Abstract
Background
The increasing demands concerning stroke rehabilitation and in-home exercise promotion grew the need for affordable and accessible assistive systems to promote patients’ compliance in therapy. These assistive systems require quantitative methods to assess patients’ quality of movement and provide feedback on their performance. However, state-of-the-art quantitative assessment approaches require expensive motion-capture devices, which might be a barrier to the development of low-cost systems.
Methods
In this work, we develop a low-cost virtual coach (VC) that requires only a laptop with a webcam to monitor three upper extremity rehabilitation exercises and provide real-time visual and audio feedback on compensatory motion patterns exclusively from image 2D positional data analysis. To assess compensation patterns quantitatively, we propose a Rule-based (RB) and a Neural Network (NN) based approaches. Using the dataset of 15 post-stroke patients, we evaluated these methods with Leave-One-Subject-Out (LOSO) and Leave-One-Exercise-Out (LOEO) cross-validation and the score that measures the accuracy (geometric mean of precision and recall) of a model to assess compensation motions. In addition, we conducted a pilot study with seven volunteers to evaluate system performance and usability.
Results
For exercise 1, the RB approach assessed four compensation patterns with a score of . For exercises 2 and 3, the NN-based approach achieved a score of and , respectively. Concerning the user study, they found that the system is enjoyable (hedonic value of 4.54/5) and relevant (utilitarian value of 4.86/5) for rehabilitation administration. Additionally, volunteers’ enjoyment and interest (Hedonic value perception) were correlated with their perceived VC performance ().
Conclusions
The VC performs analysis on 2D videos from a built-in webcam of a laptop and accurately identifies compensatory movement patterns to provide corrective feedback. In addition, we discuss some findings concerning system performance and usability.
Keywords: Upper extremity stroke rehabilitation therapy, Virtual coach, Compensation assessment, 2D video analysis
Background
Post-stroke patients often suffer from physical impairment [1], with a weakened body side [2, 3], leaving them incapable of accomplishing daily tasks [4, 5]. Rehabilitation poses a crucial strategy to reduce stroke effects, prevent disability and stroke recurrence, demanding a lot of time investment [4–6]. However, the growing number of patients lead therapists to struggle in giving them the necessary attention and rehabilitation administration [5, 7]. Therefore, therapists frequently recommend the repetition of specific exercises [4, 6, 8] as in-home rehabilitation [9] to improve patients’ functional abilities. Nonetheless, patients have difficulty with keeping their motivation and engagement with in-home exercises without professional supervision. Low adherence and incorrect execution of in-home exercises negatively affect their recovery process [2, 10].
While exercising, patients regularly exhibit compensatory motions, using additional or new body joints, to aid in task accomplishment [3, 4, 11, 12]. The most typical compensation behaviors are trunk displacements, rotation, and shoulder elevation [3, 11]. As the persistence of compensatory movements may obstruct real motor function recovery, patients require exercise instructions and feedback to reduce these movement patterns [3, 11, 12].
The escalating demands towards in-home rehabilitation [1, 5] raised the need for quantitative measures to evaluate patients’ motor performance [9, 13]. Quantitative assessment allows tracking patients’ progress and the formulation of standard therapy regimens [9, 14]. Assistive systems with quantitative assessment, as Virtual coaches (VCs), can aid patients to perform in-home exercises [15, 16]. VCs must be adequate, affordable, and accessible, with an interaction model to keep the user engaged [15–17]. Also, they must evaluate patients’ performance to provide therapists with the required data to track their progress and support clinical decisions [9, 13].
Previous works investigated computer-based solutions for in-home upper extremity rehabilitation [17–19]. The proposed systems have complex interaction models which provide visual and audio feedback [17–19]. They utilize marker-based motion capture [19] or Kinect-based [17, 18] systems to assess patients’ exercise performance through motion kinematic analysis. Exercise instructions and feedback—such as error messages and direct performance ratings—are displayed on screens [19] and tablets [17] using graphical interfaces [18].
Researchers identified kinematic variables to characterize impaired motion patterns [9, 13, 14, 20, 21]. They provided automated methods to produce assessment scores highly correlated with Fugl-Meyer Assessment (FMA) scores, a conventional assessment test. Global performance scores provide patients with exercise ratings and therapists with clinically relevant information [9, 13, 20].
In addition, research teams conducted user studies with post-stroke patients to evaluate their systems’ impact on light supervised rehabilitation sessions [17–19]. They pointed out the importance of simple technical setups and reliable performance evaluation for in-home and independent use.
Although prior works [17–19] demonstrate the potential of computer-based systems to improve movement quality, their systems’ technical setups are still very complex for massive in-home use, involving several devices and objects. Quantitative assessment methods are based on 3D pose data kinematic analysis requiring specific motion capture devices for 3D data acquisition as Kinect. Such systems are less affordable and accessible and of complicated use, being less suitable for in-home therapy. With the investigation of novel means to assess patient’s performance from built-in cameras from tablets and laptops, systems would better fit in an affordable and accessible in-home therapy. However, there has been limited investigation on low-cost quantitative assessment methods to provide real-time feedback on compensation patterns.
In this work, we present a low-cost Virtual coach (VC) for stroke rehabilitation and a preliminary study to evaluate its usability. This VC is composed of a single laptop with a built-in webcam to monitor exercises of a user and provide real-time feedback on compensatory movements to assist user engagement in therapy. We present methods to assess quantitatively in real-time motor compensation from rehabilitation exercises through 2D video analysis. To enable real-time assessment, we labeled dataset videos frame-by-frame on compensation patterns. In addition, through an exploratory user study with seven volunteers, we collect some findings on VC usability.
Virtual coach
We describe a Virtual coach (VC) that monitors upper extremity stroke rehabilitation exercises, assessing motor compensation behaviors. From the related work [15, 17–19] and therapists’ advice, we list a set of VC system requirements:
Present an exercise demonstration;
Display a patient’s image while exercising as if looking at a mirror;
Provide clear audio instructions, cues for posture correction, encouragement, and suggest task repetition;
Display visual markers indicating the arm target position and the existence of compensation.
Our VC is a Reflex Agent. It analyses body keypoints and quantitatively assesses patient’s exercises to update the state. Based on the user’s previous state, current state and a specified time interval, the agent selects an action. These actions include:
Display of position markers—the rectangle indicating patient’s valid positioning;
Display of the hand target marker;
Display of compensation indicator markers—shoulder and trunk markers;
Audio speech and respective subtitles—instructions, suggestions, encouragement, and praise.
Tables 1 and 2 describe the states and actions of the VC with their trigger rules, respectively.
Table 1.
Space state of VC state transition
| State space | Description |
|---|---|
| Out (o) | Patient not placed in the correct position |
| In (i) | Patient placed in the correct position |
| Exercise (e) | Exercise and movement trial beginning |
| Normal (n) | Normal movement pattern |
| (tr) | Patient rotates the torso |
| (se) | Patient elevates the shoulder |
| (td) | Patient displaces the torso |
| Target (tg) | Patient reaches the target position |
Table 2.
Virtual coach actions related to state transitions and also permanence in the same state
| State transition no. | Rules | Actions |
|---|---|---|
| 1 | Patient not well-positioned: VC suggests body repositioning; position rectangle in red color. | |
| 2 | Patient moves away from correct position: VC suggests body re-positioning; position rectangle in red color. | |
| 3 | Patient well-positioned: position rectangle in green color; VC gives exercise directions. | |
| 4 | Exercise beginning: VC displays target position marker (green). | |
| 5 | Patients stops moving: VC proposes movement repetition. | |
| 6 | The VC starts evaluating patient’s performance and asks one to reach the target position. | |
| 7 | Patient takes too much time reaching the target position: VC encourages patient to reach the target. | |
| 8 | Patient reaches the target: VC praises the patient; target position marker in blue color. | |
| 9 | Patient describes trunk rotation: VC suggests posture correction; it displays trunk compensation marker (red). | |
| 10 | Patient describes shoulder elevation: VC suggests correction; VC displays shoulder compensation marker (red). | |
| 11 | Patient describes displaces the torso: VC suggests posture correction; VC displays trunk compensation marker (red). |
Compensation quantitative assessment methods
To assess different compensation patterns from 2D videos, we propose an approach composed of the following steps: Body Keypoint Extraction and Selection, Data Normalization, and Classification. We investigate two classification approaches—a Rule-based (RB), our baseline method, and a Neural Network (NN) based approach. As in previous works [9], we present a set of Kinematic Variables, revealing compensation description. Kinematic variables are given as features to the RB classifier. For the NN-based classifier, we provide normalized body keypoints as features. We represent these methods with the mathematical notation specified in Table 3.
Table 3.
Mathematical notation
| Equation | Description |
|---|---|
| denotes the transposed vector of 2D coordinates in the image of a body joint j from a set of joints J (Fig. 1); t denotes the frame number | |
| Vector directed from joint to joint | |
| is the euclidean norm of vector and, alternatively, is the euclidean distance between two selected joints, and | |
| Displacement between two selected joints, and , in the X () and Y () axis | |
| Angle between two vectors, and , defined by two points, to and to |
Body keypoints extraction and selection
To extract the body joints’ 2D pose data, we use OpenPose [22], a software library that provides the 2D position of 25 body keypoints (body skeleton) in the image coordinate system, (Fig. 1). Each keypoint provided is denoted by . Here, denotes the 2D coordinates of a body keypoint j, t is the frame number, and is a confidence score of keypoint detection. Following [9], we selected the following keypoints to describe patients’ movements: Nose, Eye, Neck, MidHip, Hip, Shoulder LeftEye, RightEye, RightHip.
Fig. 1.
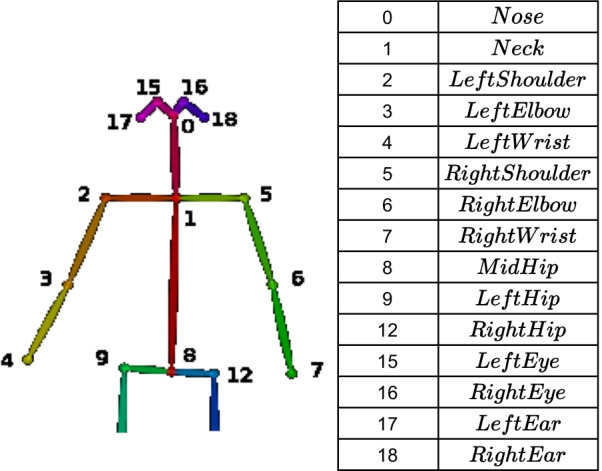
OpenPose Body keypoints
When selecting the most relevant keypoints to describe patients’ movements, we consider the three scenarios (S1, S2, and S3) concerning patient positioning in front of the camera: a patient facing the camera (S1) and with the affected arm facing the camera in a perpendicular (S2) and oblique (S3) positions. For S2 and S3, only the affected side is completely visible in the image.
Data transformation and normalization
In a real-world setting, patients have body parts of different sizes and occupy different locations regarding the camera. Accordingly, we perform keypoint normalization in three steps: transformation, normalization, and mirror. First, we apply rigid body transformation to overcome distinct patient positions. We transform each keypoint from the image coordinate system, , to the body coordinate system, , in which the patient’s joint MidHip () is the origin.
Next, we normalize each keypoint coordinates in to the patient’s spine length, , measured in , to overcome distinct body part dimensions. Finally, for the NN-based approach, to give the healthy side as a reference, we mirror the joints to the X axis, in , positive side. For the RB approach, the mirror step is not applied since each keypoint moves regarding another specified keypoint.
Kinematic variables
To assess compensation patterns from 2D body keypoints, we explore a set of measures for the three scenarios (S1, S2, and S3). From discussion with therapists, we identified four types of compensation: Trunk Forward (TF), Trunk Rotation (TR), Shoulder Elevation (SE), and Other (O) trunk compensation patterns, such as trunk moving backward and trunk tilt. Given the compensation categories, Table 4 summarizes the respective kinematic variables.
Table 4.
Kinematic variables
| Scenario | Variable | Description |
|---|---|---|
| Trunk forward/backward | ||
| S1 | Observed changes in patient’s head position, detect through patient’s head area, () | |
| S2 and S3 | Spine angular and linear displacements | |
| Trunk rotation | ||
| S1 | Simultaneous angular displacements of both shoulders | |
| S2 | Shoulder displacement regarding joint 1 in | |
| S3 | Absolute changes in the observed chest length | |
| Shoulder elevation | ||
| S1 | Shoulder elevation angle | |
| S2 and S3 | Shoulder displacement regarding joint 1 in Y | |
| Trunk tilt | ||
| S1 | Spine angular displacement | |
| S2 and S3 | Absolute changes in patient’s head size | |
Classification approaches
As we intend to identify multiple compensation patterns from video frames, we deal with a Multilabel Classification (MLC) problem. We propose two classification approaches: a Rule-based (RB) and a Neural Network (NN) based. In RB classification models, a set of if-then rules is applied to a collection of features to provide a predicted label [23]. We apply a set of independent rules to each kinematic variable from Table 4 to assess each compensation category, shown in Table 5 for each scenario (S1, S2, and S3). Table 5 details that a rule r (e.g., denotes Shoulder Elevation) predicts a label, , when a feature or set of features, , obey a certain threshold value , which limits the compensation pattern existence. Otherwise, the movement pattern is classified as Normal (). Additionally, multiple labels might be active (i.e., more than one compensation pattern happening simultaneously).
Table 5.
Rules of the RB classification method to determine the different categories of compensation: Trunk Forward (TF), ; Trunk Rotation (TR), ; Shoulder Elevation (SE), ; Other (O), . For normal movements
| Scenario | Rules |
|---|---|
| Trunk forward (TF)/Trunk backward (O) | |
| S1 | |
| S2 and S3 | |
| Trunk rotation (TR) and Shoulder elevation (SE) | |
| S1 | |
| Trunk rotation (TR) | |
| S2 | |
| S3 | |
| Shoulder elevation (SE) | |
| S2 and S3 | |
| Trunk tilt (O) | |
| S1 | |
| S2 and S3 | |
As an RB model has the advantage of easy comprehension [23–25], our VC utilizes this method to determine when a user performs compensation. Additionally, we can change rules’ threshold values (Table 5) adjusting compensation assessment detection sensitivity.
While dealing with an MLC problem, we consider two situations: multiple label occurrence and label imbalance (labels more frequent than others). We apply binarization technique/one-hot encoding to the set of labels assigned to each frame (i.e., a vector of 0 s and 1 s, with 1 encoding the active labels) [25]. Then, we apply One-vs-Rest, training a classifier for each label against all others [26] so that one label prediction does not influence the other. The model generates predictions on each label, which are then combined to produce a multilabel response.
For the NN-based approach, our classifier must be robust enough to not assign a label to a frame denoting compensation and indicate good movement quality (Normal movement patterns, i.e., without compensation). Also, we have a much higher number of samples considered Normal than frames corresponding to each compensation category. Thus, we divide our problem into two problems, a binary and a multilabel. First, a binary classifier (C1) determines compensation existence. Second, a multilabel classifier (C2) concludes the described compensation patterns from the frames with compensation detected by C1. Figure 2 represents our proposed approach.
Fig. 2.
NN-based approach to assess compensation patterns
User interface
To establish an interaction with the user, we developed a web-based UI using Flask framework [27]. The UI is composed of four web pages: Init, Menu for exercise selection (Fig. 3), Demo (exercise demonstration), and Main (Figs. 4 and 5), in which the patient exercises and interacts with the VC. The main processing to track patient’s movements (keypoint extraction and compensation assessment) is handled in a remote server, accessed via WiFi, for faster processing and result extraction.
Fig. 3.
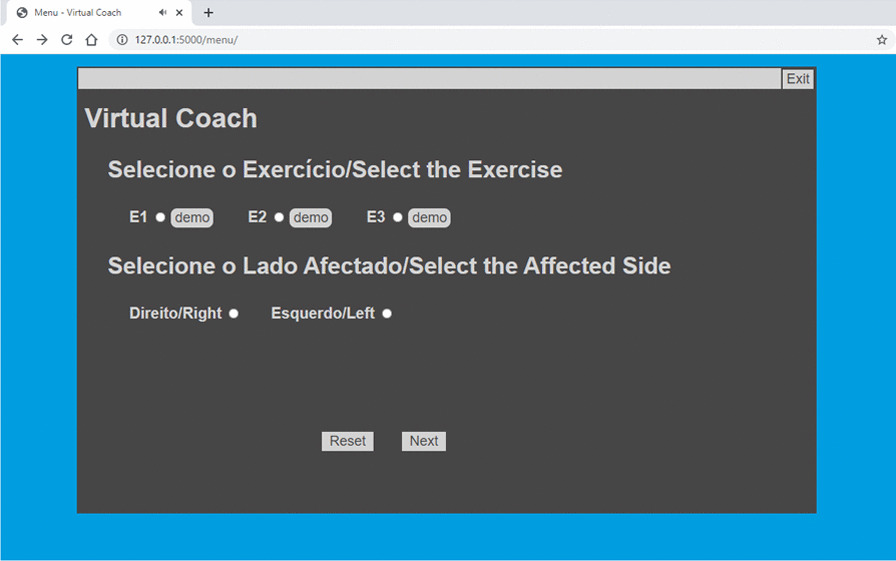
Virtual coach Menu web page
Fig. 4.
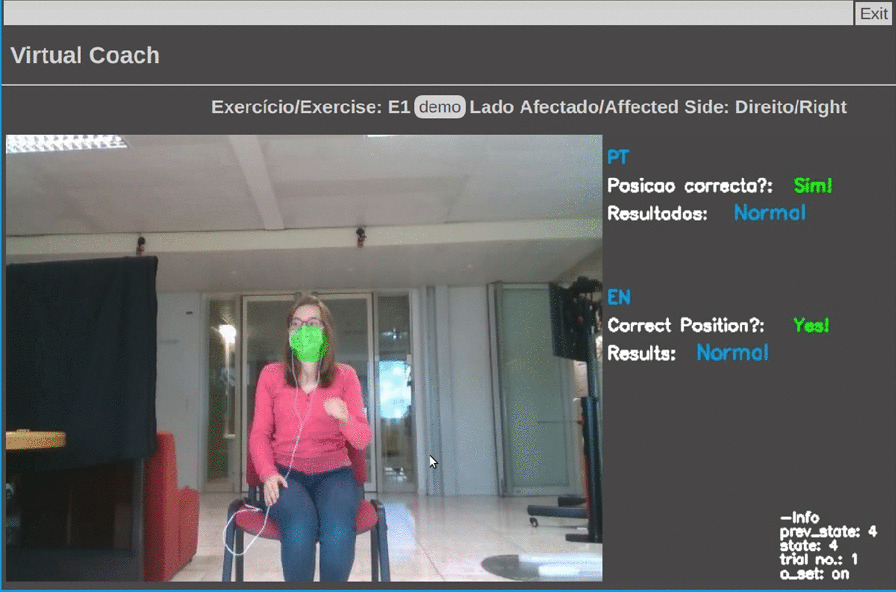
Virtual coach Main web page—display E1 target position
Fig. 5.
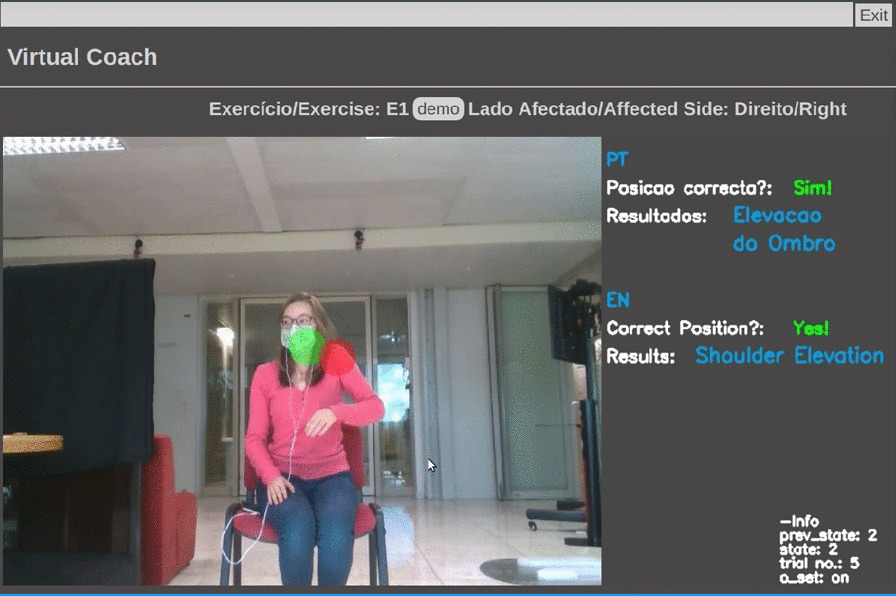
Virtual coach Main web page—shoulder elevation in E1 and display shoulder compensation marker
Once the user chooses an exercise, the user can watch each exercise demonstration. The VC describes three exercises (Table 6) and monitors user compensation behaviors during their execution. First, the VC verifies if the patient is correctly positioned to enable motion capture. Once the user is well placed, the VC gives exercise instructions, displays visual markers identifying the target position of an exercise (Fig. 4), and starts evaluating user movements. When the patient exhibits compensation, the VC suggests posture correction and displays a marker highlighting this behavior (Fig. 5). It also praises the user when one reaches the target position and encourages movement repetition.
Table 6.
The three upper extremity exercises, E1, E2, and E3. Patients’ positioning scenarios and percentage of multi-labeled frames for each exercise
| Upper extremity exercises | Positioning scenario | ||
|---|---|---|---|
| E1 | ‘Bring a Cup to the Mouth’ | S1 | |
| E2 | ‘Switch a Light On’ | S1 | |
| E3 | ‘Move a Cane Forward’ | S2 and S3 | |
Experiments
Compensation quantitative assessment methods
The upper extremity rehabilitation dataset
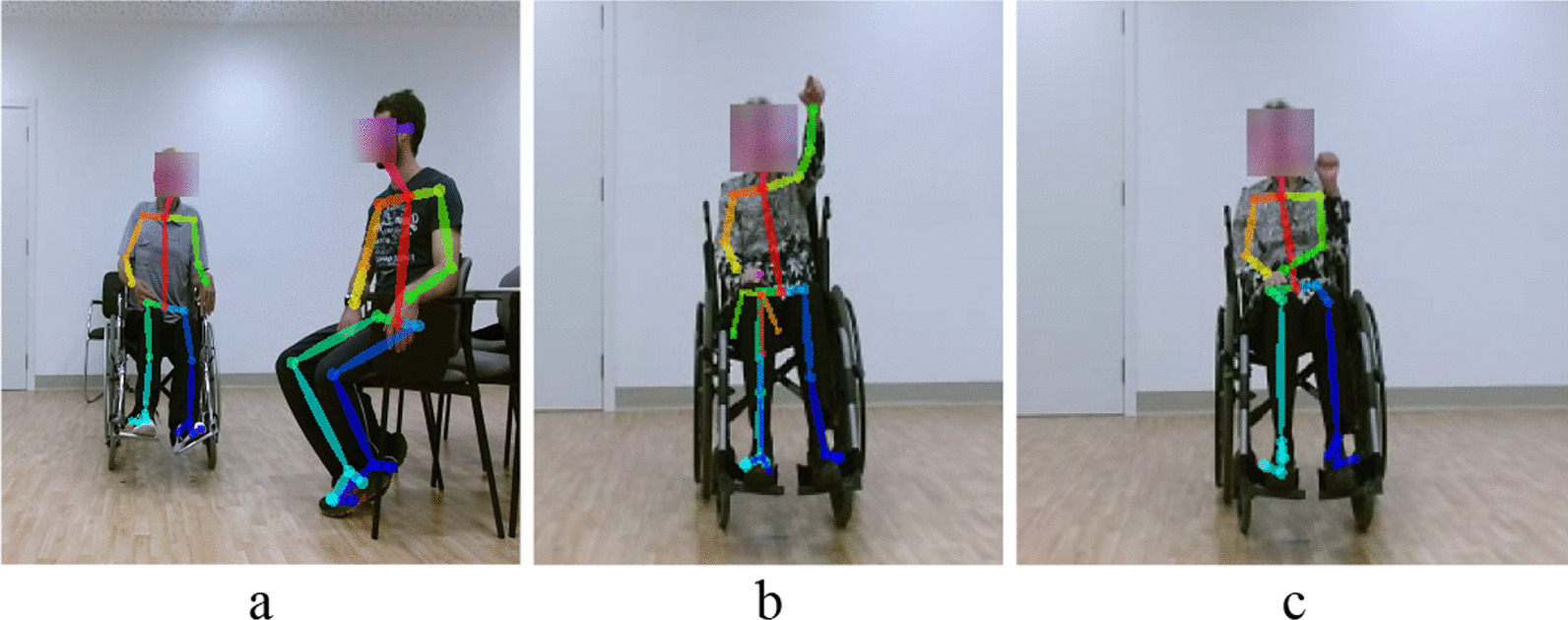
This research uses the dataset from Lee et al. [9] work for the development and validation of proposed compensation assessment methods. It is a dataset of videos of 15 post-stroke patients performing three upper extremity exercises introduced in Table 6. The post-stroke profiles and respective Fugl-Meyer Assessment scores are presented in [9]. In exercise 1 (E1), the patient simulates holding a cup and brings the hand to the mouth as drinking. In exercise 2 (E2), the patient behaves as turning on a light switch. In exercise 3 (E3), the patient moves a cane forward and then back to its initial position.Post-stroke patients with an average age of years old [9] performed an average of 10 movement trials per exercise. Table 6 relates each exercise and positioning scenario (S1, S2, and S3). Figure 6 shows examples from the dataset of E1 and E3 exercises.
Fig. 6.
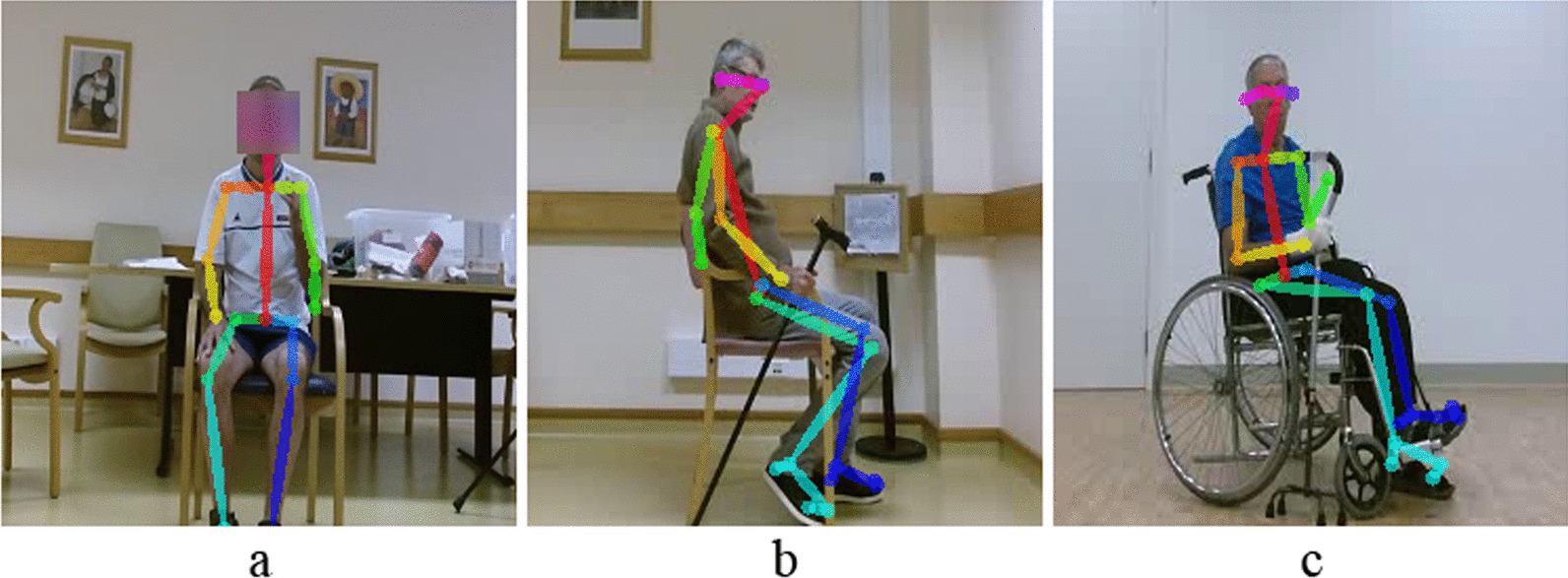
Examples of post-stroke patients performing exercises E1 and E3. E1 corresponds to S1 positioning scenario (a). In E3, patients are positioned according to S2 (b) and S3 (c) scenarios
Data labeling process
Our work explores the following four compensation categories. We specified a set of labels, Y, denoting each one—i.e., for Trunk Forward, ; Trunk Rotation, ; for Shoulder Elevation, ; for Other patterns, ; and for Normal movements, . Label denotes Normal movement patterns, i.e., without compensation. We labeled all frames of each video in agreement with Physical and Occupational therapists’ advice. We assigned one or more labels to each frame according to the visible compensation patterns.
Dataset cleansing
Once we have the body keypoints extracted with OpenPose, it is crucial to consider three distinct situations concerning body skeleton detection: the presence of other people in the image beside the patient, extra skeletons, which do not necessarily belong to a person, and body keypoint misdetection (Fig. 7).
Fig. 7.
OpenPose extra person (a) and incorrect keypoint detection, e.g., extra skeleton (b) and keypoint misdetection (c)
Considering a multi-person setting (e.g., the patient with a caregiver), the patient under evaluation is the closest person to the center of the image, measured by the distance to the image center, .
Extra skeletons often do not have spine joints (Nose, Neck, and MidHip). Therefore, their confidence score, , is zero. Thus, we removed these skeletons.
For keypoint misdetection, we consider a relevant body keypoint (affected side and opposite shoulder) was well detected if it has a confidence score higher than a specified value (). The remaining joints must have . We removed every video frame with body keypoints not meeting these conditions. In the case of frames with mispositioned body keypoints, with a detection confidence score of , we corrected keypoints’ coordinates using the MATLAB imshow function, which enables to access the coordinates of every point in the image.
Multilabel dataset characteristics
Our Multilabel Dataset (MLD) is a set of keypoints, from each video frame (sample), with one or more labels assigned denoting the compensation patterns of post-stroke patients. Before developing our classification models, we explore our MLD characteristics with two metrics: and IRLbl. Metric is the percentage of data samples with only one label active. Inversely, corresponds to the percentage of samples with more than one label assigned. As shown in Table 6, the dataset is almost single labeled, i.e., it has a low percentage of multi-labeled frames (frames with multiple compensation behaviors). Regarding label imbalance, the IRLbl metric shows the ratio between the occurrences of the most frequent label and each label [25]. Table 7 shows that, for the three exercises, label is the most frequent, . For E1 and E2, is poorly represented, , with only one patient exhibiting this compensation pattern. For E3, the less representative label is .
Table 7.
Labels for each compensation and normal movements patterns and IRLbl metric for each one, for each exercise (E1, E2, and E3)
| Compensation/Normal Pattern | Label | IRLbl | ||
|---|---|---|---|---|
| E1 | E2 | E3 | ||
| Trunk forward | – | – | 3.54 | |
| Trunk rotation | 16.23 | 19.25 | – | |
| Shoulder elevation | 2.15 | 3.03 | 15.77 | |
| Other | 4.93 | 5.55 | – | |
| Normal | 1 | 1 | 1 | |
Validation of kinematic variables for a rule-based approach
The validation of kinematic variables is crucial to determine the most suitable threshold values for the RB method and assess its efficiency in assessing compensation. We obtained the thresholds, , through an error and trial methodology by observing the kinematic variables as a starting point. In the following figures, we observe the trajectories of kinematic variables over time. We filtered the keypoints signal (joints’ position over time) with a moving average filter (filtered signal) with a window of five frames as in [9] to reduce noise.
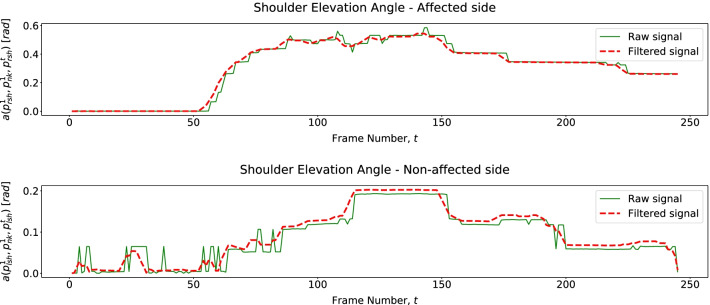
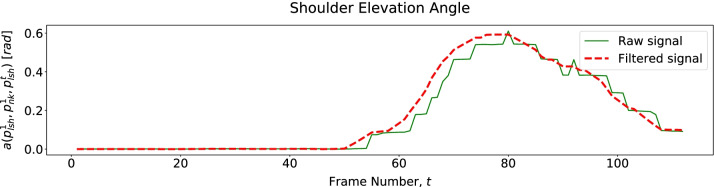
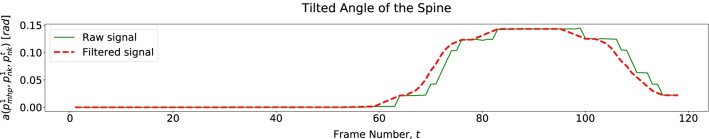
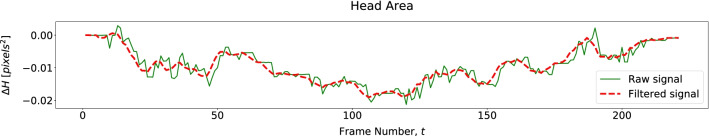
Figure 8 shows we can assess trunk rotation from 2D pose data by tracking both shoulders (affected and unaffected) angular behavior as we hypothesized in Table 4. Trajectories of both shoulders reveal elevation (affected side) and decay (unaffected side) during trunk rotation simultaneously. This shoulder behavior is valid for both exercises E1 and E2. Also, for these exercises, as in previous works [9], we assess shoulder elevation and trunk tilt (Other compensation patterns) through affected shoulder and trunk angular displacement, respectively (Figs. 9 and 10). To evaluate trunk moving backward (Other) from 2D data, we assess variations in patients head area, . Figure 11 shows that when a patient moves backward, decreases as hypothesized.
Fig. 8.
Patient shoulders’ elevation angles over time describing Trunk Rotation for E1
Fig. 9.
Patient affected shoulder elevation angle revealing Shoulder Elevation for E2
Fig. 10.
Patient tilted angle of the torso describing a trunk tilt (Other) for E2
Fig. 11.
Head area over time, revealing trunk moving backward (Other) observed in the dataset for E2
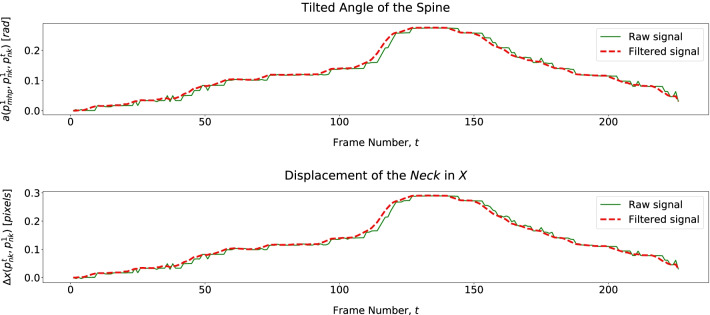
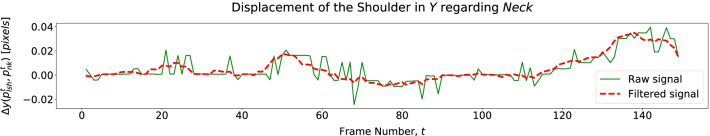
For exercise E3, we assess the torso moving forward through its linear and angular displacements (Table 4) described in Fig. 12. Since we only have 2D pose data, we assess shoulder elevation through its displacement regarding the Neck joint (). Figure 13 shows that a patient elevates the shoulder mainly when moving the cane back to its initial position.
Fig. 12.
Patient tilted and of the spine and neck displacement over time, describing Trunk Forward in E3
Fig. 13.
Patient shoulder displacement over time, describing Shoulder Elevation in E3
Neural network based approach
We explore model architectures (i.e., one to three layers with 16, 24, 32, 48, 64, 96, 128, 192, 256, 384, and 512 hidden units) for a binary classifier (C1) and a multilabel classifier (C2) for the NN-based classification approach and with adaptive learning rate with several values for the initial learning rates (i.e., 0.0001, 0.0005, 0.001, 0.005, 0.01, 0.05, and 0.1). We adopt ‘Adam Optimizer’ with a mini-batch size of 5 and a maximum of 550 iterations. For C1 we apply ‘ReLu’ activation function and for C2 ‘tanh’ activation function. We implement C1 and C2 using the ‘Scikit-learn’ Python library [26].
Evaluation metrics and validation method
We use a set of metrics appropriated to an MLC problem to evaluate our classification models’ performance. We need metrics describing that a multilabel output result might be completely correct, partially correct, or incorrect [25]. We use Precision, Recall, score, and HammingLoss [25, 26]. We calculate the first four according to a micro-averaging strategy that joins the counters of correct and incorrect predictions and then calculates the metric. This way, rare labels are diluted between the most frequent labels [25, 26].
Metric Precision is the percentage of predicted labels truly significant for the sample. Recall expresses the classifier’s ability to detect all positive samples. Score is a weighted harmonic mean Precision and Recall, which measures classification accuracy. HammingLoss reveals the portion of mispredicted labels.
We resort to cross-validation to evaluate our models’ predictive ability and ensure generalization. Cross-validation consists of partitioning the dataset into small subsets. In the validation loop, all the sets except one are used for training, and the remaining set is used for validation [26, 28]. In the end, the performance measure determined in each loop is averaged.
First, we apply Leave-One-Subject-Out (LOSO) cross-validation since all patients in a post-stroke status have their specific motion pattern, Validating the models on each patient compensation pattern enables a better understanding of their classification performance and generalization capacity. Additionally, to verify model generalization to different exercises, we apply Leave-One-Exercise-Out (LOEO) cross-validation to the NN-based approach with exercises E1 and E2, in which patients have similar positioning during data collection.
User study on the virtual coach
To achieve a preliminary evaluation of the VC usability, we performed experiments with a group of volunteers. We aim to investigate users’ perceptions of the VC on four dimensions: its Hedonic (H) value (i.e., users’ motivation and enjoyment while exercising and interacting with the VC), Utilitarian (U) value (i.e., users’ perception of the gains of exercising autonomously with the VC for post-stroke patients), System’s Performance (SP) (i.e., users’ perception of the VC’s accuracy on detecting compensation and correct feedback), and the Use Intention (IU) of VC users. In this study, we explore the following hypotheses:
Hedonic value perceptions are affected by the perception of Virtual Coach performance on monitoring exercise performance, detecting compensation, and by its interactive features;
- There is a disparity on the VC perception between:
- Post-stroke volunteer and non-stroke affected volunteers;
- Older adults and younger adults mainly concerning VC U value.
Data collection and storage is in agreement with the General Data Protection Regulation (GDPR). To ensure these conditions, the Instituto Superior Técnico Ethics Committee reviewed and approved our experimental protocol.
Volunteers
We recruited seven volunteers to exercise their limbs with our system. When selecting the participants, we aimed to gather a diverse group concerning age, sex, and experience with the stroke thematic. Volunteers signed an Informed Consent authorizing the recording of their image necessary to the normal system operation. Table 8 presents the volunteers’ profiles and general information. The post-stroke volunteer has difficulty performing specific tasks (e.g., writing). Yet, he is fully recovered and does not perform compensatory movements.
Table 8.
Profiles of the volunteers. General information: a Knows what (a) stroke is (b) Had a stroke (c) Some relative or close friend had a stroke (d) Followed the rehabilitation process closely
| VID | Age | Sex | ND/A side | (a) | (b) | (c) | (d) |
|---|---|---|---|---|---|---|---|
| V01 | 25–34 | M | Left | Y | Y | Y | Y |
| V02 | 55–64 | F | Left | Y | N | Y | Y |
| V03 | 65–74 | F | Left | Y | N | Y | Y |
| V04 | 65–74 | M | Left | Y | N | Y | Y |
| V05 | 25–34 | M | Left | Y | N | Y | N |
| V06 | 55–64 | M | Left | Y | N | Y | N |
| V07 | 25–34 | F | Left | Y | N | N | N |
VID-volunteer ID, ND-non-dominant, A-affected, F-female, M-male, Y-yes, N-no
Experimental setup
Motivated to provide an affordable and accessible solution with a simple technical infrastructure, we only use a laptop with 6GB RAM and i5-4210U 2.40 GHz 2 Cores CPU with a built-in webcam in this experiment. We use the RB classification algorithm to assess compensation, which enables easy result interpretation and the adjustment of rules’ threshold values if necessary. The sessions took place in a domestic environment spacious enough to assure the placement of the laptop from the volunteer had a distance of to capture the participant’s relevant body joints.
Experimental procedure
At the beginning of a session, the researcher placed the laptop on a table or other support, giving the volunteer the possibility to be in front of the system exercising with enough space. She introduced the study, the entire procedure, and the functionalities of the UI. The volunteers were asked to perform the three exercises (E1, E2, and E3) with the arm from their affected side due to stroke or non-dominant body side. The researcher instructed volunteers to simulate the different compensation strategies while exercising. Volunteers repeated the movements at least five times. During the exercise, volunteers followed the VC instructions, and the researcher intervened when necessary. In the end, each participant answered a questionnaire giving their feedback about the VC. The session did not exceed 30 min.
Questionnaires
We collected both quantitative and qualitative responses from study participants evaluating the VC on each dimension (H, U, SP, and IU). We collect responses on volunteers’ enjoyment, motivation, and interest during the exercise session with the VC (H value). The VC’s benefits to health, aid on physical condition improvement, utility in autonomous exercises, and as a support to diminish struggles concerning rehabilitation administration (U value). Volunteers answered questions concerning their willingness to use the system (IU) and system effectiveness and reliability (SP).
The volunteers responded to each question on a 5-point Likert scale (quantitative)—from ‘1 = Strongly Disagree’ to ‘5 = Strongly Agree’. In addition, we asked a follow-up question to gather more information about their responses.
Results
Compensation assessment results
Table 9 presents the evaluation metrics for the two proposed compensation assessment approaches,RB and NN-based, over three exercises (E1, E2, and E3). We describe the hyperparameters for the NN-based approach in Table 10. For E1, the RB classifier performed better than the NN-based classifier with an score of . For E2 and E3, the NN-based classifier had a better performance than the RB approach with and , respectively. Later, we discuss the differences in performance observed for the two approaches. In addition, LOEO cross-validation for the NN-based approach with E1 and E2, the classifier detects compensation with an score of .
Table 9.
Average results and standard deviation for the Rule-based (RB) and Neural Network (NN) methods for each exercise (E1, E2, and E3) with LOSO and LOES cross-validation
| Precision | Recall | Hamming loss | ||
|---|---|---|---|---|
| Rule-based (RB) Approach | ||||
| Leave-One-Subject-Out (LOSO) cross-validation | ||||
| 0.75 ± 0.14 | 0.78 ± 0.12 | 0.76 ± 0.12 | 0.11 ± 0.07 | |
| 0.13 ± 0.11 | ||||
| Neural Network (NN) based Approach | ||||
| Leave-One-Subject-Out (LOSO) cross-validation | ||||
| 0.73 ± 0.21 | 0.73 ± 0.19 | 0.73 ± 0.19 | 0.15 ± 0.11 | |
| 0.80 ± 0.22 | 0.80 ± 0.21 | 0.80 ± 0.22 | ||
| Leave-One-Exercise-Out (LOEO) cross-validation with E1 and E2 | ||||
The results in bold correspond to the best classifiers' performance for the different metrics for each exercise
F1 score is a measure of accuracy
Table 10.
NN based approach classifiers’ hyperparameters
| #layers | #units/layer | Learning Rate | |
|---|---|---|---|
| C1 | |||
| E1 | 1 | 16 | 0.001 |
| E2 | 2 | 16 | 0.001 |
| E3 | 1 | 96 | 0.01 |
| C2 | |||
| E1 | 1 | 64 | 0.001 |
| E2 | 1 | 16 | 0.01 |
| E3 | 1 | 16 | 0.001 |
Virtual coach validation results
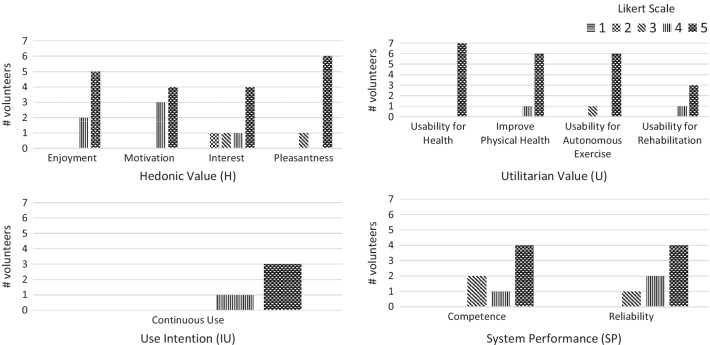
Figure 14 shows volunteers’ quantitative answers to the questionnaires on the usability and performance of the VC. Table 11 presents a set of descriptive statistics summarizing quantitative results and Pearson Correlation between dimensions.
Fig. 14.
Perceptions of the Virtual coach on four dimensions: Hedonic Value, Utilitarian Value, Use Intention, and System Performance. Only volunteers that followed a rehabilitation process previously answered use intention and usability for rehabilitation items
Table 11.
Descriptive statistics and Pearson correlation
| H | U | IU | SP | |
|---|---|---|---|---|
| H | 1 | 0.03 | 1.00 | 0.53 |
| U | 0.03 | 1 | 1.00 | 0.75 |
| IU | 1.00 | 1.000 | 1 | 1.00 |
| SP | 0.53 | 0.75 | 1.00 | 1 |
| Minimum | 3.75 | 4.00 | 4.00 | 3.00 |
| Maximum | 5.00 | 5.00 | 5.00 | 5.00 |
| Mean | 4.54 | 4.86 | 4.75 | 4.36 |
| SD | 0.51 | 0.38 | 0.50 | 0.80 |
SD-standard deviation
From Fig. 14, concerning Hedonic (H) value (), most volunteers enjoyed exercising with the VC, felt motivated and interested in the exercises, and found the established interaction pleasant. The most appreciated and motivating features of the system were the “posture corrections” (V01) and the “User Interface” (V05).
Regarding the Utilitarian (U) value, Fig. 14 shows volunteers find the system valuable for post-stroke rehabilitation. Volunteers reported the system ():
May be useful for autonomy in exercise practice. (V02)
It can help to motivate the correct exercise performance. (V03)
Concerning Use intention (IU) (), volunteers, in the case of need, revealed interest in using the system (Figure 14). A volunteer mentioned that he would use the system to “practice more” (V01), enhancing recovery.
Volunteers perceived that the system performs properly and fulfills its purpose. They expressed system’s evaluation of their motor performance was trustworthy. A mean score of 4.36 on the System’s Performance (SP) supports these affirmations. Volunteers revealed:
System proposed corrections matched the movement. (V05)
Reliable, it asks to repeat the exercise and to be perfected. (V02)
However, volunteers provided comments on aspects that need to be improved, such as the VC response time (V06) and more flexibility regarding users’ initial position:
The square that detected my body could be a little bigger because, when moving, the body could leave the square and it was necessary to repeat the exercise. (V07)
Virtual coach performance and Hedonic value
We compute the Pearson Correlation coefficient () to analyze the correlation between each dimension (H, U, SP, and IU) based on questionnaires quantitative answers (Table 11).
Table 11 shows a correlation between H and SP with a coefficient of , revealing that these dimensions are moderately correlated [29]. If the mean value of the perceived SP increases, it positively influences the perceived H. A volunteer that mentioned “the system has a slow response” also mentioned this aspect when he was asked for the most/least pleasant or interesting system features:
Slow responsive system and interaction could be more stimulating for the participant. (V06)
Stroke survivor vs. other volunteers
We compared the post-stroke survivor’s perceptions with other volunteers’ mean perceptions, shown in Table 12. Concerning H perception, the stroke survivor and other volunteers equally enjoyed the training and interact with the system (). However, stroke survivor reported a lower mean score for U () and SP () and showing a less IU ():
Certain corrections might be tricky to apply alone. (V01)
Table 12.
Stroke survivor vs. other volunteers mean perceptions
| H | U | IU | SP | |
|---|---|---|---|---|
| Stroke survivor | 4.50 | 4.0 | 4.0 | 3.0 |
| Other volunteers |
Age and utilitarian value
Additionally, we analyze how volunteers from different age groups perceive VC utilitarian value U. Table 13 shows the mean perception of two age groups: older adults, volunteers over 54 years old (), and the remaining volunteers we consider as younger adults (). Older adults found the system more useful (Table 13). However, despite the mean score difference between groups is 0.3333, this difference is not statistically significant.
Table 13.
Older and younger adults mean perceptions
| Age | n | H | U | IU | SP |
|---|---|---|---|---|---|
| 25–34 | 3 | ||||
| 55–64 | 2 | ||||
| 65–74 | 2 | ||||
| 3 | 4.67 ± 0.58 | ||||
| 4 | 5.0 ± 0.0 |
The results in bold correspond to the best classifiers' performance for the different metrics for each exercise
Discussion
Compensation assessment methods analysis
Table 9 describes the results of RB and NN-based proposed classification approaches. From LOSO cross-validation for each exercise (E1, E2, and E3), we found our methods achieved comparable performance (72–79%) to the models with 3D pose data (74–82%) [20], giving evidence that assessing compensation patterns from 2D pose data is feasible. For E1, the RB approach performs better than NN-based, and for E2 and E3, the NN-based approach presents better results than RB. An evident difference between the datasets of these exercises is their percentage of multi-labeled samples, . E1 has of multi-labeled samples. E2 and E3 have and , respectively, of samples with more than one label. This fact implies that the RB method handles multi-labeled samples better than NN-based. On the other hand, the NN-based approach is more efficient than RB with binary problems. For E3, the NN-based approach performs better. However, it has a higher value of HammingLoss, meaning that this approach provides a higher number of mispredictions.
Additionally, standard deviation values (Table 9) are related to poor representation of some compensation patterns in the dataset. The RB and the NN-based classifiers reveal an average standard deviation of and in , respectively, for the three exercises. These standard deviation values, associated with the adopted validation method, LOSO, indicate that our classifiers detect with higher accuracy some compensation patterns than others. The NN-based approach, which involves learning, has more difficulty identifying rarer compensation patterns in the dataset. This approach would benefit from more data with a homogenous representation of the different compensation patterns. However, we consider our results for the score comparable to the agreement level of annotators (i.e., for E1, for E2, and for E3) [20]. Personalized assessment techniques can improve performance evaluation from patient to patient, as in [20]. These techniques promote the generation of personalized quality of movement evaluation and corrective feedback in opposition to pre-defined rules and threshold values, which might not fit properly every patient.
Results from LOEO cross-validation for the NN-based approach ( score of ) show us that the models can generalize to other exercises as long as the setup for data collection is the same (i.e., patients’ position in front of the camera).
Virtual coach experiment analysis
From the exploratory experiment with a group of volunteers, we collected a set of findings on VC usability and performance. Quantitative scores on each dimension perceptions (Fig. 14) and volunteers’ quotes show the low-cost VC has the potential to automatically monitor participants’ exercises and provide valuable feedback on compensatory motions. In general, volunteers enjoyed the exercise session with the VC, found it beneficial, and its movement analysis trustworthy.
By analyzing the impact of System Performance on volunteers perception of Hedonic Value (H1), we found some points requiring improvement: lack of flexibility concerning volunteers initial position; the slow response of the system to users’ movements; and motion pattern mispredictions.
Volunteer V07 (Table 8) referred system’s lack of flexibility with her initial position as an unappreciated feature, negatively affecting her interest and enjoyment in the activity. In some sessions, due to space conditions, we were unable to assure subjects correct positioning to place one’s body inside the rectangle. For this reason, the system assumed the subject was incorrectly positioned to perform movement assessment.
Volunteer V06 (Table 8) pointed out the system’s slow response to his movements. In some cases, the system had a slower response when providing volunteers with feedback on their movements due to internet connection conditions since main processing steps occur in a remote server accessed through WiFi.
Additionally, during the study, we detected unexpected compensation mispredictions (RB approach). In some cases, when a user tilted the torso, the VC assumed the user was performing shoulder elevation since it detected shoulder angular displacement. This VC behavior suggests a review in rule implementation and an improvement of the RB approach to avoid the detection of shoulder compensation while a trunk compensatory movement occurs.
When comparing stroke survivor and other volunteers’ perceptions (H2.a), results reveal stroke survivor was more critical with the system than other volunteers (Table 12). The stroke survivor commented on compensation detection sensitivity. In his opinion, the VC should not be too sensitive, i.e., give feedback on compensation immediately when a patient is just beginning to perform a compensatory movement, thus very pronounced yet. It should provide patients with time and opportunity to perform the proposed movements and improve themselves without being constantly and immediately corrected.
In our population sample, older adults find the VC more useful (U) than younger adults (H2.b). This difference is expected since stroke is more prevalent among older adults and the elderly. However, the mean difference between both groups in U perception difference is only 0.3333, and the independent sample t-test revealed it to be insignificant. It is important to note that both groups have small and unequal number of subjects (young volunteers ; older volunteers ), n, a condition that can lead to an untrustworthy . To collect more significant results, we would need to conduct a user study with a larger group of volunteers and a homogeneous distribution of age categories.
Limitations and future work
To continue the investigation of motor compensation detection methods from 2D positional data, we aim to explore other assessment approaches and machine learning models. Our RB approach could be improved to avoid the detection of compensation patterns involving shoulder angular/linear displacement when trunk compensation occurs. Priority could be given to trunk displacements over shoulder elevation patterns to overcome some misdetections. Additionally, we intend to expand VC’s quality of movement assessment to other performance components, such as Range-of-Motion and Smoothness [9]. Further, we aim to achieve the generation of a performance score with clinical relevance, as in [9]. It would provide patients with exercise ratings promoting motivation and give therapists significant information to track patients’ progress.
Another relevant improvement of our VC is its response time to users’ movements (e.g., track arm movements and detect compensation), which is directly related to the connection via WiFi to the remote server in which main processing steps occur. Previous works [30] propose an architecture for a cognitive wearable assistive system that resorts to remote processing, having achieved faster response time. Additionally, to achieve faster processing, we might benefit from available frameworks, as TensorFlow Lite, and hardware accelerators for AI computing, such as Google CORAL and Inter Neural Compute Stick 2.
We could give therapists the possibility to adjust the threshold values that control the RB method rules [31], managing compensation detection sensitivity through the UI. It could enable exercise level adaptation based on compensation detection sensitivity (more sensitive, more challenging).
Additionally, once we have improved the VC according to the findings achieved in this study, the VC should be evaluated with post-stroke patients under a rehabilitation process and therapists.
Conclusions
This work contributes to the research of assistive systems for in-home rehabilitation. With the dataset of 15 post-stroke patients, we demonstrate that the proposed methods accurately assess motor compensation from 2D positional data. The proposed low-cost motion analysis approach using 2D videos can achieve comparable performance with compensatory motion assessment approaches using 3D pose data [20]. In addition, during the preliminary user study with a group of volunteers, as desired the VC provides helpful visual and audio feedback and accurately tracks users’ movements. Additionally, we identified some points for improvement and collected evidence towards the feasibility of the low-cost virtual coach (VC) for stroke rehabilitation.
Acknowledgements
The authors would like to thank the therapists from NeuroSer rehabilitation center, Mariana Mateus and Carolina Matos, for their advice and for receiving us at the center. We would like to thank our colleagues Joäo Avelino and Heitor Cardoso for their valuable help in data collection and analysis. In addition, the authors would like to thank the volunteers for having accepted the invitation to participate in this study.
Abbreviations
- 2D
Two-dimensional
- 3D
Three-dimensional
- FMA
Fugl-Meyer assessment
- WMFT
Wolf Motor function test
- RB
Rule-based
- MLC
Multilabel classification
- MLD
Multilabel dataset
- NN
Neural network
- UI
User interface
- VC
Virtual coach
Author contributions
All authors read and approved the final manuscript. ARC developed the proposed methods, analyzed the results and the user study acquired data, and drafted the manuscript. MHL critically reviewed the methods and the results, revised the manuscript, and provided valuable input to improve it. AB provided advice on research direction, evaluated study results, and critically revised the manuscript. All authors revised and approved the submitted manuscript.
Funding
This work was supported by FCT with the LARSyS - FCT Project UIDB/50009/2020 and project IntelligentCare—Intelligent Multimorbidity Management System (Reference LISBOA-01-0247-FEDER-045948), co-financed by the ERDF—European Regional Development Fund through the Lisbon Portugal Regional Operational Program—LISBOA 2020 and by the Portuguese Foundation for Science and Technology—FCT under CMU Portugal.
Availability of data and materials
The datasets and additional data gathered and/or analyzed in this study are not publicly available due to population vulnerability (i.e., patients under a post-stroke rehabilitation process) and the personal nature of video recordings captured in a hospital and domestic environments.
Declarations
Ethics approval and consent to participate
This study was approved by the Instituto Superior Técnico Ethics Committee and all user study participants signed an informed consent.
Consent for publication
Not applicable. Informed consent was obtained for data collection, analysis, and publication from all user study participants.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ana Rita Cóias, Email: ana.coias@tecnico.ulisboa.pt.
Min Hun Lee, Email: minhunl@cs.cmu.edu.
Alexandre Bernardino, Email: alex@isr.tecnico.ulisboa.pt.
References
- 1.Meadmore KL, Hallewell E, Freeman C, Hughes AM. Factors affecting rehabilitation and use of upper limb after stroke: views from healthcare professionals and stroke survivors. Top Stroke Rehabil. 2019;26(2):94–100. doi: 10.1080/10749357.2018.1544845. [DOI] [PubMed] [Google Scholar]
- 2.Billinger SA, Arena R, Bernhardt J, Eng JJ, Franklin BA, Johnson CM, Mackay-Lyons M, Macko RF, Mead GE, Roth EJ, Shaughnessy M, Tang A. Physical activity and exercise recommendations for stroke survivors: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(8):2532–2553. doi: 10.1161/STR.0000000000000022. [DOI] [PubMed] [Google Scholar]
- 3.Levin MF, Kleim JA, Wolf SL. What do motor “recovery” and “compensationg” mean in patients following stroke? Neurorehabilitation Neural Repair. 2009;23(4):313–9. doi: 10.1177/1545968308328727. [DOI] [PubMed] [Google Scholar]
- 4.Semenko B, Thalman L, Ewert E, Delorme R, Hui S, Flett H, Lavoie N. An evidence based occupational therapy toolkit for assessment and treatment of the upper extremity post stroke 2015.
- 5.Damush TM, Plue L, Bakas T, Schmid A, Williams LS. Barriers and facilitators to exercise among stroke survivors. Rehabil Nurs. 2007;32(6):253–62. doi: 10.1002/j.2048-7940.2007.tb00183.x. [DOI] [PubMed] [Google Scholar]
- 6.Rensink M, Schuurmans M, Lindeman E, Hafsteinsdóttir T. Task-oriented training in rehabilitation after stroke. J Adv Nurs. 2009;65(4):737–754. doi: 10.1111/j.1365-2648.2008.04925.x. [DOI] [PubMed] [Google Scholar]
- 7.Pollock AS, Legg L, Langhorne P, Sellars C. Barriers to achieving evidence-based stroke rehabilitation. Clin Rehabil. 2000;14(6):611–617. doi: 10.1191/0269215500cr369oa. [DOI] [PubMed] [Google Scholar]
- 8.Serrada I, McDonnell MN, Hillier SL. What is current practice for upper limb rehabilitation in the acute hospital setting following stroke? A systematic review. NeuroRehabilitation. 2016;39(3):431–438. doi: 10.3233/NRE-161374. [DOI] [PubMed] [Google Scholar]
- 9.Lee MH, Siewiorek DP, Smailagic A, Bernardino A, Badia SBi. Learning to assess the quality of stroke rehabilitation exercises. In: Proceedings of the 24th International Conference on Intelligent User Interfaces, 2019. pp. 218–228.
- 10.Maclean N, Pound P, Wolfe C, Rudd A. Qualitative analysis of stroke patients’ motivation for rehabilitation. BMJ. 2000;321(7268):1051–1054. doi: 10.1136/bmj.321.7268.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levin MF, Liebermann DG, Parmet Y, Berman S. Compensatory versus noncompensatory shoulder movements used for reaching in stroke. Neurorehabil Neural Repair. 2016;30(7):635–646. doi: 10.1177/1545968315613863. [DOI] [PubMed] [Google Scholar]
- 12.Alankus G, Kelleher C. Reducing compensatory motions in motion-based video games for stroke rehabilitation. Human-Computer Interaction. 2015;30(3–4):232–262. doi: 10.1080/07370024.2014.985826. [DOI] [Google Scholar]
- 13.Olesh EV, Yakovenko S, Gritsenko V. Automated assessment of upper extremity movement impairment due to stroke. PLoS ONE. 2014;9(8):e104487. doi: 10.1371/journal.pone.0104487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murphy MA, Willén C, Sunnerhagen KS. Kinematic variables quantifying upper-extremity performance after stroke during reaching and drinking from a glass. Neurorehabil Neural Repair. 2011;25(1):71–80. doi: 10.1177/1545968310370748. [DOI] [PubMed] [Google Scholar]
- 15.Siewiorek DP, Smailagic A, Dey A. Architecture and applications of virtual coaches. Proc IEEE. 2012;100(8):2472–2488. doi: 10.1109/JPROC.2012.2200560. [DOI] [Google Scholar]
- 16.Gimigliano F, Negrini S. The world health organization "rehabilitation 2030: a call for action". Eur J Phys Rehabil Med. 2017;53(2):155–168. doi: 10.23736/S1973-9087.17.04746-3. [DOI] [PubMed] [Google Scholar]
- 17.Rikakis T, Huang JB, Kelliher A, Kitani K, Wolf SL, Choi J, Zilevu S. Semi-automated home-based therapy for the upper extremity of stroke survivors. ACM International Conference Proceeding Series, 2018;249–256.
- 18.Brokaw EB, Eckel E, Brewer BR. Usability evaluation of a kinematics focused Kinect therapy program for individuals with stroke. Technol Health Care. 2015;23(2):143–151. doi: 10.3233/THC-140880. [DOI] [PubMed] [Google Scholar]
- 19.Duff M, Chen Y, Cheng L, Liu S-M, Blake P, Wolf SL, Rikakis T. Adaptive mixed reality rehabilitation improves quality of reaching movements more than traditional reaching therapy following stroke. Neurorehabil Neural Repair. 2012;27(4):306–315. doi: 10.1177/1545968312465195. [DOI] [PubMed] [Google Scholar]
- 20.Lee MH, Siewiorek DP, Smailagic A, Bernardino A, Badia SB. Towards personalized interaction and corrective feedback of a socially assistive robot for post-stroke rehabilitation therapy. In: 2020 29th IEEE International Conference on Robot and Human Interactive Communication (RO-MAN).2020; pp. 1366–1373 . IEEE.
- 21.Ozturk A, Tartar A, Ersoz Huseyinsinoglu B, Ertas AH. A clinically feasible kinematic assessment method of upper extremity motor function impairment after stroke. Measurement. 2016;80:207–216. doi: 10.1016/j.measurement.2015.11.026. [DOI] [Google Scholar]
- 22.Cao Z, Hidalgo Martinez G, Simon T, Wei S, Sheikh YA. Openpose: realtime multi-person 2d pose estimation using part affinity fields. IEEE Transactions on Pattern Analysis and Machine Intelligence. 2019. [DOI] [PubMed]
- 23.Lee MH, Siewiorek DP, Smailagic A, Bernardino A, Bermúdez i Badia S. An exploratory study on techniques for quantitative assessment of stroke rehabilitation exercises. In: Proceedings of the 28th ACM Conference on User Modeling, Adaptation and Personalization. 2020; pp. 303–307.
- 24.Biran O, Cotton C. Explanation and justification in machine learning: a survey. IJCAI-17 workshop on explainable AI (XAI). 2017; 8(1).
- 25.Herrera F, Charte F, Rivera AJ, Del Jesus MJ. Multilabel classification. In: Multilabel Classification.2016; pp. 17–31. Springer. [DOI] [PubMed]
- 26.Pedregosa F, Varoquaux G, Gramfort A, Michel V, Thirion B, Grisel O, Blondel M, Prettenhofer P, Weiss R, Dubourg V, Vanderplas J, Passos A, Cournapeau D, Brucher M, Perrot M, Duchesnay E. Scikit-learn: machine learning in Python. J Mach Learn Res. 2011;12:2825–2830. [Google Scholar]
- 27.Pallets: Flask Documentation. Accessed 4-Aug.-2020. https://flask.palletsprojects.com/en/1.1.x/.
- 28.Goodfellow I, Bengio Y, Courville A. Machine learning basics. Deep Learning. 2016;1:98–164. [Google Scholar]
- 29.Schober P, Boer C, Schwarte LA. Correlation coefficients: appropriate use and interpretation. Anesth Analg. 2018;126(5):1763–1768. doi: 10.1213/ANE.0000000000002864. [DOI] [PubMed] [Google Scholar]
- 30.Ha K, Chen Z, Hu W, Richter W, Pillai P, Satyanarayanan M. Towards wearable cognitive assistance. In: Proceedings of the 12th Annual International Conference on Mobile Systems, Applications, and Services. 2014; pp. 68–81.
- 31.Lee MH, Siewiorek DP, Smailagic A, Bernardino A, Bermúdez i Badia S. A human-ai collaborative approach for clinical decision making on rehabilitation assessment. In: Proceedings of the 2021 CHI Conference on Human Factors in Computing Systems.2021; pp. 1–14.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets and additional data gathered and/or analyzed in this study are not publicly available due to population vulnerability (i.e., patients under a post-stroke rehabilitation process) and the personal nature of video recordings captured in a hospital and domestic environments.