Abstract
Introduction:
Ovarian cancer is one of the most lethal neoplasms with 22,000 new cases and 14,000 deaths per year. Aggressive cytoreduction and chemotherapy have response rates as high as 80%. Most women are diagnosed with stage 3 disease or later and over 70% of women recur within 18 months. There is need for a test to determine prognosis of remission patients to allow for intervention and prolonged survival.
Methods and materials:
Women at Augusta University with ovarian cancer were enrolled between 2005 and 2015 (n=71). Blood was drawn at enrollment and follow-up visits. Patient serum collected at remission was analyzed using the SOMAscan array (n=35) to measure concentrations of 1129 proteins. The best 26 proteins were confirmed using Luminex assays in the same 35 patients and an additional 36 patients (ntotal=71) as orthogonal validation. The data from these proteins was combined with clinical factors using an elastic net multivariate model to find an optimized combination predictive of progression-free survival (PFS).
Results:
Brain Derived Neurotrophic Factor and Platelet Derived Growth Factor molecules were significant for predicting PFS on univariate and multivariate analyses. The 26 proteins were combined with clinical factors using the elastic net algorithm. Ten components were determined to predict PFS (HR of 6.55, p-value 1.12 × 10−6, CI 2.57 – 16.71). This model was named the Serous High Grade Ovarian Cancer (SHOC) Score.
Conclusion:
The SHOC score can predict patient prognosis in remission and will hopefully lead to early intervention and consolidation therapy strategies in remission patients destined to recur.
Keywords: Ovarian neoplasm, serum proteomics, biomarkers, prognosis
Introduction:
Ovarian cancer is the 5th leading cause of cancer death among women and most lethal gynecologic cancer with 14,000 deaths per year [1]. Greater than 75% of women are diagnosed with late stage disease of the serous histologic subtype, and they are often treated with aggressive cytoreduction followed by six cycles of doublet chemotherapy consisting of a platinum and taxane agent [2, 3]. Remission rates with this therapy are close to 80%. Despite excellent initial response rates, the 5-year survival for late stage ovarian cancer is estimated to be 40% with approximately 70% of women recurring in the first 18 months [4–6].
Periodic evaluation is performed by imaging with computed tomography (CT), physical exams, and monitoring of serial CA-125. However, previous studies have suggested up to 75% of patients declared in remission by the previous means will have microscopic disease noted on a second look surgery [2, 6]. This indicates that current disease evaluation modalities do a poor job of deciphering patients who are at the highest risk for residual microscopic disease post treatment. Furthermore, studies have shown these tests to monitor remission do little to inform the gynecologic oncologist which patients are at the highest risk of recurrence [5, 7]. Given this information, a test that can indicate which remission patient is at the highest risk of recurrence or death would be of great value.
Here we present a serum biomarker panel drawn at remission, which is capable of predicting patient progression free survival (PFS). The panel was initially discovered in 35 patients using the SOMAscan array, which is capable of detecting over 1,100 serum proteins. The proteins with the most significant p-value and hazard ratio on univariate analysis were selected for orthogonal validation using the Luminex assay. Luminex assay was performed using the original 35 patient sera, as well as, an additional 36 patient sera (ntotal=71). An elastic net algorithm was then applied to determine which combination of serum proteins and patient clinical characteristics would best predict patient prognosis. This resulted in a multicomponent panel providing excellent prediction of recurrence of SHOC patients who are in remission.
MATERIALS AND METHODS
Sample Collection
This was a single-institution, prospective observational study examining serial serum samples in patients with serous high grade ovarian cancer (SHOC) (N=71). This study was approved by the institutional review board at the Medical College of Georgia at Augusta University; a written informed consent was obtained from all subjects or from a legally authorized representative. The ethics committee at Augusta University approved the consenting procedure used in this study.
Serum samples were obtained at enrollment and then at subsequent follow up visits, including during treatment, remission, and recurrence. Blood samples were collected in serum separator tubes (BD Biosciences) and allowed to clot for 30 minutes at room temperature. Aliquots of serum were prepared immediately after phlebotomy into wells of 96-well plates (150µl/well) to create master plates. Daughter plates were then created by pipetting 5–25 µl of serum/well to avoid repeated freeze/thaw for all samples. Samples were aliquoted and stored in a – 80°C freezer until use. The first available remission sample from each patient was used for analyses.
Study Population
Remission was defined in accordance to Revised RECIST criteria (version 1.1) in combination with physical exam, clinical imaging, and CA125 [8, 9]. Patient records were reviewed for clinical data including demographics, treatment, chemotherapy response and disease status. Using this data, PFS was calculated for patients in remission. Progression free survival was calculated as time from blood draw to first progression.
All patients were treated with cytoreduction surgery and doublet chemotherapy with carboplatin and paclitaxel. Optimal cytoreduction was defined as less than 1 cm of disease left behind at the time of cytoreduction surgery [10]. Patients were not excluded for having received neoadjuvant chemotherapy, maintenance therapy, or were on a clinical trial, as long as they received platinum and paclitaxel.
Laboratory measurements
The SOMAscan array is a commercially available assay from the company SomaLogic (Boulder, CO USA). We sent 35 serum samples taken from SHOC patients at remission to SomaLogic. After the SOMAscan array analysis was complete, the data was sent back, which was then analyzed to determine, which proteins had the best hazard ratios and p-value for predicting time to death on univariate analysis [11]. The proteins with a log rank p-value of < 0.05 and hazard ratio greater than 2.5 were selected for in house confirmation via multiplex Luminex assay. Multivariate analysis was not performed on the significant SOMAscan proteins. The majority of significant proteins on the SOMAscan array did not have a corresponding Luminex assay available and were omitted from Luminex analysis.
Validation of SOMAScan data by Luminex assay
We elected to measure twenty-six proteins based on the SOMAscan array results as well as previously published data [12]. These proteins were Insulin-like Growth Factor Binding Proteins (IGFBP1, 2, 5, 6 and 7,) Interferon Gamma (IFNγ), interleukins (IL4, IL6, sIL6R, IL10, IL13, IL15), Macrophage Inflammatory Proteins (CCL3 and CCL4), Monocyte Chemotactic Protein 1 (MCP1/CCL2, MCP3/CCL7), Macrophage Derived Chemokine (MDC/CCL22), tissue Plasminogen activator inhibitor-1 (tPAI1), Platelet Derived Growth Factors (PDGF.AA and PDGF.ABBB), Regulated on Activation, Normal T cell Expressed and Secreted (RANTES/CCL5), soluble Glycoprotein 130 (sgp130), soluble Intercellular Adhesion Molecule 1 (sICAM1), soluble Tumor Necrosis Factor Receptors (sTNFRII and, sTNFRI), and soluble Vascular Adhesion Protein 1 (sVCAM1) [12]. These proteins were examined for their ability to predict PFS when drawn at remission.
Luminex assays for the above mentioned 26 proteins were obtained from Millipore (Millipore Inc., Billerica, MA, USA). Multiplex assays were performed according to instructions provided with the kit. Serum samples were incubated with capture antibodies immobilized on polystyrene beads for one hour. The beads were then washed and further incubated with biotinylated detection antibody cocktail for one hour. Next, beads were washed twice to remove unbound detection antibody, and then incubated with phycoerythrin-labeled streptavidin for thirty minutes. Last, beads were washed and suspended in 60µl of wash buffer.
The median fluorescence intensities (MFI) were measured using a FlexMAP 3D array reader (Millipore, Billerica, MA) with the following instrument settings: events/bead: 50, minimum events: 0, flow rate: 60µl/min, Sample size: 50ul and discriminator gate: 8000–13500. Before performing the profiling, assays were performed at different serum dilutions to ensure the MFI values of the samples were within the linear range of the standard curve.
Luminex median fluorescence intensity (MFI) data was subjected to quality control steps as described in our earlier study [13]. Described briefly, wells with low bead counts (below 30), or high bead CV (above 200) were flagged for exclusion. The coefficient of variation of replicate wells was also checked and wells with CV > 25% were not included in further analyses.
Protein concentrations were estimated using a regression fit to the standard curve with known concentration included on each plate using a serial dilution series. To achieve normal distribution, MFI and concentrations for standards were log2 transformed prior to all statistical analyses.
Statistical Analysis
All statistical analyses were performed using the R language and environment for statistical computing (RStudio version 1.1.383; R Foundation for Statistical Computing; www.r-project.org). The protein concentrations were log2 normalized after initial QC. The statistical significance of differences was set at p < 0.05, all p values were two sided. Correlation analysis between serum protein concentrations were performed using the Pearson parametric correlation test. Cox proportional hazards models were used to evaluate the impact of clinical factors and serum protein levels on PFS. These results are reported with corresponding 95% confidence intervals. Patients with no history of recurrence or death were censored at the date of last follow-up visit. Patients who died of natural causes unrelated to cancer were censored at time of death. Patient PFS was censored at 10 years. Kaplan-Meier survival analysis and log-rank test were used to compare differences in PFS between 10 groups classified based on each 10th. percentile of patients. The percentile with the best p-value and hazard ratio were subsequently chosen to be presented in this manuscript.
In order to create a comprehensive multivariate score that accounted for all clinical and serum data, we used the well-established elastic net algorithm (R package glmnet), [14]. This algorithm combines multiple predictors in a linear combination and tunes the model base on a penalty term, which is the sum of the square of the coefficients used in the model. The effect of the penalty term can be adjusted to either have no effect lambda = 0 or as lambda approaches infinity, variable coefficients approach 0. The sum of the linear combination yields a composite score for each individual patient. The number of predictors is optimized by varying an alpha value from 0 to 1. Where an alpha of 0 includes all possible predictors, while an alpha of 1, decreases the number of predictors to the lowest number possible. In our study, alpha was varied from 0 to 1 in increments of 0.01. The optimum lambda was determined using the lambda.min function in R, which automatically chooses the best lambda value to eliminate errors on cross validation. The composite score of the combined predictors for each value of alpha were then subject to survival analysis and cox proportional hazards to determine the best score for predicting PFS.
Results:
Patient Population Demographics
Seventy-one patients with SHOC were prospectively enrolled to provide serum samples during the course of their treatment and regular follow up. Of the 71 patients, 25 (35%) were used in a previous study [12]. Median follow up for these 71 patients was 5.5 years, and the median time from the date of being declared in remission to the date of the blood sample analyzed was 3 months with interquartile range of 0.19 to 16.2 months. Median age of diagnosis was 62 years old, and the majority of patients were Caucasian. Patient demographic information is summarized in Table 1.
Table 1:
Clinical and phenotype information for the study subjects
| Entire Cohort (N=109) | Percentage | Remission Patients with Blood Sample (N=71) | Percentage | |
|---|---|---|---|---|
| Stage | ||||
| Early | ||||
| 1 | 8 | 7 | ||
| 2 | 12 | 7 | ||
| Late | ||||
| 3 | 82 | 53 | ||
| 4 | 7 | 4 | ||
| Response to front line therapy | ||||
| Remission | 84 | 71 | ||
| Partial Response | 16 | NA | ||
| No Response | 9 | NA | ||
| Optimal Debulking | ||||
| Yes | 91 | 65 | ||
| No | 18 | 6 | ||
| Neoadjuvant Chemotherapy | ||||
| Yes | 25 | 12 | ||
| No | 84 | 59 | ||
| Clinical Trial | ||||
| Yes | 43 | 28 | ||
| No | 66 | 43 | ||
| Maintenance Therapy | ||||
| Yes | 33 | 22 | ||
| No | 76 | 49 |
Demographic data contributing to PFS on univariate analysis were stage (p-value: 0.004) and optimal cytoreduction (p-value: 0.037), and receiving maintenance chemotherapy (p-value: 0.05). Neoadjuvant chemotherapy (p-value: 0.22) and being on a clinical trial for first line chemotherapy (p-value: 0.058) had no impact on PFS. On multivariate analysis, stage (HR 2.00, CI 1.18 – 3.34, p-value: 0.01) remained predictive of PFS; optimal cytoreduction (HR: 0.39, CI: 0.15 – 1.002, p-value: 0.051) and receiving maintenance chemotherapy (HR 0.49, CI: 0.90 – 2.90, p-value 0.11) were not significant for predicting PFS.
SOMAscan Array Data
The SOMAscan array was used to test 35 patients and returned data on over 1,100 proteins. Our data indicate that a number of proteins are associated with patient survival. Interestingly, many of these proteins that are associated with poor survival are implicated in the inflammatory processes; whereas proteins that are associated with good survival are implicated in anti-tumor immunity. Among the best proteins identified by SOMAscan, Luminex assays were available for four proteins (BDNF, MDC, PAI1, and PDGF.AA). The SOMAscan data of these four proteins are shown in Table 2. A list of all proteins significant on the SOMAscan array are shown in Supplementary Table 1.
Table 2:
Percentile cutoff, odds ratio (OR) and p values for all 20 molecules measured by SOMAscan array.
| Overall Survival | ||||||
|---|---|---|---|---|---|---|
| Protein | Percentile Cutoff | HR | 1/HR | CI-L | CI-H | Logrank-p |
| BDNF | 50 | 0.10 | 9.82 | 0.03 | 0.36 | 0.00001 |
| GP1BA | 50 | 0.16 | 6.14 | 0.05 | 0.50 | 0.0004 |
| EPI | 50 | 0.19 | 5.26 | 0.07 | 0.53 | 0.0005 |
| WNT7A | 50 | 0.19 | 5.26 | 0.07 | 0.54 | 0.0005 |
| SCGF.beta | 50 | 0.20 | 5.04 | 0.07 | 0.56 | 0.0008 |
| GPC5 | 50 | 0.22 | 4.48 | 0.08 | 0.63 | 0.0019 |
| PDGF.AA | 50 | 0.22 | 4.55 | 0.08 | 0.62 | 0.0020 |
| Ephrin.B3 | 50 | 4.08 | 0.25 | 1.53 | 10.90 | 0.0026 |
| LCMT1 | 50 | 4.22 | 0.24 | 1.50 | 11.82 | 0.0030 |
| PRL | 50 | 4.19 | 0.24 | 1.50 | 11.76 | 0.0032 |
| Thrombopoietin.Receptor | 50 | 0.24 | 4.10 | 0.09 | 0.68 | 0.0038 |
| Thrombospondin.1 | 50 | 0.25 | 4.00 | 0.09 | 0.70 | 0.0045 |
| SREC.II | 50 | 0.25 | 3.94 | 0.09 | 0.71 | 0.0052 |
| MIP.1a | 50 | 3.72 | 0.27 | 1.39 | 9.95 | 0.0052 |
| calgranulin.B | 50 | 3.58 | 0.28 | 1.33 | 9.60 | 0.0073 |
| carbonic.anhydrase.II | 50 | 0.29 | 3.49 | 0.11 | 0.76 | 0.0074 |
| EDAR | 50 | 0.27 | 3.68 | 0.10 | 0.76 | 0.0077 |
| ALK.1 | 50 | 3.50 | 0.29 | 1.31 | 9.40 | 0.0084 |
| ON | 50 | 0.28 | 3.55 | 0.10 | 0.79 | 0.0098 |
Luminex Assay Data
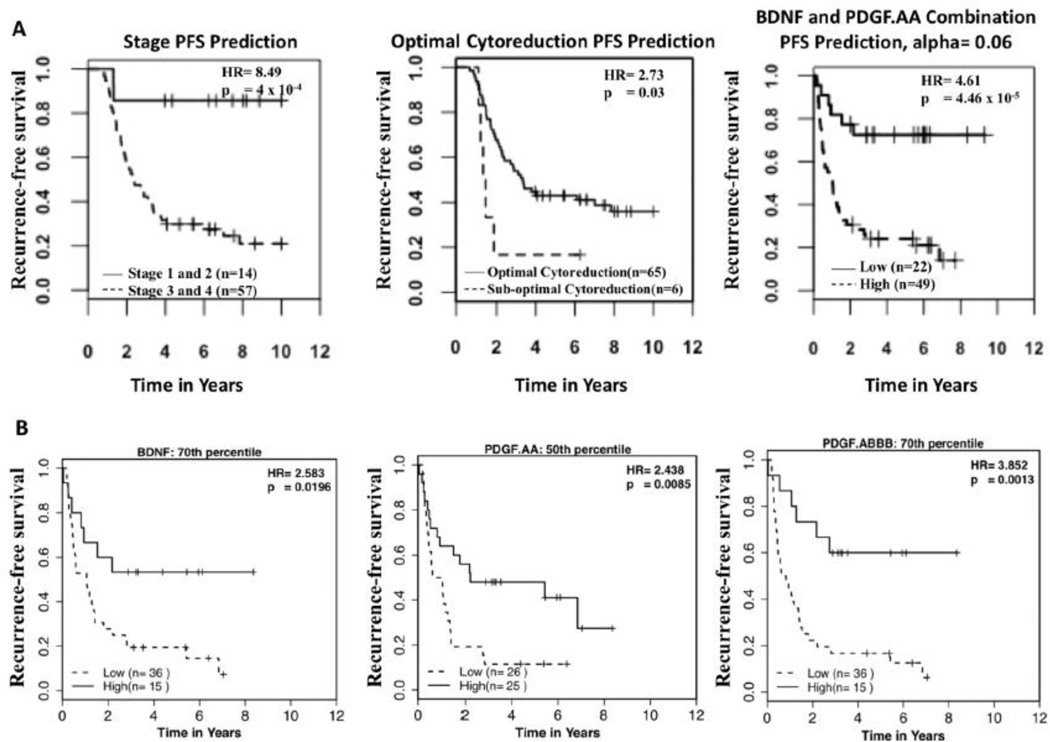
Luminex serum analyses were performed on the 71 patients who reached remission. A total of 26 proteins were analyzed by Luminex. Four of these proteins were based on the SOMAscan data (BDNF, MDC, PAI1, and PDGF.AA) while the other 22 proteins (IGFBP1, IGFBP2, IGFBP5, IGFBP6, IGFBP7, IFNG, IL4, IL6, sIL6R, IL10, IL13, IL15, MIP1A, MIP1B, MCP1, MCP3, PDG.ABBB, sICAM1, sgp130, sTNFRI, sTNFRII, sVCAM1, and RANTES) were chosen based on previous data [12]. Thirty-five of the seventy-one patients analyzed by Luminex were also patients used for the SOMAscan array. Proteins were analyzed for their ability to predict progression free survival based on different cutoffs. For example, a cutoff of 50% means half the patients are in the high concentration group while the other half are in the low concentration group. Proteins capable of predicting PFS were BDNF (HR 4.11, 95% CI: 1.73 – 9.76, p-value: 0.0005), PDGF.AA (HR 2.04, 95% CI: 1.12 – 3.73, p-value: 0.017), and PDGF.ABBB (HR 3.62, 95% CI: 1.61 – 8.14, p-value: 0.0009, Figure 1A). Interestingly, BDNF had mild correlation with both PDGF.AA and PDGF. ABBB: Pearson correlation coefficients of 0.68 (p-val < 0.001) and 0.58 (p-val <0.001), respectively. PDGF.AA and PDGF.ABBB had a strong degree of correlation: Pearson correlation coefficient of 0.82 (p-val < 0.001). Using the glmnet algorithm, we then investigated if any combination of these proteins could better predict PFS compared to individual proteins (Figure 1B). The combination of PDGF.AA and BDNF (HR 4.61, CI 1.94 – 10.94, p-value: 4.46 × 10−5) provided the best PFS prediction with BDNF being more important in determining patient prognosis. This combination was also capable of predicting recurrence within 1.5 and 3 years of the blood sampling date (Figure 1B).
Figure 1A.
Progression free survival predicted by BDNF, PDGF.AA, and PDGF.ABBB. The percentile with the best combination of hazard ratio and p-value were chosen for display.
Figure 1B. The bar graph shows both BDNF’s and PDGF.AA’s relative contribution to generating a score, which predicts PFS for each patient. The scores were determined by summing the values given post multiplying the serum level of BDNF and PDGF.AA by their respective relative contribution. These scores determined which patients were in the low and high group for the Kaplan Meyer survival prediction. Finally, ROC (receiver operator characteristic) curves were generated based on the ability of this combination to predict recurrence within 1.5 years and 3 years respectively.
It is not surprising that suboptimal cytoreduction is a predictor of PFS (HR 2.73, CI: 0.14 – 0.94, p-value: 0.037); however, only six patients had suboptimal cytoreduction (Figure 2A); and therefore, its utility in predicting PFS is limited. Stage is a better predictor of PFS in terms of HR (HR = 8.49, CI: 2.05 – 35.24, p-value: 4 × 10−4) than the best individual proteins or in combination (PDGF.AA and BDNF) (Figure 2A). Because of the known role of stage and optimal cytoreduction in predicting patient prognosis, multivariate analysis was performed using stage, optimal cytoreduction, and having received maintenance chemotherapy as covariables. BDNF (p-value: 0.013), PDGF.AA (p-value: 0.036), and PDGF.ABBB (p-value: 0.027) all remained significant for predicting PFS after correcting for these covariables.
Figure 2A.
Comparison of Kaplan Meyer PFS prediction by stage, optimal cytoreduction, and the combination of PDGF.AA and BDNF. When considering the stage Kaplan Meyer curve patients with stage 1 and 2 disease were grouped together and patients with stage 3 and 4 disease were grouped together.
Figure 2B. Progression free survival prediction of BDNF, PDGF.AA and PDGF.ABBB when considering only patients with stage 3 disease or later and an optimal debulking (n=51).
Most importantly, BDNF, PDGF.ABBB, and PDGF.AA can predict PFS in the subset of patients who have advanced stage disease (stage III and IV) and have had an optimal cytoreduction (n=51) (Figure 2B). When the glmnet algorithm was reapplied to this subset of patients, BDNF remained the main contributor to patient prognosis. However, the combination of BDNF and PDGF.ABBB resulted in better prediction than if BDNF was combined with PDGF.AA (Figure 3A). The combination of BDNF and PDGF.ABBB was also capable of predicting recurrence within 1.5 years and 3 years of blood sampling date (Figure 3B). These results strongly suggest that BDNF, PDGF.AA, and PDGF.ABBB have prognostic value independent of the known clinical variables.
Figure 3A.
Bar graph showing the contribution of both BDNF and PDGF.ABBB to determining PFS in patients with greater than stage 3 disease and an optimal debulking (n=51). The corresponding Kaplan Meyer curve is made by dividing patients based on the score generated for each patient based on the relative contribution bar graph. The score is determined specifically by summing the values generated post multiplying the serum level of BDNF and PDGF.ABBB by their respective relative contribution.
Figure 3B. Finally, ROC (receiver operator characteristic) curves were generated based on the ability of this combination to predict recurrence within 1.5 years and 3 years respectively.
Finally, we used the glmnet algorithm to create a composite serous high grade ovarian cancer score (SHOCS) consisting of serum proteins in combination with clinical variables (Figure 4A). The best combination was at an alpha of 0.03 and consisted of two clinical factors (stage and optimal debulking) along with 8 different serum proteins (HR 6.55, CI: 2.57 – 16.71, p-value: 1.12 × 10−6). The three most important factors for determining time to recurrence are stage, serum BDNF level and then cytoreduction status. The SHOC score provides the best prediction of time from blood draw to recurrence within 1.5 and 3 years, with AUC values of 0.78 and 0.83, respectively (Figure 4B). Notably, these AUC values were much better than the AUC values of stage alone when predicting recurrence at 1.5 and 3 years (stage AUC 0.52, and 0.61, respectively) (Figure 4B).
Figure 4A.
Bar graph showing the contributions of stage, optimal cytoreduction, BDNF, sIL6R, sgp130, sTNFRI, IGFBP6, MIP1B, IL6, and IFNG to determining PFS among all patients in the entire cohort (n=71). The corresponding Kaplan Meyer curve is made by dividing patients based on the score generated for each patient based on the relative contribution bar graph. The score is determined specifically by summing the values generated post multiplying the stage (1–4), optimal cytoreduction (0–1) and each serum protein level by their respective relative contribution.
Figure 4B. SHOCS and stage ROC curves were then generated and compared based on their ability to predict recurrence within 1.5 years and 3 years, respectively. SHOCS had superior prediction ability at both 1.5 and 3 years.
Discussion
Here we present data on multiple serum proteins which were predictive of PFS in high grade serous ovarian neoplasms. BDNF and PDGF.AA were validated by two different proteomic technologies (SOMAscan and Luminex). Interestingly, higher levels of both of these proteins, as well as, PDGF.ABBB were associated with better PFS, suggesting that these proteins most likely have protective roles in anti-tumor immunity. Indeed, this is supported by BDNF, PDGF.AA, and PDGF.ABBB are known to play major roles in immune functions related to anti-tumor immunity [11, 15]. It is possible that patients with low protein levels do not efficiently destroy residual microscopic cancer and are therefore more likely to have recurrent disease.
Of the significant proteins found in the study, BDNF is the least studied serum protein in ovarian cancer, compared to PDGF.AA, and PDGF.ABBB, but arguably the best predictor of PFS in our study. There is not a large amount of literature published about role of BDNF in ovarian cancer; however, BDNF has been shown to play a role in the immune system with a previous study showing activated T cells and B cells secrete BDNF [11]. Supporting this conclusion, in a study by Cao et al, [16] mice with higher serum levels of BDNF were shown to have superiorly functioning immune systems and increased survival in both colon and melanoma cancer models in mice. This coincides with our results that with increasing BDNF level there is an increase in PFS even when considering only advanced stage optimally debulked patients (30th percentile HR: 1.85, p-value: 0.06; 50th percentile HR: 2.16, p-value: 0.02; 80th percentile HR: 2.93, p-value 0.03). Given this information, it is essential to validate the findings on BDNF in a larger scale study. If validated, BDNF could provide a key piece of insight for early intervention in ovarian cancer patients, while in remission.
The PDGF molecules may also serve as valuable serum markers for potential immune deficiency resulting in the inability to resolve microscopic cancer. PDGF has been shown to be secreted by platelets to stimulate dendritic cell differentiation, T-cell migration, and early T-cell activation [15]. Consistent with the findings in this study, previously our group showed that PDGF molecules are higher in controls compared to patients with ovarian cancer [12]. In line with this reasoning, it has also been shown that some tumors overexpressing PDGF.BB have impaired growth [17]. However, PDGF has a variety of other functions, which have been shown to potentiate malignancy. The most prominent of these functions being angiogenesis [18]. However, our results indicate that elevated levels of serum PDGF are protective. This may indicate a possible improved immune response against residual microscopic cancer. Additional studies are required for further validation of the PDGF molecules as predictors of prognosis in ovarian cancer, as well as, differentiating in which situations their functions act to inhibit or promote cancer.
The combination of BDNF and PDGF.AA (HR 4.61, CI 1.94 – 10.94, p-value: 4.46 × 10−5) showed encouraging results when combined to predict prognosis among all patients. However, BDNF levels appear to be the main determinant of patient prognosis in this combination. Interestingly, when considering advanced stage ovarian cancer patients with an optimal debulking; it was BDNF and PDGF.ABBB that were most predictive of prognosis (HR 4.21 p-value: 0.001). The fact that BDNF and the PDGF molecules predict prognosis and have a positively correlation in expression supports that they could have a potential functional and interactive role in promoting a prolonged remission. Thus, these molecules should be investigated further in the future. may also eventually aide in choosing when and when not to intervene in a patient who appears to be in remission. However, based on the known contribution of stage and optimal cytoreduction to patient prognosis, we then made a separate model, Serous High-Grade Ovarian Cancer Score (SHOCS), which could be applied to all patients in remission based on a combination of clinical factors and serum protein concentration.
The SHOC score, consisted of two clinical components: stage and optimal debulking, as well as, serum levels of 8 different proteins: BDNF, sIL6R, sgp130, sTNFRI, IGFBP6, MIP1B, IL6, and IFNG. This combination had the best ability to predict PFS (HR 6.55, p-value 1.12 × 10−6) when considering all patients in the entire cohort (n=71). This score correctly identified 40 of the 45 patients who had a recurrence. Although stage and optimal debulking were key parts of the score, protein components were far from neglible. BDNF carried almost twice the relative impact of optimal cytoreduction. Furthermore, proteins with small relative contribution when considered collectively have a large impact. Interestingly of the 23 patients in the low score group, 10 had stage 3 disease. The SHOC score also did correctly identify the lone stage 2 patient who had a recurrence. Because of this, it seems that a favorable or unfavorable proteomic profile can indeed greatly impact a patient’s overall prognosis despite their clinical determinants. It is also worth noting, of the four patients who had a recurrence but were predicted to not recur by the SHOC score, one had stage 1 disease and four had stage 3 disease. Validation is needed to see on a larger scale how the SHOC score would consistently perform when evaluating early stage patients. Despite the concerns about early stage patients, this score was remarkably successful in predicting recurrence among advanced stage patients. Because of this score’s success in identifying patients at the highest risk of recurrence, in the future the SHOC score may aide in determining which remission patients need early treatment and intervention.
Although these results are exciting, our study has a number of limitations that need to be addressed with future studies. One of the largest limitations is the lack of samples obtained from patients in the pretreatment setting. A pretreatment serum value for all proteins measured in this paper would provide a more complete picture of each protein’s role, change over time, and allow better comparison to other papers discussing biomarkers in ovarian cancer. Another limitation is that this paper only consisted of 71 total remission patients with half of the patients being used in the discovery data set. We suspect this small sample number of patients in the SOMAscan array and the overall small number of patients in the entire study most likely contributed to the large decrease in hazard ratio when comparing SOMAscan results (35 patients), to the Luminex study (71 patients). However, the number of analyzed patients is similar to other published data sets of ovarian cancer patients [19–22]. Further studies should also focus on using assays capable of analyzing a wider variety of serum proteins compared to Luminex. Example assays include mass spectrometry or using the SOMAscan array exclusively; both offer accurate and efficient detection of a large number of proteins. By analyzing concentrations of molecules part of numerous physiologic pathways, such as those involved in anti-tumor immunity or general inflammation, in a larger patient population, we would hopefully be able to provide more evidence proteomic biopsies in remission can predict prognosis. Perhaps, we may eventually be able to make inferences into possible effective intervention. Despite these pitfalls, there are also a number of positives to this study.
One of the major strengths of this study is its longitudinal nature. Another positive is that the combined SHOC score consists of two clinical components, stage and optimal cytoreduction, which are already used by gynecologic oncologists [23]. Furthermore, the eight proteins can be tested using a platform has been previously validated and is high throughput. Finally, the SHOC score is used to determine risk of recurrence at a unique time point, remission.
Remission since the development of targeted biologic therapy, immunotherapy, and the SOLO-1 trial has become of increasing interest as a time of intervention [24]. Currently, gynecologic oncologists have no way to accurately monitor microscopic cancer and its molecular workings in a remission patient. The SHOC score demonstrated the ability to predict prognosis during the remission time period, when only microscopic cancer is present indicating it is representative of aggressive microscopic disease. We hope this study will help lay the ground work for future investigations focusing on proteomic biopsies capable of both detecting microscopic disease and guiding personalized maintenance therapy interventions.
Translational Relevance:
Too often, remission is a reactive period of repeat CT scans waiting for cancer growth as evidence of recurrence. This leaves patients and doctors with anxiety as there is no way to know when the cancer may return. We present a panel of proteomic markers drawn during remission; that when combined with clinical factors are able to predict time to recurrence in serous high grade ovarian neoplasms. Because this combination identifies patients with residual microscopic disease, undetectable by CT scan, it will hopefully lead to successful intervention during remission. This is especially relevant in the advent of numerous biologic therapies, which are primed to be used as consolidation therapy because of their unique mechanisms of action and often preferable side effect profile compared to traditional chemotherapy. Hopefully, this combination of predictors will help spur trials investigating which therapeutic options are best to eradicate residual microscopic cancer undetectable by conventional imaging.
Acknowledgements
We are thankful for the patients who bravely volunteered to participate in this study. Without them, we would be unable to push forward the forefront of medicine.
Support:
This project was supported by the general fund from Dr. Jin-Xiong She
Footnotes
Conflict of Interest: The authors declare no potential conflicts of interest.
Uncategorized References
- 1.Jemal A, et al. , Cancer statistics, 2006. CA Cancer J Clin, 2006. 56(2): p. 106–30. [DOI] [PubMed] [Google Scholar]
- 2.Cannistra SA, Cancer of the ovary. N Engl J Med, 2004. 351(24): p. 2519–29. [DOI] [PubMed] [Google Scholar]
- 3.Morgan RJ Jr., et al. , Ovarian Cancer, Version 1.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw, 2016. 14(9): p. 1134–63. [DOI] [PubMed] [Google Scholar]
- 4.Jayson GC, et al. , Ovarian cancer. Lancet, 2014. 384(9951): p. 1376–88. [DOI] [PubMed] [Google Scholar]
- 5.Marcus CS, et al. , Current approaches and challenges in managing and monitoring treatment response in ovarian cancer. J Cancer, 2014. 5(1): p. 25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Armstrong DK, Relapsed ovarian cancer: challenges and management strategies for a chronic disease. Oncologist, 2002. 7 Suppl 5: p. 20–8. [DOI] [PubMed] [Google Scholar]
- 7.Gupta D and Lis CG, Role of CA125 in predicting ovarian cancer survival - a review of the epidemiological literature. J Ovarian Res, 2009. 2: p. 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eisenhauer EA, et al. , New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer, 2009. 45(2): p. 228–47. [DOI] [PubMed] [Google Scholar]
- 9.Rustin GJ, et al. , Definitions for response and progression in ovarian cancer clinical trials incorporating RECIST 1.1 and CA 125 agreed by the Gynecological Cancer Intergroup (GCIG). Int J Gynecol Cancer, 2011. 21(2): p. 419–23. [DOI] [PubMed] [Google Scholar]
- 10.Chi DS, et al. , What is the optimal goal of primary cytoreductive surgery for bulky stage IIIC epithelial ovarian carcinoma (EOC)? Gynecol Oncol, 2006. 103(2): p. 559–64. [DOI] [PubMed] [Google Scholar]
- 11.Kerschensteiner M, et al. , Activated human T cells, B cells, and monocytes produce brain-derived neurotrophic factor in vitro and in inflammatory brain lesions: a neuroprotective role of inflammation? J Exp Med, 1999. 189(5): p. 865–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang J, et al. , Serum protein profile at remission can accurately assess therapeutic outcomes and survival for serous ovarian cancer. PLoS One, 2013. 8(11): p. e78393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Purohit S, et al. , Large-Scale Discovery and Validation Studies Demonstrate Significant Reductions in Circulating Levels of IL8, IL-1Ra, MCP-1, and MIP-1beta in Patients With Type 1 Diabetes. J Clin Endocrinol Metab, 2015. 100(9): p. E1179–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedman JH, Hastie T, and Tibshirani R, Regularization Paths for Generalized Linear Models via Coordinate Descent. 2010, 2010. 33(1): p. 22. [PMC free article] [PubMed] [Google Scholar]
- 15.Morrell CN, et al. , Emerging roles for platelets as immune and inflammatory cells. Blood, 2014. 123(18): p. 2759–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cao L, et al. , Environmental and genetic activation of a brain-adipocyte BDNF/leptin axis causes cancer remission and inhibition. Cell, 2010. 142(1): p. 52–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCarty MF, et al. , Overexpression of PDGF-BB decreases colorectal and pancreatic cancer growth by increasing tumor pericyte content. J Clin Invest, 2007. 117(8): p. 2114–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heldin CH, Lennartsson J, and Westermark B, Involvement of platelet-derived growth factor ligands and receptors in tumorigenesis. J Intern Med, 2018. 283(1): p. 16–44. [DOI] [PubMed] [Google Scholar]
- 19.Oikonomopoulou K, et al. , Prediction of ovarian cancer prognosis and response to chemotherapy by a serum-based multiparametric biomarker panel. Br J Cancer, 2008. 99(7): p. 1103–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Madsen CV, et al. , Serial measurements of serum PDGF-AA, PDGF-BB, FGF2, and VEGF in multiresistant ovarian cancer patients treated with bevacizumab. J Ovarian Res, 2012. 5(1): p. 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abendstein B, et al. , Predictive value of uPA, PAI-1, HER-2 and VEGF in the serum of ovarian cancer patients. Anticancer Res, 2000. 20(1B): p. 569–72. [PubMed] [Google Scholar]
- 22.Dupont J, et al. , Early detection and prognosis of ovarian cancer using serum YKL-40. J Clin Oncol, 2004. 22(16): p. 3330–9. [DOI] [PubMed] [Google Scholar]
- 23.Sioulas VD, et al. , Optimal primary management of bulky stage IIIC ovarian, fallopian tube and peritoneal carcinoma: Are the only options complete gross resection at primary debulking surgery or neoadjuvant chemotherapy? Gynecol Oncol, 2017. 145(1): p. 15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moore K, et al. , Maintenance Olaparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N Engl J Med, 2018. [DOI] [PubMed] [Google Scholar]