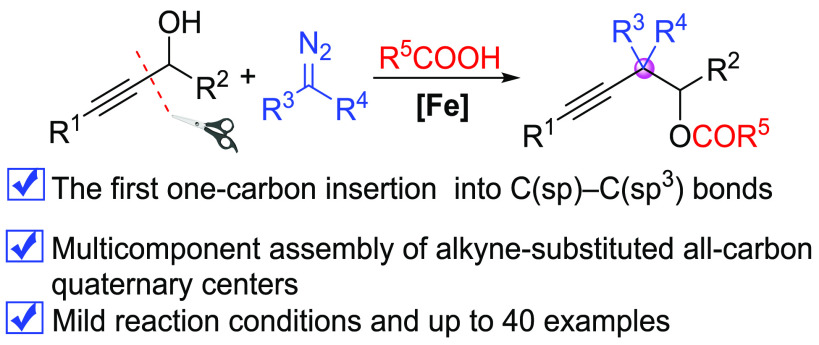
Abstract
The construction of all-carbon quaternary centers, especially those containing an alkyne-substituted framework, represents an important challenge in organic synthesis. Here we present a novel Fe-catalyzed selective formal insertion of diazo compounds into C(sp)–C(sp3) bonds of propargyl alcohols under mild conditions that enables the streamlined construction of alkyne-substituted all-carbon quaternary centers. This unique strategy starts with in situ generation of an ester group in the presence of carboxylic acids, followed by insertion of metal-carbene into C(sp)–C(sp3) bonds, which may open up a new reaction mode for exploring metal-carbene insertion into acyclic C–C bonds.
Short abstract
A novel Fe-catalyzed selective formal insertion of diazo compounds into C(sp)−C(sp3) bonds of propargyl alcohols for the streamlined construction of alkyne-substituted all-carbon quaternary centers.
1. Introduction
All-carbon quaternary centers have rigidity and structural diversity and are key structural units in many natural products, pharmaceuticals, as well as biologically active molecules.1−7 Hence, the construction of all-carbon quaternary centers is quite attractive for organic synthetic chemists; structures containing alkyne-substituted framework are versatile intermediates and basic structural motifs in organic transformations.8,9 The most common method for obtaining alkyne-substituted all-carbon quaternary centers is through the whole assembly of alkynyl groups into other substrates (Figure 1a), such as Sonogashira coupling,10−12 electrophilic alkynylation,13−16 enantioselective conjugate alkynylation,17−19 and so on.20,21 Major obstacles to these aforementioned transformations include dimerization of terminal alkynes, reliance on functionalized precursors, and the β-H elimination of branched tertiary alkyl units. In this case, as an alternative strategy to solve these problems, we questioned whether a specific protocol could be realized through selective cleavage of C(sp)–C(sp3) bonds of internal alkynes and subsequent insertion of carbon sources to construct alkyne-substituted all-carbon quaternary centers (Figure 1b).22−25
Figure 1.
Construction of alkyne-substituted all-carbon quaternary centers and the insertion of dizao compounds into C–C bonds.
The selective cleavage of inherently inert C–C bonds and subsequent functionalization are considered as a formidable synthetic challenge due to the thermodynamic stability and kinetic inertness of C–C bonds.26−30 Nonetheless, significant advances have been made over the past decades, specifically in the insertion of dizao compounds into C–C bonds. In these transformations, acid or base can promote homologation of diazo compounds with ketones, which insert into C–C bonds through a carbene-free process.31 Notably, transition metals with diazo compounds are able to form energetic carbenoids that can formally insert into the C–C bonds, producing homologues plus one carbon.32 This one-carbon insertion strategy not only allows selective cleavage of inert C–C bonds but also forms functionally all-carbon quaternary centers.33,34 However, the strained system is commonly required as it can provide a pivotal thermodynamic driving force through strain release.35−39 In 2018, Bi and co-workers reported the first Ag-catalyzed one-carbon insertion into the unstrained C(CO)–C bonds of 1,3-dicarbonyl compounds using diazoes (the left of Figure 1c).40,41 Subsequently, Cheng’s group developed an unparalleled Rh-catalyzed multicomponent assembly reaction of 1,3-diones, diazoesters, and N,N-dimethylformamide via insertion of O–C(sp3)–C(sp2) into unstrained C(CO)–C bonds (the right of Figure 1c).42 These two extremely rare examples successfully realized the insertion of diazoes into acyclic C–C bonds, but the range of substrates were limited to 1,3-dicarbonyl species. Moreover, to the best of our knowledge, the insertion of diazo-derived metal-carbene into C(sp)–C(sp3) bonds of internal alkynes is still unknown.43−46
Inspired by Fe-catalyzed carbene transfer/insertion reactions,47−52 we envision the use of iron catalyst to mediate C–C bond insertion for the construction of important alkyne-substituted all-carbon quaternary centers. Herein, we present a novel Fe-catalyzed selective formal one-carbon insertion of α-diazoacetates into C(sp)–C(sp3) bonds of propargyl alcohols in the presence of carboxylic acids, providing a convenient route for the formation of alkyne-substituted all-carbon quaternary centers (Figure 1d). This transformation starts with in situ generation of ester groups by esterification of propargyl alcohol with carboxylic acid, avoiding the limitation of the ketone group, followed by insertion of diazo metal-carbene into the C(sp)–C(sp3) bond in the presence of FeCl3 and carboxylic acid.
2. Results and Discussion
Reaction Development
We commenced our investigation by using propargyl alcohol 1a and α-diazoacetate 2a (Caution! Diazo compound is a flammable as well as explosive substance; thus all manipulations should be performed on the practical scale) as the model substrates (Table 1). After extensive evaluation, 20 mol % of FeCl3 as the catalyst and 10.0 equiv of HOAc as the additive and reaction partner in EtOAc under the argon (Ar) atmosphere at room temperature (r.t.) proved to be the optimal conditions, and afford the desired product 3a in 88% isolated yield (entry 1). The target product 3a was not detected without FeCl3 or HOAc, indicating the importance of FeCl3 and HOAc for this one-carbon insertion reaction (entries 2–3). Whether HOAc only plays as a source of ester group remains a question that deserves to be explored later. Subsequently, a series of iron catalysts like FeCl2, FeBr3, Fe(acac)3, and Fe(NO3)3 were examined, but only FeCl2 and FeBr3 promoted the reaction in satisfactory yields (entries 4–7). Notably, copper or silver salt was not a suitable catalyst for this transformation (entries 8–9). Finally, reducing the loading of FeCl3 resulted in a slight decrease in the yield of 3a (entry 10).
Table 1. Optimization of the Reaction Conditionsa.
| entry | variations from standard conditions | yieldb |
|---|---|---|
| 1 | None | 88% |
| 2 | No FeCl3 | N.Dc |
| 3 | No HOAc | N.Dc |
| 4 | FeCl2 instead of FeCl3 | 71% |
| 5 | FeBr3 instead of FeCl3 | 81% |
| 6 | Fe(acac)3 instead of FeCl3 | 11% |
| 7 | Fe(NO3)3 instead of FeCl3 | 7% |
| 8 | CuBr instead of FeCl3 | 6% |
| 9 | AgOAc instead of FeCl3 | 10% |
| 10 | 10 mol % of FeCl3 | 72% |
Reaction conditions: 1a (0.2 mmol), 2a (2.0 equiv), FeCl3 (20 mol %), HOAc (10.0 equiv), and EtOAc (2.0 mL) at r.t. under Ar for 8 h. The d.r. of 3a was about 9:1.
Isolated yields.
N.D. = Not detected.
Next, to emphasize the reproducibility of the transformation, we examined the reaction-condition-based sensitivity assessment (for details, see the Supporting Information).53,54 The radar diagram of sensitivity assessment (Figure 2) demonstrated that this reaction features low sensitivity, having outstanding reproducibility under various conditions. Moreover, this method can be scaled up to gram scale smoothly.
Figure 2.
Sensitivity assessments.
Substrate Scope of α-Diazoacetates
With the optimized reaction conditions in hand, we then proceed to investigate the scope of α-diazoacetates to explore the adaptability of this Fe-catalyzed selective formal one-carbon insertion, which was summed up in Scheme 1. To begin with, the effect of substituents on the aromatic ring of α-phenyldiazoacetates was examined. Gratifyingly, all electron-donating or electron-withdrawing groups on the aromatic ring, including tert-butyl (t-Bu), nitryl, halide (F, Cl, and Br) groups, were well tolerated, providing the corresponding desired products (3b–3f) in good yields. Then, different ester moieties at the substituted α-aryldiazoacetates were surveyed. Varying the methyl at ester moieties to isopropyl (i-Pr), benzyl (Bn), and allyl moieties furnished the desired one-carbon insertion products (3g–3i) with slightly lower yields. Inspiringly, the one-carbon insertion reaction was also suitable for α-H diazoester (3j).
Scheme 1. Substrate Scope with Regard to α-Diazoacetates.
Reaction conditions: 1 (0.2 mmol), 2 (2.0 equiv), FeCl3 (20 mol %), HOAc (10.0 equiv), and EtOAc (2.0 mL) at r.t. under Ar for 8 h.
Substrate Scope of Propargyl Alcohols
The substrate scope of propargyl alcohols was next examined (Scheme 2). We were delighted to find that the Fe-catalyzed selective formal one-carbon insertion proceeded well with a wide range of propargyl alcohols, affording access to a diverse array of alkyne-substituted all-carbon quaternary centers. First, we set out to evaluate the effect of the neighboring hydroxyl position (R2) of the propargyl alcohols. When the substituents on the aryl group of R2 were altered (3k–3r), the yields were kept at good to very good level. Importantly, the steric hindrance effect of the substrate was negligible, as demonstrated by the use of α-aryldiazoacetate or α-H diazoester as insertion partners (3n–3q). Moreover, the structure of product 3p was definitely confirmed by X-ray crystallography (CCDC 2149750). Substrates containing thienyl and alkyl were smoothly converted to the desired products 3s and 3t, respectively. Subsequently, we turned our attention to examining the substituents at the alkynyl position (R1) of the propargyl alcohols. Several substituents, namely, Me, n-butyl (n-Bu), Cl, and Br, on the aromatic ring of R1 were well tolerated (3u–3ab). In this, α-aryldiazoacetate was more efficient than α-H diazoester for this reaction under optimal conditions. Luckily, propargyl alcohol with thienyl was proved to be a suitable substrate, giving product 3ac in 82% yield. Aside from aromatic propargyl alcohols, a series of aliphatic substituted propargyl alcohols also gave the desired products 3ad–3af in slightly lower yields. Moreover, propargyl alcohol with trimethylsilyl (TMS) substituent at the alkynyl position was also easily transformed into the desired product 3ag in 72% yield. Finally, the reaction between propargyl alcohols with α-H diazoester could also be successfully converted to one-carbon insertion products 3ah–3ai in satisfactory yields.
Scheme 2. Substrate Scope with Regard to Propargyl Alcohols.
Reaction conditions: 1 (0.2 mmol), 2 (2.0 equiv), FeCl3 (20 mol %), HOAc (10.0 equiv), and EtOAc (2.0 mL) at r.t. under Ar for 8 h.
Substrate Scope of Carboxylic Acids
Our attention then turned toward the development of one-carbon insertion based on other carboxylic acids (Scheme 3). We selected a few representative substrates to react with HCOOH (Caution! HCOOH is a corrosive and irritating liquid; contact with skin should be strictly avoided). Pleasingly, 1a readily took part in this reaction with HCOOH and gave the target product 3aj in 82% yield. Propargyl alcohol with Cl substituent was tolerated in this transformation (3ak). A variety of substituted α-diazoacetates were suitable partners and transformed into the corresponding products in moderate to good yields (3al–3an). Disappointingly, no desired product (3ao) could be generated when pivalic acid was employed as the acid source.
Scheme 3. Substrate Scope with Regard to Carboxylic Acids.
Reaction conditions: 1 (0.2 mmol), 2 (2.0 equiv), FeCl3 (20 mol %), HOAc (10.0 equiv), and EtOAc (2.0 mL) at r.t. under Ar for 8 h.
Mechanistic Investigation
To obtain more information about the reaction pathway of the described transformation, several additional experiments were conducted (Scheme 4). The one-carbon insertion proceeded smoothly when 2,2,6,6-tetramethylpiperidinooxy (TEMPO) or butylated hydroxytoluene (BHT) was introduced into the reaction system, suggesting that the radical pathway may not be involved in this reaction (Scheme 4a). The role of HOAc in this reaction was investigated next. The 1b could be converted to propargyl ester 4a under standard conditions in the absence of diazo substrate, which confirmed the presence of an esterification process (Scheme 4b). Next, propargyl ester 4a was treated with 2a under the standard reaction conditions; the desired product 3m was obtained in 94% yield (Scheme 4c). This result demonstrated the significant role of the ester group introduced by esterification reaction with carboxylic acid.
Scheme 4. Mechanistic Studies.
Then, the correlation between the yield of 3m and the amount of HOAc was investigated by the use of 1b or 4a as the reaction partner (Figure 3). As shown in the red curve (1b as the reaction partner), the yield of 3m increased slowly and then rapidly with the increase of HOAc concentration, which was probably due to the initial involvement of esterification reaction before 5.0 equiv of HOAc. As presented in the blue curve (4a as the reaction partner), 7% of 3m was still detected when no HOAc was in the reaction system, but the yield of 3m increased rapidly with the increase of HOAc concentration. In addition, it is noteworthy that the yield of 3m approached the maximum when 7.0 equiv of HOAc was added. These results suggested that HOAc not only acted as a source of the ester group to initiate this multicomponent reaction but also promoted diazo metal-carbene insertion into the C(sp)–C(sp3) bond of propargyl alcohol.
Figure 3.
Correlation between the yield of 3m and the amount of HOAc.
Proposed Mechanism
Based on the density functional theory (DFT) calculations (for details, see the Supporting Information) and literature precedents,20,47,49,55,56 we propose a possible reaction pathway (Figure 4). Initially, in the presence of Lewis acid FeCl3, esterification product 4 is in situ formed from the propargyl alcohol 1 with HOAc. In parallel, Fe3+ catalyst can be reduced to Fe2+ by the diazo compound. Subsequently, the Fe2+ complex attacks the negatively charged diazo compound 2, leading to the formation of iron carbene complex A after irreversible removal of N2. Then, the coordination of A with 4 gives complex B. Afterward, intermediate B is first deprotonated from the carbon atom adjacent to the ester group under the assistance of a Cl anion along with release of the HCl molecule to give intermediate D, which undergoes a migration of the alkynyl group to the iron catalyst through the transition state TS-D followed by reduction elimination (TS-E) to generate species F. Next, the tertiary carbon atom attacks the carbonyl carbon through TS-F to yield intermediate G. Finally, intermediate G undergoes a protonation process with the assistance of the previous step releasing HCl or HOAc in this reaction system to afford the desired product. Notably, the activation barrier is 23.1 kcal/mol for this pathway, so these computational results are consistent with the experimental observations in the Fe-catalyzed insertion reactions.
Figure 4.
Plausible reaction mechanism with DFT calculations.
3. Conclusion
In conclusion, we have developed a novel Fe-catalyzed selective formal one-carbon insertion of C(sp)–C(sp3) bonds between propargyl alcohols and α-diazoacetates using carboxylic acids under mild conditions. This approach starts with in situ generation of an ester group, followed by the insertion of diazo metal-carbene into the C(sp)–C(sp3) bond, which enables the convenient construction of alkyne-substituted all-carbon quaternary centers. Controlled experiments demonstrated that carboxylic acid not only serves as a source of ester group to initiate this multicomponent reaction, but also promotes diazo metal-carbene insertion into the C(sp)–C(sp3) bond. Notably, this unique strategy represents the first example of C(sp)–C(sp3) bond cleavage and subsequent functionalization for the assembly of alkyne-substituted all-carbon quaternary centers through the metal-carbene insertion process, which potentially pioneer a new reaction mode for exploring metal-carbene insertion into acyclic C–C bonds. Further studies on the other types of diazo metal-carbene insertion and asymmetric catalysis are currently investigated in our laboratory.
Acknowledgments
We thank the Fundamental Research Funds for the Provincial Universities of Zhejiang (SJLY2021004), and the National Natural Science Foundation of China (Grants 21801142) for financial support.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acscentsci.2c00204.
Synthetic procedures, sensitivity assessments, compound characterization (copies of 1 H, 13C, and 19F NMR spectra), density functional theory calculations, and additional data (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Xue W.; Jia X.; Wang X.; Tao X.; Yin Z.; Gong H. Nickel-Catalyzed Formation of Quaternary Carbon Centers Using Tertiary Alkyl Electrophiles. Chem. Soc. Rev. 2021, 50, 4162–4184. 10.1039/D0CS01107J. [DOI] [PubMed] [Google Scholar]
- Yang Z. Navigating the Pauson–Khand Reaction in Total Syntheses of Complex Natural Products. Acc. Chem. Res. 2021, 54, 556–568. 10.1021/acs.accounts.0c00709. [DOI] [PubMed] [Google Scholar]
- Wang B.; Tu Y. Q. Stereoselective Construction of Quaternary Carbon Stereocenters via a Semipinacol Rearrangement Strategy. Acc. Chem. Res. 2011, 44, 1207–1222. 10.1021/ar200082p. [DOI] [PubMed] [Google Scholar]
- Zeng X.-P.; Cao Z.-Y.; Wang Y.-H.; Zhou F.; Zhou J. Catalytic Enantioselective Desymmetrization Reactions to All-Carbon Quaternary Stereocenters. Chem. Rev. 2016, 116, 7330–7396. 10.1021/acs.chemrev.6b00094. [DOI] [PubMed] [Google Scholar]
- Long R.; Huang J.; Gong J.; Yang Z. Direct Construction of Vicinal All-Carbon Quaternary Stereocenters in Natural Product Synthesis. Nat. Prod. Rep 2015, 32, 1584–1601. 10.1039/C5NP00046G. [DOI] [PubMed] [Google Scholar]
- Quasdorf K. W.; Overman L. E. Catalytic Enantioselective Synthesis of Quaternary Carbon Stereocentres. Nature 2014, 516, 181–191. 10.1038/nature14007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu P.; Chi H. M.; DeBacker K. C.; Gong X.; Keim J. H.; Hsu I. T.; Snyder S. A. Quaternary-Centre-Guided Synthesis of Complex Polycyclic Terpenes. Nature 2019, 569, 703–707. 10.1038/s41586-019-1179-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulsen T. B.; Bernardi L.; Alemán J.; Overgaard J.; Jørgensen K. A. Organocatalytic Asymmetric Direct α-Alkynylation of Cyclic β-Ketoesters. J. Am. Chem. Soc. 2007, 129, 441–449. 10.1021/ja067289q. [DOI] [PubMed] [Google Scholar]
- Haughey M. B.; Christensen K. E.; Poole D. L.; Donohoe T. J. Development of an Enolate Alkynylation Approach Towards the Synthesis of the Taiwanschirin Natural Products. Chem. Sci. 2021, 12, 13392–13397. 10.1039/D1SC04247E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W.; Li L.; Li C.-J. Empowering a Transition-Metal-Free Coupling between Alkyne and Alkyl Iodide with Light in Water. Nat. Commun. 2015, 6, 6526–6531. 10.1038/ncomms7526. [DOI] [PubMed] [Google Scholar]
- Yamane Y.; Miwa N.; Nishikata T. Copper-Catalyzed Functionalized Tertiary-Alkylative Sonogashira Type Couplings via Copper Acetylide at Room Temperature. ACS Catal. 2017, 7, 6872–6876. 10.1021/acscatal.7b02615. [DOI] [Google Scholar]
- Hazra A.; Lee M. T.; Chiu J. F.; Lalic G. Photoinduced Copper-Catalyzed Coupling of Terminal Alkynes and Alkyl Iodides. Angew. Chem. Int. Ed 2018, 57, 5492–5496. 10.1002/anie.201801085. [DOI] [PubMed] [Google Scholar]
- Fernández González D.; Brand J. P.; Waser J. Ethynyl-1,2-Benziodoxol-3(1H)-One (EBX): An Exceptional Reagent for the Ethynylation of Keto, Cyano, and Nitro Esters. Chem.—Eur. J. 2010, 16, 9457–9461. 10.1002/chem.201001539. [DOI] [PubMed] [Google Scholar]
- Fernández González D.; Brand J. P.; Mondière R.; Waser J. Ethynylbenziodoxolones (EBX) as Reagents for the Ethynylation of Stabilized Enolates. Adv. Synth. Catal 2013, 355, 1631–1639. 10.1002/adsc.201300266. [DOI] [Google Scholar]
- Utaka A.; Cavalcanti L. N.; Silva L. F. Electrophilic Alkynylation of Ketones Using Hypervalent Iodine. Chem. Commun. 2014, 50, 3810–3813. 10.1039/C4CC00608A. [DOI] [PubMed] [Google Scholar]
- Teodoro B. V. M.; Silva L. F. Metal-Free Synthesis of Homopropargylic Alcohols from Aldehydes. J. Org. Chem. 2017, 82, 11787–11791. 10.1021/acs.joc.7b01629. [DOI] [PubMed] [Google Scholar]
- Dabrowski J. A.; Gao F.; Hoveyda A. H. Enantioselective Synthesis of Alkyne-Substituted Quaternary Carbon Stereogenic Centers through NHC–Cu-Catalyzed Allylic Substitution Reactions with (i-Bu)2(Alkynyl)Aluminum Reagents. J. Am. Chem. Soc. 2011, 133, 4778–4781. 10.1021/ja2010829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z.-X.; Li B.-J. Construction of Acyclic Quaternary Carbon Stereocenters by Catalytic Asymmetric Hydroalkynylation of Unactivated Alkenes. J. Am. Chem. Soc. 2019, 141, 9312–9320. 10.1021/jacs.9b03027. [DOI] [PubMed] [Google Scholar]
- Zhang S.-L.; Zhang W.-W.; Li B.-J. Ir-Catalyzed Regio- and Enantioselective Hydroalkynylation of Trisubstituted Alkene to Access All-Carbon Quaternary Stereocenters. J. Am. Chem. Soc. 2021, 143, 9639–9647. 10.1021/jacs.1c04493. [DOI] [PubMed] [Google Scholar]
- Tian M.-Q.; Shen Z.-Y.; Zhao X.; Walsh P. J.; Hu X.-H. Iron-Catalyzed Tertiary Alkylation of Terminal Alkynes with 1,3-Diesters via a Functionalized Alkyl Radical. Angew. Chem. Int. Ed 2021, 60, 9706–9711. 10.1002/anie.202100641. [DOI] [PubMed] [Google Scholar]
- Xia Y.; Feng S.; Liu Z.; Zhang Y.; Wang J. Rhodium(I)-Catalyzed Sequential C(Sp)–C(Sp3) and C(Sp3)–C(Sp3) Bond Formation through Migratory Carbene Insertion. Angew. Chem. Int. Ed 2015, 54, 7891–7894. 10.1002/anie.201503140. [DOI] [PubMed] [Google Scholar]
- Nishimura T.; Katoh T.; Takatsu K.; Shintani R.; Hayashi T. Rhodium-Catalyzed Asymmetric Rearrangement of Alkynyl Alkenyl Carbinols: Synthetic Equivalent to Asymmetric Conjugate Alkynylation of Enones. J. Am. Chem. Soc. 2007, 129, 14158–14159. 10.1021/ja076346s. [DOI] [PubMed] [Google Scholar]
- Shintani R.; Takatsu K.; Katoh T.; Nishimura T.; Hayashi T. Rhodium-Catalyzed Rearrangement of Aryl Bis(alkynyl) Carbinols to 3-Alkynyl-1-Indanones. Angew. Chem. Int. Ed 2008, 47, 1447–1449. 10.1002/anie.200704818. [DOI] [PubMed] [Google Scholar]
- Dou X.; Huang Y.; Hayashi T. Asymmetric Conjugate Alkynylation of Cyclic A,B-Unsaturated Carbonyl Compounds with a Chiral Diene Rhodium Catalyst. Angew. Chem. Int. Ed 2016, 55, 1133–1137. 10.1002/anie.201509778. [DOI] [PubMed] [Google Scholar]
- Yasui K.; Chatani N.; Tobisu M. Rhodium-Catalyzed C–O Bond Alkynylation of Aryl Carbamates with Propargyl Alcohols. Org. Lett. 2018, 20, 2108–2111. 10.1021/acs.orglett.8b00674. [DOI] [PubMed] [Google Scholar]
- Chen F.; Wang T.; Jiao N. Recent Advances in Transition-Metal-Catalyzed Functionalization of Unstrained Carbon-Carbon Bonds. Chem. Rev. 2014, 114, 8613–8661. 10.1021/cr400628s. [DOI] [PubMed] [Google Scholar]
- Lutz M. D. R.; Morandi B. Metal-Catalyzed Carbon–Carbon Bond Cleavage of Unstrained Alcohols. Chem. Rev. 2021, 121, 300–326. 10.1021/acs.chemrev.0c00154. [DOI] [PubMed] [Google Scholar]
- Murakami M.; Ishida N. Cleavage of Carbon–Carbon σ-Bonds of Four-Membered Rings. Chem. Rev. 2021, 121, 264–299. 10.1021/acs.chemrev.0c00569. [DOI] [PubMed] [Google Scholar]
- Song F.; Gou T.; Wang B.-Q.; Shi Z.-J. Catalytic Activations of Unstrained C-C Bond Involving Organometallic Intermediates. Chem. Soc. Rev. 2018, 47, 7078–7115. 10.1039/C8CS00253C. [DOI] [PubMed] [Google Scholar]
- Chen P.-h.; Billett B. A.; Tsukamoto T.; Dong G. Cut and Sew“Transformations via Transition-Metal-Catalyzed Carbon–Carbon Bond Activation. ACS Catal. 2017, 7, 1340–1360. 10.1021/acscatal.6b03210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candeias N. R.; Paterna R.; Gois P. M. P. Homologation Reaction of Ketones with Diazo Compounds. Chem. Rev. 2016, 116, 2937–2981. 10.1021/acs.chemrev.5b00381. [DOI] [PubMed] [Google Scholar]
- Huang M.-Y.; Zhu S.-F. Uncommon Carbene Insertion Reactions. Chem. Sci. 2021, 12, 15790–15801. 10.1039/D1SC03328J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y.; Qiu D.; Wang J. Transition-Metal-Catalyzed Cross-Couplings through Carbene Migratory Insertion. Chem. Rev. 2017, 117, 13810–13889. 10.1021/acs.chemrev.7b00382. [DOI] [PubMed] [Google Scholar]
- Yang Z.; Stivanin M. L.; Jurberg I. D.; Koenigs R. M. Visible Light-Promoted Reactions with Diazo Compounds: A Mild and Practical Strategy Towards Free Carbene Intermediates. Chem. Soc. Rev. 2020, 49, 6833–6847. 10.1039/D0CS00224K. [DOI] [PubMed] [Google Scholar]
- Hashimoto T.; Naganawa Y.; Maruoka K. Desymmetrizing Asymmetric Ring Expansion of Cyclohexanones with α-Diazoacetates Catalyzed by Chiral Aluminum Lewis Acid. J. Am. Chem. Soc. 2011, 133, 8834–8837. 10.1021/ja202070j. [DOI] [PubMed] [Google Scholar]
- Li W.; Liu X.; Hao X.; Cai Y.; Lin L.; Feng X. A Catalytic Asymmetric Ring-Expansion Reaction of Isatins and α-Alkyl-α-Diazoesters: Highly Efficient Synthesis of Functionalized 2-Quinolone Derivatives. Angew. Chem. Int. Ed 2012, 51, 8644–8647. 10.1002/anie.201204594. [DOI] [PubMed] [Google Scholar]
- Tan F.; Pu M.; He J.; Li J.; Yang J.; Dong S.; Liu X.; Wu Y.-D.; Feng X. Catalytic Asymmetric Homologation of Ketones with α-Alkyl α-Diazo Esters. J. Am. Chem. Soc. 2021, 143, 2394–2402. 10.1021/jacs.0c12683. [DOI] [PubMed] [Google Scholar]
- Xia Y.; Liu Z.; Liu Z.; Ge R.; Ye F.; Hossain M.; Zhang Y.; Wang J. Formal Carbene Insertion into C–C Bond: Rh(I)-Catalyzed Reaction of Benzocyclobutenols with Diazoesters. J. Am. Chem. Soc. 2014, 136, 3013–3015. 10.1021/ja500118w. [DOI] [PubMed] [Google Scholar]
- Yada A.; Fujita S.; Murakami M. Enantioselective Insertion of a Carbenoid Carbon into a C–C Bond to Expand Cyclobutanols to Cyclopentanols. J. Am. Chem. Soc. 2014, 136, 7217–7220. 10.1021/ja502229c. [DOI] [PubMed] [Google Scholar]
- Liu Z.; Sivaguru P.; Zanoni G.; Anderson E. A.; Bi X. Catalyst-Dependent Chemoselective Formal Insertion of Diazo Compounds into C–C or C–H Bonds of 1,3-Dicarbonyl Compounds. Angew. Chem. Int. Ed 2018, 57, 8927–8931. 10.1002/anie.201802834. [DOI] [PubMed] [Google Scholar]
- Liu Z.; Zhang X.; Virelli M.; Zanoni G.; Anderson E. A.; Bi X. Silver-Catalyzed Regio- and Stereoselective Formal Carbene Insertion into Unstrained C–C σ-Bonds of 1,3-Dicarbonyls. iScience 2018, 8, 54–60. 10.1016/j.isci.2018.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ba D.; Wen S.; Tian Q.; Chen Y.; Lv W.; Cheng G. Rhodium(II)-Catalyzed Multicomponent Assembly of α,α,α-Trisubstituted Esters via Formal Insertion of O–C(Sp3)–C(Sp2) into C–C Bonds. Nat. Commun. 2020, 11, 4219–4224. 10.1038/s41467-020-17990-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ping Y.; Wang R.; Wang Q.; Chang T.; Huo J.; Lei M.; Wang J. Synthesis of Alkenylboronates from N-Tosylhydrazones through Palladium-Catalyzed Carbene Migratory Insertion. J. Am. Chem. Soc. 2021, 143, 9769–9780. 10.1021/jacs.1c02331. [DOI] [PubMed] [Google Scholar]
- Ning Y.; Song Q.; Sivaguru P.; Wu L.; Anderson E. A.; Bi X. Ag-Catalyzed Insertion of Alkynyl Carbenes into C–C Bonds of β-Ketocarbonyls: A Formal C(Sp2) Insertion. Org. Lett. 2022, 24, 631–636. 10.1021/acs.orglett.1c04081. [DOI] [PubMed] [Google Scholar]
- Luo K.; Mao S.; He K.; Yu X.; Pan J.; Lin J.; Shao Z.; Jin Y. Highly Regioselective Synthesis of Multisubstituted Pyrroles via Ag-Catalyzed [4 + 1C]Insert Cascade. ACS Catal. 2020, 10, 3733–3740. 10.1021/acscatal.9b05360. [DOI] [Google Scholar]
- Huo J.; Zhong K.; Xue Y.; Lyu M.; Ping Y.; Liu Z.; Lan Y.; Wang J. Palladium-Catalyzed Enantioselective Carbene Insertion into Carbon-Silicon Bonds of Silacyclobutanes. J. Am. Chem. Soc. 2021, 143, 12968–12973. 10.1021/jacs.1c05879. [DOI] [PubMed] [Google Scholar]
- Conde A.; Sabenya G.; Rodríguez M.; Postils V.; Luis J. M.; Díaz-Requejo M. M.; Costas M.; Pérez P. J. Iron and Manganese Catalysts for the Selective Functionalization of Arene C(Sp2)–H Bonds by Carbene Insertion. Angew. Chem. Int. Ed 2016, 55, 6530–6534. 10.1002/anie.201601750. [DOI] [PubMed] [Google Scholar]
- Griffin J. R.; Wendell C. I.; Garwin J. A.; White M. C. Catalytic C(Sp3)–H Alkylation via an Iron Carbene Intermediate. J. Am. Chem. Soc. 2017, 139, 13624–13627. 10.1021/jacs.7b07602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postils V.; Rodríguez M.; Sabenya G.; Conde A.; Díaz-Requejo M. M.; Pérez P. J.; Costas M.; Solà M.; Luis J. M. Mechanism of the Selective Fe-Catalyzed Arene Carbon-Hydrogen Bond Functionalization. ACS Catal. 2018, 8, 4313–4322. 10.1021/acscatal.7b03935. [DOI] [Google Scholar]
- Hernán-Gómez A.; Rodríguez M.; Parella T.; Costas M. Electrophilic Iron Catalyst Paired with a Lithium Cation Enables Selective Functionalization of Non-Activated Aliphatic C–H Bonds via Metallocarbene Intermediates. Angew. Chem. Int. Ed 2019, 58, 13904–13911. 10.1002/anie.201905986. [DOI] [PubMed] [Google Scholar]
- Zhang R. K.; Chen K.; Huang X.; Wohlschlager L.; Renata H.; Arnold F. H. Enzymatic Assembly of Carbon-Carbon Bonds via Iron-Catalysed Sp3 C–H Functionalization. Nature 2019, 565, 67–72. 10.1038/s41586-018-0808-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou A. Z.; Chen K.; Arnold F. H. Enzymatic Lactone-Carbene C–H Insertion to Build Contiguous Chiral Centers. ACS Catal. 2020, 10, 5393–5398. 10.1021/acscatal.0c01349. [DOI] [Google Scholar]
- Pitzer L.; Schäfers F.; Glorius F. Rapid Assessment of the Reaction-Condition-Based Sensitivity of Chemical Transformations. Angew. Chem. Int. Ed 2019, 58, 8572–8576. 10.1002/anie.201901935. [DOI] [PubMed] [Google Scholar]
- Cembellín S.; Dalton T.; Pinkert T.; Schäfers F.; Glorius F. Highly Selective Synthesis of 1,3-Enynes, Pyrroles, and Furans by Manganese(I)-Catalyzed C–H Activation. ACS Catal. 2020, 10, 197–202. 10.1021/acscatal.9b03965. [DOI] [Google Scholar]
- Chen Z.-S.; Duan X.-H.; Zhou P.-X.; Ali S.; Luo J.-Y.; Liang Y.-M. Palladium-Catalyzed Divergent Reactions of α-Diazocarbonyl Compounds with Allylic Esters: Construction of Quaternary Carbon Centers. Angew. Chem. Int. Ed 2012, 51, 1370–1374. 10.1002/anie.201106619. [DOI] [PubMed] [Google Scholar]
- Jiang Z.; Niu S.-L.; Zeng Q.; Qin O.; Chen Y.-C.; Xiao Q. Selective Alkynylallylation of the C–C σ Bond of Cyclopropenes. Angew. Chem. Int. Ed 2021, 60, 297–303. 10.1002/anie.202008886. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.