Abstract
Amphotericin B (AmB) is a powerful but toxic fungicide that operates via enigmatic small molecule–small molecule interactions. This mechanism has challenged the frontiers of structural biology for half a century. We recently showed AmB primarily forms extramembranous aggregates that kill yeast by extracting ergosterol from membranes. Here, we report key structural features of these antifungal ‘sponges’ illuminated by high-resolution magic-angle spinning solid-state NMR, in concert with simulated annealing and molecular dynamics computations. The minimal unit of assembly is an asymmetric head-to-tail homodimer: one molecule adopts an all-trans C1–C13 motif, the other a C6–C7-gauche conformation. These homodimers are staggered in a clathrate-like lattice with large void volumes similar to the size of sterols. These results illuminate the atomistic interactions that underlie fungicidal assemblies of AmB and suggest this natural product may form biologically active clathrates that host sterol guests.
AmB is an archetypical clinically approved drug that exerts its biological activities via small molecule–small molecule interactions and higher-order assembly. Small molecule aggregation further impacts the aqueous solubility, plasma compatibility, bioavailability and toxicity of many other drugs, and causes a wide range of nonspecific biological effects in in vitro assays that can confound the drug discovery process1. It also plays key roles in the lateral heterogeneity of lipid bilayers2, supramolecular chemistry3 and molecular prosthetics4,5. The structural and biophysical underpinnings of small molecule aggregation in biologically relevant contexts remain poorly understood, and this has broadly limited rational drug discovery and optimization in this important space. This is particularly true in the case of AmB.
Over one million invasive fungal infections occur annually, primarily affecting immunocompromised patients6. In recent years, this at-risk patient population has substantially grown due to improved therapies for diseases such as cancer and human immunodeficiency virus6. First-line treatments are well tolerated, but are susceptible to microbial resistance7. For example, the current first-line therapeutic for invasive aspergillosis, as well as most other invasive mold infections, is the triazole antifungal voriconazole8. However, pan-triazole resistance in Aspergillus is as high as 30% in some locations and certain high-risk patient groups9. The polyene macrolide natural product AmB (Fig. 1a) has remained the last line of defense for treating life-threatening invasive fungal infections due to its broad-spectrum activity and lack of clinically impactful resistance10. However, AmB is also highly toxic, especially to the kidneys, which invariably limits its dose and therefore efficacy. Liposomal AmB preparations with somewhat reduced renal toxicity have been developed, but remaining toxicity issues and disparate access to the more expensive formulations result in regional mortality rates ranging from 30% to 70%6,11,12. An international expert panel recently concluded that novel therapeutic approaches centered around AmB are urgently needed13.
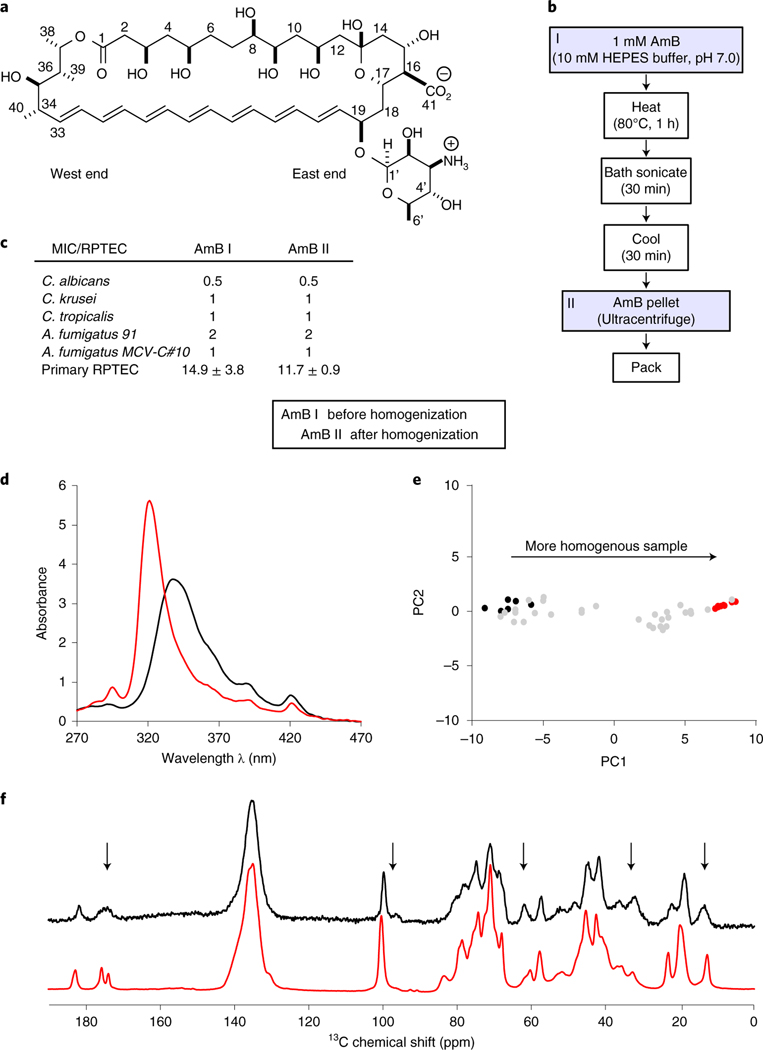
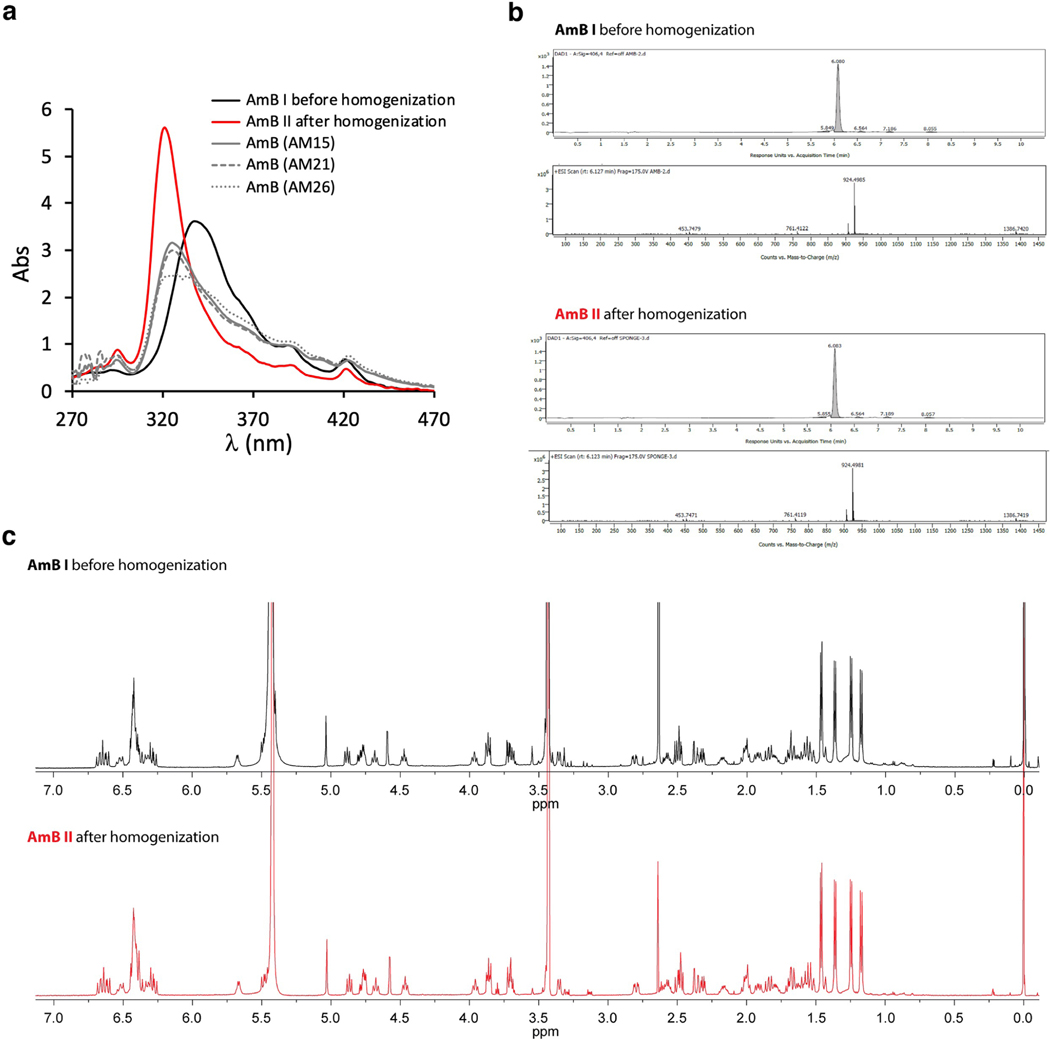
Fig. 1 |. An improved AmB sponge preparation technique that reproducibly yields homogeneous SSNMR samples.
a, Molecular structure of AmB polyene macrolide. b, Schematic representation of the process for obtaining homogenous AmB assemblies for SSNMR spectroscopy experiments. First, 1 mM AmB aggregate in 10 mM HEPES buffer pH 7.0 is prepared. This sample is then heated at 80 °C for 1 h, followed by bath sonication for 30 min and cooling at room temperature for another 30 min. Next, the sample is pelleted in the ultracentrifuge and spectrophotometrically analyzed before packing it into the SSNMR rotor. c, Biological analysis (MIC and MTC) of the AmB aggregates before (AmB I) and after (AmB II) homogenization. MIC against yeast pathogens (C. albicans, C. krusei, C. tropicalis and A. fumigatus) and MTC values causing 90% toxicity against human primary RPTECs ± percent error standard deviation. MIC and MTC values are given in μM. d, UV–Vis spectra of AmB aggregates before (black) and after (red) homogenization. e, Sample quality validation by PCA of the UV spectrum. PC1/PC2 projection of the heterogeneous (black) and homogenous (red) samples that were obtained after homogenization. f, 13C 1D SSNMR spectra (with 50 Hz line broadening) of the [U-13C]AmB before (black) and after (red) homogenization. Black arrows represent carbon sites with changes in resolution.
More than half a century of widespread efforts to improve the therapeutic index of AmB via chemical modification have failed to yield a clinically viable solution. Until recently, many of these efforts were guided by a mechanistic model in which membrane permeabilization was thought to be primarily responsible for both the fungicidal and toxic side effects of AmB. Extensive biophysical and computational modeling studies were interpreted through the lens of this mechanism, yielding a range of structural models for the corresponding AmB ion channel complex that were widely used to guide drug discovery efforts14–17.
Challenging this membrane permeabilization model, we deployed Lego-like synthesis with MIDA boronate building blocks to prepare a C35 deoxygenated derivative of AmB that binds to ergosterol but does not form ion channels18. Remarkably, this non-channel forming variant of AmB retains robust fungicidal activity. This showed that, in contrast to the long-standing mechanistic model, membrane permeabilization is not required for the antifungal action of AmB. It also suggested that sterol binding was sufficient18–20. To further probe the latter, we performed a series of solid-state NMR (SSNMR), transmission electron microscopy (TEM) and cell-based experiments that demonstrated AmB primarily forms large extramembranous aggregates that extract ergosterol from lipid bilayers. We further showed in yeast that AmB-mediated ergosterol extraction is fast (>80% of membrane ergosterol is removed in less than 30 min) and quickly followed by fungal cell death. Precomplexing AmB with ergosterol blocked both ergosterol extraction and fungicidal action21. Recent studies showed that this ‘sterol sponge’ mechanism is conserved throughout the very large family of glycosylated polyene macrolide natural products22.
Here, we report an extensive series of high-resolution SSNMR experiments in concert with density functional theory (DFT) chemical shift calculations23,24, XPLOR-NIH simulated annealing25 and molecular dynamics calculations that yielded a high-resolution structure of the small molecule–small molecule interactions that underlie fungicidal AmB sponges. The minimal unit of assembly is an asymmetric head-to-tail homodimer, in which one subunit adopts an all-trans conformation for the C1–C13 region and the other adopts a C6–C7-gauche conformation. Unambiguous intermolecular restraints, including numerous 13C–13C and precise 14N–13C distances that identified interdimer salt bridges, further revealed a staggered organization of these asymmetric homodimers in a clathrate-like lattice with large void volumes similar in size to sterol molecules. These key structural features collectively suggest that AmB forms biologically active clathrates that host sterol guests, thus providing a structural basis for the sterol sponge mechanism of action.
Results
Homogeneous preparations of fungicidal AmB.
We developed a new method to obtain microscopically well-ordered, homogeneous AmB samples with >80% 13C incorporation21 (Methods) that retain full fungicidal activity and also yield high-quality resolved SSNMR data suitable for structure determination. The protocol (Fig. 1b and Methods) utilizes heating, sonication, cooling and ultracentrifugation to yield milligram quantities of biologically active AmB sponges.
To test the effect of this sample preparation on the biological activity of AmB, we investigated the in vitro antifungal activity and toxicity of homogenized AmB sponges. Minimum inhibitory concentrations (MICs) of AmB were evaluated against various strains of Candida and Aspergillus and determined to be the same (within experimental error) before and after sponge homogenization (Fig. 1c). The toxicity of AmB was evaluated against primary human renal proximal tubule epithelial cells (RPTECs) and again determined to be the same within error (Fig. 1c). Thus, homogenized AmB sponges retain both biological activities. We also found that this homogenization procedure does not cause chemical modification of AmB (Extended Data Fig. 1).
We measured the ultraviolet and visible (UV–Vis) spectra of each sample batch (Fig. 1d) and analyzed the data objectively by principal component analysis (PCA) (Fig. 1e). Principal component 1 (PC1) corresponded to a major characteristic shift and narrowing of the absorption band, from 342 nm (black line Fig. 1d) in the case of less-homogeneous AmB samples to 321 nm in the homogeneous samples (red line Fig. 1d). Thus, the PCA of AmB sample UV–Vis spectra conveniently allowed us to use the hypsochromic shift in UV–Vis absorption as a validation of sample homogeneity before SSNMR analysis. The SSNMR spectra before and after homogenization (Fig. 1f) show similar overall patterns of peak positions and intensities, indicating a conservation of the major structural features. Importantly, however, the homogenized sample yields narrower 13C SSNMR linewidths, higher signal-to-noise ratios, reduction in the intensities of signals arising from minor species and an overall improvement in spectral quality, enabling a full structure determination using state-of-the-art SSNMR methods.
SSNMR chemical shifts reveal two conformations.
NMR chemical shifts depend strongly upon conformation, and this phenomenon has been widely used to study protein structure by NMR26,27. In the case of small molecules, quantum chemistry can elucidate exceptional levels of detail in the hybridization, conformation and ionization events that drive molecular interactions28. Thus, we utilized the computationally rigorous approach of performing ab initio quantum mechanical shielding calculations29, which are especially sensitive to conformation along sp3-hybridized chains of carbons29,30. An important early example is the observation of conformation-dependent 13C chemical shifts from so-called ‘gamma gauche’ effects31. Thus, isotropic chemical shifts alone provide powerful structural insights, and we obtained the 13C assignments for AmB through a series of two-dimensional (2D) and three-dimensional (3D) spectra for unambiguous assignment. We also performed measurements to emphasize only the one- and two-bond correlations (utilizing double quantum super-cycled permutationally offset stabilized C element (SPC) mixing at defined times32,33) as well as longer broadband zero quantum mixing schemes34 to identify correlations over three to six bonds. The resulting high-resolution spectra clearly distinguish two unique, complete chains of C1–C13 resonances (Fig. 2a).
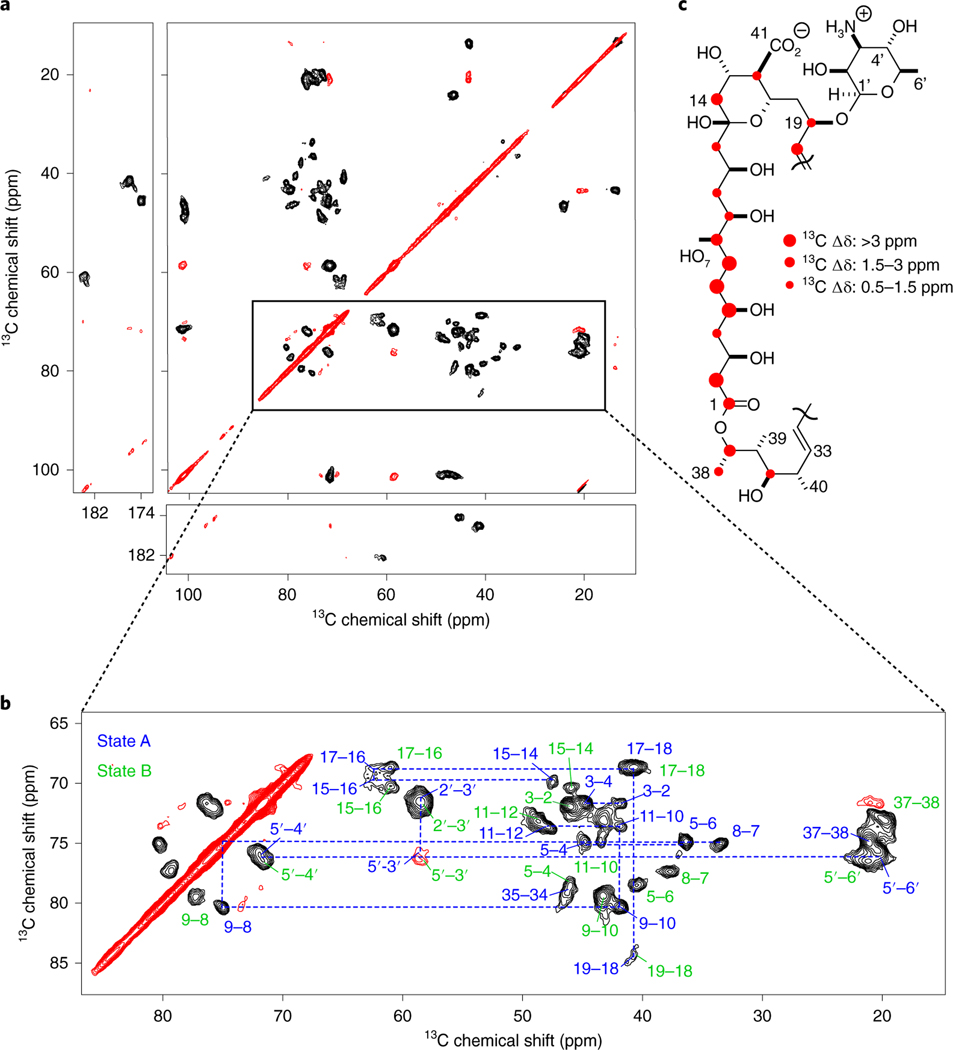
Fig. 2 |. AmB exists in two states.
a, 13C–13C 2D SPC mixing spectrum of the homogenous AmB sample. b, AmB C1–C13 region showing 1- and 2-bond correlations that allowed for assigning two AmB populations. Chemical shift assignments of state A and state B are presented in blue and green, respectively. c, Graphical representation of the 13C chemical shift differences between two states of AmB. The biggest changes are observed on carbons 2, 5, 6 and 7 of the C1–C13 region.
Most immediately evident in Fig. 2b, two distinct C8–C9 correlations (arising from the unique pair of neighboring, hydroxy-bearing carbons) establish a starting point for tracing the 13C assignments throughout each C1–C13 region. Thus, we assigned two complete sets of resonances, corresponding to two states (A and B). Interestingly, the two states exhibit large differences in chemical shifts along the C1–C13 region, especially for C2, C5, C6 and C7. These chemical shift differences (Fig. 2c) of >3 ppm clearly indicate a different conformation of the two C1–C13 chains. By contrast, signals for the remainder of AmB show only modest differences, indicating that in both states A and B the polyene, the termini and mycosamine rings have similar conformations.
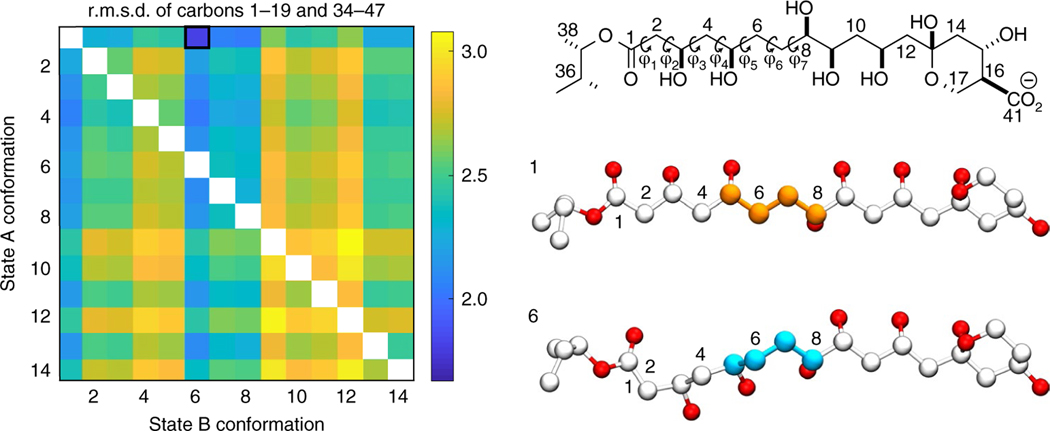
To interpret the observation of two distinct conformations in the C1–C13 regions, we performed DFT calculations of the feasible C1–C13 conformations. Specifically, we generated a library of 14 conformers for each molecule (the complete set of energetically feasible conformations) and calculated their 13C chemical shifts using the mPW1PW91 (ref. 35) level of theory and 6–311G(d,p)36 basis set. Among the 196 possible combinations of theoretical chemical shifts (14 for each molecule in the asymmetric pair), we found an exceptionally good match with the observed experimental shifts for a unique pair of model structures (Fig. 3 and Supplementary Fig. 3): one molecule in an all-trans C1–C13 conformation, (structure number 1, hereafter referred to as state A) and a second with a prominent gamma gauche effect; that is, at the C5–C6–C7–C8 dihedral (structure number 6, hereafter referred to as state B).
Fig. 3 |. DFT calculations of AmB state A and state B conformations.
Heatmap displaying the r.m.s.d. (from blue as the lowest and yellow as the highest r.m.s.d.) between experimentally measured and calculated chemical shifts for carbon atoms 1–19 and 34–47 for pairs of AmB structures with varied conformations of the C1–C13 regions labeled 1–14. Based on the r.m.s.d. comparisons in the heatmap, the best pair of structures contains structure number 1 as state A and structure number 6 as state B. Carbons 5–8 are highlighted to illustrate the difference in C1–C13 region dihedral geometry between state A and state B.
Head-to-tail and end-to-mid-C1–C13 region interactions.
With the secondary structure in hand, we next performed an extensive series of dipolar SSNMR measurements to obtain more than 200 unique 13C–13C and 14N–13C distance restraints (Table 1). First, we used dipolar-assisted rotational resonance (DARR) spectra34 to resolve 933 cross-peaks and included mixing times (500 ms) long enough to detect distances up to at least 6.5 Å. We confirmed the medium range distances with more precise proton-assisted recoupling (PAR)37 spectra, targeting C1–C13 region–polyene and inter-C1–C13 region interactions. We assigned and classified 1,373 total peaks into distance ranges based on observed intensities: very strong (<4.0 Å), strong (<5.5 Å), medium (<6 Å) and weak (<6.5 Å). As a final confirmation for a select set of especially important carbons, we also prepared AmB from 1-13C-glucose, yielding fractional (∼16% 13C) labeling patterns that we quantified by solution NMR (Supplementary Fig. 2b). Then, with 2D 13C–13C spectra at very long mixing times (1,000 ms) we again observed more than 200 specific peaks with higher certainty. In total, 14 high-resolution SSNMR spectra on three samples revealed >5,000 cross-peaks and enabled independent validation of the assignments.
Table 1 |.
NMR and refinement statistics
| AmB lattice | |
|---|---|
| NMR distance and dihedral restraints per lattice | |
| Distance restraints | |
| Total | 221 |
| 13C–13C restraints | 214 |
| Up to 5.5 Å | 64 |
| Up to 6.5 Å | 150 |
| Precise PM-RESPDOR 13C–14N | 3 |
| Unambiguous intermolecular (intradimer) restraints | 4 |
| Total dihedral-angle restraints | 94 |
| Structure statistics from 10% lowest energy structures | |
| Violations (mean ± s.d.) | 9.2 ± 1.4 |
| Distance restraints ≥ 5 Å (Å) | 1.3 ± 1.4 |
| Dihedral-angle restraints ≥ 5° (°) | 0.0 ± 0.0 |
| Bond angle restraints | 7.9 ± 0.3 |
| Improper restraints | 0.0 ± 0.0 |
| Deviations from idealized geometry | |
| Max dihedral-angle violation (°) | 10.11 |
| Average r.m.s.d. of 10 lowest energy structures (Å) | 0.65a |
Overlayed over segid 111A (the center monomer A of the 3 × 3 × 3 lattice).
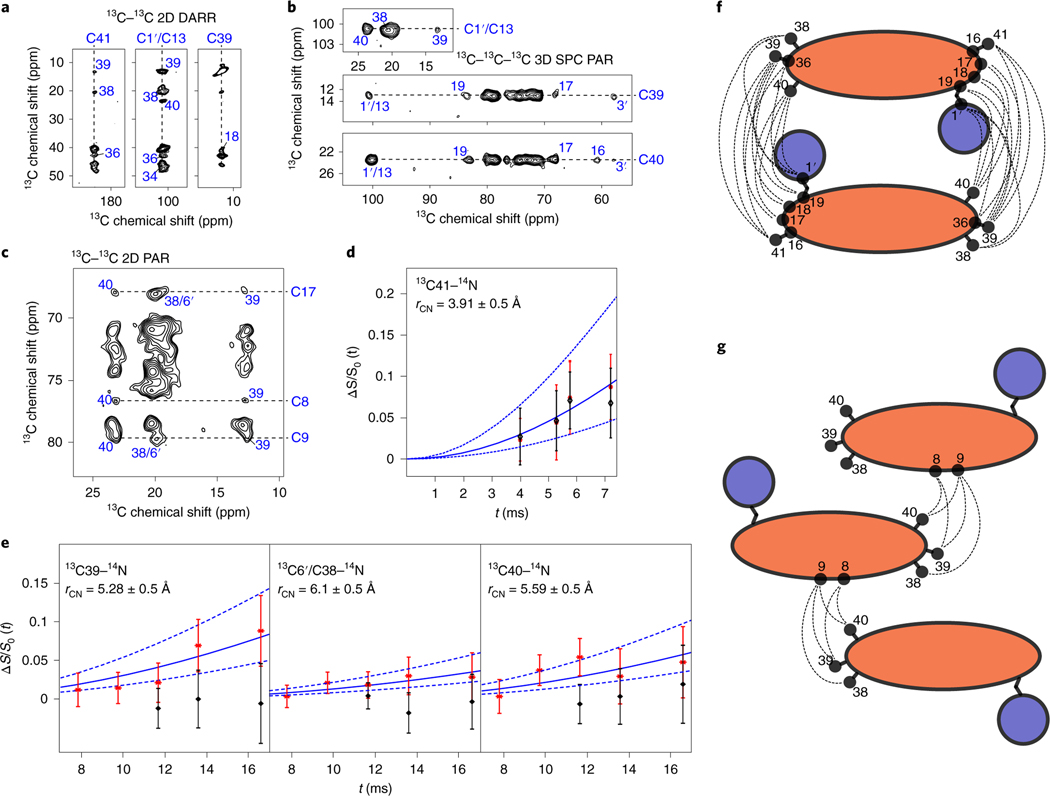
Notably, many of the strong peaks directly report on intermolecular distances. For example, cross-peaks between the west-end methyl groups (C38, C39 and C40) and the east-end carboxylate group (C41) and carbons in the tetrahydropyran ring (C17 and C19) are repeatedly observed (Fig. 4a-c and supplementary Fig. 4); the intramolecular distances between the west- and east-end carbons are >15 Å, well beyond the range of the measurements performed, and so these peaks strongly suggested a head-to-tail arrangement of AmB homodimers. Because we observed two separate sets of chemical shifts for state A and state B, we could also identify additional inter- and intramolecular restraints that would otherwise have required differentially labeled AmB molecules. For example, we observed cross-peaks, only at long mixing times, between the C1–C13 region of state A and the C1–C13 region of state B, as well as the west end of state A to the C1–C13 region of state B and vice versa. A series of specialized pseudo 2D phase-modulated rotational-echo saturation-pulse double resonance (PM-RESPDOR) (or modified low-amplitude rotational-echo double resonance (LA-REDOR) experiments38,39 (Supplementary Fig. 5a) were also performed that revealed carbon–nitrogen distances between 13C41, 13C38/13C6′, 13C39 and 13C40, and the 14N on the mycosamine ring. These patterns could not be fully explained with simple dimer models containing only two molecules; indeed, many of these cross-peaks between molecules were greatly diminished in intensity in a preparation of 13C AmB diluted in natural abundance AmB. Therefore, it was essential to consider explicitly the arrangement of the sponge lattice using advanced computational approaches.
Fig. 4 |. SSNMR experiments indicate an asymmetric AmB homodimer structure.
a–c, Representative regions of the SSNMR spectra: 13C–13C 2D 1,000 ms DARR spectrum of the homogenous [13C] skip-labeled AmB (a); 13C–13C–13C 3D SPC PAR spectrum of the homogenous [U-13C]AmB (b); and 13C–13C 2D 11 ms PAR spectrum of the homogenous [U-13C]AmB showing head-to-tail interactions between AmB molecules as well as interactions between AmB C1–C13 middle region and the tail methyls (c); namely, between C41 and C36, C38, C39; C1′/C13 and C34, C36, C38, C39, C40; C39 and C18 (a); between C1′/C13 and C38, C39, C40; C39 and C1′/C13, C3′, C17, C19; C40 and C1′/C13, C3′, C16, C17, C19 (b); and between C17 and C38, C39, C40; C8 and C39, C40; C9 and C40, C38, C39 (c). d,e, PM-RESPDOR experiments of homogenized [13C] skip-labeled AmB. d, experimental ΔS/S0(t) dipolar curves of 13C41 (183 ppm)–14N under two different lengths of PM-RESPDOR pulses: tPM = 14TR and tPM = 24TR (black diamonds). Solid blue lines represent Bessel function (equation (1.1)) with optimal distance of 3.91 Å (D = 36.49 Hz). ν1 = 25kHz, νR = 12.5kHz. Dashed blue lines represent the cases when rCN = 3.41 and 4.41 Å, respectively. e, experimental ΔS/S0(t) curves (red stars) of 13C39 (11 ppm)–14N, 13C6′/13C38 (18 ppm)–14N and 13C40 (23 ppm)–14N, and their comparison with Bessel function (blue solid line) with optimal distances: r = 5.28 Å (D = 14.82 Hz), r = 6.1 Å (D = 9.61 Hz) and r = 5.59 Å (D = 12.49 Hz), respectively. For d and e, distances were found using least squares fitting (Levenberg–Marquardt method). Blue dashed lines represent Bessel functions with ±0.5 Å of deviation in each case, which were estimated according to the experimental errors. experimental parameters for red stars were: v1 = 25 kHz and tPM = 14TR. The first two black diamond points represent the experimental curves with v1= 0 kHz during S part. The last point (for each methyl) was obtained when the PM-RESDOR pulse was applied before proton excitation. The experimental errors were defined as the noise level intensities of the 1D 13C spectra, where the zero signals were expected. f,g, Graphical illustrations of the intermolecular interactions: f for panels a and b, and g for panel c.
Head-to-tail AmB homodimers are staggered in a lattice.
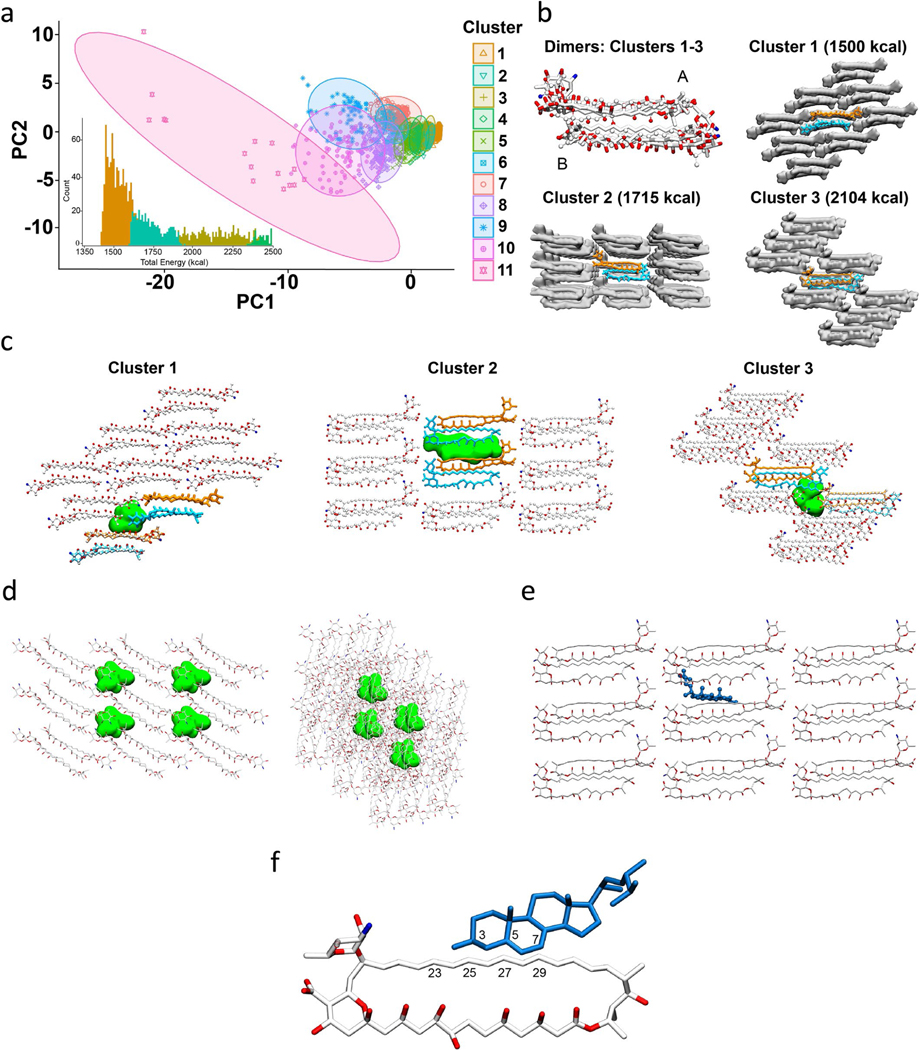
To compute the lattice structure of AmB from SSNMR distance and chemical shift restraints, we performed simulated annealing calculations in XPLOR-NIH25 utilizing the strict symmetry facility recently developed by Schwieters and coworkers40. We thereby incorporated 54 AmB monomers (27 of state A and 27 of state B) as the initial condition for the XPLOR-NIH calculation, with a random distance and orientation for each molecule. Symmetry conditions were defined (see Supporting Information for details) to comply with the knowledge that only two states were present, and the SSNMR distances served as the potential energy function to drive assembly of the sponge lattice. This procedure yielded structures that accounted for >95% of the observed experimental distances. Violations were inspected and removed according to standard NMR data analysis procedures. To classify the resulting data, we performed partitioning around medoids clustering analysis41 on the XPLOR-NIH lowest energy AmB lattices (Extended Data Fig. 2a and Supplementary Fig. 6a). The three lowest energy clusters captured more than 50% of all structures. Although there are small differences in the dimer packing, the overall arrangements within the three lowest energy lattices are similar (Extended Data Fig. 2b and Supplementary Fig. 6b).
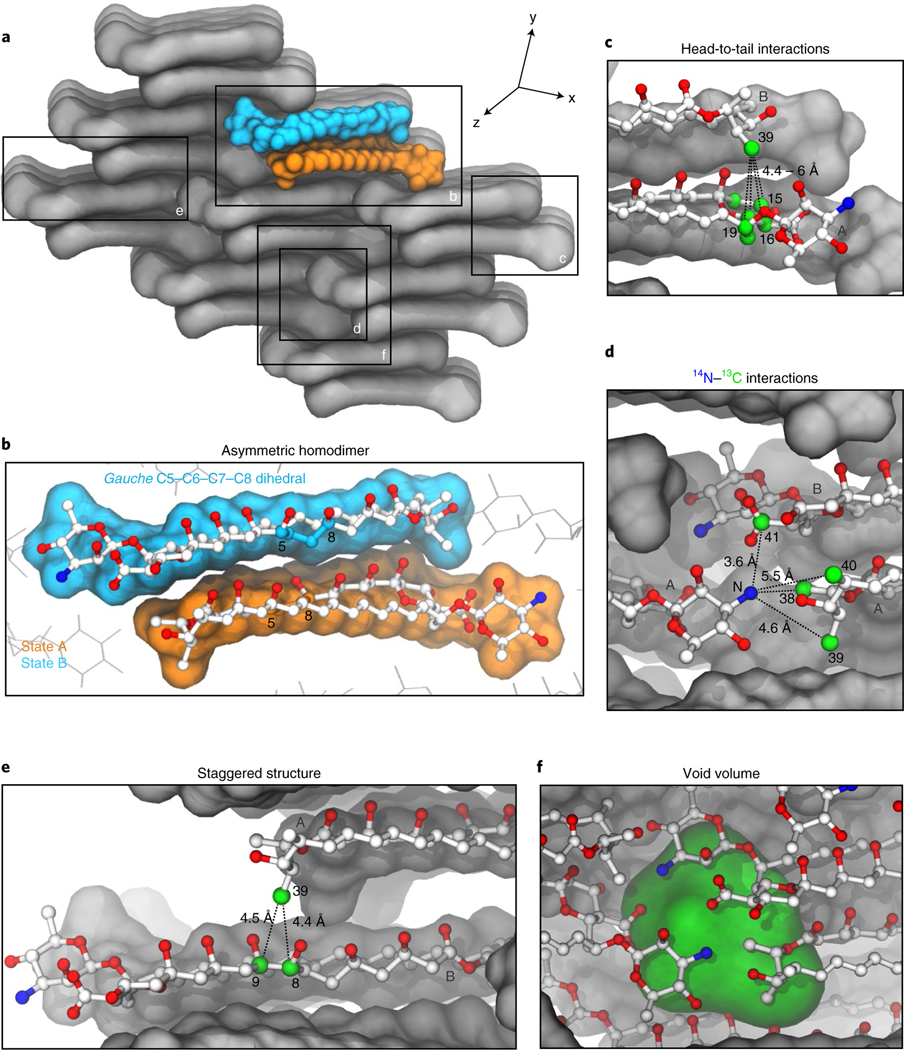
A structure, representative of the ten lowest energy solutions, of the AmB sponge is shown in Fig. 5 (see also Extended Data Fig. 2 and Supplementary Fig. 6). Although all structural analysis techniques, including SSNMR, have limitations, this structure is consistent with the extensive set of restraints that were generated in this study. The calculation was free to consider many possible oligomerization states, and all ten of the lowest energy structures that were calculated support dimers of AmB arranged into a higher-order clathrate-like lattice.
Fig. 5 |. XPLoR-NIH AmB sponge structure.
a, Lowest distance restraint energy (6.8 kcal) XPLOR-NIH structure of the AmB sponge (3 × 3 × 3) lattice which consists of asymmetric head-to-tail homodimers. b, Minimal head-to-tail asymmetric unit of the AmB sponge. State A is shown in orange and state B in blue. In state B, the gauche C5–C6–C7–C8 dihedral is outlined in blue. c, Representative head-to-tail interactions between AmB molecules in the lattice derived from 13C–13C SSNMR experiments (Fig. 4a–c and Supplementary Fig. 4). d, Representative interactions between 14N and 13C derived from PM-RESPDOR experiments (Fig. 4d). e, Representative staggered interactions between AmB molecules in the lattice derived from 13C–13C SSNMR experiments (Fig. 4c). f, Representative void volume in the AmB lattice.
Remarkably, this structure satisfies all of the qualitative features anticipated above, as well as details reflecting the highly evolved details of AmB chemistry. First of all, the minimal unit of the AmB sponge is asymmetric, with one A unit and one B unit, head-to-tail homodimer (Fig. 5a). Similar asymmetric head-to-tail homodimers were observed as the core unit of all ten of the lowest energy solutions. The sponge exhibits both intradimer (Fig. 5b,c) and interdimer head-to-tail interactions, including well-defined intermolecular mycosamine–carboxylate (N–C41) salt bridges (Fig. 5d). Neighboring homodimers are staggered along the y axis by approximately half the length of AmB (Fig. 5e). This staggered arrangement yields large void volumes between homodimer units that are similar in size to sterol molecules (Fig. 5d, Extended Data Fig. 2c and Supplementary Fig. 6c). Moreover, alignment of the medoid structures for clusters 1–3 revealed similar, low-energy dimer structures with variations in the register shifts and packing orientation of dimers in the resulting lattice structures (Extended Data Fig. 2b and Supplementary Fig. 6b). This variation in registry translates into variations in size and shape of the corresponding void volumes (Extended Data Fig. 2c and Supplementary Fig. 6c), and an ergosterol molecule was readily docked into the void volume of the medoid structure for cluster 2 (Extended Data Fig. 2e,f and Supplementary Fig. 6e,f). This finding is consistent with our previously observed experimental data, showing that, in a complex of AmB and ergosterol, the polyene region of AmB has strong cross-peaks to ergosterol21.
Thus, the AmB sponge is comprised of asymmetric homodimers that are staggered in a higher-order clathrate-like lattice that may host sterol guests. The observation of structurally and energetically similar lattice structures with registry-shifted homodimers and correspondingly varied void volumes suggest that plasticity of the sponge lattice in concert with sterol binding, reminiscent of ligand-based conformational selection, may play an important role in the fungicidal mechanism of action of AmB.
Discussion
Understanding the fungicidal action of AmB has challenged the frontiers of structural biology for over 50 years. It has long been clear that small molecule–small molecule interactions are important, but relative to peptides and proteins, tools and methods for structurally characterizing biologically active small molecule assemblies, like the AmB–sterol sponge complex described herein that is responsible for the fungicidal activity of AmB. Given that AmB is also a unique example of a clinically used antimicrobial that has evaded resistance for half a century, the fact that its severe toxicity to humans continues to limit its efficacy in the clinic, and recent evidence that it may hold promise for the development of a molecular prosthetics approach for treating cystic fibrosis4,42, it is increasingly important to better understand the structural underpinnings that underlie the biological function of this extraordinary natural product.
Extensive protocols, databases and computational tools are available for solving the structures of protein complexes43. Nevertheless, structure determination of large, highly repetitive protein aggregates, such as amyloid fibrils44, remains challenging. Relative to proteins, even fewer established resources are available to support structure determination of small molecule aggregates. Thus, three points of innovation were necessary for this study. First, we found a new way to prepare AmB sponges that are both biologically active and sufficiently homogenous to enable high-resolution SSNMR analyses. This sample preparation methodology paired with our previously reported21 procedures for producing both uniformly and fractionally labeled AmB allowed us to obtain sufficient intra- and intermolecular SSNMR restraints. Second, we adapted and optimized SSNMR methods typically used in protein science, including 2D 13C–13C PAR experiments and 3D 13C–13C–13C SPC PAR experiments, to obtain intermolecular AmB aggregate restraints. We also advanced the PM-RESPDOR technique38,39 to obtain precise salt bridge restraints between 13C and the naturally abundant 14N of the mycosamine (AmB is difficult to 15N label). Third, we adapted XPLOR-NIH, a tool established primarily for determining protein structure by NMR, to perform simulated annealing calculations on this small molecule-based assembly. Our specific implementation of the XPLOR-NIH strict symmetry function on a lattice of AmB dimers (detailed in Methods and Supporting Information) provides a first-in-class framework for calculating the structure of a small molecule assembly characterized by a high degree of symmetry. All three of these innovations could readily be extended to other small molecule-based structural problems, including other polyene macrolides and polyene macrolactams22,45 that also interact with membrane sterols to further illuminate the resistance-refractory mode(s) of action of these natural products.
A key feature of the AmB sterol sponge clathrate structure is the presence of large void volumes the size of sterols. Specifically, the void volumes in clusters 1 and 2 are about the size of 1 and 2 ergosterol molecules, respectively. This suggests that the ratio of AmB to ergosterol in the bound state is either 1:1 or 1:2. We previously reported that there is an excess of AmB relative to ergosterol molecules per yeast cell at the MIC of AmB18. This further suggests an excess of ergosterol binding capacity under fungicidal conditions.
Previous SSNMR studies of AmB have been interpreted through the lens of the classic ion channel model14,15. Our findings enable a fresh look at these data through the alternative lens of the sterol sponge model21. For example, a previous SSNMR study identified large aggregates (20 nm) of AmB in the presence of lipid bilayers based on rotational correlation measurements46. This size is consistent with the large AmB sponge assemblies analyzed here as well as that described in our previous work21. Another SSNMR study using 13C–19F REDOR experiments identified both head-to-tail and head-to-head interactions of AmB molecules16 in the presence of sterol-free membranes. Both of these interactions are consistent with our structure of the AmB sponge in which head-to-tail homodimers of AmB can further interact to yield head-to-head interdimer interactions. Another key feature noted in previous studies is the role of the conspicuous amphoteric C3′ ammonium and C41 carboxylate groups of AmB, and it was suggested that these groups participate in salt bridges that stabilize ion channel assembly17,47. Here, we show that alternatively these groups form salt bridges between asymmetric homodimers in the higher-order AmB lattice. We also previously showed via synthesis and study of a C41-methyl variant of AmB, that such salt bridges are not critical for potent fungicidal activity19,20. Thus, although the observed interdimer salt bridges appear to be stabilizing, they are likely not required for the assembly or function of the fungicidal AmB sponge.
There are two reports describing X-ray crystal structures of an N-iodoacylated derivative of AmB (N-IA-AmB) (Supplementary Fig. 7a)48,49. We synthesized and tested the biological activity of this derivative and found that it has little or no fungicidal activity (Supplementary Fig. 7b). Notably, there are both similarities and differences between the previously reported crystal structures of N-IA-AmB and the herein reported structure of the biologically active AmB sponge.
With respect to similarities, in the crystal structures, the N-IA-AmB molecules are also arranged in head-to-tail homodimers stacked in a higher-order 3D lattice that contains large void volumes (Supplementary Fig. 7e,f). There are also five important differences. (1) In the AmB sponge structure the AmB molecules exist in two distinct conformations (state A with an all-trans configuration of the C1–C13 region and state B with C6–C7-gauche conformation) that are paired in asymmetric homodimers (Fig. 5a and Supplementary Fig. 7d), whereas in the crystal structure the N-IA-AmB monomers all exist in a single conformation with an all-trans configuration of the C1–C13 region (similar to state A) (Supplementary Fig. 7c). (2) In the AmB sponge the registry of AmB homodimers places the tail of the AmB molecule in one homodimer near the middle of the AmB in the neighboring homodimer (Fig. 5b), whereas in the crystal structure of N-IA-AmB the registry is shifted toward increased separation such that the tail of an N-IA-AmB molecule in one homodimer lies closer to the head of an N-IA-AmB molecule in the neighboring homodimer (Supplementary Fig. 7e). (3) The void volumes in the AmB sponge are discrete (Extended Data Fig. 2d and Supplementary Fig. 6d), whereas in the crystal structure of N-IA-AmB the void volumes are contiguous and form channels through the entire crystal lattice (Supplementary Fig. 7f). (4) The size of the void volumes in the AmB sponge range from 390 to 830 Å3 (Extended Data Fig. 2c and Supplementary Fig. 6c), whereas each of the void volumes in the crystal structure of N-IA-AmB are substantially larger (∼2,700 Å3) (Supplementary Fig. 7f). (5) The number density of the AmB sponge lattice is 6.36 molecules per 10,000 Å3, whereas the lattice in the crystal structure of N-IA-AmB is less dense (number density = 5.85 molecules per 10,000 Å3).
These differences provide further support for the aforementioned plasticity of the AmB sponge lattice and for potential conformational changes upon sterol binding. Specifically, they suggest that covalent addition of the sterically bulky N-iodoacyl group promotes selection of energetically accessible macrocycle and lattice conformers analogous to that which might occur upon sterol binding. We also note that the void volumes in the crystal structure of N-IA-AmB are all occupied by highly ordered tetrahydrofuran guest molecules (Supplementary Fig. 7g), and we have previously shown that mixing AmB with ergosterol yields a complex with mitigated capacity for ergosterol extraction and yeast killing21.
The herein reported structure of the AmB sponge also provides important insights into how derivatives of AmB might enable the selective binding of ergosterol over cholesterol and thereby improve the therapeutic index of this clinically vital fungicidal agent. We have previously shown that the mycosamine appendage is required for sterol binding18–20, and studies by Matsumori and coworkers have suggested that modifying the orientation of the mycosamine appendage relative to the macrocycle core50 could impact sterol binding selectivity. We also reported the serendipitous discovery that a synthesized derivative of AmB lacking a hydroxyl group at the C2′ position of the mycosamine ring, called C2′deOAmB, retained binding to ergosterol but showed no binding to cholesterol51. This derivative also retained potent antifungal activity but showed no toxicity to human red blood cells or human primary renal epithelial cells in vitro. Although synthetic challenges have precluded further development of this derivative, it is a critical first example showing that the binding of ergosterol and cholesterol can be separated, and that achieving this goal leads to selective killing of yeast over human cells. Understanding the structural underpinnings of these observations should help guide the rational development of more synthetically accessible derivatives of AmB that achieve the same selectivity profile.
We previously suggested that elimination of this hydroxyl group may cause a ligand-selective allosteric effect, akin to effects seen for protein–small molecule interactions52,53. Specifically, we proposed that the C2′-OH stabilizes a conformer of AmB that binds both ergosterol and cholesterol, and that elimination of this hydroxyl group causes a change in conformation that favors ergosterol binding. We further noted that in the crystal structure of N-IA-AmB48,49, there is a prominent intramolecular water-bridged hydrogen bond between the C2′ hydroxyl group and the C13 hydroxyl group of the macrocycle, which may serve to reinforce a particular conformation of the mycosamine ring relative to the polyene macrolide core (Supplementary Fig. 7h). Remarkably, in state A of our AmB sponge structure, the orientation of the mycosamine appendage relative to the polyene macrolide core is very similar to the conformation observed in the monomers in the N-IA-AmB48,49 crystal structure (Supplementary Fig. 7i). Moreover, we readily docked in a water molecule to form a similar intramolecular water-bridged hydrogen bond between the C2′-OH on the mycosamine and the C13-OH of the macrocycle (Supplementary Fig. 7i). These observations strengthen the case that this type of water-bridged hydrogen bond in the biologically active AmB sponge may stabilize a conformation of AmB that promotes binding to both ergosterol and cholesterol, thus driving the sterol sponge-mediated toxicity to both yeast and human cells. They further suggest that modifications at the C2′, C13 and/or neighboring positions that perturb this intramolecular water-bridged hydrogen bond network may lead to ergosterol-selective allosteric effects that increase the therapeutic index of AmB.
In summary, we have presented a first-in-class structure of a biologically active small molecule aggregate. These results advance our fundamental understanding of the structural and biophysical underpinnings driving AmB function, and small molecule aggregates in general. The strategies, tactics and tools that were developed and deployed to achieve this result should enable the structural characterization and/or future design of other small molecules that similarly operate via higher-order aggregation4,5. Better understanding the aggregation of drug-like small molecules in aqueous solutions also has broad implications for rational drug design, as aggregation is also often associated with undesired effects such as off-target promiscuity and toxicity1. Further studies of AmB derivatives or other polyene antifungals22 will advance understanding of the unique and resistance-refractory mode of action of these natural products and/or further enable their use as prosthetic ion channels4,5. Additional work is ongoing to apply these same techniques to the study of AmB–sterol complexes, which could provide further insights to guide the pursuit of AmB derivatives with improved therapeutic indices.
Online content
Any methods, additional references, Nature Research reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at https://doi.org/10.1038/s41594-021-00685-4.
Methods
General methods.
Materials.
Commercially available materials were purchased from Sigma-Aldrich, Cambridge Isotope Laboratories or ThermoFisher Scientific and were used without future purification unless stated otherwise.
Isotopic expression and purification of AmB.
Isotopically 13C-enriched AmB was expressed as previously described in Anderson et al.21 from cultures of Streptomyces nodosus (ATCC, catalog number 14899). Cultures were pooled and centrifuged at 8,000g, 4 °C for 15 min in 50-ml Nalgene Oak Ridge High-Speed centrifuge tubes (Nalge Nunc).
The supernatant was pooled in a round-bottom flask and was rotary evaporated until it was a dried solid. AmB was extracted by resuspending the dried media component in methanol (MeOH) and pelleting out the solid material (8,000g, 4 °C, 15 min). The supernatant was collected, and the extraction process was repeated until there were no detectable amounts of AmB present in MeOH. The MeOH solutions were pooled and rotary evaporated. The crude AmB was then stored at −80 °C until purification.
Cell pellets were first lyophilized and AmB was extracted from the pellets by homogenizing the cell pellets in dimethylsulfoxide (DMSO), where AmB goes into solution and the insoluble cellular debris is pelleted out by centrifugation (8,000g, 4 °C, 15 min). The supernatant was collected, and the process was repeated multiple times until all AmB was extracted. The solutions were then pooled and DMSO was removed by lyophilization.
Crude AmB was adsorbed onto Celite 545 by first resuspending the crude material in a minimal amount of N,N-dimethylformamide. Celite 545 was added and the mixture was homogenized with MeOH and rotary evaporated. The process was repeated several times until the sample appeared to be a homogeneous powder. The sample was then placed under high vacuum for at least 14 h before being packed into a sample cartridge. The crude AmB was then subjected to medium-pressure liquid chromatography using a CombiFlash Rf system (Teledyne Isco) with reverse-phase C18 silica gel as the column resin. The medium-pressure liquid chromatography column was equilibrated in 95% water (containing 0.1% formic acid)/5% MeOH (containing 0.1% formic acid). Sample was loaded and eluted off the column by running isocratic with 95% water (containing 0.1% formic acid)/5% MeOH (containing 0.1% formic acid) for 5 min, with ramping from 5% to 100% MeOH (containing 0.1% formic acid) over 10 min, and then running isocratic at 100% MeOH (containing 0.1 % formic acid) for 20 min at a flow rate of 30 ml min−1.
Fractions that had a yellow hue were collected and the solvent was removed using rotary evaporation. Residual formic acid was removed from the sample by resuspending and bath sonicating the solid sample in water and rotary evaporating the sample, azeotroping with excess toluene. This process was repeated at least three times before placing the samples under high vacuum for at least 14 h before HPLC purification. HPLC purification was performed as previously described21.
Preparation of samples for SSNMR.
Preparation of stock solutions.
A fresh stock solution of HPLC-purified AmB (natural abundance, [U-13C]AmB or [13C] skip-labeled AmB) was prepared for each experiment by dissolving ∼10–12 mg of AmB in ∼400 μl of DMSO to reach a final concentration of 25–30 mM. The concentration was measured in triplicate by dilution in MeOH and measuring the absorbance at 406 nm (ε406 = 164,000 M−1cm−1)19.
Preparation of AmB samples for SSNMR.
A small amount of a concentrated DMSO stock solution of AmB was diluted with 10 mM HEPES buffer, pH 7.0 to a final concentration of 1 mM (in 1 ml volume). This step was repeated several times to assure enough material to fill the SSNMR 3.2 mm rotor (typically 10× 1 ml). The samples were then heated at 80 °C for 1 h in a water bath. After heating, the samples were placed into a bath sonicator for 30 min and then allowed to cool to room temperature in the bath sonicator for another 30 min. Next, the UV–Vis spectrum was recorded to confirm the transition in maximum absorbance from 342 nm (heterogeneous aggregate) to 321 nm (homogenous aggregate). The samples were then pelleted (260,000g, 4 °C, 30 min) in a 1.7-ml ultracentrifuge tube using a TLA-100.3 centrifuge rotor (Beckman Coulter, catalog number 349481). The supernatant was removed, and the material in each Eppendorf tube was resuspended in 400 μl of HEPES. The final UV–Vis spectrum was recorded for PCA (Methods). Afterwards, the samples underwent an additional centrifugation step (260,000g, 4 °C, 30 min), were resuspended in a small amount of 10 mM HEPES and were transferred to 200-μl ultracentrifuge tubes. The material was then pelleted (280,000g; 4 °C, 1 h) using a TLA-100 centrifuge rotor and packed into a SSNMR 3.2 mm or 1.6 mm rotor using a rotor-packing device54.
WST-8 cell proliferation assays.
Primary renal proximal tubule epithelial cells growth conditions.
Primary human RPTECs were purchased from ATCC and immediately cultured upon receipt. Complete growth media was prepared using renal epithelial cell basal medium (ATCC, catalog number PCS-400–030), renal epithelial cell growth kit (ATCC, catalog number PCS-400–040) and penicillin/streptomycin (10 units ml−1 and 10 μg ml−1). Complete media was stored at 4 °C in the dark and used within 28 days. Primary RPTECs were grown in a CO2 incubator at 37 °C with an atmosphere of 95% air/5% CO2.
WST-8 assay.
The WST-8 cell proliferation assay kit was purchased from Cayman Chemical Company (catalog number 10010199) and WST-8 reagent was prepared and stored following known procedures51. A suspension of primary RPTECs in complete growth medium was brought to a concentration of 1 × 105 cells ml−1. A 96-well plate was seeded with 99 μl of the cell suspension and incubated at 37 °C with an atmosphere of 95% air/5% CO2 for 3 h. AmB stock solution, ∼10 mM, was prepared in DMSO, and serially diluted to the following concentrations with DMSO: 4,000, 3,000, 2,000, 1,500, 1,000, 800, 600, 400, 200, 100, 50, 25 and 12.5 μM. One-microliter aliquots of each solution were added to the 96-well plate in triplicate. QA positive control was prepared by adding 1 μl of DMSO. One hundred microliters of the complete media was used as negative control. The 96-well plate was incubated at 37 °C with an atmosphere of 95% air/5% CO2 for 24 h. After incubation, the media was aspirated and 100 μl of serum-free media and 10 μl of the WST-8 reagent solution were added to each well. The 96-well plate was mixed in a shaking incubator at 200 r.p.m. for 1 min and incubated at 37 °C with an atmosphere of 95% air/5% CO2 for 2 h. Following incubation, the 96-well plate was mixed in a shaking incubator at 200 r.p.m. for 1 min and absorbance was read at 450 nm using a BioTek H1 Synergy Hybrid Reader. Experiments were performed in triplicate and the reported cytotoxicity represents an average of three experiments. Percent viability was determined using following equation.
Concentration versus percentage viability was plotted and fitted to four-parameter logistic dose–response fit using OriginPro 8.6 (ref. 55). The minimum toxic concentration (MTC) was defined as the concentration required to cause a 90% loss of cell viability.
Growth conditions and MIC assay for Candida albicans, Candida tropicalis and Candida glabrata.
The organisms were maintained with yeast peptone dextrose growth media consisting of 10 g l−1 of yeast extract, 20 g l−1 of peptone, 20 g l−1 of dextrose and 20 g l−1 of agar for solid media. The media was sterilized by autoclaving at 120 °C for 30 min. Dextrose was subsequently added as a sterile 40% w/v solution in water (dextrose solutions were filter sterilized). Solid media was prepared by pouring sterile media containing agar (20 g l−1) onto Corning 100 × 20 mm polystyrene plates. Liquid cultures were incubated at 37 °C on a rotary shaker and solid cultures were maintained at 37 °C in an incubator. MIC determinations were performed in triplicate on at least two occasions using the Clinical and Laboratory Standards Institute M27-A2 microbroth methodology (CLSI 2002).
Growth conditions and MIC assay for Aspergillus fumigatus.
The organisms were maintained, grown and subcultured in a similar manner on Sabouraud Dextrose media consisting of 10 g l−1 of peptone, 40 g l−1 of dextrose and 15 g l−1 of agar for solid media. The final pH of media was adjusted to 5.6. MIC determinations were performed in duplicate on at least two occasions adopting the method from Clinical and Laboratory Standards Institute M28-A2 microbroth methodology at 48 h (CLSI 2007).
Principal component analysis of UV–Vis spectra.
All UV–Vis spectra of AmB were collected using a NanoDrop 2000c Spectrophotometer (ThermoFisher Scientific) with a 1.0 cm path length quartz cell at ambient temperature. After baseline subtraction and normalization, the 285–430 nm regions of the spectra were analyzed using the probabilistic PCA method implemented in Matlab56. The spectra were resolved into two principal components and the first principal component (PC1) was used to characterize the progress of the sample preparation.
Density functional theory calculations.
Trial structures were generated by first using the geometry optimization extension in Avogadro57 with torsion angle restraints. The dihedrals C5–C6–C7–C8 and C7–C8–C9–C10 were restrained as either −60 (gauche−), +60 (gauche+) or 180 (trans), and the dihedral O37–C1– C2–C3 was restrained as either 180 or −60. Initial positions of hydroxyl protons of the C1–C13 region were also varied among six different configurations. The results of these initial optimizations were further optimized using Gaussian 09 (ref. 58), keeping the dihedrals O37–C1–C2–C3, C5–C6–C7–C8 and C7–C8–C9–C10 fixed using the ModRedundant option. The coordinates of the protons on the amino group were similarly fixed to prevent proton transfer to the carboxylate. Gaussian geometry optimizations used the B3LYP59 level of theory and 6–31 G(d)60 basis set. 13C isotropic shielding tensors were then calculated for each structure with Gaussian using the mPW1PW91 (ref. 35) level of theory and 6–311 G(d,p)36 basis set and the SCRF/PCM61 solvent method to include water. Linear regression analysis was used to convert the calculated 13C isotropic shielding tensors into 13C chemical shift values. Calculated chemical shift values were related to experimental shifts by regression analysis in Matlab15 and pairs of DFT structures were scored based on the root mean squared deviation (r.m.s.d.) between their DFT-calculated shifts and the experimental shifts of states A and B. The r.m.s.d. values of all carbon atoms, excluding the polyene region, were used to develop an initial heatmap for all 14 studied structures (Fig. 3a). The resultant structures with the lowest r.m.s.d. values were then evaluated based only on the carbon shifts of the C1–C13 region (Fig. 3b). The resulting matrices are not symmetric because each structure is more characteristic of either state A or B, and thus has a smaller r.m.s.d. for one state over the other.
Solid-state NMR spectroscopy.
Magic-angle spinning (MAS) SSNMR experiments were conducted at 11.7 T (500 MHz 1H frequency), 14.1 T (600 MHz) and 17.6 T (750 MHz) on Varian Instruments VNMRS (750 MHz and 500 MHz) and InfinityPlus (600 MHz) spectrometers using 3.2 mm Balun and 1.6 mm FastMAS probes. Spinning was controlled with a Varian MAS controller to 22,222 Hz (for experiments at 500 MHz 1H frequency), 12,500 Hz and 26,316 Hz (for 600 MHz 1H frequency) and 28,249 Hz (for 750 MHz 1H frequency). Typical pulse widths were ∼1.7–2.5 μs for 1H, and ∼1.5–3 μs for 13C, using high-power decoupling during evolution and acquisition. Sample temperature was maintained at 0 ± 5 °C. Spectra were processed with 20 Hz and 50–75 Hz line broadening for one-dimensional (1D), 2D and 3D experiments, respectively (see details in Supplementary Table 1). Chemical shifts were externally referenced using adamantane with the downfield peak set to 40.48 ppm62. Chemical shifts for each sample were obtained from 2D spectra using broadband DARR34 mixing at a series of mixing times, as well as 2D 13C–13C SPC-5, SPC-7, SPC-11 with short mixing time to observe one- and two-bond correlations33. Distance restraints were obtained from longer mixing time DARR spectra, 2D 13C–13C PAR37 and 3D 13C–13C–13C SPC-N PAR mixing experiments (see details in Supplementary Table 1). For the 13C–13C SPC-N spectra of the [U-13C]AmB sample, the 1H–13C CP contact time was set to 1.3 ms. For the 13C–13C DARR spectra of the [13C] skip-labeled AmB sample, the 1H–13C CP contact time was set to 1 ms to emphasize the signals of the rigid polyene and C1–C13 regions of AmB. For the 13C–13C PAR spectra of the [U-13C]AmB sample, the 1H–13C CP contact time was set to 1.8 ms. To maximize double quantum filtered signal intensity, the SPC-5, SPC-7 and SPC-11 mixing times were set to 800 μs, 960 μs and 1.28 ms, respectively. DARR mixing time was set to 50, 100, 200, 500, 900, 1,000 and 1,500 ms, and PAR mixing was set to 5.5 and 11 ms. Spectra were processed with NMRPipe with back linear prediction and polynomial baseline correction applied to the direct dimension. Lorentzian to Gaussian apodization, phase-shifted sine bells and zero filling were applied to both dimensions before Fourier transformation and phase correction. The spectra were analyzed using NMRFAM-Sparky.
PM-RESPDOR experiments.
The experiments were performed at 14.1 T (600 MHz) on a Varian InfinityPlus spectrometer with a 3.2-mm T3 HXY MAS probe tuned to 1H–13C–14N using the low-gamma box capability for the Y channel. For all experiments, a spinning rate of 12.5 kHz was chosen, and sample temperature was 10 ± 5 °C. 1H–13C cross-polarization was performed with 45.5 kHz on 13C channel and 69 kHz on 1H channel with a 1.2 ms contact time. TPPM and SPINAL-64 were used during evolution and acquisition times with 83.3 kHz of proton decoupling, respectively. The 13C rf field during 180° pulses were 50.4 kHz. The 14N radio-frequency (rf) nutation frequency of PM-RESPDOR pulse was 25 kHz (Direct Polarization of 14N in NH3Cl was used for rf field calibration). A 2-s delay was used between scans.
The PM-RESPDOR sequence38,39,63 is a Pseudo 2D REDOR-based experiment, which is shown in Supplementary Fig. 5a. The PM-RESPDOR pulse sequence consists of 68 pulses with the same rf field, but different phases and lengths38. The PM-RESPDOR experiment consists of two parts37: S0 and S. For the S0 experiment, the rf field is applied on the detected channel only by applying a train of π pulses every half rotor period with an empty window in the middle. This sequence eliminates the influence of all internal interactions of the detected spins (assuming small values of homonuclear dipolar interaction and offset) except spin–spin relaxation. For the S experiment, PM-RESPDOR pulses are also applied on the second channel. The normalized signal, ΔS/S0 = 1 − S/S0 is a dipolar recoupled curve, which can be computed with Bessel functions38,64.
The PM-RESPDOR experiment was divided into two parts to decrease the influence of carbon offsets. In the first part, the observed frequency was set on C41 (183 ppm) and the data (four points) were collected from this carbon signal only. For this 13C–14N pair, the PM-RESPDOR experiment was done twice using a 14TR pulse length for the first experiment, and 24TR for the second experiment. Supplementary Table 3 summarizes the number of replicas (NR) and number of scans (NS) used for each data point.
The 14TR pulse length experiment was signal averaged for 3 days, and the 24TR experiment was signal averaged for 4 days. In the second part the observed frequency was set at 17 ppm and the data (five points) were collected from the methyl carbons C39 (13 ppm), C6′/C38 (20 ppm) and C40 (23 ppm). This PM-RESPDOR experiment was only performed using the 14TR pulse length. Supplementary Table 4 summarizes the number of replicas and number of scans used for each collected point.
Xplor-NIH calculations.
Xplor-NIH v.2.53.x was used for structure calculations25. We used portions of the probabilistic assignment algorithm for automated structure determination combined with manually assigned cross-peaks to generate restraint tables. Fifty-four AmB monomers were arranged into a 3 × 3 × 3 lattice of 27 dimers with each dimer composed of one AmB monomer in the state A conformation and one monomer in the state B conformation (see Solid-state NMR spectroscopy in Methods). Each state corresponded to a unique segment ID in the XPLOR-NIH calculations. This dimer was used as the primary subunit, from which the coordinates of the remaining 26 dimers were generated by translations along the three Cartesian axes within Xplor-NIH’s strict symmetry implementation40. Distance modeling restraints were used to maintain lattice packing such that monomers within the same dimer were within 10 Å of each other, and the monomers were packed in an alternating A–B–A–B arrangement along the z axis. Unambiguous intermolecular distance restraints from experimental data were used to maintain the head-to-tail orientation of the A and B monomers within each dimer. Ambiguous 3C–13C and 13C–14N distance restraints from PAR and PM-RESPDOR experiments, respectively, were also included in the calculation. Owing to the use of strict symmetry, all distance restraints were entered with respect to the primary dimer subunit at the center of the lattice (segid 111) for both the ambiguous and unambiguous restraints.
The annealing calculation of 1,200 structures started at 4,000 K with a high-temperature dynamics run for 100 ps or 1,000 steps, whichever came first. Simulated annealing followed the high-temperature calculation, where molecular dynamics was run for the shorter of 100 steps or 0.2 ps at temperatures of 4,000 K to 20 K in steps of 20 K. A gyration volume term65 was applied to all subunits to disfavor the formation of voids in our calculations.
Torsion angle restraints from DFT calculations were included in the calculation in addition to terms for bond length, bond angle and improper dihedral angles. Force constants for the restraint terms were ramped as follows during simulated annealing: angle restraints were ramped from 0.4 to 1 kcal mol−1 rad−2, improper restraints were ramped from 0.1 to 1 kcal mol−1 rad−2, while the gyration volume was scaled from 10−6 to 0.1.
Reporting Summary.
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
Data that support the findings of this study are available within the paper and its Supplementary Information. Source data are provided in BMRbig, entry ID bmrbig28. Atomic coordinates are deposited in BMRB, entry ID 21097. Further requests can be directed to the corresponding authors.
Extended Data
Extended Data Fig. 1 |. AmB homogenization validation.
(a) UV-Vis spectra of AmB aggregates before (black) and after (red) homogenization. Grey UV-Vis spectra correspond to representative samples from the middle of the PC1 range (Fig. 1e). (b) LC-MS spectra of AmB before and after homogenization. (c) 1H NMR spectra of AmB before (black line) and after (red line) homogenization.
Extended Data Fig. 2 |. AmB cluster analysis and Erg docking.
(a) Principal component analysis (PCA) of the 9-dimensional data used for dimer structure clustering analysis for all 11 cluster ensembles. The inset shows the lowest clusters’ energies overlaid on the total energy histogram. (b) Alignment of medoid dimer structures of clusters 1–3 and lattice structures of medoid structures from the lowest energy cluster; states A and B of AmB are marked. (c) Medoid lattices of clusters 1–3 showing representative void volumes in green. Approximate volumes for each void in clusters 1–3 are 390 Å3, 830 Å3, and 530 Å3, respectively. The volume of ergosterol is estimated to be 427 Å3 21. (d) Snapshots of all four void pockets found in cluster 1 lattice - front view (left) and side view (right). The volume of each pocket is approximately 390 Å3. The number density of this lattice is 6.36 molecules per 10,000 Å3. (e) Snapshot of an ergosterol molecule (cpk-blue) docked to the cluster 2 medoid lattice – front view. (f) Close-up snapshot of AmB (white) – ergosterol (blue) docked interaction as seen in (e). Select atom numbers near interaction site are labeled for both AmB and ergosterol molecules.
Supplementary Material
Acknowledgements
This work was supported by the US National Institutes of Health (NIH) R01-GM112845 and R01-GM123455 to C.M.R. and R35-GM118185 to M.D.B. This study made use of the National Magnetic Resonance Facility at Madison, including the technology development program which is supported by NIH grant P41GM136463. C.D.S. was supported by the National Institutes of Health Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases. We would like to dedicate this paper to the memory of our coauthor, A. Khandelwal, who passed away during the course of this investigation.
Peer review information Nature Structural & Molecular Biology thanks Hartmut Oschkinat and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Florian Ullrich was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Footnotes
Competing interests
The authors declare no competing interests.
Additional information
Extended data is available for this paper at https://doi.org/10.1038/s41594-021-00685-4.
Supplementary information The online version contains supplementary material available at https://doi.org/10.1038/s41594-021-00685-4.
References
- 1.LaPlante SR et al. Compound aggregation in drug discovery: implementing a practical NMR assay for medicinal chemists. J. Med. Chem. 56, 5142–5150 (2013). [DOI] [PubMed] [Google Scholar]
- 2.Sezgin E, Levental I, Mayor S & Eggeling C The mystery of membrane organization: composition, regulation and physiological relevance of lipid rafts. Nat. Rev. Mol. Cell Biol. 18, 361–374 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lehn JM Towards complex matter: supramolecular chemistry and self-organization. Eur. Rev. 17, 263–280 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muraglia KA et al. Small-molecule ion channels increase host defences in cystic fibrosis airway epithelia. Nature 567, 405–408 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grillo AS et al. Restored iron transport by a small molecule promotes absorption and hemoglobinization in animals. Science 616, 608–616 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bongomin F, Gago S, Oladele RO & Denning DW Global and multi-national prevalence of fungal diseases—estimate precision. J. Fungi 3, 57 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanglard D Resistance of human fungal pathogens to antifungal drugs. Curr. Opin. Microbiol. 5, 379–385 (2002). [DOI] [PubMed] [Google Scholar]
- 8.Patterson TF et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 63, e1–e60 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fuhren J et al. High prevalence of azole resistance in Aspergillus fumigatus isolates from high-risk patients. J. Antimicrob. Chemother. 70, 2894–2898 (2015). [DOI] [PubMed] [Google Scholar]
- 10.Perfect JR The antifungal pipeline: a reality check. Nat. Rev. Drug Discov. 16, 603–616 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cleveland AA et al. Changes in incidence and antifungal drug resistance in candidemia: results from population-based laboratory surveillance in Atlanta and Baltimore, 2008–2011. Clin. Infect. Dis. 55, 1352–1361 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kreusch A & Karstaedt AS Candidemia among adults in Soweto, South Africa, 1990–2007. Int. J. Infect. Dis. 17, e621–e623 (2013). [DOI] [PubMed] [Google Scholar]
- 13.Verweij PE et al. International expert opinion on the management of infection caused by azole-resistant Aspergillus fumigatus. Drug Resist. Updat. 21–22, 30–40 (2015). [DOI] [PubMed] [Google Scholar]
- 14.Ermishkin LN, Kasumov KM & Potzeluyev VM Single ionic channels induced in lipid bilayers by polyene antibiotics amphotericin-B and nystatine. Nature 262, 698–699 (1976). [DOI] [PubMed] [Google Scholar]
- 15.Katzung BG, Masters SB & Trevor AJ Basic & Clinical Pharmacology 12th edn (McGraw-Hill, 2012). [Google Scholar]
- 16.Umegawa Y, Matsumori N, Oishi T & Murata M Ergosterol increases the intermolecular distance of amphotericin B in the membrane-bound assembly as evidenced by solid-state NMR. Biochemistry 47, 13463–13469 (2008). [DOI] [PubMed] [Google Scholar]
- 17.Khutorsky VE Structures of amphotericin B–cholesterol complex. Biochim. Biophys. Acta 1108, 123–127 (1992). [DOI] [PubMed] [Google Scholar]
- 18.Gray KC et al. Amphotericin primarily kills yeast by simply binding ergosterol. Proc. Natl Acad. Sci. USA 109, 2234–2239 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palacios DS, Anderson TM & Burke MD A post-PKS oxidation of the amphotericin B skeleton predicted to be critical for channel formation is not required for potent antifungal activity. J. Am. Chem. Soc. 129, 13804–13805 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palacios DS, Dailey I, Siebert DM, Wilcock BC & Burke MD Synthesis-enabled functional group deletions reveal key underpinnings of amphotericin B ion channel and antifungal activities. Proc. Natl Acad. Sci. USA 108, 6733–6738 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anderson TM et al. Amphotericin forms an extramembranous and fungicidal sterol sponge. Nat. Chem. Biol. 10, 400–406 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo XR et al. Sterol sponge mechanism is conserved for glycosylated polyene macrolides. ACS Cent. Sci. 7, 781–791 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun D, Rosokha SV, Lindeman SV & Kochi JK Intervalence (charge-resonance) transitions in organic mixed-valence systems. Through-space versus through-bond electron transfer between bridged aromatic (redox) centers. J. Am. Chem. Soc. 125, 15950–15963 (2003). [DOI] [PubMed] [Google Scholar]
- 24.Wylie BJ, Franks WT, Graesser DT & Rienstra CM Site-specific 13C chemical shift anisotropy measurements in a uniformly 15N,13C-labeled microcrystalline protein by 3D magic-angle spinning NMR spectroscopy. J. Am. Chem. Soc. 127, 11946–11947 (2005). [DOI] [PubMed] [Google Scholar]
- 25.Schwieters CD, Kuszewski JJ & Marius Clore G Using Xplor-NIH for NMR molecular structure determination. Prog. Nucl. Magn. Reson. Spectrosc. 48, 47–62 (2006). [Google Scholar]
- 26.Ulrich EL et al. BioMagResBank. Nucleic Acids Res. 36, D402–D408 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shen Y, Delaglio F, Cornilescu G & Bax A TALOS+: a hybrid method for predicting protein backbone torsion angles from NMR chemical shifts. J. Biomol. NMR 44, 213–223 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caulkins BG et al. NMR crystallography of a carbanionic intermediate in tryptophan synthase: chemical structure, tautomerization, and reaction specificity. J. Am. Chem. Soc. 138, 15214–15226 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wylie BJ, Schwieters CD, Oldfield E & Rienstra CM Protein structure refinement using 13C alpha chemical shift tensors. J. Am. Chem. Soc. 131, 985–992 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wylie BJ et al. Ultrahigh resolution protein structures using NMR chemical shift tensors. Proc. Natl Acad. Sci. USA 108, 16974–16979 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dalling DK & Grant DM Carbon-13 magnetic resonance. IX. The methylcyclohexanes. J. Am. Chem. Soc. 760, 6612–6622 (1967). [Google Scholar]
- 32.Hohwy M, Rienstra CM, Jaroniec CP & Griffin RG Fivefold symmetric homonuclear dipolar recoupling in rotating solids: application to double quantum spectroscopy. J. Chem. Phys. 110, 7983–7992 (1999). [Google Scholar]
- 33.Reif B, Jaroniec CP, Rienstra CM, Hohwy M & Griffin RG 1H–1H MAS correlation spectroscopy and distance measurements in a deuterated peptide. J. Magn. Reson. 151, 320–327 (2001). [DOI] [PubMed] [Google Scholar]
- 34.Takegoshi K, Nakamura S & Terao T 13C–1H dipolar-assisted rotational resonance in magic-angle spinning NMR. Chem. Phys. Lett. 344, 631–637 (2001). [Google Scholar]
- 35.Adamo C & Barone V Exchange functionals with improved long-range behavior and adiabatic connection methods without adjustable parameters: the mPW and mPW1PW models. J. Chem. Phys. 108, 664–675 (1998). [Google Scholar]
- 36.Krishnan R, Binkley JS, Seeger R & Pople JA Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys. 72, 650–654 (1980). [Google Scholar]
- 37.Paëpe GDE, Lewandowski JR, Loquet A, Böckmann A & Griffin RG Proton assisted recoupling and protein structure determination. J. Chem. Phys. 129, 245101 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nimerovsky E et al. Phase-modulated LA-REDOR: a robust, accurate and efficient solid-state NMR technique for distance measurements between a spin-1/2 and a quadrupole spin. J. Magn. Reson. 244, 107–113 (2014). [DOI] [PubMed] [Google Scholar]
- 39.Makrinich M, Nimerovsky E & Goldbourt A Pushing the limit of NMR-based distance measurements – retrieving dipolar couplings to spins with extensively large quadrupolar frequencies. Solid State Nucl. Magn. Reson. 92, 19–24 (2018). [DOI] [PubMed] [Google Scholar]
- 40.Schwieters CD, Bermejo GA & Clore GM Xplor-NIH for molecular structure determination from NMR and other data sources. Protein Sci. 27, 26–40 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rousseeuw P et al. cluster: ‘Finding Groups in Data’: Cluster Analysis Extended Rousseeuw et al. R package version 2.1.2 https://rdrr.io/cran/cluster/ (2019). [Google Scholar]
- 42.Chorghade RS et al. Amphotericin B induces epithelial voltage responses in people with cystic fibrosis. J. Cyst. Fibros. 20, 540–550 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Richardson D, Vriend G & Allison J Tools 2020: a compilation of tools for protein science. Protein Sci. 29, 5–7 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tuttle MD Solid-state NMR structure of a pathogenic fibril of full-length human α-synuclein. Nat. Struct. Mol. Bio. 23, 409–415 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nishimura S & Matsumori N Chemical diversity and mode of action of natural products targeting lipids in the eukaryotic cell membrane. Nat. Prod. Rep. 37, 677–702 (2020). [DOI] [PubMed] [Google Scholar]
- 46.Matsumori N, Sawada Y & Murata M Large molecular assembly of amphotericin B formed in ergosterol-containing membrane evidenced by solid-state NMR of intramolecular bridged derivative. J. Am. Chem. Soc. 128, 11977–11984 (2006). [DOI] [PubMed] [Google Scholar]
- 47.Baginski M, Resat H & Borowski E Comparative molecular dynamics simulations of amphotericin B–cholesterol/ergosterol membrane channels. Biochim. Biophys. Acta - Biomembr. 1567, 63–78 (2002). [DOI] [PubMed] [Google Scholar]
- 48.Ganis P, Avitabil G, Mechlins W & Schaffne C Polyene macrolide antibiotic amphotericin-B – crystal structure of N-iodoacetyl derivative. J. Am. Chem. Soc. 93, 4560–4564 (1971). [DOI] [PubMed] [Google Scholar]
- 49.Jarzembska KN et al. Controlled crystallization, structure, and molecular properties of iodoacetylamphotericin B. Cryst. Growth Des. 12, 2336–2345 (2012). [Google Scholar]
- 50.Matsumori N., Sawada Y. & Murata M. Mycosamine orientation of amphotericin B controlling interaction with ergosterol: sterol-dependent activity of conformation-restricted derivatives with an amino-carbonyl bridge. J. Am. Chem. Soc. 127, 10667–10675 (2005). [DOI] [PubMed] [Google Scholar]
- 51.Wilcock BC, Endo MM, Uno BE & Burke MD C2′-OH of amphotericin B plays an important role in binding the primary sterol of human cells but not yeast cells. J. Am. Chem. Soc. 135, 8488–8491 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Knight ZA & Shokat KM Features of selective kinase inhibitors. Chem. Biol. 12, 621–637 (2005). [DOI] [PubMed] [Google Scholar]
- 53.Duggan KC et al. (R)-Profens are substrate-selective inhibitors of endocannabinoid oxygenation by COX-2. Nat. Chem. Biol. 7, 803–809 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hisao GS et al. An efficient method and device for transfer of semisolid materials into solid-state NMR spectroscopy rotors. J. Magn. Reson. 265, 172–176 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sebaugh JL Guidelines for accurate EC50/IC50 estimation. Pharm. Stat. 10, 128–134 (2011). [DOI] [PubMed] [Google Scholar]
- 56.MATLAB v.R2015a. (MathWorks, 2015).
- 57.Hanwell MD et al. Avogadro: an advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. 4, 17 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Frisch MJ et al. Gaussian v.9 (Gaussian Inc., 2009). [Google Scholar]
- 59.Stephens PJ, Devlin FJ, Chabalowski CF & Frisch MJ Ab initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J. Phys. Chem. 98, 11623–11627 (1994). [Google Scholar]
- 60.Hariharan PC & Pople JA The influence of polarization functions on molecular orbital hydrogenation energies. Theor. Chim. Acta 28, 213–222 (1973). [Google Scholar]
- 61.Tomasi J, Mennucci B & Cammi R Quantum mechanical continuum solvation models. Chem. Rev. 105, 2999–3093 (2005). [DOI] [PubMed] [Google Scholar]
- 62.Morcombe CR & Zilm KW Chemical shift referencing in MAS solid state NMR. J. Magn. Reson. 162, 479–486 (2003). [DOI] [PubMed] [Google Scholar]
- 63.Gullion T Measurement of dipolar interactions between spin-12 and quadrupolar nuclei by rotational-echo, adiabatic-passage, double-resonance NMR. Chem. Phys. Lett. 246, 325–330 (1995). [Google Scholar]
- 64.Chen L et al. Distance measurement between a spin-1/2 and a half-integer quadrupolar nuclei by solid-state NMR using exact analytical expressions. J. Magn. Reson. 206, 269–273 (2010). [DOI] [PubMed] [Google Scholar]
- 65.Schwieters CD & Clore GM A pseudopotential for improving the packing of ellipsoidal protein structures determined from NMR data. J. Phys. Chem. B 112, 6070–6073 (2008). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data that support the findings of this study are available within the paper and its Supplementary Information. Source data are provided in BMRbig, entry ID bmrbig28. Atomic coordinates are deposited in BMRB, entry ID 21097. Further requests can be directed to the corresponding authors.