Abstract
Objective:
To compare the effectiveness of the Healthy Caregivers-Healthy Children (HC2) phase 1 (2011–2014) and 2 (2015–2018) child care center (CCC)-based obesity prevention intervention(s) on child dietary practices and body mass index percentile (PBMI) outcomes over 2 years. Phase 1 was implemented via a university-based research team, and phase 2 was delivered via a train-the-trainers approach (university-based research team trains preschool-based coaches, who in turn train CCC teachers to implement and disseminate HC2).
Methods:
Phase 1 and 2 were both cluster randomized controlled trials of the HC2 obesity prevention intervention. Phase 1 was composed of 1224 children in 28 CCCs (12 intervention and 16 control). Phase 2 was composed of 825 children in 24 CCCs (12 intervention and 12 control). Both phases included CCCs serving low-resource, predominantly ethnic minority families.
Results:
The mean rate of weekly fruit consumption significantly increased (β = 0.16, p = 0.001) in phase 1, whereas vegetable intake significantly increased (β = 0.16, p = 0.002) in phase 2 intervention CCCs. Fried (β = −0.36, p < 0.001), fast (β = −0.16, p = 0.001), and other unhealthy food (β = −0.57, p < 0.001) consumption significantly decreased in phase 1 only. The mean rate of snack food consumption significantly decreased in phase 2 (β = −0.97, p < 0.001). Mean child PBMI remained in the healthy range over 2 years for all groups in both study phases.
Conclusion:
A university-based research team implementation and dissemination approach seemed to be more effective than a train-the-trainers implementation method in improving dietary intake patterns. This finding suggests that CCCs may need robust educational support beyond their existing internal resources for long-term positive dietary intake pattern changes.
Keywords: obesity, preschool, child care center, prevention, dissemination, implementation
One in 4 US children younger than the age of 5 years is either classified as overweight [≥85th to <95th body mass index percentile (PBMI) for age and sex] or obese (≥95th PBMI for age and sex), with ethnic minority children being disproportionately affected.1,2 These statistics are of particular concern because preschool-aged children with obesity are 5 times more likely to be overweight during adolescence3 and 5 times more likely as adults to have obesity compared with their normal weight counterparts.4 These data show that, contrary to popular belief, children do not “grow out of” their “baby fat.” In fact, excessive weight gain in the first years of life can alter developing neural, metabolic, and behavioral systems in ways that increase the risk for not only obesity but also chronic disease later in life, such as cardiovascular disease, hypertension, type 2 diabetes, stroke, osteoarthritis, asthma, and certain cancers.5 As such, for the first time in decades, it has been suggested that the life expectancy of Americans has decreased as a consequence of obesity.6 Indeed, the first years of life may present the best opportunity for obesity prevention. During early childhood (defined here as ages 2–5), it may be important to promote health behaviors via positive parent and teacher role modeling as a means to change or break existing unhealthy habits later in life.7-10 Child care settings offer a potentially powerful infrastructure to implement early childhood health interventions because (1) 70% of preschool-aged children are enrolled in daily out-of-home child care,11 (2) low-income children consume 50% to 100% of their Recommended Dietary Allowances in the child care setting,12 and (3) many children spend most of their waking hours in a child care setting.13 However, the quality and structure of early child care environments varies considerably.14
Although several studies have targeted obesity prevention in the child care setting,12-14 no studies have compared implementation strategies using a university-based research team with those using preschool-based coaches. Furthermore, little is known about the relationship between the quality of implementation and dissemination efforts and the outcomes obtained among young children in the child care setting. This is even more evident in low-resource settings and for populations traditionally underrepresented in obesity prevention research, for which dissemination and implementation may not be a simple process. Based on the results of preliminary research and a history of working in the Miami-Dade County early childhood community, federal funding was secured in 2009 to conduct “Healthy Caregivers-Healthy Children (HC2) Phase 1,” a 3-year group-randomized controlled trial (NCT017220321) among 28 child care centers (CCCs) in both urban and rural Miami-Dade County.15 This was followed in 2015 by the HC2 phase 2 efficacy trial (NCT02697565), the results of which we have reported elsewhere.16 In the first trial, we implemented a multicomponent CCC-based obesity prevention program targeting teachers, parents, and children in English and Spanish that resulted in increased reported child dietary intake of fruits and vegetables.17,18 The first trial was implemented and disseminated primarily by a university-based research team (Master Trainers). In the second efficacy trial, we delivered HC2 via a train-the-trainers (TTTs) delivery method in CCCs that were part of the Quality Rating Improvement System (QRIS)19 to maximize sustainability. QRIS coaches are professionals who provide technical assistance to improve program quality and are each responsible for a number of centers. QRIS coaches were trained by university Master Trainers to train CCC teachers, who, in turn, were the primary HC2 disseminators and implementers in the classroom to children in phase 2. The TTT approach has successfully disseminated interventions across a variety of health issues (e.g., HIV, substance use, and mental health)20-22 but has been underused in the preschool setting to deliver health and wellness interventions. The TTT model was selected to implement and disseminate the HC2 program, given that (1) it is shown to be an effective and cost-effective means of dissemination and (2) it has been used to teach public health professionals and cooperative extension employees how to train CCC providers to promote healthy lifestyles.23
Here, we compare the primary outcomes in changes in dietary intake habits and BMI trajectories by study phase. It was hypothesized that those children in CCCs randomized to HC2 would show more improvement in healthy lifestyle dietary intake versus control centers in both phases and that the TTT dissemination and implementation approach (phase 2) would result in the same fruit and vegetable intake as for the phase 1 child participants randomized to HC2. Specifically, we hypothesized the following: (1) there would be similar changes in fruit and vegetable intake in both phase 1 and phase 2 because the intervention that addressed this component remained unchanged; (2) there would be a change from unhealthy food and snack food consumption to healthy food and snack food consumption in both phases for the intervention group; and (3) children in the intervention group across both phases would have healthier BMI growth trajectories as compared with those in the control group.
Healthy Caregivers-Healthy Children Theoretical Framework
To effectively promote health behavior change at the individual, family, and community level, multiple strategies including education, advocacy, policy change, and environmental change should be considered. The Social Cognitive Theory (SCT) describes specific mediating factors that affect an individual’s decision to make health behavior changes including perceived benefits of making the behavior change (positive health benefits), perceived control (who is in charge of food shopping and preparation), and self-efficacy for making changes (including confidence that barriers can be overcome).24 In particular, preschool children’s health behaviors are ultimately modified through observational learning and imitation of parents and teachers as they make changes in their eating and physical activity habits (principal underpinnings of SCT).
Dissemination and Implementation Approach
Currently, the science needed to promote successful implementation of evidence-based early childhood obesity prevention practices in real time, under naturally occurring conditions, is not well developed. There is a dearth of information comparing effective dissemination and implementation strategies, programs, and/or policies to understand how health promotion and disease prevention is best advanced. Yet, there are studies that have investigated the methods used for the dissemination of cancer control and prevention interventions that promote behavior change. One study concluded that there was not enough evidence to suggest 1 dissemination strategy was superior compared with the other.25 A systematic review of dissemination and implementation in the topic areas of smoking, healthy diet, physical activity, and sun protection concluded that there was much heterogeneity across studies in reported mediators, moderators, and outcomes, suggesting the need for standardized reporting criteria and active and multimodal strategies.26 Although cancer control is obviously a different health context from the studies reported here, it nevertheless illuminates the need for more robust and rigorous study designs to test potentially effective prevention outcomes that can then in turn be widely disseminated to support population-level health.
The RE-AIM (Reach, Effectiveness, Adoption, Implementation, Maintenance) framework was used to guide our implementation science. The dimensions of the framework all have applicability to the proposed project27: (1) Reach (the absolute number, proportion, and representativeness of individuals who are willing to participate), (2) Effectiveness (impact of an intervention on outcomes, including potential negative effects and quality of life), (3) Adoption (absolute number, proportion, and representativeness of settings and intervention agents who are willing to initiate a program), (4) Implementation (fidelity to the various elements of an intervention’s protocol including consistency of delivery as intended and the time and cost of the intervention), and (5) Maintenance (extent to which a program or policy becomes institutionalized or part of the routine organizational practices and policies but also has individual-level outcomes). RE-AIM was initially designed to help evaluate interventions and public health programs, to produce a more balanced approach to internal and external validity, and to address key issues important for dissemination and generalization.27 More recently, it has been applied to policies28 and community-based multilevel interventions29 as well as to the reduction of health disparities,30 which is ideal for our local populations and project goals. As such, RE-AIM provided the framework for both phases on this study.
METHODS
Phase 1 and Phase 2 Similarities
Study Design
Both phase 1 and phase 2 randomized, controlled trials were conducted among child care centers (CCCs) serving low-income, ethnically diverse families in Miami-Dade County (MDC), Florida. Random assignment of individuals to treatments was not feasible because of the school-based setting (no ability to randomize individuals within classrooms to treatments); rather, we randomly assigned CCCs to 1 of 2 treatments (either intervention or attention control). Thus, this study was designed as a group-randomized study, often referred to as a cluster randomized trial (CRT).31 In CRTs, clusters of people or intact social units (i.e., schools or CCCs) rather than individuals are randomized to intervention and control groups, and the outcomes are measured on individuals within those clusters. CCCs were randomly assigned (via a random number table) to either study arm. Both arms were followed for 2 school years (approximately 10 months each) and received the same dosage of services [Healthy Caregivers-Healthy Children (HC2) for the intervention group and Safety Sam for the control group]. The outcome measures described below were collected at 3 key time points: the beginning of school year 1 (September-October) and the ends of school years 1 and 2 (April–May). The University of Miami Institutional Review Board approved both phase 1 and phase 2 study protocols, and each child’s parent or legal guardian provided informed consent to participate in the studies.
Participants
Child care centers must have met the following criteria to be included in the phase 1 or phase 2 study: (1) have ≥50 children enrolled who are 18 to 66 months in age, (2) serve low-income families (i.e., the child meets state eligibility as receiving school readiness funds), (3) reflect the ethnic diversity of the MDC Public School System (63% Hispanic, 19% non-Hispanic Black, and 18% non-Hispanic White), and (4) obtain agreement from CCC directors and teachers to participate. Phase 1 and phase 2 CCCs were mutually exclusive. In other words, no phase 1 CCCs were included in the phase 2 trial. Parents and their 18 months to 5-year-old child at the CCC were invited to participate and were given consent forms. CCC directors and teachers consented to participate at the beginning of the study. CCCs were excluded if they did not meet the inclusion criteria and if they had a high prevalence of special needs children (i.e., a child with a diagnosed disability with an Individual Education Plan). Special needs did not include children with food allergies and sensitivities; these children were included if their parent consented to study participation. Children who brought their own meals because of diet restrictions and those who were identified by parents on the demographic form as failure to thrive [<fifth body mass index or body mass index percentile (PBMI)] were also excluded.
Healthy Caregivers-Healthy Children Phase 1 and Phase 2 Intervention Content
The HC2 toolkit consists of material designed to incorporate all current nutrition and physical activity policy requirements for preschool children in Florida and embrace best-practice guidelines from Caring for Children,32 The Institute of Medicine,33 Healthy Kids, Healthy Future (formerly Let’s Move!34), and USDA Team Nutrition.35
Tier 1: Environmental Changes, Policy Component
The policies that serve as the foundation of the HC2 toolkit are as follows: (1) Snack Policy—fresh fruits and vegetables and whole grains (no sweets and high-fat foods) are the snack foods of choice/preferred foods; (2) Beverage Policy—serve low-fat (1%) or nonfat milk only (no whole milk), serve 100% fruit juice only 1 time per week, make water available all day, and encourage it as the beverage of choice; (3) Physical Activity Policy—at least 90 minutes of physical activity every day; and (4) Screen Time Policy—screen time less than 30 minutes per week.
Lesson plans were developed to support the policies as described below. In addition, a registered dietitian added a component to the teacher training to enhance sustainability by including the steps needed to implement menu changes/planning. The dietitian provided guidelines for menu planning that were (1) consistent with HC2 policy guidelines (i.e., fruits and vegetables with no added sugar, salt, or fat and low or nonfat milk), (2) consistent with the Dietary Guidelines for Americans35 and the Child and Adult Care Food Program36 meal patterns, and (3) cost neutral. The intention of HC2’s policy piece was not a legislative action but rather (1) the incorporation of the HC2 standards into future benchmarks when rating the quality of centers and (2) the implementation of nutrition and physical activity guidelines at CCCs with the assistance of the toolkit.
Child Curriculum to Support Policies
The child curriculum has lesson plans for instructional needs that are consistent with the policies above (3 plans focus on beverage/snack policies and 3 plans focus on physical activity/screen time policies) and incorporate Caring for Children, Third Edition32 standards and messaging from the Let’s Move campaign.34 The lesson plans are designed to support center policy adoption via monthly technical assistance to CCC teachers. To increase the ease of use and reduce burden, the lesson plans consist of physical activities and health-oriented messages that can be seamlessly incorporated into everyday activities as they include cognitive, fine motor, and self-help instructional components required in preschool curriculums.
Tier 2 (Teacher) and Tier 3 (Parent) Role Modeling Curriculum
The HC2 role modeling curriculum for parents and teachers was based on Project Mothers & Others & MyPyramid (M.O.M.).30,37 and the principles of the “nutritional gatekeeper” concept developed by the USDA.38 The parent curriculum consisted of 6 monthly workshops that were related to the core lesson plan principles. The University of Florida Institute of Food and Agricultural Sciences Extension Family Nutrition Program staff delivered all curriculum content to parents. A similar percentage of parents participated in the role modeling trainings across both phases (approximately 30%).
Phase 1 and 2 Control Arms
Child care centers randomized to the control arms in both phase 1 and phase 2 trials received an attention control consisting of Safety Sam, a character who delivers a safety curriculum. Parallel to the intervention arms’ dissemination and implementation approaches, this curriculum was delivered via a university-based research team in phase 1 and via a train-the-trainer (TTT) model in phase 2. The control arms in both phases received the Safety Sam content at the same level of exposure and contact time as the intervention arms. Control CCCs received all the same premeasures and postmeasures and incentives as the intervention arms to ensure retention/reduce loss to follow-up.
Healthy Caregivers-Healthy Children Phase 1 and Phase 2 Measurements
Child Measures
Child measures included Consumption of Fruit/Vegetables and Consumption of Unhealthy Foods (fried, fast, snack, etc.). These measures were based on questions from the Healthy Kids Checklist,39 a 32-item rating scale targeted at children in preschool via parental responses on their behalf. The Consumption of Fruit/Vegetables was measured using 5 survey questions, such as “my child eats fruit” and “my child eats vegetables at his main meal.” The Consumption of Unhealthy Food was measured using 8 survey questions, such as “my child eats fast food,” “my child drinks soda or sugared drinks with meals,” and “my child eats chips for snacks.” The outcome scores were based on a 7-point continuous scale ranging from once per week (1 day) to 7 times per week (every day). Snack food consumption was similarly measured on a continuous scale of frequency of consumption per week, ranging from once per week (1 day) to 7 times per week (every day). Snacks included cookies, chips, and candy and did not include fruits or vegetables.
Child Body Mass Index
Height (stadiometer) and weight (digital scale) were used to calculate raw body mass index (BMI) via BMI = [] and then converted to age- and sex-adjusted PBMIs. Data collection methods are based on the US Health and Human Services (HHS) guidelines.40 Healthy weight was defined as a BMI <85th percentile for age and sex, and unhealthy weight was defined as ≥85th percentile for age and sex.
Phase 1 and Phase 2 Differences
Phase 1
The phase 1 data resulted from a randomized controlled intervention trial (NCT017220321) that took place from 2011 to 2013. Details of the phase 1 randomized controlled trial methods and the results are published elsewhere.17,18,41 In summary, the HC2 content was delivered over 2 school years to CCCs randomized to the intervention arm (n = 12) or an attention control arm (n = 16) by a university-based research team. The theoretical framework and intervention content are described below and are the same for phase 1 and phase 2 (Table 1).
Table 1.
Similarities and Differences Between Phase 1 and Phase 2 of HC2
| HC2 Phase 1 | HC2 Phase 2 | |
|---|---|---|
| No. of centers | 28 | 24 |
| Implementation strategy | University-based research staff-implemented intervention | Train-the-Trainer cascading approach; Quality Rating Improvement System implemented the intervention |
| Intervention content | Same in both | |
| Measures used | Same in both | |
| No. of parent sessions | Same in both | |
| No. of teacher sessions | Same in both |
HC2, Healthy Caregivers-Healthy Children.
Phase 2
The phase 2 data resulted from a randomized controlled intervention trial (NCT02697565) that took place from 2015 to 2018 among 24 CCCs (12 HC2 intervention and 12 attention control). Details of the study design can be found elsewhere.16 HC2 phase 2 was delivered via a TTT cascading approach.42,43 Cascade refers to the flow of delivery over the tiers of knowledge, curriculums, and program (see below for details of each HC2 tier content).
The TTT approach developed for HC2 addressed the following 3 principles from the Social Cognitive Theory and adult learning theory: (1) knowledge, (2) self-efficacy, and (3) follow-up support (proactive technical assistance),44 with the primary goal of ensuring that HC2 “coaches” (QRIS employees who are embedded in the CCCs) are as motivated and effective as a university-based research team (Master Trainers).
Recent studies in the emerging field of implementation science strongly suggest that the successful incorporation of interventions into existing practice requires pervasive changes at multiple levels, including changes in policy, administrative procedures, and delivery of frontline practice.45 Furthermore, to implement new interventions and sustain them in the context of routine practice, factors such as a sense of ownership, collaboration, user-friendly communication and assessments, and compatibility with users’ needs and goals have proven important. HC2 builds on this model’s capacity to promote sustainability, given that QRIS coaches have existing relationships with CCCs, the HC2 toolkit fills teachers’ needs for evidence-based programming, and HC2 emphasizes collaboration via proactive technical assistance to promote sustainability.
Specifically, HC2 content was delivered by university-based Master Trainers to QRIS coaches. University Master Trainers had a master’s degree in education or related field, as did QRIS coaches. The university Master Trainers and the QRIS coaches were both trained on the HC2 curriculum in the same way. Six 3-hour monthly workshops were used to deliver the content of each of the monthly lesson plans in a small group setting with 3 to 5 teachers. The workshop format included an introductory session, followed by didactic presentations, role-playing, interaction among learners in small group breakouts, a presentation practice session, and a final question and answer session. QRIS coaches in turn disseminated this information and trained teachers in their QRIS network schools (each were responsible for approximately 5 schools). Adult learning model constructs included using university-based Master Trainers to teach the course (e.g., learning from professional experts), providing feedback on practice presentations, and recognizing the diverse backgrounds of the target audience in terms of subject matter and cultural practices.23
Statistical Analyses
To analyze the Reach dimension of RE-AIM (Reach, Effectiveness, Adoption, Implementation, Maintenance), descriptive characteristics (mean and percent) of child and parent demographics were calculated and included child age, sex, and race/ethnicity, and caregiver age, relationship to child, language spoken at home, level of education, and birthplace. To analyze the Effectiveness and Maintenance impact of the HC2 intervention on change in the outcome measures (fruits snacks, consumption of unhealthy food such as chips, and PBMI) over time, growth curve analysis was conducted. Four time points were included in the models: for phase 1, time 1 (T1) (baseline or beginning of the 2011–2012 school year), T2 (end of 2011–2012 school year), T3 (beginning of 2012–2013 school year), and T4 (end of 2012–2013 school year); for phase 2, T1 (baseline or beginning of the 2015–2016 school year), T2 (end of 2015–2016 school year), T3 (beginning of 2016–2017 school year), and T4 (end of 2016–2017 school year). Growth curves were chosen as the analytical unit to compare phase 1 and phase 2 to properly take into consideration the within and between variances while modeling the longitudinal process. Hsu et al.46 showed that both the multilevel latent growth curve model and the maximum model may result in unbiased estimates when the interindividual variability is taken into consideration, as was conducted here. Research shows that growth curves can correct some of the flaws of type III unbalanced designs such as when every person is observed at a potentially different set of time points and individuals are repeatedly measured on 3 occasions but at different sets of ages.47
A separate growth curve was created for the consumption of fruit snacks, consumption of unhealthy food such as chips, and PBMI. The growth model for nominal outcomes fruit snacks and unhealthy food such as chips were based on generalized estimation equation. Each model included an intercept(s) and a slope(s) estimate, and the impact of intervention CCC assignment on the intercept and slope was tested. For outcomes with a statistically significant relationship between intervention CCCs and slope, model estimated means were calculated at each time point for the intervention and control CCCs. For outcomes with a statistically significant interaction term, stratified analysis was conducted. Model estimates for the impact of intervention CCC assignment on the intercept and the slope of the growth curve are reported for each stratum. Model estimate means for PBMI were calculated at each time point for the intervention and control group for each stratum. All models controlled for gender, race/ethnicity, and age. All models also accounted for clustering of students within centers. Statistical significance was defined as p < 0.05 for all statistical tests. Analysis was conducted in SAS software version 9.4 (SAS Institute, Cary, NC).
RESULTS
Consistent with the RE-AIM (Reach, Effectiveness, Adoption, Implementation, Maintenance) framework, phase 1 and phase 2 data were collected and analyzed within the dimensions of Reach, Effectiveness, Adoption, Implementation, and Maintenance. For the purposes of comparison for this study, the dimensions of Reach and Effectiveness and Maintenance are presented because they are most relevant to phase 1 and phase 2. Reach is described in Table 2, which displays the demographic characteristics of the parents and children in both phases. Effectiveness is described in Table 3, showing outcomes of dietary intake. Maintenance is displayed in Figure 1, showing the body mass index percentile (PBMI) outcomes over 2 years.
Table 2.
Demographic Characteristics of Children and Parents for Healthy Caregivers-Healthy Children Phase 1 and Phase 2 by Intervention Arm
| Phase 1 |
Phase 2 |
|||||||
|---|---|---|---|---|---|---|---|---|
| Characteristics | Entire Sample (n = 1224) |
Intervention Group (n = 767) |
Control Group (n = 457) |
p | Entire Sample (n = 825) |
Intervention Group (n = 465) |
Control Group (n = 360) |
p |
| Child sex | ||||||||
| Girl | 596 (49.8) | 371 (49.5) | 225 (50.3) | 0.7882 | 429 (52.5) | 247 (30.2) | 182 (22.3) | 0.5831 |
| Boy | 600 (50.2) | 378 (50.5) | 222 (49.7) | 388 (47.5) | 216 (26.4) | 172 (21.1) | ||
| Child race/ethnicity | ||||||||
| Hispanic Cuban | 162 (15.9) | 75 (7.4) | 87 (8.5) | 0.0000 | 112 (20.5) | 75 (13.7) | 37 (6.8) | 0.0001 |
| Other Hispanic | 431 (42.3) | 326 (32) | 105 (10.3) | 238 (43.5) | 92 (16.9) | 146 (26.7) | ||
| Non-Hispanic Black | 300 (29.4) | 180 (17.6) | 120 (11.8) | 138 (25.2) | 85 (15.5) | 53 (9.7) | ||
| Non-Hispanic White | 29 (2.9) | 15 (1.5) | 14 (1.4) | 27 (4.9) | 14 (2.5) | 13 (2.4) | ||
| Other | 97 (9.5) | 71 (7) | 26 (2.5) | 32 (5.8) | 21 (3.8) | 11 (2) | ||
| Child age, mo, mean | 49.5 (11.2) | 52.6 (7.9) | 44.4 (13.7) | 0.0000 | 43.4 (13.3) | 42.5 (13.0) | 44.6 (13.5) | 0.0184 |
| Caregiver age, yrs | ||||||||
| 18–24 | 148 (12.1) | 84 (6.9) | 64 (5.2) | 0.0963 | 57 (6.9) | 19 (2.3) | 38 (4.6) | 0.0001 |
| 25–30 | 370 (30.2) | 249 (20.3) | 121 (9.9) | 174 (21.1) | 85 (10.3) | 89 (10.8) | ||
| 31–40 | 376 (30.7) | 246 (20.1) | 130 (10.6) | 248 (30.1) | 150 (18.2) | 98 (11.9) | ||
| 41–50 | 85 (6.9) | 62 (5) | 23 (1.9) | 55 (6.7) | 26 (3.2) | 29 (3.5) | ||
| 51+ | 12 (1) | 7 (0.6) | 5 (0.4) | 12 (1.5) | 8 (1) | 5 (0.5) | ||
| Missing | 233 (19) | 119 (9.7) | 114 (9.3) | 279 (33.8) | 174 (21.1) | 105 (12.7) | ||
| Caregiver relationship to child | ||||||||
| Mother | 863 (70.5) | 552 (45.1) | 311 (25.4) | 0.0000 | 457 (55.4) | 232 (28.1) | 225 (27.3) | 0.0001 |
| Father | 104 (8.5) | 76 (6.2) | 28 (2.3) | 52 (6.3) | 33 (4.0) | 19 (2.3) | ||
| Other | 257 (21) | 139 (11.4) | 118 (9.6) | 316 (38.3) | 200 (24.2) | 116 (14.1) | ||
| Caregiver language spoken in home | ||||||||
| English only | 413 (33.7) | 214 (17.5) | 199 (16.2) | 0.0000 | 366 (44.4) | 220 (26.7) | 146 (17.7) | 0.0000 |
| Spanish only | 461 (37.7) | 345 (28.2) | 116 (9.5) | 186 (22.5) | 75 (9) | 111 (13.5) | ||
| Other | 350 (28.6) | 208 (17) | 142 (11.6) | 273 (33.1) | 170 (20.1) | 103 (12.5) | ||
| Caregiver level of education | ||||||||
| Less than 12th grade or GED | 363 (29.7) | 302 (24.7) | 61 (5) | 0.0000 | 44 (5.3) | 15 (1.8) | 29 (3.5) | 0.0002 |
| Completed high school | 258 (21.1) | 171 (14) | 87 (7.1) | 138 (16.7) | 60 (7.2) | 78 (9.5) | ||
| Completed education >high school | 406 (33.2) | 196 (16) | 210 (17.2) | 363 (66.6) | 216 (26.2) | 147 (17.8) | ||
| Missing | 197 (16.1) | 98 (8) | 99 (8.1) | 280 (31.4) | 172 (19.8) | 101 (11.6) | ||
| Birthplace of caregiver | ||||||||
| US born | 740 (60.5) | 537 (43.9) | 203 (16.6) | 0.0000 | 299 (34.5) | 164 (18.9) | 135 (15.6) | 0.0004 |
| Foreign born | 320 (26.1) | 159 (13) | 161 (13.1) | 303 (34.9) | 142 (16.4) | 161 (18.5) | ||
| Missing | 164 (13.4) | 71 (5.8) | 93 (7.6) | 266 (30.7) | 97 (11.2) | 169 (19.5) | ||
| Outcome variables of interest | ||||||||
| PBMI (mean) | 65.9 (28.8) | 65.8 (28.2) | 66.2 (28.4) | 0.8330 | 62.3 (28.9) | 60.1 (29.4) | 64.1 (28.2) | 0.1253 |
| BMI category | ||||||||
| Normal weight | 577 (47) | 367 (30) | 210 (17) | 0.5206 | 557 (67.5) | 319 (38.7) | 238 (28.8) | 0.4492 |
| Overweight or obese | 647 (52.9) | 400 (32.7) | 247 (20.2) | 268 (32.5) | 146 (17.7) | 122 (14.8) | ||
GED, general education development; PBMI, body mass index percentile.
Table 3.
Mean Comparison of Nutrition Outcomes by Healthy Caregivers-Healthy Children Phase 1 and Phase 2 by Intervention Arm
| Phase 1 | |||||
|---|---|---|---|---|---|
| Visit 1 | Visit 2 | Visit 3 | Visit 4 | Overall Change | |
| Rate of vegetable consumption per week | |||||
| Control | 3.49 (1.73) | 3.41 (1.42) | 3.92 (1.39) | 3.14 (1.27) | −0.35 |
| Intervention | 3.70 (1.62) | 3.78 (1.53) | 3.59 (1.43) | 3.48 (1.31) | −0.22 |
| Rate of fruit consumption per week | |||||
| Control | 4.18 (1.77) | 4.46 (1.89) | 4.74 (1.65) | 4.69 (1.87) | +0.51 |
| Intervention | 4.23 (1.86) | 4.35 (1.76) | 4.69 (1.74) | 4.64 (1.84) | +0.41 |
| Rate of fried food consumption per week | |||||
| Control | 1.98 (3.64) | 1.38 (1.17) | 1.39 (1.04) | 1.49 (0.94) | −0.49 |
| Intervention | 2.50 (4.08) | 2.05 (3.05) | 1.65 (1.56) | 1.42 (1.61) | −1.08 |
| Rate of fast food consumption per week | |||||
| Control | 1.46 (2.79) | 1.62 (0.89) | 1.21 (1.08) | 1.29 (0.90) | −0.17 |
| Intervention | 1.35 (2.32) | 1.21 (1.59) | 1.02 (0.80) | 0.96 (0.79) | −0.39 |
| Rate of snack food consumption per week | |||||
| Control | 1.45 (1.69) | 1.49 (1.68) | 2.61 (1.72) | 2.70 (1.76) | +1.25 |
| Intervention | 1.27 (1.47) | 1.11 (1.19) | 2.29 (1.68) | 2.32 (1.74) | +1.05 |
| Rate of soda consumption per week | |||||
| Control | 0.68 (1.14) | 0.61 (1.01) | 0.68 (1.25) | 0.73 (1.25) | +0.05 |
| Intervention | 0.76 (1.17) | 0.72 (1.04) | 0.72 (1.41) | 0.74 (1.42) | −0.02 |
| Phase 2 | |||||
| Visit 1 | Visit 2 | Visit 3 | Visit 4 | Overall Change | |
| Rate of vegetable consumption per week | |||||
| Control | 3.14 (1.37) | 3.35 (1.51) | 3.57 (1.71) | 3.14 (1.27) | 0 |
| Intervention | 3.06 (1.37) | 3.01 (1.46) | 3.40 (1.33) | 3.23 (1.34) | +0.17 |
| Rate of fruit consumption per week | |||||
| Control | 4.81 (1.76) | 4.96 (1.77) | 4.92 (1.57) | 4.67 (1.68) | −0.14 |
| Intervention | 5.05 (1.82) | 5.00 (1.69) | 5.08 (1.80) | 5.04 (2.03) | −0.01 |
| Rate of fried food consumption per week | |||||
| Control | 1.56 (1.25) | 1.53 (1.09) | 1.41 (1.29) | 1.57 (1.24) | +0.01 |
| Intervention | 1.41 (1.04) | 1.58 (1.30) | 1.45 (1.19) | 1.40 (1.15) | −0.01 |
| Rate of fast food consumption per week | |||||
| Control | 1.45 (1.31) | 1.39 (1.18) | 1.23 (1.01) | 1.69 (1.99) | +0.24 |
| Intervention | 1.23 (1.06) | 1.21 (0.99) | 1.14 (0.98) | 1.44 (1.11) | +0.21 |
| Rate of snack food consumption per week | |||||
| Control | 4.92 (2.03) | 2.04 (1.62) | 2.57 (1.19) | 2.42 (1.76) | −2.50 |
| Intervention | 4.55 (1.91) | 4.78 (2.75) | 1.79 (1.10) | 2.39 (1.05) | −2.16 |
| Rate of soda consumption per week | |||||
| Control | 0.84 (1.18) | 1.06 (1.01) | 0.75 (1.13) | 0.93 (1.28) | +0.09 |
| Intervention | 0.76 (1.16) | 0.79 (1.18) | 0.54 (1.17) | 0.83 (1.09) | +0.07 |
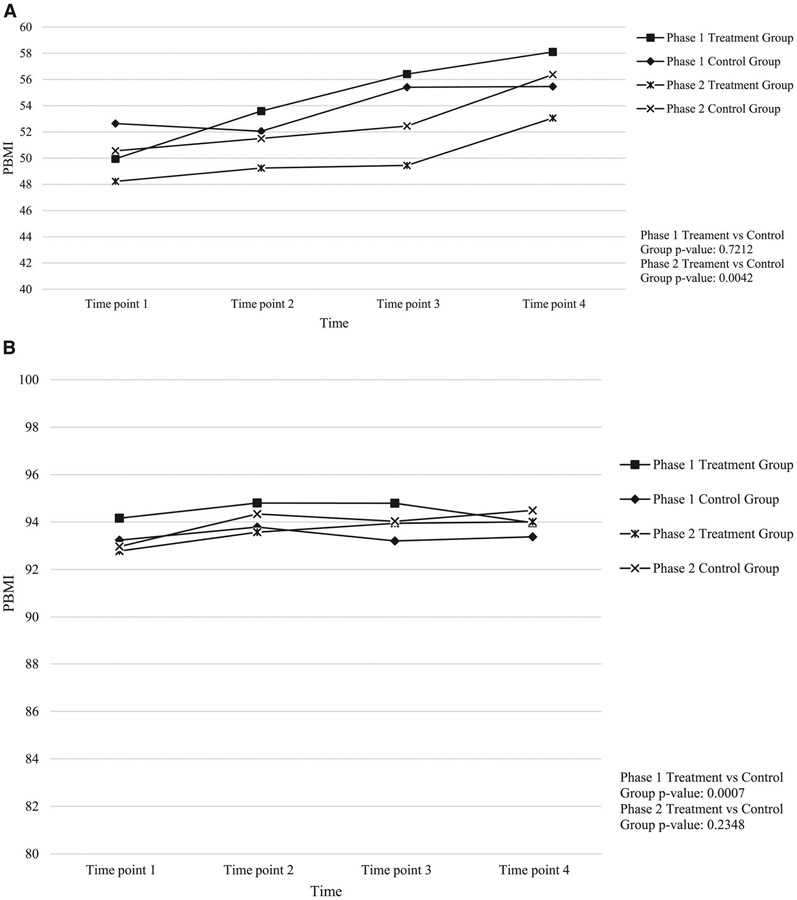
Figure 1.
A, PBMI change over 2 years among normal weight participants, HC2 phase 1 and phase 2 by intervention arm. B, PBMI change over 2 years among unhealthy weight participants, HC2 phase 1, and phase 2 by intervention arm. HC2, Healthy Caregivers-Healthy Children; PBMI, body mass index percentile.
As noted in Table 2, phase 1 and phase 2 caregivers were a majority of “other Hispanic” ethnicity, spoke Spanish only at home, and completed education beyond high school. Phase 2 caregivers were evenly distributed across US born and foreign born, with a high percentage of missing data. The average age of phase 1 children was 4 years and 1 month, whereas the average age of phase 2 children was younger by approximately 7 months. Less than 5% of children were excluded because their parent did not provide consent. There was a statistically significant difference between the intervention group and the control group across both phases for child race/ethnicity, child age, caregiver age, caregiver relationship to child, caregiver language spoken in home, caregiver level of education, and birthplace of caregiver.
Effectiveness is described in Table 3, which displays the mean results for all primary dietary intake outcomes for both phases. There was a statistically significant difference between the intervention and control child care centers (CCCs) for the rate of mean vegetable consumption per week in phase 1, with the intervention CCCs having a higher rate of mean vegetable consumption versus the control CCCs; however, this was not the case in phase 2. The rate of mean fruit consumption was higher in phase 2 than phase 1; however, neither phases saw statistical significance between the control and intervention CCCs. The intervention CCCs in phase 1 had a higher mean rate of fried food consumption than the control CCCs, whereas the intervention CCCs in phase 2 had a lower mean rate of fried food consumption than the control CCCs. There was no significant difference in the mean rate of soda consumption between the intervention and control groups for phase 1. Mean soda consumption in phase 1 was higher in the intervention CCCs than in the control CCCs (NS, nonsignificant), and vice versa for phase 2.
The rate of fruit consumption decreased in phase 1 in the intervention compared with the control CCCs; however, across time, it increased significantly (β = 0.16, p = 0.001). Most of the results over time for phase 2 were not statistically significant, with the exception of an increased rate of vegetable consumption (β = 0.16, p = 0.002) and a reduction in snack food consumption (β = −0.97, p < 0.001). Phase 1 showed significantly decreased intake of vegetables, fried foods, soda and sugary drinks, fast food, unhealthy foods (cookies and chips), and snack foods (prepackaged, simple carbohydrate cookies, cakes, etc.). For the overall comparison of phase 1 and phase 2 for each dietary intake outcome, all were statistically different with the exception of fast food and unhealthy (e.g., highly processed convenience items) food consumption. The trend for PBMI in the aggregate samples (not stratified by BMI group, not shown on tables) decreased by 0.01 points in the intervention group compared with the control group in phase 1 over 2 years (NS) and increased by 0.16 points in phase 2 (p = 0.002).
Maintenance is illustrated in Figure 1, panel A, which shows the change in PBMI over 2 years for normal weight children by study phase, and Figure 1, panel B, shows the same for unhealthy weight (≥85th PBMI for age and sex) participants. The figure does not display any healthy weight children leaving the healthy weight PBMI range into the unhealthy range, nor does it show any unhealthy weight children decreasing into healthy weight percentiles. Both groups stayed in their range. Once the groups were stratified, the results for the intervention group in phase I showed that healthy PBMI increased by 9 points from time point 1 to time point 4 (p = 0.0032), whereas unhealthy PBMI decreased during the same time period (NS). The increase of 9 points among the healthy weight children showed an increase into a potential unhealthy weight percentile range. In phase 2, healthy PBMIs for the intervention and control groups also increased from time point 1 to time point 4 by 5 points and 6 points (p = 0.050 and 0.027), respectively. Unhealthy PBMI increased in both the phase 2 intervention (NS) and control group (p = 0.023).
In both phases of the intervention, the overall mean child BMI remained in the healthy range throughout the 4 time points for both intervention and control groups.
DISCUSSION
This study compared the results of the Healthy Caregivers-Healthy Children (HC2) phase 1 and phase 2 CCC-based obesity prevention intervention(s) on child dietary practices and body mass index percentile (PBMI) outcomes over 2 years. Phase 1 was delivered via a university-based research team, and phase 2 was delivered via a train-the-trainers (TTTs) model. Using the RE-AIM (Reach, Effectiveness, Adoption, Implementation, Maintenance) framework, Effectiveness was demonstrated in phase 1 intervention CCCs showing significantly increased fruit consumption and in phase 2 intervention CCCs showing increased vegetable intake. Fried, fast, and other unhealthy food consumption significantly decreased in phase 1 only, whereas the mean rate of snack food consumption significantly decreased in phase 2 only. In summary, 75% (6 of 8) of phase 1 and 50% (4 of 8) of phase 2 dietary intake behaviors in HC2 CCCs showed positive dietary intake trends over 2 years. Overall, the phase 1 university-based research team-implemented HC2 was shown to be more effective than a TTT implementation method in improving dietary intake patterns. Although the TTT approach did make some improvements in dietary intake behaviors, the results suggest that CCCs may need additional support, perhaps university-based or through other educational organizations beyond their internal, existing Quality Rating Improvement System (QRIS) resources. Furthermore, regarding Maintenance dimensions of RE-AIM, long-term positive BMIs were not noted in either group, suggesting that BMI growth trajectories may be more difficult to affect.
The results here show that partnering with universities with the appropriate expertise may be a better approach to disseminating and implementing childhood obesity prevention programs in CCCs. Although BMI was not affected, the nutrition patterns of the centers did change to a greater degree when university Master Trainers were present. These results indicate the complexity of the issue of addressing obesity prevention and the time it takes to see the impacts on BMI, as seen in previous research.48 Although our results show that with a supportive framework/network such as QRIS, some quality dietary intake achievements can be seen, those results also suggest the need for more robust support of academic staff who may be more versed in content. The university Master Trainers had only 1 job focus, which was to deliver the HC2 intervention. The QRIS staff had multiple job demands while working with a CCC, which included not only delivering the HC2 intervention but also assisting the centers with quality control issues. This may have interfered with the uptake of the intervention. Therefore, we can conjecture that perhaps a more intentional unilateral focus on health education could assist CCC teachers in sustaining long-term positive health trends. Furthermore, given the differences in the demographic characteristics of the participants in the phases, it is important to consider that these characteristics (i.e., education levels) could have also influenced the results.
This study was one of the first to use a CCC-based TTT model with QRIS coaches and teachers as the primary dissemination and implementation agents or facilitators to promote healthy lifestyle behaviors. Other studies have shown that TTT dissemination models may not be effective if the staff do not have a lot of expertise on a specific topic.49 Modifications were made based on the literature, experience in the field, and focus groups that pointed to some of the difficulties CCC staff had in carrying out HC2. This led to a more realistic and efficient TTT mechanism to avoid the potential pitfalls of the TTT cascade model. Specifically, (1) healthy lifestyle knowledge was transferred in workshop training formats that included an introduction session, followed by game-based activities, role playing, interaction among learners in small group breakouts, a presentation practice session, and a final barriers/solutions brainstorming session; (2) self-efficacy was enhanced by collaborating with teachers and coaches and through the use of reinforcement/rewards; (3) observational learning took place with teams of teachers paired with QRIS coaches who role modeled healthy behaviors and choices through their daily interactions and pledged to consume healthy meals while at the CCCs; and (4) proactive technical assistance was used, including responsive consultation after training to enhance the adoption and use of HC2. Future research efforts could also take advantage of the QRIS framework, especially because it has the capacity to be scalable at a national level. However, the results shown here suggest that the QRIS network alone may not be enough to sustain long-term, positive dietary intake patterns in CCC attendees. Rather, partnering with universities or other community-based organizations with dietary and nutrition intake and education delivery expertise may enhance long-term health outcomes.
Limitations and Strengths
As with any community-based study using self-reported data, it could be challenging to gather accurate information from participants. However, both the phase 1 and 2 trials used valid and structured questionnaires that were easy to comprehend by study participants and were delivered by trained researchers and Master Trainers. In addition, the utilization of a control group helped address this limitation because both received the same surveys, so any inaccurate reporting was distributed across both groups. Furthermore, we consider it a strength that both state and county partnerships were used, and the QRIS framework provides a scalable and sustainable framework to build on.
Another limitation was that although teacher fidelity measures were collected in phase 1 and phase 2, they were collected inconsistently, so data could not be compared across phases. Differences in fidelity could have thus affected the results across the phases. The parent training component could have also affected the outcomes, given that parents who attended more trainings could have been better versed in program content, thus resulting in children with better nutritional outcomes.
Two major limitations are that both phases showed demographic sample differences and temporal differences between phase 1 and 2. The differences in caregiver demographics (relationship to child, level of education, birthplace, etc.) may have accounted for the differences observed between phase 1 and 2. It is possible that children had fewer significant dietary changes in phase 2 because the caregivers had higher levels of education at baseline. However, both phases were randomized controlled trials, and thus, it would be expected that demographic characteristics would be balanced. We are aware that this did not occur as anticipated, and thus, in the analyses, we tried to control for demographic differences by using statistical procedures that took into consideration the within-phase and between-phase variability. Furthermore, similar to the way meta-analysis research is conducted when comparing different studies, we did analyze the phases as random effects. This will not completely reduce the bias because of participants not being randomly assigned to either phase but will assist in reducing the bias. In addition, it is important to note that CCCs were the main unit of analysis, and there were different participants in each phase so that there was no overlap. However, given some of the complications that arose with randomization, this study helps to justify the need for a 2-stage randomization, such as stratified randomization, when conducting this type of research.
CONCLUSIONS
A university-based research team implementation and dissemination approach seemed to be more effective than a train-the-trainers implementation method in improving dietary intake patterns through a child care center (CCC)-based obesity prevention intervention. This finding suggests that CCCs may need robust educational support beyond their existing internal resources for long-term positive dietary intake pattern changes. Healthy body mass index growth trajectory development among their child attendees may also need a longer intervention period to show significant changes.
Acknowledgments
This work was funded by the United States Department of Agriculture NRI/AFRI (grant numbers #2009-05065 and #2014-08403) and National Institutes of Health (F31DK116533).
Footnotes
Disclosure: The authors declare no conflict of interest.
REFERENCES
- 1.Ogden CL, Fryar CD, Hales CM, et al. Differences in obesity prevalence by demographics and urbanization in US children and adolescents, 2013–2016. JAMA. 2018;319:2410–2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hales CM, Fryar CD, Carroll MD, et al. Trends in obesity and severe obesity prevalence in US youth and adults by sex and age, 2007–2008 to 2015–2016. JAMA. 2018;319:1723–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nader PR, O’Brien M, Houts R, et al. Identifying risk for obesity in early childhood. Pediatrics. 2006;118:e594–e601. [DOI] [PubMed] [Google Scholar]
- 4.Birch LL, Anzman SL. Learning to eat in an obesogenic environment: a developmental systems perspective on childhood obesity. Child Dev Persp. 2010;4:138–143. [Google Scholar]
- 5.Gluckman PD, Hanson MA, Cooper C, et al. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 2008;359:61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu JQ, Murphy SL, Kochanek KD, et al. Mortality in the United States, 2015: NCHS Data Brief, No. 267. Hyattsville, MD: National Center for Health Statistics; 2016. Available at: https://www.cdc.gov/nchs/products/databriefs/db267.htm. Accessed January 31, 2020. [Google Scholar]
- 7.Rhee K Childhood overweight and the relationship between parent behaviors, parenting style, and family functioning. Ann Am Acad Pol Soc Sci. 2008;615:11–37. [Google Scholar]
- 8.Lindsay AC, Sussner KM, Kim J, et al. The role of parents in preventing childhood obesity. Future Child. 2006;16:169–186. [DOI] [PubMed] [Google Scholar]
- 9.McBean LD, Miller GD. Enhancing the nutrition of America’s youth. J Am Coll Nutr. 1999;18:563–571. [DOI] [PubMed] [Google Scholar]
- 10.Stice E, Shaw H, Marti CN. A meta-analytic review of obesity prevention programs for children and adolescents: the skinny on interventions that work. Psychol Bull. 2006;132:667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Federal Interagency Forum on Child and Family Statistics. America’s Children: Key National Indicators of Well-Being. Washington, DC: U.S. Government Printing Office; 2007. [Google Scholar]
- 12.Fox M, Glantz F, Endahl J. Early Childhood and Childcare Study. Alexandria, VA: U.S. Department of Agriculture; 1997. [Google Scholar]
- 13.Fitzgibbon ML, Stolley MR, Dyer AR, et al. A community-based obesity prevention program for minority children: rationale and study design for Hip-Hop to Health Jr. Prev Med. 2002;34:289–297. [DOI] [PubMed] [Google Scholar]
- 14.Ward DS, Vaughn AE, Burney RV, et al. Recruitment of family child care homes for an obesity prevention intervention study. Contemp Clin Trials Commun. 2016;3:131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Natale R, Scott SH, Messiah SE, et al. Design and methods for evaluating an early childhood obesity prevention program in the childcare center setting. BMC Public Health. 2013;13:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Messiah SE, Lebron C, Moise R, et al. Healthy caregivers-healthy children (HC2) phase 2: integrating culturally sensitive childhood obesity prevention strategies into childcare center policies. Contemp Clin Trials. 2017;53:60–67. [DOI] [PubMed] [Google Scholar]
- 17.Natale RA, Messiah SE, Asfour L, et al. Role modeling as an early childhood obesity prevention strategy: effect of parents and teachers on preschool children’s healthy lifestyle habits. J Dev Behav Pediatr. 2014;35:378–387. [DOI] [PubMed] [Google Scholar]
- 18.Natale RA, Messiah SE, Asfour LS, et al. Obesity prevention program in childcare centers: two-year follow-up. Am J Health Promot. 2017;31:502–510. [DOI] [PubMed] [Google Scholar]
- 19.National Center on Early Childhood Quality Assurance. QRIS Resource Guide: About QRIS. Available at: https://qrisguide.acf.hhs.gov/about-qris. Accessed October 2, 2017.
- 20.Corelli RL, Fenlon CM, Kroon LA, et al. Evaluation of a train-the-trainer program for tobacco cessation. Am J Pharm Educ. 2007;71:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Beurs DP, de Groot MH, de Keijser J, et al. The effect of an e-learning supported Train-the-Trainer programme on implementation of suicide guidelines in mental health care. J Affect Dis. 2015;175:446–453. [DOI] [PubMed] [Google Scholar]
- 22.Hiner CA, Mandel BG, Weaver MR, et al. Effectiveness of a training-of-trainers model in a HIV counseling and testing program in the Caribbean Region. Hum Resour Health. 2009;7:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Orfaly RA, Frances JC, Campbell P, et al. Train-the-trainer as an educational model in public health preparedness. J Public Health Manag Pract. 2005;11:S123–S127. [DOI] [PubMed] [Google Scholar]
- 24.Bagherniya M, Taghipour A, Sharma M, et al. Obesity intervention programs among adolescents using social cognitive theory: a systematic literature review. Health Educ Res. 2018;33:26–39. [DOI] [PubMed] [Google Scholar]
- 25.Ellis P, Robinson P, Ciliska D, et al. A systematic review of studies evaluating diffusion and dissemination of selected cancer control interventions. Health Psychol. 2005;24:488. [DOI] [PubMed] [Google Scholar]
- 26.Rabin BA, Glasgow RE, Kerner JF, et al. Dissemination and implementation research on community-based cancer prevention: a systematic review. Am J Prev Med. 2010;38:443–456. [DOI] [PubMed] [Google Scholar]
- 27.Gaglio B, Shoup JA, Glasgow RE. The RE-AIM framework: a systematic review of use over time. Am J Public Health. 2013;103:e38–e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Glasgow RE, Vogt TM, Boles SM. Evaluating the public health impact of health promotion interventions: the RE-AIM framework. Am J Public Health. 1999;89:1322–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Glasgow RE, Dickinson P, Fisher L, et al. Use of RE-AIM to develop a multi-media facilitation tool for the patient-centered medical home. Implement Sci. 2011;6:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jilcott S, Ammerman A, Sommers J, et al. Applying the RE-AIM framework to assess the public health impact of policy change. Ann Behav Med. 2007;34:105–114. [DOI] [PubMed] [Google Scholar]
- 31.Eldridge S, Kerry S. A Practical Guide to Cluster Randomised Trials in Health Services Research. Vol 120. Hoboken, NJ: John Wiley & Sons; 2012. [Google Scholar]
- 32.American Academy of Pediatrics. Caring for Our Children: National Health and Safety Performance Standards; Guidelines for Early Care and Education Programs. Aurora, CO: National Resource Center for Health and Safety in Child Care and Early Education; 2011. [Google Scholar]
- 33.Institute of Medicine (IOM). Early Childhood Obesity Prevention Policies. Washington, DC: The National Academies Press; 2011: 2156–5376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.The Nemours Foundation. Healthy Kids, Healthy Future. 2018. Available at: https://healthykidshealthyfuture.org/. Accessed June 15, 2014.
- 35.Dietary Guidelines Advisory Committee. Report of the Dietary Guidelines Advisory Committee on the Dietary Guidelines for Americans, 2010, to the Secretary of Agriculture and the Secretary of Health and Human Services. Washington, DC: Agricultural Research Service; 2010. [Google Scholar]
- 36.USDA Food and Nutrition Service. Child and Adult Care Food Program (CACFP). Available at: https://www.fns.usda.gov/cacfp/child-and-adult-care-food-program. Accessed February 14, 2017.
- 37.Wasnick B Project M.O.M.: Mothers & Others & MyPyramid. J Am Diet Assoc. 2008;108:1302–1304. [DOI] [PubMed] [Google Scholar]
- 38.United States Department of Agriculture. Nutritional gatekeepers: begin a journey to healthy eating. Available at: https://articles.extension.org/pages/68791/nutritional-gatekeepers:-begin-a-journey-to-healthy-eating. Accessed July 12, 2019.
- 39.Townsend M, Sylva K, Shilts M, et al. Healthy Kids checklist. Available at: http://townsendlab.ucdavis.edu. Accessed February 20, 2014.
- 40.Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC growth charts for the United States: methods and development. Vital Health Stat 11. 2002;246:1–190. [PubMed] [Google Scholar]
- 41.Natale R, Messiah S, Asfor L, et al. Healthy Caregivers-Healthy Children (HC2): a childcare center based obesity prevention. J Nutr Educ Behav. 2013;45:S86–S87. [Google Scholar]
- 42.Wedell M Cascading training down into the classroom: the need for parallel planning. Int J Educ Dev. 2005;25:637–651. [Google Scholar]
- 43.Ray ML, Wilson MM, Wandersman A, et al. Using a training-of-trainers approach and proactive technical assistance to bring evidence based programs to scale: an operationalization of the interactive systems framework’s support system. Am J Community Psychol. 2012;50:415–427. [DOI] [PubMed] [Google Scholar]
- 44.Dunn C, Thomas C, Ward D, et al. Design and implementation of a nutrition and physical activity curriculum for child care settings. Prev Chronic Dis. 2006;3:A58. [PMC free article] [PubMed] [Google Scholar]
- 45.Chinman M, Woodward EN, Curran GM, et al. Harnessing implementation science to increase the impact of health disparity research. Med Care. 2017;55(suppl 9 2):S16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hsu HY, Lin JJ, Skidmore ST, et al. Evaluating fit indices in a multilevel latent growth curve model: a Monte Carlo study. Behav Res Methods. 2019;51:172–194. [DOI] [PubMed] [Google Scholar]
- 47.Wu W, West SG, Taylor AB. Evaluating model fit for growth curve models: integration of fit indices from SEM and MLM frameworks. Psychol Methods. 2009;14:183. [DOI] [PubMed] [Google Scholar]
- 48.Taveras EM, Gortmaker SL, Hohman KH, et al. Randomized controlled trial to improve primary care to prevent and manage childhood obesity: the High Five for Kids study. Arch Pediatr Adolesc Med. 2011;165:714–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Burr CK, Storm DS, Gross E. A faculty trainer model: increasing knowledge and changing practice to improve perinatal HIV prevention and care. AIDS Patient Care STDS. 2006;20:183–192. [DOI] [PubMed] [Google Scholar]