Abstract
Background
Migrants face an increased risk of HIV infection and late presentation for HIV care.
Aim
To examine delays in HIV diagnosis, linkage to care (LTC), and risk of late presentation for migrants living with HIV in Denmark.
Methods
We conducted a population-based, nationwide study of adult migrants (n = 2,166) presenting for HIV care between 1 January 1995 and 31 December 2020 in Denmark. Time from immigration to HIV diagnosis and from diagnosis to LTC, and late presentation were assessed, stratified by migrants’ geographical regions of origin, using descriptive statistics.
Results
The demographics of the migrant population changed over time. Overall, migrants diagnosed with HIV after immigration to Denmark resided a median of 3.7 (IQR: 0.8–10.2) years in Denmark before diagnosis. Median time from HIV diagnosis to LTC was 6 (IQR: 0–24) days. Migrants diagnosed with HIV infection before immigration had a median of 38 (IQR: 0–105) days from arrival in Denmark to LTC. The corresponding median times for 2015–20 alone were 4.1 (IQR: 0.9–13.1) years, 0 (IQR: 0–8) days, and 62 (IQR: 25–152) days, respectively. The overall proportion of late presentation among migrants diagnosed with HIV after immigration was 60%, and highest among migrants from sub-Saharan Africa and East and South Asia.
Conclusion
HIV diagnosis is still substantially delayed in Danish migrants, while LTC is timely. The proportions with late presentation are high. These results call for targeted interventions to reduce the number of migrants with undiagnosed HIV infections and of late presenters.
Keywords: HIV, transients and migrants, delayed diagnosis, continuity of patient care, health services
Introduction
Migrants in Europe are disproportionately affected by the HIV epidemic [1,2] and are recognised by the United Nations (UN) as one of the most vulnerable populations for HIV acquisition [1]. In Europe, migrants constitute an estimated 10% of the population, a proportion which is growing [3]. In Denmark, which has an estimated prevalence of HIV infection of 0.11%, migrants comprised 52% of incident cases presenting for HIV care in 2019 [4], whereas they constituted 10% of the Danish population [5]. Many migrants originate from countries with a high prevalence of HIV infection. Several other factors, which reflect the socioeconomic status of migrants before, during, and after migration, may also contribute to migrants’ excess vulnerability to HIV acquisition [1,6,7].
Migrants in Europe [2,8] and Denmark [9] face not only an increased risk of being infected with HIV, but also of presenting late for HIV care. Several studies have shown how barriers such as legal status and fear of legal repercussions, racism, and stigma may prevent migrants living with HIV (MLWH) from seeking timely HIV care [10-12]. Late presenters (LPs) face increased risk of adverse outcomes, including increased short-term mortality, related to their HIV infection [8,13] and miss the health benefits of the recommended early initiation of antiretroviral therapy (ART) [14]. Thus, the medical consequences of late presentation exacerbate inequality in healthcare. Late presentation also presents a challenge for public health efforts to use treatment as prevention of new HIV infections [15,16].
Timely HIV care starts with timely HIV diagnosis, as described in the HIV Continuum of Care [17]. A diagnosis of HIV infection, however, does not guarantee linkage to care (LTC). The HIV epidemic among MSM (men who have sex with men) in Denmark has been thoroughly mapped by previous research from the Danish HIV Cohort [18,19]. However, there is lack of information about the dynamics of the HIV epidemic and the HIV continuum of care among MLWH, and about how it has changed over the past 25 years, where there have been considerable changes in migration dynamics, policies, diagnostic practices, and access to care.
Internationally, little work has been done to study time from migration to diagnosis and LTC among MLWH [20,21]. Determining this is essential to understand how delays in the HIV continuum of care after immigration may contribute to late presentation among MLWH. Such knowledge could guide targeted interventions to reduce the number of undiagnosed and untreated patients with HIV infections.
We aimed to determine the time from immigration to diagnosis of HIV infection and from HIV diagnosis to LTC and to examine late presentation, stratified by migrant geographical region of origin and calendar period, in Denmark over a 25-year period.
Methods
We conducted a nationwide, population-based, retrospective, observational study of all MLWH registered in the Danish HIV Cohort Study (DHCS) between 1995 and 2020.
Setting and testing strategies
In Denmark, all HIV-infected individuals are referred to one of eight specialised HIV healthcare centres, where they are seen on outpatient visits at proposed 12 to 48-week intervals. HIV testing, care and ART are offered free of charge to all individuals living with HIV who have legal residence in Denmark. The Box shows an overview of the Danish HIV testing strategies [9,22].
Box. The four primary HIV testing strategies, Denmark, 1995–2020.
General practice
• Available for migrants with legal residence
• Recommended optional HIV test at first visit to GP for migrants from high HIV prevalence regions
• Recommended optional yearly HIV test for all MSM
• Routine optional pregnancy screening since 2010
• Symptom-based testing
Hospital
• Available for all migrants with legal residence and, in case of emergency treatment, also for undocumented migrants
• HIV test offered to individuals presenting with possibly HIV-related symptoms
• HIV test for undocumented migrants as part of emergency treatment
Community-based clinics in the four largest cities
• Available for migrants, including undocumented, from Africa, Asia, Latin America and Eastern Europe, and all migrants who are MSM or transgender since 2008
• Drop-in, anonymous HIV test
Red Cross clinic/refugee camp
• Available for asylum seekers/quota refugees
• Voluntary health assessments at Red Cross clinics for asylum seekers (do not routinely include HIV test)
• Mandatory health assessments by IOM in refugee camps abroad for quota refugees (include routine optional HIV test)
GP: general practice; IOM: International Organization for Migration; MSM: men who have sex with men.
Data sources
The DHCS, described in detail elsewhere [23], is a nationwide, population-based cohort study of all individuals living with HIV treated at Danish HIV healthcare centres since 1 January 1995. Individuals are continuously enrolled. Data including demographics, date of HIV diagnosis, first contact with a Danish HIV centre, AIDS-defining events, and ART are updated yearly.
The Danish Civil Registry System (CRS), which contains demographic data and vital status on all Danish residents, was accessed to obtain information on immigration and emigration dates and countries of origin. The Danish National Hospital Registry (DNHR), containing information on all non-psychiatric hospital admissions since 1977 and all outpatient visits since 1995, was used to obtain information on dates of first visits to specialised infectious diseases departments. Data were linked through the unique personal identification numbers given to all residents in Denmark.
Study population
We included all individuals with HIV-1 in DHCS who (i) immigrated to Denmark and obtained residence, (ii) were aged 16 years or older at time of first contact with an HIV healthcare centre and (iii) had first contact with a Danish HIV healthcare centre between 1 January 1995 and 31 December 2020. Undocumented migrants are not registered in the CRS and were not included.
Definitions
Data on country of origin of MLWH were obtained from the CRS. We categorised MLWH into five geographical regions of origin based on the United Nations Standard country and Area codes classifications [24]: (i) sub-Saharan Africa (SSA), (ii) western countries including northern, southern, and western Europe, United States (US) and Canada, and Australia and New Zealand, (iii) East and South Asia, (iv) eastern Europe and (v) other (North Africa, the Middle East, Latin America, the Caribbean and MLWH with missing information on country of origin) (Supplementary Material: Appendix 1. Country codes).
The date of HIV diagnosis was obtained from DHCS. It was defined as the date of the first positive HIV test, either in Denmark or abroad, as self-reported by MLWH. LTC was defined as date of first contact with a Danish HIV healthcare centre. This date was identified as either (i) date of first HIV RNA viral load (VL) measurement, (ii) date of first CD4+ T-cell measurement, (iii) date of ART initiation if ART was initiated in Denmark or (iv) date of first visit to a Danish specialised department of infectious diseases after the first positive HIV test and/or with HIV infection as the primary diagnosis as registered in the DNHR, whichever came first. If a measurement of VL or CD4+ T-cell count was conducted within 90 days after LTC, it was counted as a measurement of VL/CD4+ T-cells at time of LTC.
Migrants’ periods of residence in Denmark were defined by dates of immigration and subsequent dates of emigration obtained from the CRS. If the diagnosis of HIV infection occurred while the MLWH was residing in Denmark, the MLWH was categorised as diagnosed with HIV after immigration. If the diagnosis of HIV infection occurred while the MLWH was residing abroad, the MLWH was categorised as diagnosed with HIV before immigration.
According to the European consensus definition [25], late presentation (LP) was defined as presenting for HIV care with a CD4+ T-cell count < 350 cells/µl or presenting with an AIDS-defining event regardless of CD4+ T-cell count. Presentation with advanced HIV disease (AHD) was defined as presenting with a CD4+ T-cell count < 200 cells/µl or with an AIDS-defining event regardless of CD4+ T-cell count [25]. Presenting with AIDS was defined as having developed an AIDS-defining event no later than 30 days after LTC. Viral suppression was defined as a VL < 200 copies/ml and was used as a marker for being on ART.
Outcomes
For MLWH diagnosed with HIV infection after immigration to Denmark, we examined time from immigration to HIV diagnosis and time from HIV diagnosis to LTC. For MLWH diagnosed before immigration, we examined time from the first date of immigration following HIV diagnosis to LTC. We examined the proportions of LP and AHD.
Statistics
All results were stratified according to MLWHs’ status as either diagnosed after or before immigration to Denmark.
MLWH could have several migrations in and out of Denmark before HIV diagnosis or LTC, and thus more than one period of residence in Denmark. Only time spent residing in Denmark contributed time to the outcomes.
We stratified our analyses according to calendar period and region of origin. The calendar periods were (i) 1 January 1995–31 December 1999, (ii) 1 January 2000–31 December 2004, (iii) 1 January 2005–31 December 2009, (iv) 1 January 2010–31 December 2014 (v) 1 January 2015–31 December 2020, as defined by the date of LTC. We chose 5-year periods to balance between having a sufficient number of individuals in each calendar period, while at the same time having enough calendar periods to be able to show possible trends over time. We conducted subanalyses restricted to LPs and MLWH with AHD and to MLWH from SSA and East and South Asia, stratified by calendar period. We performed a trend analysis using logistic regression to evaluate the developments over time in percentages of LP.
A preliminary analysis revealed that a small proportion (n = 212) of MLWH diagnosed before immigration were registered with a date of immigration to Denmark, which occurred after linkage to Danish HIV care. They were assumed to be MLWH living in Denmark and receiving Danish HIV care before obtaining residence. In the calculations, their time from immigration to LTC was defined as 0 days.
A small number of MLWH had missing data on VL (n = 264) or CD4+ T-cell (n = 192) measurements. They were excluded from the analyses of VL, CD4+ T-cells and LP/AHD.
All calculations were done using descriptive statistics. SPSS statistical software version 25 was used for data analysis (IBM).
Results
We identified 2,166 MLWH who fulfilled the inclusion criteria. Of these, 1,191 (55%) were men, and the median age was 33 (IQR: 28–40). Overall, 1,514 MLWH (70%) were diagnosed with HIV after immigration to Denmark. Of these, 202 (13%) had multiple periods of residence in Denmark before diagnosis. Of all MLWH, 652 (30%) were diagnosed with HIV before immigration. Over time, the number of MLWH diagnosed after immigration was constant, while the number diagnosed before immigration increased. Thus, the percentage of MLWH diagnosed before immigration to Denmark increased from 22% in 1995–99 to 41% in 2015–20. Characteristics of the two study populations are presented, stratified by region of origin (Table 1) and by calendar period (Table 2).
Table 1. Characteristics of migrants living with HIV, diagnosed before or after immigration, stratified by regions of origin, Denmark, 1995–2020 (n = 2,166).
| Characteristics | Region of origin | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All n = 2,166 |
Sub-Saharan Africa n = 891 |
Western countries n = 446 |
East and South Asia n = 403 |
Eastern Europe n = 171 |
Other n = 255 |
||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | ||
| Diagnosed after immigration | 1,514 | 70 | 655 | 74 | 250 | 56 | 293 | 73 | 128 | 75 | 188 | 74 | |
| Age in years, median (IQR) | 33 (28–40) | 32 (28–38) | 37 (31–43) | 34 (28–40) | 31 (27–37) | 34 (28–41) | |||||||
| Sexa | Males | 830 | 55 | 214 | 33 | 219 | 88 | 152 | 52 | 89 | 70 | 156 | 83 |
| Females | 682 | 45 | 440 | 67 | 31 | 12 | 141 | 48 | 39 | 30 | 31 | 16 | |
| Reported HIV transmission modes | Sex between men | 388 | 26 | 16 | 2 | 151 | 60 | 85 | 29 | 54 | 42 | 82 | 44 |
| Sex between men and women | 873 | 58 | 551 | 84 | 62 | 25 | 156 | 53 | 41 | 32 | 63 | 34 | |
| Injecting drug use | 50 | 3 | 5 | 1 | 16 | 6 | 8 | 3 | 9 | 7 | 12 | 6 | |
| Other/unknown | 203 | 13 | 83 | 13 | 21 | 8 | 44 | 15 | 24 | 19 | 31 | 16 | |
| HIV-RNA valuesb | Measurement at LTC | 1,335 | 88 | 562 | 86 | 215 | 86 | 277 | 95 | 115 | 90 | 166 | 88 |
| HIV-RNA (log10 copies/ml), median (IQR) | 4.7 (3.9–5.2) | 4.5 (3.7–5.2) | 4.8 (4.0–5.4) | 4.8 (4.0–5.3) | 4.6 (4.0–5.3) | 4.7 (4.1–5.2) | |||||||
| HIV-RNA < 200 copies/ml | 78 | 6 | 42 | 7 | 15 | 7 | 13 | 5 | ≤ 4 | ≤ 3 | ≤ 4 | ≤ 2 | |
| CD4+ T-cell countsb | Measurement at LTC | 1,421 | 94 | 615 | 94 | 229 | 92 | 283 | 97 | 120 | 94 | 174 | 93 |
| CD4+ T-cell count (cells/µL), median (IQR)c | 290 (130–460) | 250 (120–410) | 362 (170–557) | 276 (75–439) | 341 (189–524) | 341 (177–510) | |||||||
| Late-stage HIV | Late presentersd | 856 | 60 | 416 | 68 | 107 | 47 | 181 | 64 | 63 | 53 | 89 | 51 |
| Advanced HIV diseasee | 530 | 37 | 259 | 42 | 66 | 29 | 123 | 43 | 33 | 28 | 49 | 28 | |
| AIDS | 175 | 12 | 74 | 11 | 21 | 8 | 55 | 19 | 8 | 6 | 17 | 9 | |
| Diagnosed before immigration | 652 | 30 | 236 | 26 | 196 | 44 | 110 | 27 | 43 | 25 | 67 | 26 | |
| Age in years, median (IQR) | 33 (28–40) | 33 (28–38) | 36 (31–46) | 31 (27–37) | 30 (26–38) | 32 (26–41) | |||||||
| Sex | Males | 361 | 55 | 70 | 30 | 165 | 84 | 47 | 43 | 25 | NA | 54 | 81 |
| Females | 291 | 45 | 166 | 70 | 31 | 16 | 63 | 57 | 18 | NA | 13 | 19 | |
| Reported HIV transmission modes | Sex between men | 202 | 31 | 10 | 4 | 114 | 58 | 26 | 24 | 16 | NA | 36 | 54 |
| Sex between men and women | 279 | 43 | 172 | 73 | 29 | 15 | 55 | 50 | 12 | NA | 11 | 16 | |
| Injecting drug use | 20 | 3 | ≤ 4 | ≤ 2 | 9 | 5 | ≤ 4 | ≤ 4 | 5 | NA | ≤ 4 | ≤ 6 | |
| Other/unknown | 151 | 23 | 52 | 22 | 44 | 22 | 26 | 24 | 10 | NA | 19 | 28 | |
| HIV-RNA valuesf | Measurement at LTC | 567 | 87 | 199 | 84 | 174 | 89 | 98 | 89 | 39 | NA | 57 | 85 |
| HIV-RNA (log10 copies/ml), median (IQR) | 2.1 (1.3–4.4) | 3.6 (1.3–4.7) | 1.3 (1.3–3.5) | 2.7 (1.3–4.7) | 1.4 (1.3–4.0) | 1.6 (1.3–3.5) | |||||||
| HIV-RNA < 200 copies/ml | 296 | 52 | 68 | 34 | 119 | 68 | 45 | 46 | 25 | NA | 39 | 68 | |
| CD4+ T-cell countsf | Measurement at LTC | 553 | 85 | 200 | 85 | 164 | 84 | 99 | 90 | 34 | NA | 56 | 84 |
| CD4+ T-cell count (cells/µL), median (IQR)c | 430 (250–630) | 311 (160–462) | 525 (350–770) | 440 (260–610) | 550 (373–757) | 521 (361–670) | |||||||
| Late-stage HIV | CD4+ T-cell < 350 cells/µL/AIDSf | 218 | 39 | 118 | 59 | 44 | 27 | 35 | 35 | 8 | NA | 13 | 23 |
| CD4+ T-cell < 200 cells/µL/AIDSf | 122 | 22 | 70 | 35 | 22 | 13 | 21 | 21 | ≤ 4 | NA | 5 | 9 | |
| AIDS | 48 | 7 | 24 | 10 | 11 | 6 | 9 | 8 | ≤ 4 | NA | ≤ 4 | ≤ 6 | |
AIDS: acquired immunodeficiency syndrome; IQR: interquartile range; LTC: linkage to care; NA: not applicable.
All characteristics were collected at date of LTC.
a Missing information on sex was ≤ 4 (≤ 0.2%) in SSA and in Other.
b Data were missing for some MLWH for the following variables: HIV-RNA (n = 179) and CD4+ T-cell count (n = 93) and were excluded from analyses of HIV-RNA, CD4+ T-cell count and late presentation/advanced HIV disease.
c CD4+ T-cell normal range: 500–1,500 cells/µl.
d Presenting with CD4+ T-cell count < 350 cells/µl or with an AIDS-defining event regardless of CD4+ T-cell count.
e Presenting with CD4+ T-cell count < 200 cells/µl or with an AIDS-defining event regardless of CD4+ T-cell count.
f Data were missing for some MLWH for the following variables: HIV-RNA (n = 85) and CD4+ T-cell count (n = 99) and were excluded from analyses of HIV-RNA and CD4+ T-cell count.
Table 2. Characteristics of migrants living with HIV, diagnosed before or after immigration, stratified by calendar periods, Denmark, 1995–2020 (n = 2,166).
| Characteristics | Calendar period | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1995–99 n = 394 |
2000–04 n = 414 |
2005–09 n = 426 |
2010–14 n = 456 |
2015–20 n = 476 |
|||||||
| n | % | n | % | n | % | n | % | n | % | ||
| Diagnosed after immigration (n = 1,514) | 308 | 78 | 316 | 76 | 317 | 74 | 291 | 64 | 282 | 59 | |
| Age in years, median (IQR) | 33 (28–38) | 32 (29 – 38) | 35 (28–41) | 35 (30–41) | 33 (28–41) | ||||||
| Sexa | Males | 154 | 50 | 150 | 47 | 164 | 52 | 170 | 58 | 192 | 68 |
| Females | 154 | 50 | 166 | 53 | 153 | 48 | 121 | 42 | 88 | 31 | |
| Region of origin | Sub-Saharan Africa | 161 | 52 | 169 | 53 | 137 | 43 | 108 | 37 | 80 | 28 |
| Western Countries | 57 | 19 | 46 | 15 | 46 | 15 | 55 | 19 | 46 | 16 | |
| East and South Asia | 45 | 15 | 63 | 20 | 71 | 22 | 58 | 20 | 56 | 20 | |
| Eastern Europe | 5 | 2 | 12 | 4 | 27 | 9 | 36 | 12 | 48 | 17 | |
| Other | 40 | 13 | 26 | 8 | 36 | 11 | 34 | 12 | 52 | 18 | |
| Reported HIV transmission modes | Sex between men | 54 | 18 | 54 | 17 | 76 | 24 | 92 | 32 | 112 | 40 |
| Sex between men and women | 215 | 70 | 224 | 71 | 199 | 63 | 154 | 53 | 81 | 29 | |
| Injecting drug use | 11 | 4 | 17 | 5 | 9 | 3 | 10 | 3 | ≤ 4 | ≤ 1 | |
| Other/unknown | 28 | 9 | 21 | 7 | 33 | 10 | 35 | 12 | 86 | 30 | |
| HIV-RNA valuesb | HIV-RNA measurement at LTC | 185 | 60 | 306 | 97 | 294 | 93 | 284 | 98 | 266 | 94 |
| HIV-RNA (log10 copies/ml), median (IQR) | 4.6 (3.9–5.2) | 4.7 (4.1 – 5.3) | 4.5 (3.7–5.1) | 4.7 (4.0–5.3) | 4.7 (3.8–5.3) | ||||||
| HIV-RNA < 200 copies/ml | 5 | 3 | 12 | 4 | 13 | 4 | 19 | 7 | 29 | 11 | |
| CD4+ T-cell countsb | CD4+ T-cell count at LTC | 294 | 95 | 307 | 97 | 289 | 91 | 285 | 98 | 246 | 87 |
| CD4+ T-cell count (cells/µL), median (IQR)c | 253 (95–400) | 220 (89–440) | 310 (163–441) | 334 (152–524) | 330 (179–502) | ||||||
| Late-stage HIV | Late presentersd | 197 | 67 | 205 | 67 | 168 | 58 | 151 | 53 | 135 | 55 |
| Advanced HIV diseasee | 124 | 42 | 147 | 48 | 91 | 31 | 98 | 34 | 70 | 28 | |
| AIDS | 45 | 15 | 47 | 15 | 38 | 12 | 33 | 11 | 12 | 12 | |
| Diagnosed before immigration (n = 652) | 86 | 22 | 98 | 24 | 109 | 26 | 165 | 36 | 194 | 41 | |
| Age in years, median (IQR) | 32 (28–38) | 31 (26–37) | 35 (30–41) | 34 (28–41) | 33 (29–41) | ||||||
| Sex | Males | 39 | 45 | 36 | 37 | 62 | 57 | 101 | 61 | 123 | 63 |
| Females | 47 | 55 | 62 | 63 | 47 | 43 | 64 | 39 | 71 | 37 | |
| Region of origin | Sub-Saharan Africa | 46 | 53 | 48 | 49 | 43 | 39 | 55 | 33 | 44 | 23 |
| Western Countries | 21 | 24 | 19 | 19 | 34 | 31 | 51 | 31 | 71 | 37 | |
| East and South Asia | 15 | 17 | 24 | 24 | 14 | 13 | 28 | 17 | 29 | 15 | |
| Eastern Europe | ≤ 4 | ≤ 5 | ≤ 4 | ≤ 4 | 5 | 5 | 17 | 10 | 19 | 10 | |
| Other | ≤ 4 | ≤ 5 | 6 | 6 | 13 | 12 | 14 | 8 | 31 | 16 | |
| Reported HIV transmission modes | Sex between men | 20 | 23 | 18 | 18 | 41 | 38 | 63 | 38 | 60 | 31 |
| Sex between men and women | 51 | 59 | 69 | 70 | 61 | 56 | 56 | 34 | 42 | 22 | |
| Injecting drug use | ≤ 4 | ≤ 5 | ≤ 4 | ≤ 4 | 5 | 5 | 7 | 4 | ≤ 4 | ≤ 2 | |
| Other/unknown | 11 | 13 | 7 | 7 | ≤ 4 | ≤ 4 | 39 | 24 | 92 | 47 | |
| HIV-RNA valuesf | HIV-RNA measurement at LTC | 53 | 62 | 85 | 87 | 97 | 89 | 151 | 92 | 181 | 93 |
| HIV-RNA (log10 copies/ml), median (IQR) | 4.3 (2.8–5.1) | 4.4 (3.4–5.0) | 2.6 (1.6–4.3) | 1.9 (1.3–4.4) | 1.3 (1.3–1.5) | ||||||
| HIV-RNA < 200 copies/ml | 6 | 11 | 10 | 12 | 45 | 46 | 84 | 56 | 151 | 83 | |
| CD4+ T-cell countsf | CD4+ T-cell count at LTC | 74 | 86 | 93 | 94 | 93 | 85 | 148 | 90 | 145 | 75 |
| CD4+ T-cell count (cells/µL), median (IQR)c | 270 (133–488) | 262 (128–472) | 376 (224–560) | 435 (290–603) | 600 (446–774) | ||||||
| Late-stage HIV | CD4+ T-cell < 350 cells/µL/AIDSf | 44 | 59 | 57 | 61 | 40 | 43 | 58 | 39 | 19 | 13 |
| CD4+ T-cell < 200 cells/µL/AIDSf | 28 | 38 | 35 | 38 | 21 | 23 | 30 | 20 | 8 | 6 | |
| AIDS | 10 | 12 | 14 | 14 | 6 | 6 | 12 | 7 | 6 | 3 | |
AIDS: acquired immunodeficiency syndrome; IQR: interquartile range; LTC: linkage to care; NA: not applicable.
All characteristics were collected at date of LTC.
a Missing information on sex on ≤ 4 (≤ 0.2%) in 2015–20.
b Data were missing for some MLWH for the following variables: HIV-RNA (n = 179) and CD4+ T-cell count (n = 93) and were excluded from analyses of HIV-RNA, CD4+ T-cell count and late presentation/advanced HIV disease.
c CD4+ T-cell normal range: 500–1,500 cells/µl.
d Presenting with CD4+ T-cell count < 350 cells/µl or with an AIDS-defining event regardless of CD4+ T-cell count.
e Presenting with CD4+ T-cell count < 200 cells/µl or with an AIDS-defining event regardless of CD4+ T-cell count.
f Data were missing for some MLWH for the following variables: HIV-RNA (n = 85) and CD4+ T-cell count (n = 99) and were excluded from analyses of HIV-RNA and CD4+ T-cell count.
Migrants diagnosed with HIV infection after immigration
Among MLWH diagnosed with HIV after immigration, 830/1,514 (55%) were men and the median age at LTC was 33 (IQR: 28–40) years (Table 1). The demographic and HIV-related characteristics changed over time. From 1995–99, MLWH migrated predominantly from SSA (52%), 50% were women, and a high proportion (70%) reported heterosexual transmission. Over time, the regions of origin became more diverse, with an increasing proportion of MLWH from eastern Europe. More MLWH were men (68%) and MSM was more frequently reported as the route of infection (40%) (Table 2). In general, MLWH originating from SSA and East and South Asia were more frequently women and heterosexually infected and had lower CD4+ T-cell counts at LTC (< 300 cells/uL), whereas MLWH from Western countries and eastern Europe were predominantly men and MSM and had higher CD4+ T-cell (> 300 cells/uL) counts at LTC (Table 1).
Time from immigration to HIV diagnosis and from HIV diagnosis to LTC
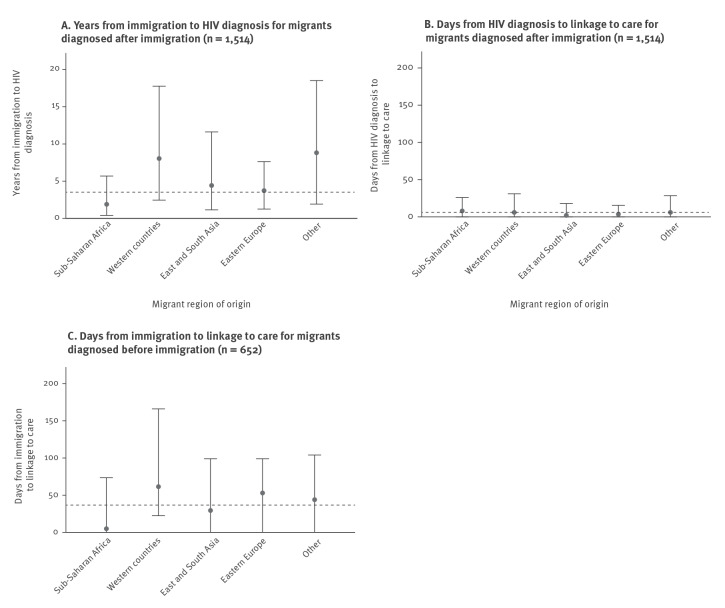
The median time from immigration to HIV diagnosis was 3.7 (IQR: 0.8–10.2) years. There were notable geographical differences: time from immigration to HIV diagnosis was 1.9 (IQR: 0.4–5.7) years for MLWH from SSA and 8.0 (IQR: 2.5–17.8) years for MLWH from Western countries (Figure 1).
Figure 1.
A. Time to HIV diagnosis and time to linkage to care for B. migrants diagnosed with HIV after immigration and C. migrants diagnosed with HIV before immigrationa stratified by migrant region of origin, Denmark, 1995–2020 (n = 2,166)
a A total of 212 migrants were registered with a date of immigration which occurred after date of linkage to Danish HIV care. Their time from immigration to linkage to care was defined as 0 days.
Graphs show median time (points), overall median (dashed line) and interquartile range (whiskers). Migrants’ region of origin was defined by United Nations standard country and area codes classifications [24].
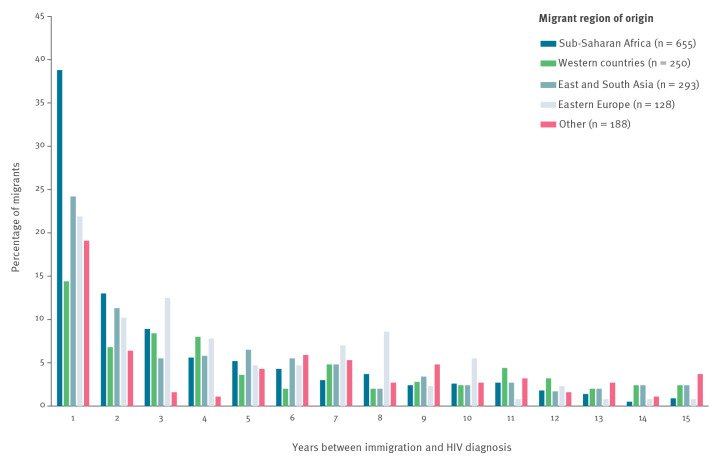
The proportion of MLWH diagnosed with HIV less than 1 year after immigration to Denmark ranged from 39% among MLWH from SSA to 14% among MLWH from Western countries (Figure 2).
Figure 2.
Distribution of migrants’ length of stay in years before HIV diagnosisa, Denmark, 1995–2020 (n = 1,514)
a MLWH with > 15 years between migration and HIV diagnosis (n = 237) are not shown in the figure, but they were included in all calculations.
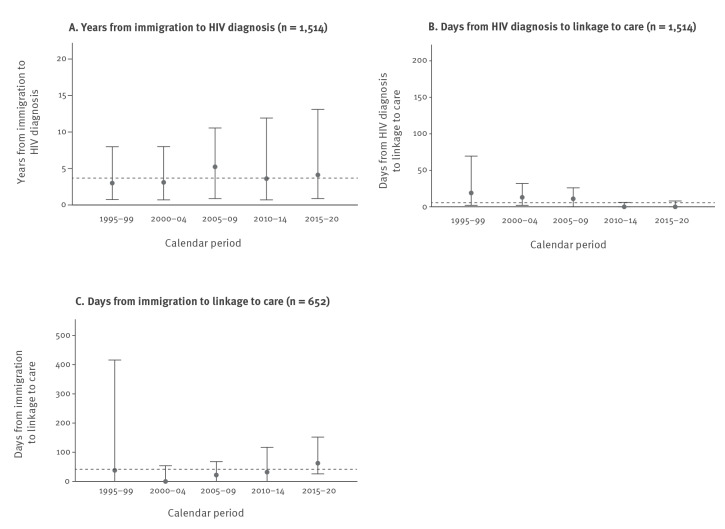
For the total population (Figure 3), as well as for MLWH from SSA and East and South Asia specifically (Supplementary Figure S1: Years (median, IQR) from immigration to HIV-diagnosis for MLWH from Sub-Saharan Africa and East and South Asia stratified by calendar period), time from immigration to HIV diagnosis was stable throughout the study period. In all analyses, we found a greater range in the duration of time from immigration to HIV diagnosis in the later calendar periods (Figure 3A).
Figure 3.
A. Time to HIV diagnosis and time to linkage to care for B. migrants diagnosed with HIV after immigration and C. migrants diagnosed with HIV before immigrationa, stratified by calendar period, Denmark, 1995–2020 (n = 2,166)
a A total of 212 migrants were registered with a date of immigration which occurred after date of linkage to Danish HIV care. Their time from immigration to linkage to care was defined as 0 days.
Graphs show median time (points), overall median (dashed line) and interquartile range (whiskers). Migrants’ region of origin as defined by United Nations standard country and area codes classifications [24].
For all MLWH diagnosed after immigration, the median time from HIV diagnosis to LTC was 6 (IQR: 0–24) days. Between 223/250 (89%) from Western countries and 278/293 (95%) from East and South Asia and 122/128 (95%) from eastern Europe were linked to care within 90 days of HIV diagnosis. Time from HIV diagnosis to LTC was overall shortest in 2010–20 (Figure 3B).
Late presentation
MLWH from SSA and East and South Asia had the highest proportions of LP (68% and 64%, respectively) (Table 1). MLWH from East and South Asia had the highest proportion of AIDS at LTC (19%). Over the studied time, we observed a decline in the number and percentages of LP and AHD for the total MLWH population. In 1995–99, 197/294 (67%) were LPs and 124/294 (42%) had AHD compared with 135/246 (55%) and 70/246 (28%) respectively in 2015–20 (Table 2). However, the proportions of LP and AHD among MLWH from SSA and East and South Asia remained high over time. Among MLWH from SSA, 106 (69%) were LPs and 67 (44%) had AHD in 1995–99 compared with 42 (60%) and 25 (36%) in 2015–20, respectively. There was a slight but significant downwards trend in the percentage of LP (p value = 0.02). Among MLWH from East and South Asia, there was no significant change in the percentage of LP over time (p value = 0.45). In 1995–99, 33 (73%) were LPs and 18 (40%) had AHD compared with 36 (71%) LPs and 22 (43%) with AHD in 2015–20. (Supplementary Table S1: Late presentation and presentation with advanced HIV disease among migrants from Sub-Saharan Africa and East and South Asia stratified by calendar periods).
Among women with available data on CD4+ T-cell counts (n = 646), 442/646 (68%) were LPs and 272/646 (42%) had AHD compared with 413/773 (53%) LPs and 258/773 (33%) with AHD among men with available data on CD4+ T-cell counts (n = 773). In 2015–20 among women, 50/76 (66%) were LPs and 28/76 (37%) had AHD compared with 85/168 (51%) LPs and 42/168 (25%) with AHD among men.
Time from immigration to HIV diagnosis for late presenters and migrants presenting with advanced HIV disease
For LPs and MLWH with AHD, the median times from immigration to HIV diagnosis were 3.5 (IQR: 0.9–9.3) and 3.9 (IQR: 1.0–9.4) years. Time from immigration to HIV diagnosis was stable over calendar time (Supplementary Figure S2: Years (median, IQR) from immigration to HIV-diagnosis for late presenters, stratified by calendar period).
Migrants diagnosed with HIV infection before immigration
Among MLWH diagnosed with HIV infection before immigration, 361/652 (55%) were men and the median age was 33 (IQR: 28–40) years. Overall, SSA was the predominant region of origin (Table 1), but over time, Western countries became the predominant region of origin (Table 2). The proportion of men also increased over time. The demographic characteristics for each region resembled those of MLWH diagnosed after immigration, as described above. The median CD4+ T-cell count increased from 270 (IQR: 133–488) cells/µL in 1995–99 to 600 (IQR: 446–774) cells/µL in 2015–20. The number of MLWH presenting to Danish HIV care with a CD4+ T-cell count < 350 cells/µL or AIDS decreased from 44 (59%) in 1995–99 to 19 (13%) in 2015–20. Likewise, the proportion of MLWH presenting with VL < 200 copies/ml increased from 11% in 1995–99 to 83% in 2015–20.
Time from immigration to LTC
The overall median time from immigration to LTC was 38 (IQR: 0–105) days, ranging from 5 (IQR: 0–74) days for MLWH from SSA to 62 (IQR: 22–169) days for MLWH from Western countries (Figure 1). A sensitivity analysis excluding the 212 MLWH with LTC in Denmark occurring before official immigration changed the estimate to 75 days (IQR: 35–186). The proportion of MLWH with LTC within 90 days of immigration ranged from 79% (SSA) to 62% (Western countries). Time from immigration to LTC increased from 2000 to 2004 and onward (Figure 3).
Discussion
In this nationwide study, we demonstrated that MLWH had resided a median of 3.7 years in Denmark before diagnosis of HIV infection with no change over time from 1995 to 2020. However, regardless of whether migrants were diagnosed with HIV infection in Denmark or arrived in Denmark with a known HIV diagnosis, linkage to care was timely for a high (62%–95%) proportion of MLWH. Consistent with other studies [9,26], we found high proportions of LP and AHD among MLWH diagnosed after immigration. In comparison, the proportion of LP among Danish-born individuals has been estimated to be 42% [9].
Although the median time between immigration and diagnosis remained unchanged, the proportions of LP, AHD and AIDS at time of LTC decreased in later calendar periods. Consistent with this, the CD4+ T-cell counts at LTC were higher in the later calendar periods. These changes mainly reflect that the composition of the migrant population changed over time, with fewer migrants from SSA following increasingly restrictive migration policies in Denmark [27]. Furthermore, the global HIV pandemic has changed following the increased focus on early and regular HIV testing and immediate ART provision. The proportion of MLWH – especially from Western countries – who were already diagnosed with HIV and on effective ART at time of immigration increased over time. This might explain why time from immigration to LTC increased over time, as MLWH already on ART at immigration might bring medicine and have no need of immediate healthcare.
In line with other studies [9], our findings illustrate that MLWH constitute a heterogenous population with different trends across geographical regions of origin. In addition, the interpretation and clinical implications of measuring time from immigration to HIV diagnosis are different for subpopulations of MLWH. We presume that MLWH from the high prevalence regions of SSA and East and South Asia were mainly infected with HIV before immigration to Denmark [7,9,20] and, consequently, most of the time spent in Denmark before HIV diagnosis reflected a diagnostic delay. In both subpopulations, we found consistently high proportions of LP and AHD. This makes it crucial to reduce the diagnostic delays, which even during 2015–20 exceeded 2 years for MLWH from SSA and 4 years for MLWH from East and South Asia. Other studies have also identified migrants from SSA and East and South Asia to be at high risk of late presentation [2,8,9], and modelling studies have identified risk of delays in the HIV continuum of care for these migrant subpopulations [20,28]. We saw a high correlation between geographic region of origin and sex. MLWH from SSA and East and South Asia were more likely to be women, and in line with this, we found high proportions of LP and ADH among women.
Compared with migrants from SSA and East and South, MLWH from Western countries are more likely to become infected with HIV after immigration, especially among MSM [7]. Thus, for migrants from Western countries, the observed large dispersion and sometimes long time periods between immigration and HIV diagnosis probably represent a combination of diagnostic delays and post-migration HIV transmission. As a consequence, healthcare efforts towards this migrant subpopulation should focus on prevention of new infections [7] in addition to continuous HIV testing efforts.
MLWH from eastern Europe constituted an increasing proportion of the total population of MLWH over calendar time. Eastern Europe is one of the few regions where the HIV epidemic is growing with a high incidence:prevalence ratio of 10% [29]. Our results underline that the risk of HIV infection among key populations in eastern Europe should be targeted not only in the region but also recognised among migrants from the region.
Over time, the proportion of MSM MLWH increased. This reinforces that HIV prevention and testing efforts should ensure initiatives aimed at MSM migrants.
Healthcare is free of charge in Denmark, and healthcare efforts have been very effective in reducing the HIV epidemic among Danish-born MSM [18,19,30]. Nevertheless, we still face a problem with migrants’ access to HIV testing. It is essential to make anonymous, voluntary HIV testing available free of charge to everyone, both at a community level and at primary healthcare providers, and to ensure provider-initiated (voluntary) testing, so that migrants who do not perceive themselves at risk of HIV are still offered tests. However, several studies have shown how structural barriers prevent migrants from seeking timely HIV diagnosis and care [10-12]. Furthermore, a recent study has pointed to many missed opportunities for provider-initiated HIV testing at contact with the primary healthcare system for people later diagnosed with HIV in Denmark [31]. Our study demonstrates that the barriers to HIV testing in combination with missed testing opportunities potentially cause diagnostic delays of several years.
Other studies have also shown how migrants are at increased risk of late presentation [2,8,9], our study further elucidates that while some MLWH arrive as LPs, others likely become LPs after immigration because of diagnostic delays. This implies that increased and diverse HIV testing efforts in the migrants’ country of arrival, combined with the breakdown of barriers, have the potential to reduce morbidity and mortality associated with presentation with advanced HIV disease.
This population-based study provides new insights into the extend of the delays in the first steps in the HIV continuum of care among migrants. The strengths of our study include the population-based and nationwide design. In contrast to another Danish study, which only included refugees and family-reunified immigrants [9], our study included all migrants with residence in the country. The use of a population-based clinical database linked to Danish health and administrative registers allowed us to trace patients retrospectively from date of LTC without recall bias. Although this work was planned as a descriptive study and we did not include a multivariate analysis, the large population enabled us to stratify our analysis according to region of origin, calendar period and late presentation.
Our study had some limitations. The study design and number of migrants did not allow us to take socioeconomic differences within regions and migrant status into account. CRS registers arrival in Denmark as the date of obtaining official residence, which means we were unable to study undocumented migrants living in Denmark without residence and with limited access to healthcare. This may have led us to underestimate the true delay in HIV diagnosis among MLWH. We were unable to take post-migration HIV transmission fully into account, and the assumption that migrants from high prevalence regions are mostly HIV-infected before immigration may not always be correct [32]. Our study design only allowed us to include MLWH once they were linked to care. The number of MLWH diagnosed with HIV but never linked to care is most likely very small. It is mandatory to report HIV infections, and HIV care is very structured in Denmark. However, presumably, a number of MLWH with short stays in Denmark have maintained contact with the healthcare system in their home country, and consequently their HIV status would not be registered in Danish national registries.
Conclusion
This nationwide study of MLWH in Denmark demonstrates that, while almost every aspect of HIV healthcare has improved greatly over the last decades, we still face the challenge that many MLWH, including those with late presentation and presentation with advanced HIV disease, have resided years in Denmark before being diagnosed with HIV infection. These results point to missed opportunities for both detection and reducing late presentation among MLWH in Denmark. Our findings call for an increased effort to systematically implement voluntary HIV testing, and to make it more accessible for migrants – especially for those arriving in Denmark from high HIV prevalence areas and for women. Work should be done to investigate and combat the barriers preventing migrants from seeking timely HIV testing, to close this crucial gap in the HIV continuum of care.
Ethical statement
According to Danish law, registry-based studies do not require approval from an ethics board. The study was approved by the Danish Data Protection Agency (registration number: P-2021-446). All data were anonymised.
Supplementary Data
Conflict of interest: OB has received financial support for participation in an HIV conference from MSD. The other authors declare no competing interests.
Authors’ contributions: OB, LHO, NO, and AEH conceived the idea and designed the study. JG, CSL, ISJ, SL, JJ, and NO collected the data. OB and LHO did the statistical analysis. OB and AEH wrote the first draft of the manuscript. All other authors revised the manuscript critically for important intellectual content. All authors approved the final version of the manuscript.
References
- 1. Del Amo J, Likatavičius G, Pérez-Cachafeiro S, Hernando V, González C, Jarrín I, et al. The epidemiology of HIV and AIDS reports in migrants in the 27 European Union countries, Norway and Iceland: 1999-2006. Eur J Public Health. 2011;21(5):620-6. 10.1093/eurpub/ckq150 [DOI] [PubMed] [Google Scholar]
- 2. Hernando V, Alvárez-del Arco D, Alejos B, Monge S, Amato-Gauci AJ, Noori T, et al. HIV Infection in Migrant Populations in the European Union and European Economic Area in 2007-2012: An Epidemic on the Move. J Acquir Immune Defic Syndr. 2015;70(2):204-11. 10.1097/QAI.0000000000000717 [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization (WHO). World Health Organization Regional Office for Europe: Migration and Health. Geneva: WHO. [Accessed: 9 Mar 2022]. Available from: https://www.who.int/europe/health-topics/refugee-and-migrant-health#tab=tab_1
- 4.Statens Serum Institut (SSI). Hiv - opgørelse over sygdomsforekomst 2019. [HIV - inventory of disease incidence 2019]. Copenhagen: SSI. [Accessed: 12 Apr 2021]. Danish. Available from: https://www.ssi.dk/sygdomme-beredskab-og-forskning/sygdomsovervaagning/h/hiv-2019
- 5.Danmarks Statistik. Danmarks Statistik: Folketal den 1. i kvartalet efter tid og herkomst. [FOLK1E: Population at the first day of the quarter by region, sex, age and ancestry.] Copenhagen: StatBank Denmark. [ Accessed: 12 April 2021]. Available from: https://www.statbank.dk/statbank5a/SelectVarVal/Define.asp?Maintable=FOLK1E&PLanguage=0
- 6. Desgrees-du-Lou A, Pannetier J, Ravalihasy A, Le Guen M, Gosselin A, Panjo H, et al. Is hardship during migration a determinant of HIV infection? Results from the ANRS PARCOURS study of sub-Saharan African migrants in France. AIDS. 2016;30(4):645-56. 10.1097/QAD.0000000000000957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fakoya I, Álvarez-del Arco D, Woode-Owusu M, Monge S, Rivero-Montesdeoca Y, Delpech V, et al. A systematic review of post-migration acquisition of HIV among migrants from countries with generalised HIV epidemics living in Europe: mplications for effectively managing HIV prevention programmes and policy. BMC Public Health. 2015;15(1):561. 10.1186/s12889-015-1852-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mocroft A, Lundgren JD, Sabin ML, Monforte A, Brockmeyer N, Casabona J, et al. Risk factors and outcomes for late presentation for HIV-positive persons in Europe: results from the Collaboration of Observational HIV Epidemiological Research Europe Study (COHERE). PLoS Med. 2013;10(9):e1001510. 10.1371/journal.pmed.1001510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Deen L, Cowan S, Wejse C, Petersen JH, Norredam M, ESCMID Study Group for Infections in Travellers and Migrants . Refugees and family-reunified immigrants have a high incidence of HIV diagnosis and late presentation compared with Danish born: a nationwide register-based cohort study. Infection. 2018;46(5):659-67. 10.1007/s15010-018-1167-8 [DOI] [PubMed] [Google Scholar]
- 10. Alvarez-del Arco D, Monge S, Azcoaga A, Rio I, Hernando V, Gonzalez C, et al. HIV testing and counselling for migrant populations living in high-income countries: a systematic review. Eur J Public Health. 2013;23(6):1039-45. 10.1093/eurpub/cks130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fakoya I, Álvarez-Del Arco D, Copas AJ, Teixeira B, Block K, Gennotte AF, et al. Factors associated with access to HIV testing and primary care among migrants living in Europe: Cross-sectional survey. JMIR Public Health Surveill. 2017;3(4):e84. 10.2196/publichealth.7741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nkulu Kalengayi FK, Hurtig A-K, Ahlm C, Krantz I. Fear of deportation may limit legal immigrants’ access to HIV/AIDS-related care: a survey of Swedish language school students in Northern Sweden. J Immigr Minor Health. 2012;14(1):39-47. 10.1007/s10903-011-9509-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sobrino-Vegas P, Moreno S, Rubio R, Viciana P, Bernardino JI, Blanco JR, et al. Impact of late presentation of HIV infection on short-, mid- and long-term mortality and causes of death in a multicenter national cohort: 2004-2013. J Infect. 2016;72(5):587-96. 10.1016/j.jinf.2016.01.017 [DOI] [PubMed] [Google Scholar]
- 14. Lundgren JD, Babiker AG, Gordin F, Emery S, Grund B, Sharma S, et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med. 2015;373(9):795-807. 10.1056/NEJMoa1506816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Deblonde J, Sasse A, Del Amo J, Burns F, Delpech V, Cowan S, et al. Restricted access to antiretroviral treatment for undocumented migrants: a bottle neck to control the HIV epidemic in the EU/EEA. BMC Public Health. 2015;15(1):1228. 10.1186/s12889-015-2571-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493-505. 10.1056/NEJMoa1105243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joint United Nations Programme on HIV/AIDS (UNAIDS). 90-90-90 - An ambitious treatment target to help end the AIDS epidemic. Geneva: UNAIDS. 2014. Available from: https://www.unaids.org/sites/default/files/media_asset/90-90-90_en.pdf
- 18. Okano JT, Robbins D, Palk L, Gerstoft J, Obel N, Blower S. Testing the hypothesis that treatment can eliminate HIV: a nationwide, population-based study of the Danish HIV epidemic in men who have sex with men. Lancet Infect Dis. 2016;16(7):789-96. 10.1016/S1473-3099(16)30022-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Okano JT, Gerstoft J, Obel N, Blower S. HIV elimination and population viral load. Lancet HIV. 2016;3(11):e507-9. 10.1016/S2352-3018(16)30174-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Whittaker R, Case KK, Nilsen Ø, Blystad H, Cowan S, Kløvstad H, et al. Monitoring progress towards the first UNAIDS 90-90-90 target in key populations living with HIV in Norway. BMC Infect Dis. 2020;20(1):451. 10.1186/s12879-020-05178-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kronfli N, Linthwaite B, Sheehan N, Cox J, Hardy I, Lebouché B, et al. Delayed linkage to HIV care among asylum seekers in Quebec, Canada. BMC Public Health. 2019;19(1):1683. 10.1186/s12889-019-8052-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sundhedsstyrelsen. Vejledning om HIV (human immundefekt virus) og hepatitis b og c virus. Forebyggelse af blodbåren smitte, diagnostik og håndtering i sundhedsvæsenet og på andre arbejdspladser. [Prevention of bloodborne transmission, diagnostics and management in the healthcare system and in other workplaces]. Copenhagen: Sundhedsstyrelsen; 2013. Available from: https://www.sst.dk/-/media/Udgivelser/2013/Publ2013/Vejledning-om-HIV-hepatitis-B-og-C-virus.ashx
- 23. Omland LH, Ahlström MG, Obel N. Cohort profile update: the Danish HIV Cohort Study (DHCS). Int J Epidemiol. 2014;43(6):1769-9e. 10.1093/ije/dyu153 [DOI] [PubMed] [Google Scholar]
- 24.United Nations (UN) Statistics Division. Methodology. Standard country or area codes for statistical use (M49). Countries or areas / geographical regions. New York: UN. [Accessed: 30 Oct 2019]. Available from: https://unstats.un.org/unsd/methodology/m49
- 25. Antinori A, Coenen T, Costagiola D, Dedes N, Ellefson M, Gatell J, et al. Late presentation of HIV infection: a consensus definition. HIV Med. 2011;12(1):61-4. 10.1111/j.1468-1293.2010.00857.x [DOI] [PubMed] [Google Scholar]
- 26. Brännström J, Svedhem Johansson V, Marrone G, Wendahl S, Yilmaz A, Blaxhult A, et al. Deficiencies in the health care system contribute to a high rate of late HIV diagnosis in Sweden. HIV Med. 2016;17(6):425-35. 10.1111/hiv.12321 [DOI] [PubMed] [Google Scholar]
- 27.Statista Research Department. Migration flow in Denmark from 2011 to 2021. Statista. [Accessed: 9 Mar 2022]. Available from: https://www.statista.com/statistics/575189/migration-flow-in-denmark
- 28. Marukutira T, Gray RT, Douglass C, El-Hayek C, Moreira C, Asselin J, et al. Gaps in the HIV diagnosis and care cascade for migrants in Australia, 2013-2017: A cross-sectional study. PLoS Med. 2020;17(3):e1003044. 10.1371/journal.pmed.1003044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Joint United Nations Programme on HIV/AIDS (UNAIDS). UNAIDS data 2020. Geneva: UNAIDS; 2020. Available from: https://www.unaids.org/sites/default/files/media_asset/2020_aids-data-book_en.pdf [PubMed]
- 30. Qvist T, Cowan SA, Graugaard C, Helleberg M. High linkage to care in a community-based rapid HIV testing and counseling project among men who have sex with men in Copenhagen. Sex Transm Dis. 2014;41(3):209-14. 10.1097/OLQ.0000000000000096 [DOI] [PubMed] [Google Scholar]
- 31. Martin-Iguacel R, Pedersen C, Llibre JM, Søndergaard J, Jensen J, Omland LH, et al. Primary health care: an opportunity for early identification of people living with undiagnosed HIV infection. HIV Med. 2019;20(6):404-17. 10.1111/hiv.12735 [DOI] [PubMed] [Google Scholar]
- 32. Yin Z, Brown AE, Rice BD, Marrone G, Sönnerborg A, Suligoi B, et al. Post-migration acquisition of HIV: Estimates from four European countries, 2007 to 2016. Euro Surveill. 2021;26(33):26. 10.2807/1560-7917.ES.2021.26.33.2000161 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.