies at late points, using molecular imaging to identify neurodegenerative processes (essentially reflecting neuropathology in vivo) and their effect on progressive anatomical and microstructural changes in the brain after TBI. These data could aid the design and implementation of clinical trials of new therapies aimed at this cohort of patients, and selection of patients for therapies that emerge as successful from such evaluation.
Acknowledgements
The work was done as part of the CENTER-TBI (Comparative European Neurotrauma Effectiveness Research in Traumatic Brain Injury) project, and was supported by the Framework 7 programme of the European Union (602150-2). We thank Andrew Maas for his guidance and comments on draft manuscripts. The funding source had no input into the conception or execution of this paper.
Panel: Major long-term consequences of traumatic brain injury
Function
Disease
Mortality
Search strategy and selection criteria
We searched PubMed for the period Jan 1, 2010, to June 22, 2017, for papers published in English, with the search terms: “traumatic brain injury” or “head injury”, “long-term”, “survivors”, “follow-up”, “mortality”, “longitudinal”, “dementia”, “Alzheimer”, “Parkinson”, and “degenerative”. We identified studies concerning adults with traumatic brain injury that included follow-up to 5 years or more after injury. Studies were selected that addressed the central theme of long-term deterioration after TBI, including the topics of functional decline, mortality, and neurodegenerative disease. The search was supplemented by personal files and by citations in the identified articles. The final selection was made on the basis of relevance to the specific topics selected for the Series paper
Footnotes
Declarations of interest
We declare no competing interests.
References
- 1.Masel BE, DeWitt DS. Traumatic brain injury: a disease process, not an event. J Neurotrauma 2010; 27: 1529–40. [DOI] [PubMed] [Google Scholar]
- 2.Zaloshnja E, Miller T, Langlois JA, Selassie AW. Prevalence of long-term disability from traumatic brain injury in the civilian population of the United States, 2005. J Head Trauma Rehabil 2008; 23:394–400. [DOI] [PubMed] [Google Scholar]
- 3.Whiteneck GG, Cuthbert JP, Corrigan JD, Bogner JA. Prevalence of self-reported lifetime history of traumatic brain injury and associated disability: a statewide population-based survey. J Head Trauma Rehabil 2016; 31: E55–62. [DOI] [PubMed] [Google Scholar]
- 4.Corrigan JD, Hammond FM. Traumatic brain injury as a chronic health condition. Arch Phys Med Rehabil 2013; 94: 1199–201. [DOI] [PubMed] [Google Scholar]
- 5.Green RE. Editorial: brain injury as a neurodegenerative disorder. Front Hum Neurosci 2015; 9: 615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gooch CL, Pracht E, Borenstein AR. The burden of neurological disease in the United States: a summary report and call to action. Ann Neurol 2017: 81; 479–84. [DOI] [PubMed] [Google Scholar]
- 7.Temkin NR, Corrigan JD, Dikmen SS, Machamer J. Social functioning after traumatic brain injury. J Head Trauma Rehabil 2009; 24: 460–7. [DOI] [PubMed] [Google Scholar]
- 8.Dikmen SS, Machamer JE, Powell JM, Temkin NR. Outcome 3 to 5 years after moderate to severe traumatic brain injury. Arch Phys Med Rehabil 2003; 84: 1449–57. [DOI] [PubMed] [Google Scholar]
- 9.Himanen L, Portin R, Isoniemi H, Helenius H, Kurki T, Tenovuo O. Longitudinal cognitive changes in traumatic brain injury: a 30-year follow-up study. Neurology 2006; 66: 187–92. [DOI] [PubMed] [Google Scholar]
- 10.Perry DC, Sturm VE, Peterson MJ, et al. Association of traumatic brain injury with subsequent neurological and psychiatric disease: a meta-analysis. J Neurosurg 2016; 124: 511–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baguley IJ, Cooper J, Felmingham K. Aggressive behavior following traumatic brain injury: how common is common? J Head Trauma Rehabil 2006; 21: 45–56. [DOI] [PubMed] [Google Scholar]
- 12.Moretti L, Cristofori I, Weaver SM, Chau A, Portelli JN, Grafman J. Cognitive decline in older adults with a history of traumatic brain injury. Lancet Neurol 2012; 11:1103–12. [DOI] [PubMed] [Google Scholar]
- 13.Shively S, Scher Al, Perl DP, Diaz-Arrastia R. Dementia resulting from traumatic brain injury: what is the pathology? Arch Neurol 2012; 69: 1245–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Plassman BL, Grafman J. Traumatic brain injury and late-life dementia. Handb Clin Neurol 2015; 128: 711–22. [DOI] [PubMed] [Google Scholar]
- 15.Jafari S, Etminan M, Aminzadeh F, Samii A. Head injury and risk of Parkinson disease: a systematic review and meta-analysis. Mov Disord 2013; 28: 1222–9. [DOI] [PubMed] [Google Scholar]
- 16.Crane PK, Gibbons LE, Dams-O’Connor K, et al. Association of traumatic brain injury with late-life neurodegenerative conditions and neuropathologic findings. JAMA Neurol 2016; 73:1062–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Washington PM, Villapol S, Burns MP. Polypathology and dementia after brain trauma: does brain injury trigger distinct neurodegenerative diseases, or should they be classified together as traumatic encephalopathy? Exp Neurol 2016; 275: 381–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lehman EJ, Hein MJ, Baron SL, Gersic CM. Neurodegenerative causes of death among retired National Football League players. Neurology 2012; 79: 1970–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McKee AC, Stern RA, Nowinski CJ, et al. The spectrum of disease in chronic traumatic encephalopathy. Brain 2013; 136: 43–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vaaramo K, Puljula J, Tetri S, Juvela S, Hillbom M. Predictors of new-onset seizures: a 10-year follow-up of head trauma subjects with and without traumatic brain injury. J Neurol Neurosurg Psychiatry 2014; 85: 598–602. [DOI] [PubMed] [Google Scholar]
- 21.Najafi MR, Tabesh H, Hosseini H, Akbari M, Najafi MA. Early and late posttraumatic seizures following traumatic brain injury: a five-year follow-up survival study. Adv Biomed Res 2015; 4: 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen YH, Kang JH, Lin HC. Patients with traumatic brain injury: population-based study suggests increased risk of stroke. Stroke 2011; 42: 2733–9. [DOI] [PubMed] [Google Scholar]
- 23.Burke JF, Stulc JL, Skolarus LE, Sears ED, Zahuranec DB, Morgenstern LB. Traumatic brain injury may be an independent risk factor for stroke. Neurology 2013; 81: 33–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Behan LA, Phillips J, Thompson CJ, Agha A. Neuroendocrine disorders after traumatic brain injury. J Neurol Neurosurg Psychiatry 2008; 79: 753–9. [DOI] [PubMed] [Google Scholar]
- 25.Bazarian JJ, Cernak I, Noble-Haeusslein L, Potolicchio S, Temkin N. Long-term neurologic outcomes after traumatic brain injury. J Head Trauma Rehabil 2009; 24: 439–51. [DOI] [PubMed] [Google Scholar]
- 26.Hesdorffer DC, Rauch SL, Tamminga CA. Long-term psychiatric outcomes following traumatic brain injury: a review of the literature. J Head Trauma Rehabil 2009; 24: 452–9. [DOI] [PubMed] [Google Scholar]
- 27.Harrison-Felix C, Kreider SE, Arango-Lasprilla JC, et al. Life expectancy following rehabilitation: a NIDRR Traumatic Brain Injury Model Systems study. J Head Trauma Rehabil 2012; 27: E69–80. [DOI] [PubMed] [Google Scholar]
- 28.McMillan TM, Weir CJ, Wainman-Lefley J. Mortality and morbidity 15 years after hospital admission with mild head injury: a prospective case-controlled population study. J Neurol Neurosurg Psychiatry 2014; 85:1214–20. [DOI] [PubMed] [Google Scholar]
- 29.Kolakowsky-Hayner SA, Hammond FM, Wright J, et al. Ageing and traumatic brain injury: age, decline in function and level of assistance over the first 10 years post-injury. Brain Inj 2012; 26: 1328–37. [DOI] [PubMed] [Google Scholar]
- 30.Schulz-Heik RJ, Poole JH, Dahdah MN, et al. Service needs and barriers to care five or more years after moderate to severe TBI among Veterans. Brain Inj 2017: doi: 10.1080/02699052.2017.1307449. [DOI] [PubMed] [Google Scholar]
- 31.Pearce N, Gallo V, McElvenny D. Head trauma in sport and neurodegenerative disease: an issue whose time has come? Neurobiol Aging 2015; 36: 1383–9. [DOI] [PubMed] [Google Scholar]
- 32.Gardner RC, Yaffe K. Epidemiology of mild traumatic brain injury and neurodegenerative disease. Mol Cell Neurosci 2015; 66: 75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith DH, Johnson VE, Stewart W. Chronic neuropathologies of single and repetitive TBI: substrates of dementia? Nat Rev Neurol 2013; 9: 211–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hay J, Johnson VE, Smith DH, Stewart W. Chronic traumatic encephalopathy: the neuropathological legacy of traumatic brain injury. Annu Rev Pathol 2016; 11: 21–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bigler ED. Traumatic brain injury, neuroimaging, and neurodegeneration. Front Hum Neurosci 2013; 7: 395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stocchetti N, Zanier ER. Chronic impact of traumatic brain injury on outcome and quality of life: a narrative review. Crit Care 2016; 20:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Starkstein SE, Jorge R. Dementia after traumatic brain injury. Int Psychogeriatr 2005; 17 (suppl 1): S93–107. [DOI] [PubMed] [Google Scholar]
- 38.Institute of Medicine. Gulf War and health. Volume 7: long-term consequences of traumatic brain injury. Washington (DC): National Academy Press; 2009. [PubMed] [Google Scholar]
- 39.Whitnall L, McMillan TM, Murray GD, Teasdale GM. Disability in young people and adults after head injury: 5–7 year follow up of a prospective cohort study. J Neurol Neurosurg Psychiatry 2006; 77:640–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McMillan TM, Teasdale GM, Stewart E. Disability in young people and adults after head injury: 12–14 year follow-up of a prospective cohort. J Neurol Neurosurg Psychiatry 2012; 83:1086–91. [DOI] [PubMed] [Google Scholar]
- 41.Pretz CR, Dams-O’Connor K. Longitudinal description of the Glasgow Outcome Scale-Extended for individuals in the Traumatic Brain Injury Model Systems National Database: a National Institute on Disability and Rehabilitation Research Traumatic Brain Injury Model Systems study. Arch Phys Med Rehabil 2013; 94: 2486–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dams-O’Connor K, Pretz C, Billah T, Hammond FM, Harrison-Felix C. Global outcome trajectories after TBI among survivors and nonsurvivors: a National Institute on Disability and Rehabilitation Research Traumatic Brain Injury Model Systems study. J Head Trauma Rehabil 2015; 30: E1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Corrigan JD, Cuthbert JP, Harrison-Felix C, et al. US population estimates of health and social outcomes 5 years after rehabilitation for traumatic brain injury. J Head Trauma Rehabil 2014; 29: E1–9 [DOI] [PubMed] [Google Scholar]
- 44.Harrison-Felix C, Kolakowsky-Hayner SA, Hammond FM, et al. Mortality after surviving traumatic brain injury: risks based on age groups. J Head Trauma Rehabil 2012; 27: E45–56. [DOI] [PubMed] [Google Scholar]
- 45.Baguley IJ, Nott MT, Howle AA, et al. Late mortality after severe traumatic brain injury in New South Wales: a multicentre study. Med J Aust 2012; 196:40–5. [DOI] [PubMed] [Google Scholar]
- 46.Nott MT, Gates TM, Baguley IJ. Age-related trends in late mortality following traumatic brain injury: a multicentre inception cohort study. Australas J Ageing 2015; 34: E1–6. [DOI] [PubMed] [Google Scholar]
- 47.Flaada JT, Leibson CL, Mandrekar JN, et al. Relative risk of mortality after traumatic brain injury: a population-based study of the role of age and injury severity. J Neurotrauma 2007; 24: 435–45. [DOI] [PubMed] [Google Scholar]
- 48.Dams-O’Connor K, Gibbons LE, Bowen JD, McCurry SM, Larson EB, Crane PK. Risk for late-life re-injury, dementia and death among individuals with traumatic brain injury: a population-based study. J Neurol Neurosurg Psychiatry 2013; 84: 177–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thornhill S, Teasdale GM, Murray GD, McEwen J, Roy CW, Penny Kl. Disability in young people and adults one year after head injury: prospective cohort study. BMJ 2000; 320:1631–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dijkers MP, Harrison-Felix C, Marwitz JH. The Traumatic Brain Injury Model Systems: history and contributions to clinical service and research. J Head Trauma Rehabil 2010; 25: 81–91. [DOI] [PubMed] [Google Scholar]
- 51.Marquez de la Plata CD, Hart T, Hammond FM, et al. Impact of age on long-term recovery from traumatic brain injury. Arch Phys Med Rehabil 2008; 89: 896–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gary KW, Ketchum JM, Arango-Lasprilla JC, et al. Differences in employment outcomes 10 years after traumatic brain injury among racial and ethnic minority groups. J Vocat Rehabil 2010; 33: 65–75. [Google Scholar]
- 53.Novack TA, Labbe D, Grote M, et al. Return to driving within 5 years of moderate-severe traumatic brain injury. Brain Inj 2010; 24: 464–71. [DOI] [PubMed] [Google Scholar]
- 54.Hawley CA, Ward AB, Magnay AR, Long J. Outcomes following childhood head injury: a population study. J Neurol Neurosurg Psychiatry 2004; 75: 737–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McKinlay A, Dalrymple-Alford JC, Horwood LJ, Fergusson DM. Long term psychosocial outcomes after mild head injury in early childhood. J Neurol Neurosurg Psychiatry 2002; 73: 281–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sariaslan A, Sharp DJ, D’Onofrio BM, Larsson H, Fazel S. Long-term outcomes associated with traumatic brain injury in childhood and adolescence: a nationwide Swedish cohort study of a wide range of medical and social outcomes. PLoS Med 2016; 13: e1002103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Anderson V, Catroppa C, Morse S, Haritou F, Rosenfeld J. Functional plasticity or vulnerability after early brain injury? Pediatrics 2005; 116: 1374–82. [DOI] [PubMed] [Google Scholar]
- 58.Stein SC, Georgoff P, Meghan S, Mizra K, Sonnad SS. 150 years of treating severe traumatic brain injury: a systematic review of progress in mortality. J Neurotrauma 2010; 27: 1343–53. [DOI] [PubMed] [Google Scholar]
- 59.Ventura T, Harrison-Felix C, Carlson N, et al. Mortality after discharge from acute care hospitalization with traumatic brain injury: a population-based study. Arch Phys Med Rehabil 2010; 91: 20–9. [DOI] [PubMed] [Google Scholar]
- 60.Guskiewicz KM, Marshall SW, Bailes J, et al. Association between recurrent concussion and late-life cognitive impairment in retired professional football players. Neurosurgery 2005; 57: 719–26 [DOI] [PubMed] [Google Scholar]
- 61.Nordström P, Michaëlsson K, Gustafson Y, Nordström A. Traumatic brain injury and young onset dementia: a nationwide cohort study. Ann Neurol 2014; 75: 374–81. [DOI] [PubMed] [Google Scholar]
- 62.Gardner RC, Burke JF, Nettiksimmons J, Kaup A, Barnes DE, Yaffe K. Dementia risk after traumatic brain injury vs nonbrain trauma: the role of age and severity. JAMA Neurol 2014; 71:1490–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gardner RC, Burke JF, Nettiksimmons J, Goldman S, Tanner CM, Yaffe K. Traumatic brain injury in later life increases risk for Parkinson disease. Ann Neurol 2015; 77: 987–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang HK, Lin SH, Sung PS, et al. Population based study on patients with traumatic brain injury suggests increased risk of dementia. J Neurol Neurosurg Psychiatry 2012; 83: 1080–5. [DOI] [PubMed] [Google Scholar]
- 65.Chio A, Benzi G, Dossena M, Mutani R, Mora G. Severely increased risk of amyotrophic lateral sclerosis among Italian professional football players. Brain 2005; 128:472–6. [DOI] [PubMed] [Google Scholar]
- 66.McMillan TM, McSkimming P, Wainman-Lefley J, et al. Long-term health outcomes after exposure to repeated concussion in elite level: rugby union players. J Neurol Neurosurg Psychiatry 2017; 88, 505–11. [DOI] [PubMed] [Google Scholar]
- 67.Fleminger S, Oliver DL, Lovestone S, Rabe-Hesketh S, Giora A. Head injury as a risk factor for Alzheimer’s disease: the evidence 10 years on; a partial replication. J Neurol Neurosurg Psychiatry 2003; 74: 857–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guo Z, Cupples LA, Kurz A, et al. Head injury and the risk of AD in the MIRAGE study. Neurology 2000; 54:1316–23. [DOI] [PubMed] [Google Scholar]
- 69.Dams-O’Connor K, Guetta G, Hahn-Ketter AE, Fedor A. Traumatic brain injury as a risk factor for Alzheimer’s disease: current knowledge and future directions. Neurodegener Dis Manag 2016; 6: 417–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bennett DA, Schneider JA, Arvanitakis Z, Wilson RS. Overview and findings from the religious orders study. Current Alzheimer research 2012; 9: 628–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bennett DA, Schneider JA, Buchman AS, Barnes LL, Boyle PA, Wilson RS. Overview and findings from the rush Memory and Aging Project. Current Alzheimer research 2012; 9: 646–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Corsellis JA, Bruton CJ, Freeman-Browne D. The aftermath of boxing. Psychol Med 1973; 3: 270–303. [DOI] [PubMed] [Google Scholar]
- 73.Giza CC, Prins ML, Hovda DA. It’s not all fun and games: sports, concussions, and neuroscience. Neuron 2017; 94: 1051–5. [DOI] [PubMed] [Google Scholar]
- 74.Hume PA, Theadom A, Lewis GN, et al. A comparison of cognitive function in former Rugby Union players compared with former non-contact-sport players and the impact of concussion history. Sports Med 2017; 47:1209–20. [DOI] [PubMed] [Google Scholar]
- 75.Decq P, Gault N, Blandeau M, et al. Long-term consequences of recurrent sports concussion. Acta Neurochir 2016; 158: 289–300. [DOI] [PubMed] [Google Scholar]
- 76.Chio A, Calvo A, Dossena M, Ghiglione P, Mutani R, Mora G. ALS in Italian professional soccer players: the risk is still present and could be soccer-specific. Amyotroph Lateral Scler 2009; 10: 205–9. [DOI] [PubMed] [Google Scholar]
- 77.Di Virgilio TG, Hunter A, Wilson L, et al. Evidence for acute electrophysiological and cognitive changes following routine soccer heading. EBioMedicine 2016; 13: 66–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McCrory P, Meeuwisse WH, Kutcher JS, Jordan BD, Gardner A. What is the evidence for chronic concussion-related changes in retired athletes: behavioural, pathological and clinical outcomes? Br J Sports Med 2013; 47: 327–30. [DOI] [PubMed] [Google Scholar]
- 79.Levin HS, Diaz-Arrastia RR. Diagnosis, prognosis, and clinical management of mild traumatic brain injury. Lancet Neurol 2015; 14: 506–17. [DOI] [PubMed] [Google Scholar]
- 80.Johnson VE, Stewart W, Smith DH. Axonal pathology in traumatic brain injury. Exp Neurol 2013; 246: 35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Morris NA, Cool J, Merkler AE, Kamel H. Subarachnoid hemorrhage and long-term stroke risk after traumatic brain injury. The Neurohospitalist 2017; 7:122–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Brandenburg W, Hallervorden J. Dementia pugilistica mit anatomischem Befund. Virchows Arch Pathol Anat Physiol Klin Med 1954; 325(6): 680–709. [DOI] [PubMed] [Google Scholar]
- 83.Omalu BI, DeKosky ST, Minster RL, Kamboh MI, Hamilton RL, Wecht CH. Chronic traumatic encephalopathy in a National Football League player. Neurosurgery 2005; 57: 128–33. [DOI] [PubMed] [Google Scholar]
- 84.Ling H, Morris HR, Neal JW, et al. Mixed pathologies including chronic traumatic encephalopathy account for dementia in retired association football (soccer) players. Acta Neuropathol 2017: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Stewart W, McNamara PH, Lawlor B, Hutchinson S, Farrell M. Chronic traumatic encephalopathy: a potential late and under recognized consequence of rugby union? QJM 2016; 109: 11–5. [DOI] [PubMed] [Google Scholar]
- 86.Goldstein LE, Fisher AM, Tagge CA, et al. Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model. Sci Transl Med 2012; 4: 134ra60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Johnson VE, Stewart W, Smith DH. Widespread tau and amyloid-beta pathology many years after a single traumatic brain injury in humans. Brain Pathol 2012; 22: 142–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.McKee AC, Cairns NJ, Dickson DW, et al. The first NINDS/NIBIB consensus meeting to define neuropathological criteria for the diagnosis of chronic traumatic encephalopathy. Acta Neuropathol 2016; 131: 75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ling H, Holton JL, Shaw K, Davey K, Lashley T, Revesz T. Histological evidence of chronic traumatic encephalopathy in a large series of neurodegenerative diseases. Acta Neuropathol 2015; 130: 891–3. [DOI] [PubMed] [Google Scholar]
- 90.Doherty CP, O’Keefe E, Wallace E, et al. Blood-brain barrier dysfunction as a hallmark pathology in chronic traumatic encephalopathy. J Neuropathol Exp Neurol 2016; 75: 656–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Johnson VE, Stewart JE, Begbie FD, Trojanowski JQ, Smith DH, Stewart W. Inflammation and white matter degeneration persist for years after a single traumatic brain injury. Brain 2013; 136: 28–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hay JR, Johnson VE, Young AM, Smith DH, Stewart W. Blood-brain barrier disruption is an early event that may persist for many years after traumatic brain injury in humans. J Neuropathol Exp Neurol 2015; 74: 1147–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dams-O’Connor K, Spielman L, Hammond FM, Sayed N, Culver C, Diaz-Arrastia R. An exploration of clinical dementia phenotypes among individuals with and without traumatic brain injury. NeuroRehabilitation 2013; 32: 199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sayed N, Culver C, Dams-O’Connor K, Hammond F, Diaz-Arrastia R. Clinical phenotype of dementia after traumatic brain injury. J Neurotrauma 2013; 30:1117–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sidaros A, Engberg AW, Sidaros K, et al. Diffusion tensor imaging during recovery from severe traumatic brain injury and relation to clinical outcome: a longitudinal study. Brain 2008; 131: 559–72. [DOI] [PubMed] [Google Scholar]
- 96.Newcombe VF, Correia MM, Ledig C, et al. Dynamic changes in white matter abnormalities correlate with late improvement and deterioration following TBI: a diffusion tensor imaging study. Neurorehabil Neural Repair 2016; 30: 49–62. [DOI] [PubMed] [Google Scholar]
- 97.Haberg AK, Olsen A, Moen KG, et al. White matter microstructure in chronic moderate-to-severe traumatic brain injury: impact of acute-phase injury-related variables and associations with outcome measures. J Neurosci Res 2015; 93: 1109–26. [DOI] [PubMed] [Google Scholar]
- 98.Kraus MF, Susmaras T, Caughlin BP, Walker CJ, Sweeney JA, Little DM. White matter integrity and cognition in chronic traumatic brain injury: a diffusion tensor imaging study. Brain 2007; 130: 2508–19. [DOI] [PubMed] [Google Scholar]
- 99.Hayes JP, Logue MW, Sadeh N, et al. Mild traumatic brain injury is associated with reduced cortical thickness in those at risk for Alzheimer’s disease. Brain 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.MacDonald CL, Barber J, Andre J, et al. 5-Year imaging sequelae of concussive blast injury and relation to early clinical outcome. Neuroimage Clinical 2017; 14: 371–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gatson JW, Stebbins C, Mathews D, et al. Evidence of increased brain amyloid in severe TBI survivors at 1, 12, and 24 months after injury: report of 2 cases. J Neurosurg 2016; 124(6): 1646–53. [DOI] [PubMed] [Google Scholar]
- 102.Scott G, Ramlackhansingh AF, Edison P, et al. Amyloid pathology and axonal injury after brain trauma. Neurology 2016; 86: 821–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yang ST, Hsiao IT, Hsieh CJ, et al. Accumulation of amyloid in cognitive impairment after mild traumatic brain injury. J Neurol Sci 2015; 349: 99–104. [DOI] [PubMed] [Google Scholar]
- 104.Mitsis EM, Riggio S, Kostakoglu L, et al. Tauopathy PET and amyloid PET in the diagnosis of chronic traumatic encephalopathies: studies of a retired NFL player and of a man with FTD and a severe head injury. Transl Psychiatry 2014; 4: e441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hong YT, Veenith T, Dewar D, et al. Amyloid imaging with carbon 11-labeled Pittsburgh compound B for traumatic brain injury. JAMA Neurol 2014; 71: 23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Barrio JR, Small GW, Wong KP, et al. In vivo characterization of chronic traumatic encephalopathy using [F-18]FDDNP PET brain imaging. Proc Natl Acad Sci USA 2015; 112: E2039–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Coughlin JM, Wang Y, Munro CA, et al. Neuroinflammation and brain atrophy in former NFL players: an in vivo multimodal imaging pilot study. Neurobiol Dis 2015; 74: 58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ramlackhansingh AF, Brooks DJ, Greenwood RJ, et al. Inflammation after trauma: microglial activation and traumatic brain injury. Ann Neurol 2011; 70: 374–83. [DOI] [PubMed] [Google Scholar]
- 109.Folkersma H, Boellaard R, Yaqub M, et al. Widespread and prolonged increase in (R)-(11)C-PK11195 binding after traumatic brain injury. J Nucl Med 2011; 52: 1235–9. [DOI] [PubMed] [Google Scholar]
- 110.Jack CR, Knopman DS, Jagust WJ, et al. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurology 2013; 12: 207–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Brown AW, Moessner AM, Mandrekar J, Diehl NN, Leibson CL, Malec JF. A survey of very-long-term outcomes after traumatic brain injury among members of a population-based incident cohort. J Neurotrauma 2011; 28:167–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Maas AI, Menon DK, Steyerberg EW, et al. Collaborative European NeuroTrauma Effectiveness Research in Traumatic Brain Injury (CENTER-TBI): a prospective longitudinal observational study. Neurosurgery 2015; 76: 67–80. [DOI] [PubMed] [Google Scholar]
- 113.Shaw LM, Korecka M, Clark CM, Lee VM, Trojanowski JQ. Biomarkers of neurodegeneration for diagnosis and monitoring therapeutics. Nat Rev Drug Discov 2007; 6: 295–303. [DOI] [PubMed] [Google Scholar]


