Abstract
Some microorganisms can transform methyl ricinoleate into γ-decalactone, a valuable aroma compound, but yields of the bioconversion are low due to (i) incomplete conversion of ricinoleate (C18) to the C10 precursor of γ-decalactone, (ii) accumulation of other lactones (3-hydroxy-γ-decalactone and 2- and 3-decen-4-olide), and (iii) γ-decalactone reconsumption. We evaluated acyl coenzyme A (acyl-CoA) oxidase activity (encoded by the POX1 through POX5 genes) in Yarrowia lipolytica in lactone accumulation and γ-decalactone reconsumption in POX mutants. Mutants with no acyl-CoA oxidase activity could not reconsume γ-decalactone, and mutants with a disruption of pox3, which encodes the short-chain acyl-CoA oxidase, reconsumed it more slowly. 3-Hydroxy-γ-decalactone accumulation during transformation of methyl ricinoleate suggests that, in wild-type strains, β-oxidation is controlled by 3-hydroxyacyl-CoA dehydrogenase. In mutants with low acyl-CoA oxidase activity, however, the acyl-CoA oxidase controls the β-oxidation flux. We also identified mutant strains that produced 26 times more γ-decalactone than the wild-type parents.
γ-Decalactone is an aroma compound present naturally in many fruits and fermented products. It is particularly important in the formulation of peach, apricot, and strawberry flavors. Microbial processes to produce this compound have been patented (8, 17), although the metabolic pathways involved are not yet completely defined (6). Yeasts are used for industrial production, but the yields of this biotransformation commonly are poor, rarely reaching concentrations over 4 to 5 g/liter of fermentation broth (10).
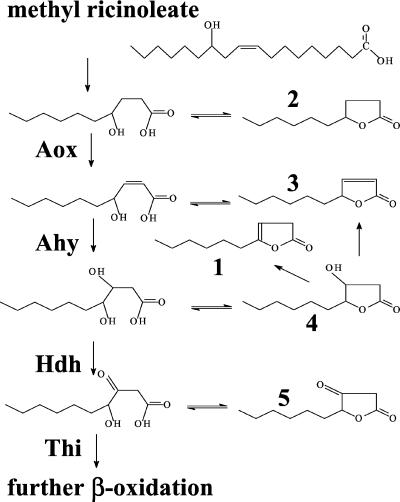
There are two hypotheses for these poor yields. First, the yeast may reconsume some of the γ-decalactone. Second, only a portion of the methyl ricinoleate (methyl δ-12-hydroxy-cis-9-octadecenoate) is oxidized to the C10 level, and the C10 product serves as the precursor for several γ-decalactones (9, 11). Many hypotheses have been proposed to explain the reconsumption, all of which require β-oxidation (11, 15), with some including ω-oxidation or delactonization as the first or limiting steps (6). β-Oxidation fluxes also are not well understood, and in the transformation of ricinoleyl coenzyme A (ricinoleyl-CoA) to acetyl-CoA, up to 27 intermediates may be formed. Mitochondrial β-oxidation is very efficient and well organized (5), usually converting acyl-CoA to acetyl-CoA (2, 16). By comparison, peroxisomal β-oxidation, which is utilized by yeasts (6), does not proceed via channelization (16), and β-oxidation intermediates may accumulate, depending on the substrate and CoA concentrations (3, 16). With the yeast Yarrowia lipolytica, Gatfield et al. (11) identified other C10 lactones (Fig. 1). These lactones could result from a single critical enzymatic step in β-oxidation, with γ-decalactone resulting from the activity of acyl-CoA oxidase (Aox) and 3-hydroxy-γ-decalactone and decenolides resulting from the activity of the multifunctional enzyme (acyl-CoA hydratase and 3-hydroxy-acyl-CoA dehydrogenase).
FIG. 1.
Potential intermediates of the β-oxidation of methyl ricinoleate at the C10 level. 1, dec-3-en-4-olide; 2, γ-decalactone; 3, dec-2-en-4-olide; 4, 3-hydroxy-γ-decalactone; and 5, 3-keto-γ-decalactone. β-Oxidation enzymatic activities: Ahy, acyl-CoA hydratase; Hdh, 3-hydroxy-acyl-CoA dehydrogenase; and Thi, 3-keto-acyl-CoA-thiolase. β-Oxidation enzymes catalyze reactions between acyl-CoA esters. It is not known whether CoA esters or fatty acids are subject to lactonization, so fatty acids are shown.
Y. lipolytica possesses a five-gene family (named POX1 to POX5) that encodes Aox1 to 5 (24). These peroxisomal enzymes appear to have an important role in γ-decalactone production from methyl ricinoleate, particularly the short-chain-specific Aox (Aox3) (13), which, when present, lowers yields (22).
Our objectives in this study were to determine if Aox was involved in γ-decalactone reconsumption and the synthesis of other C10 lactones. We found that (i) Aox, especially the short-chain Aox, is involved in γ-decalactone reconsumption and (ii), with decreased Aox activity, some POX mutants produce γ-decalactone instead of 3-hydroxy-γ-decalactone. By explaining metabolic fluxes, these results suggest new strategies to improve conversion yields of γ-decalactone or other medium-chain compounds through selective disruption of pox genes or inhibition of their products.
MATERIALS AND METHODS
Experimental rationale.
γ-Decalactone reconsumption and the production of diverse lactones during methyl ricinoleate metabolism were investigated by using a set of mutants in which one or several Aox-encoding genes (pox) were disrupted. After a preculture, cells were cultured on methyl ricinoleate in a bioreactor to determine lactone accumulation or incubated in a medium containing γ-decalactone in Erlenmeyer flasks to evaluate lactone consumption.
Strains and culture conditions.
We used Y. lipolytica W29 (ATCC 20460) or derived mutants that were disrupted in genes coding for one or more Aox (POX) (Table 1) (23, 24).
TABLE 1.
Strains of Y. lipolytica used in this work
| Strain name | Name in reference | Nature of disruption | Properties | Reference(s) |
|---|---|---|---|---|
| WT | W29 | No disruption: wild type | Reference | 20 |
| Δpox2 | MTLY16 | Long-chain Aox | Normal growth on methyl ricinoleate | 20 |
| Δpox3 | MTLY17 | Short-chain Aox | Normal growth on methyl ricinoleate | 19 |
| Δpox2pox3 | MTLY20 | 2-chain-length specific Aox | Growth altered on methyl ricinoleate and no growth on methyl decanoate | 18, 20 |
| Δpox2pox3pox5 | MTLY35 | 3 out of 4 active Aox | No detectable activity or growth altered on fatty acids | 20 |
| Δpox2pox3pox4pox5 | MTLY37 | 4 active Aox | No growth on fatty acids | 20 |
All strains were cultured for 48 h on malt extract agar (Difco, Osi, Paris, France) at 27°C and used to inoculate a 500-ml baffled Erlenmeyer flask containing 200 ml of glucose medium (22) to an optical density at 600 nm (OD600) of 0.25 (6 × 106 cells/ml). Flasks were shaken at 140 rpm for 18 h until the cultures reached the late logarithmic growth phase. Cells were harvested (10,000 × g for 5 min), washed twice with phosphate buffer (50 mM, pH 7.4), and, for culture on methyl ricinoleate, resuspended in a 2-liter Setric reactor (NBS, Toulouse, France) containing the methyl ricinoleate medium (22) with 5 g of methyl ricinoleate per liter, 6.7 g of yeast nitrogen base per liter, and 5 g of NH4Cl per liter, emulsified by agitation in the presence of 0.2 g of Tween 80/liter. Agitation and aeration were at 300 rpm and 0.44 volume of air per volume of reactor per min, respectively. Lactone degradation was monitored with cells resuspended (OD600 = 1) in baffled Erlenmeyer flasks containing 200 ml of distilled water with 50 mg of γ-decalactone/liter and 9 g of NaCl/liter. All chemicals were purchased from Sigma Aldrich (Saint-Quentin Fallavier, France) except methyl ricinoleate (Stearinerie Dubois, Boulogne, France).
Analyses.
For lactone quantification, 1.5-ml samples were removed from the methyl ricinoleate medium. These samples were centrifuged (10,000 × g, 5 min), and the supernatants (both aqueous and oil phases) were mixed. An internal standard, γ-undecalactone, was added to reach a final concentration of 100 mg/liter, and the mixture was extracted with diethyl ether, in 4-ml glass vials, by shaking for 90 s. The ether phase was analyzed in an HP6890 gas chromatograph (Agilent Technologies, Lyon, France) with an HP-INNOWax capillary column (Agilent) (30.0 m by 320 μm by 0.25 μm) with N2 as a carrier gas at a linear flow rate of 4.3 ml/min. The split injector (split ratio, 7.1:1) temperature was set to 250°C, and that of the flame ionization detector was set to 300°C. The oven temperature was programmed to increase from 60 to 145°C at a rate of 5°C/min and then at a rate of 2°C/min to 215°C. Mass spectra were obtained through a gas chromatography-mass spectrometry analysis with an HP5890 gas chromatograph (Agilent) with He as the carrier gas and an HP MSD 5970 mass spectrometer (Agilent) using ionization with a 70-eV electronic impact.
RESULTS
Lactone degradation by Aox altered mutants.
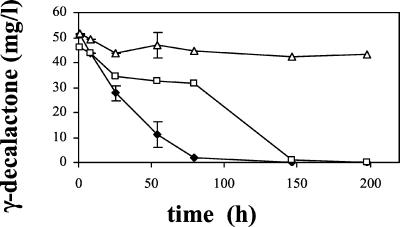
We monitored lactone degradation in mutants of Y. lipolytica disrupted for one or several acyl-CoA-encoding genes. The Δpox2pox3pox4pox5 mutant strain had no detectable Aox activity and could not grow on fatty acid methyl esters (24). The Δpox2pox3 mutant could grow on methyl ricinoleate and transform it to γ-decalactone but could not grow on medium- and short-chain methyl esters (22). The Δpox3 mutant had decreased short-chain Aox activity (24). Mutants without detectable Aox activity could not degrade γ-decalactone, even after 8 days, whereas the degradation was complete for the wild type after 3 days (Fig. 2). The Δpox2pox3 mutant could not grow on C10 but degraded lactone after a 3-day lag. The behavior of the Δpox3 and Δpox2 mutants was not significantly different from that of the wild type (data not shown).
FIG. 2.
γ-Decalactone degradation at 27°C in water containing 9 g of NaCl/liter in the presence of Y. lipolytica cells (OD600 = 1) in Erlenmeyer flasks. ⧫, wild-type strain; □, Δpox2pox3; and ▵, Δpox2pox3pox4pox5. Error bars indicate the standard deviation (if higher than 10%) based on three independent experiments.
Lactone reconsumption during methyl ricinoleate transformation.
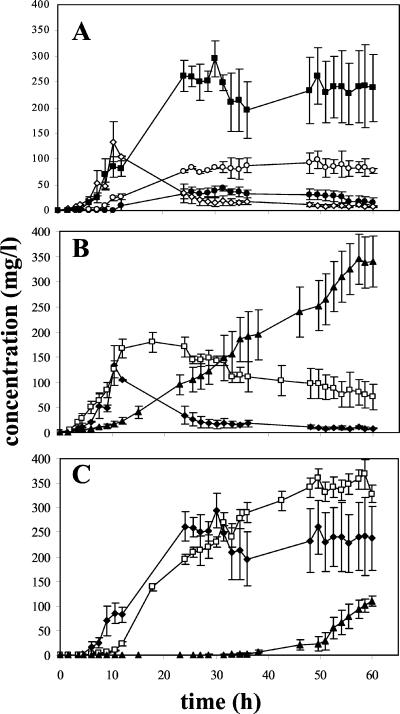
In the reactor with the wild type and Δpox2 (results not shown), the lactone concentration increased rapidly to 130 mg/liter after 11 h and then fell to about 30 mg/liter after 24 h (Fig. 3B). For the mutant disrupted for the short-chain Aox-encoding gene (pox3), the concentration increased to 170 mg/liter after 12 h, was stable for the next 12 h, and then decreased slowly (Fig. 3B). For Δpox2pox3 the lactone concentration increased steadily throughout the culture, yielding 350 mg/liter (corresponding to 17.4% molar conversion) (Fig. 3B).
FIG. 3.
Profile of γ-decalactone accumulation during a culture on methyl ricinoleate in a 2-liter bioreactor. (A) Accumulation of C10 lactones during culture of the wild type. ◊, γ-Decalactone; ▪, 3-hydroxy-γ-decalactone; ○, dec-2-en-4-olide; and ●, dec-3-en-4-olide. (B and C) Accumulation profile of γ-decalactone (B) and 3-hydroxy-γ-decalactone (C) for the wild type (⧫), Δpox3 (□), and Δpox2pox3 (▴). The results presented are means of three or four independent experiments.
Accumulation of other decalactones.
The main peaks of the mass spectra (frequency in percent) are as follows: compound 1, 168 (11), 139 (9), 125 (10), 111 (100), 98 (92), 83 (21), 70 (45), 55 (84), and 41 (42); compound 3, 168 (3), 139 (22), 126 (11), 113 (14), 108 (16), 97 (30), 84 (90), 69 (15), 55 (70), and 43 (100); and for compound 4, 158 (1), 144 (1), 115 (45), 97 (79), 88 (5), 83 (9), 69 (22), 55 (100), and 43 (58). These mass spectra correspond almost exactly to those obtained by Gatfield et al. (11) for 3-decen-4-olide (compound 1), 2-decen-4-olide (compound 3), and 3-hydroxy-γ-decalactone (compound 4) and are published here for the first time.
Lactone production profiles for various strains.
For the wild type, 3-hydroxy-γ-decalactone accumulated as γ-decalactone disappeared, reaching molar conversion yields of 12.3%. There was a similar pattern for 2- and 3-decen-4-olide but with lesser amounts (Fig. 3A). Δpox3 had a hydroxylactone accumulation similar to that of the wild type (Fig. 3C), whereas the γ-decalactone concentration was more constant (Fig. 3B). For Δpox2pox3, which was quite similar to Δpox2pox3pox5 (not shown), the concentration of 3-hydroxy-γ-decalactone was low until 50 h of culture, when it began to increase sharply (Fig. 3C). For all the strains, decenolides had the same general profile as the hydroxylactone but with amounts three to four times lower for 2-decen-4-olide and eight to ten times lower for 3-decen-4-olide (Fig. 3A, shown only for the wild type).
DISCUSSION
Y. lipolytica (synonym, Candida lipolytica) can efficiently degrade hydrophobic substrates. It is used for lipase production (18, 19), decontamination of diesel-contaminated soils (14) and olive-mill wastewaters (21), and γ-decalactone production (6, 9, 10).
Y. lipolytica can reconsume lactone as it is synthesized. The reconsumption pathway is not yet defined, but the presence of β-oxidation intermediates shorter than C10 (11, 15) suggests that β-oxidation is occurring to the lactone precursor. Gatfield et al. (11) observed concomitant lactone disappearance and accumulation of 3-hydroxy-γ-decalactone and decen-4-olides. They hypothesized that β-oxidation hydroxylation of 4-hydroxy decanoic acid led to 3,4-dihydroxy decanoic acid, which was lactonized to 3-hydroxy-γ-decalactone, which was in turn dehydrated to the two corresponding 4-decenolides. However, Endrizzi-Joran (7) observed that strains of Candida spp. with or without peroxisome induction were degrading lactone the same way. She suggested that, since only strains possessing a cytochrome P-450 could reconsume lactone, ω-oxidation occurred first, followed by β-oxidation. A similar mechanism was proposed by Ratledge et al. (20) for α-ω-dioic acids and was described by Abbott et al. (1) for nabilone degradation by Nocardia salmonicolor. Endrizzi et al. (6, 7) hypothesized that a lactonase was the potential rate-limiting step, since in strains producing both the lactone and acid forms, the rate of product disappearance was twice as high for the acid as it was for the lactone.
In mammals, lactone metabolism begins with the lactonase-catalyzed opening of the lactone ring and the resulting 4-hydroxy-decanoic acid goes through one cycle of β-oxidation, followed by decarboxylation to resolve the steric hindrance caused by the hydroxy group. The resulting heptanoic acid is then β oxidized to acetyl- and succinyl-CoA.
In this study, we confirmed the involvement of Aox and thus of β-oxidation in the degradation pathway. However, the degradation profiles were similar for the wild-type, Δpox2, Δpox3, and Δpox2pox3 strains, with only an increased lag phase for the latter. These results suggest that Aox is not the rate-limiting step of the pathway, which instead could be the opening of the lactone ring or the CoA esterification.
When culture occurs on methyl ricinoleate, lactone reconsumption is difficult to assess, as production and degradation occur concomitantly. Both we (Fig. 3) and Gatfield et al. (11) found that the wild type accumulated hydroxylated and unsaturated lactones as γ-decalactone was degraded. However, a strain lacking the short-chain Aox (Aox3) behaved similarly, except that this mutant reconsumed γ-decalactone very slowly. Thus, 3-hydroxy-γ-decalactone is not exclusively a product of γ-decalactone degradation. Y. lipolytica β-oxidation appears to follow established pathways (4), but at the C10 level when the hydroxy group is at the γ-carbon, there is competition between the next β-oxidation reaction and the hydrolysis of the CoA ester or lactonization (Fig. 1). The latter reaction is reversible, so γ-decalactones can enter back into the β-oxidation loop, probably in a manner dependent upon the actual fluxes. The accumulation of 3-hydroxy-γ-decalactone suggests that the competition between the third β-oxidation reaction, catalyzed by Hdh and lactonization, favors the 3-hydroxy-γ-decalactone. This accumulation of 3-hydroxy-acyl-CoA has been described in both mitochondrial and peroxisomal in vitro β-oxidation systems (2, 16). In some cases, this accumulation has been attributed to poor reoxidation of NADH, which could inhibit Hdh (3). Δ-2-Enoyl-CoA also may accumulate through a reversal of the enoyl-CoA hydratase-catalyzed reaction. This compound is a powerful inhibitor of Aox (16). The accumulation of β-oxidation intermediates is still unclear, especially in mitochondrial β-oxidation, where 3-hydroxy intermediates may accumulate in systems with a high NAD/NADH ratio and a specific activity higher for the multifunctional enzyme than for acyl-CoA dehydrogenase, the mitochondrial counterpart of Aox (2). We attribute the accumulation of 3-hydroxy-γ-decalactone in the wild type to the high Aox efficiency resulting from the five isoforms of this enzyme. Mutants disrupted in several Aox-encoding genes do not accumulate the hydroxylactone and instead accumulate γ-decalactone, showing the key role of Aox in the reaction.
3-Hydroxy-γ-decalactone does not seem to be degraded in the same manner as γ-decalactone. The two decenolides may be formed either from the hydroxylated lactone in the cell or from its precursor. If they are formed extracellularly, then the rate of formation is lower than for the hydroxylated lactone formation.
In this study, we show that, although they are not the rate-limiting enzymes, Aox isozymes are involved in lactone reconsumption and that mutants with lower β-oxidation fluxes in short-chain acyl-CoA have significantly less γ-decalactone reconsumption. To completely block γ-decalactone reconsumption by Y. lipolytica would require a strain with no Aox activity below C10. For Y. lipolytica, such a strain would carry mutants in all of the POX genes except POX2, which encodes the long-chain Aox. Unfortunately, such a strain hardly grows on fatty acid (data not shown).
Aox is usually considered, particularly in mammals, to be the rate-limiting enzyme of the β-oxidation pathway (12). Wild-type Y. lipolytica is the only yeast so far in which β-oxidation is controlled by Hdh. The wild type produces primarily 3-hydroxy-γ-decalactone, but by decreasing Aox activity in a strain, we could obtain γ-decalactone instead of 3-hydroxy-γ-decalactone (Fig. 1). γ-Decalactone production also can be increased by forcing acyl-CoA to exit β-oxidation at the C10 level. This increase can be achieved with the nonreconsuming strains described above or could eventually result from a strain possessing a high-activity decanoyl-CoA specific hydrolase.
Lactonization reporting the efficiency of the enzymes in the breakdown pathway, the metabolism of ricinoleic acid or of other hydroxylated fatty acids, appears to be a good model for the in vivo study of β-oxidation. We are presently investigating the impact of environmental conditions on the β-oxidation fluxes in order to use the ability of the wild type to accumulate 3-hydroxy products to resolve a commonly encountered problem in the production of polyhydroxyalkanoates by yeasts: the poor synthesis of monomers. From the set of mutants used in this study, we also are trying to construct a strain with high activity on long-chain substrates with the pathway blocked for short-chain fatty acids, since such a strain would be efficient not only for γ-decalactone production but also for all kinds of lipid-derived medium-chain-length products.
REFERENCES
- 1.Abbott B J, Fukuda D S, Archer R A. Microbiological transformation of cannabinoids. Experientia. 1977;33:718–720. doi: 10.1007/BF01944147. [DOI] [PubMed] [Google Scholar]
- 2.Bartlett K, Eaton S. Intermediates of mitochondrial β-oxidation. Biochem Soc Trans. 1994;22:432–436. doi: 10.1042/bst0220432. [DOI] [PubMed] [Google Scholar]
- 3.Bartlett K, Hovik R, Eaton S, Watmough N J, Osmundsen H. Intermediates of peroxisomal β-oxidation. Biochem J. 1990;270:175–180. doi: 10.1042/bj2700175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blin-Perrin C, Molle D, Dufossé L, Le-Quéré J-L, Viel C, Mauvais G, Feron G. Metabolism of ricinoleic acid into γ-decalactone: β-oxidation and long chain acyl intermediates of ricinoleic acid in the genus Sporidiobolus sp. FEMS Microbiol Lett. 2000;188:69–74. doi: 10.1111/j.1574-6968.2000.tb09170.x. [DOI] [PubMed] [Google Scholar]
- 5.Eaton S, Bursby T, Middleton B, Pourfarzam M, Mills K, Johnson A W, Bartlett K. The mitochondrial trifunctional protein: centre of a β-oxidation metabolon? Biochem Soc Trans. 2000;28:177–182. doi: 10.1042/bst0280177. [DOI] [PubMed] [Google Scholar]
- 6.Endrizzi A, Pagot Y, Le Clainche A, Nicaud J-M, Belin J-M. Production of lactones and peroxisomal β-oxidation in yeasts. Crit Rev Biotechnol. 1996;16:301–329. doi: 10.3109/07388559609147424. [DOI] [PubMed] [Google Scholar]
- 7.Endrizzi-Joran A. Ph.D. thesis. Dijon, France: Université de Bourgogne; 1994. [Google Scholar]
- 8.Farbood, M., and B. Willis. 1983. Production of gamma-decalactone. International patent PCT WO83/01072.
- 9.Farbood, M., J. A. Morris, M. A. Sprecker, L. J. Bienkowski, K. P. Miller, M. H. Vock, and M. L. Hagerdorn. 1989. Process for preparing compositions containing unsaturated lactones, products thereby and organoleptic uses of said products. European patent 0 354 000.
- 10.Gatfield I L. Biotechnological production of natural flavor materials. In: Teranishi R, Wick E L, Hornstein I, editors. Flavor chemistry, thirty years of progress. New York, N.Y: Plenum Press; 1999. pp. 211–227. [Google Scholar]
- 11.Gatfield I L, Güntert M, Sommer H, Werkhoff P. Some aspects of the microbiological production of flavor-active lactones with particular reference to γ-decalactone. Chem Mikrobiol Technol Lebensm. 1993;15:165–170. [Google Scholar]
- 12.Inestrosa N C, Bronfman M, Leighton F. Purification of the peroxisomal fatty acyl-CoA oxidase from rat liver. Biochem Biophys Res Commun. 1980;95:7–12. doi: 10.1016/0006-291x(80)90696-8. [DOI] [PubMed] [Google Scholar]
- 13.Luo Y S, Wang H J, Gopalan K V, Srivastava D K, Nicaud J M, Chardot T. Purification and characterization of the recombinant form of Acyl-CoA oxidase 3 from the yeast Yarrowia lipolytica. Arch Biochem Biophys. 2000;384:1–8. doi: 10.1006/abbi.2000.2079. [DOI] [PubMed] [Google Scholar]
- 14.Margesin R, Schinner F. Effect of temperature on oil degradation by a psychrotrophic yeast in liquid culture and in soil. FEMS Microbiol Ecol. 1997;24:243–249. [Google Scholar]
- 15.Okui S, Uchiyama M, Mizugaki M. Metabolism of hydroxy fatty acids: 2. Intermediates of the oxidative breakdown of ricinoleic acid by genus Candida. J Biochem. 1963;54:536–540. doi: 10.1093/oxfordjournals.jbchem.a127827. [DOI] [PubMed] [Google Scholar]
- 16.Osmundsen H, Hovik R, Bartlett K, Pourfazam M. Regulation of flux of acyl-CoA esters through peroxisomal β-oxidation. Biochem Soc Trans. 1994;22:436–441. doi: 10.1042/bst0220436. [DOI] [PubMed] [Google Scholar]
- 17.Page, G. V., and R. G. Eilerman. 1989. Process for the preparation of γ- and δ-lactones. International patent PCT WO 89/12104.
- 18.Pignède G, Wang H, Fudalej F, Gaillardin C, Seman M, Nicaud J-M. Characterization of an extracellular lipase encoded by LIP2 in Yarrowia lipolytica. J Bacteriol. 2000;182:2802–2810. doi: 10.1128/jb.182.10.2802-2810.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pignède G, Wang H, Fudalej F, Seman M, Gaillardin C, Nicaud J-M. Autocloning and amplification of LIP2 in Yarrowia lipolytica. Appl Environ Microbiol. 2000;66:3283–3289. doi: 10.1128/aem.66.8.3283-3289.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ratledge C. Microbial conversions of alkanes and fatty acids. J Am Oil Chem Soc. 1984;61:447–453. [Google Scholar]
- 21.Scioli C, Vollaro L. The use of Yarrowia lipolytica to reduce pollution in olive mill wastewaters. Water Res. 1997;31:2520–2524. [Google Scholar]
- 22.Waché Y, Laroche C, Bergmark K, Møller-Andersen C, Aguedo M, Le Dall M-T, Wang H, Nicaud J-M, Belin J-M. Involvement of acyl coenzyme A oxidase isozymes in biotransformation of methyl ricinoleate into γ-decalactone by Yarrowia lipolytica. Appl Environ Microbiol. 2000;66:1233–1236. doi: 10.1128/aem.66.3.1233-1236.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang H, Le Clainche A, Le Dall M-T, Waché Y, Pagot Y, Belin J M, Gaillardin C, Nicaud J M. Cloning and characterization of the peroxisomal acyl CoA oxidase ACO3 gene from the alkane-utilizing yeast Yarrowia lipolytica. Yeast. 1998;14:1373–1386. doi: 10.1002/(SICI)1097-0061(199811)14:15<1373::AID-YEA332>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 24.Wang H J, Le Dall M-T, Waché Y, Laroche C, Belin J-M, Gaillardin C, Nicaud J-M. Evaluation of acyl coenzyme A oxidase (Aox) isozyme function in the n-alkane-assimilating yeast Yarrowia lipolytica. J Bacteriol. 1999;181:5140–5148. doi: 10.1128/jb.181.17.5140-5148.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]