DEAR EDITOR,
Cognitive flexibility is crucial for animal survival but is frequently impaired in neuropsychiatric disorders. Although many brain structures and functional networks are involved in cognitive flexibility, the neural mechanisms underlying cooperation among specific functional networks remain unclear from a global perspective. In this study, [18F]-fluorodeoxyglucose positron emission tomography (FDG-PET) was performed on 19 male tree shrews after four different visual discrimination tasks, including baseline, learning expert (LE), reversal naive (RN), and reversal expert (RE). Voxel-based analysis was used to identify the specific brain clusters corresponding to RN, LE, and RE, and the corresponding metabolic networks were subsequently constructed. Finally, potential dynamic combinations of brain structures dealing with contingencies and normal situations were explored. Intergroup comparison showed that the left nucleus accumbens (NAc) was activated in RN, and the network derived from the left NAc contained performance monitoring and executive control components of prefrontal cortex (PFC) regions as key nodes. The LE and RE networks contained key components of the memory system, including the amygdala and hippocampus, and PFC executive control systems that overlapped with the RN network. The reversal learning (RL) and learning processes were mediated by the interactions of multiple functional networks associated with performance monitoring, executive control, and memory systems. Notably, the NAc and PFC networks may act as functional interfaces in different systems to deal with contingencies and normal situations flexibly and effectively.
RL paradigms can be used to explore the neural mechanisms underpinning cognitive flexibility, which is crucial for animal survival and often disturbed in neuropsychiatric disorders. Previous studies have shown that efficient RL performance requires the participation of various functional brain systems, including neural correlates associated with internal and external environmental monitoring, reward, memory, and behavioral execution (Uddin, 2021). However, the core brain regions that trigger RL and how these functional systems cooperate to achieve RL remain unclear.
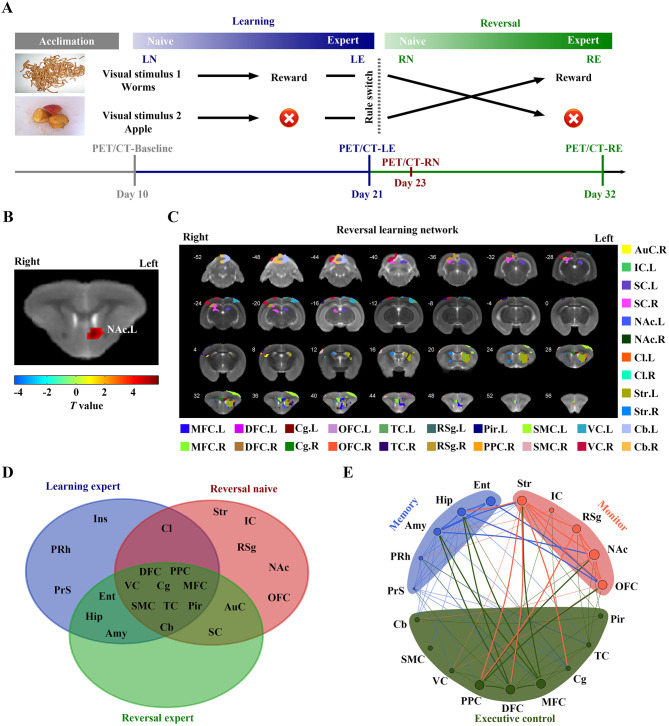
Here, we employed an RL paradigm based on visual discrimination using Bussey-Saksida Touch Screen Chambers (Campden Instruments Ltd., UK) (Mar et al, 2013) and [18F]-fluorodeoxyglucose positron emission tomography (FDG-PET) brain imaging to explore longitudinal metabolic activity variation patterns in brain-wide networks. Chinese tree shrews (Tupaia belangeri chinensis), a close relative of primates with a well-developed brain structure and visual system, were applied as an animal model for the visual-based cognitive tasks (Schumacher et al, 2021; Yao, 2017). Considering the potential effects of sex hormones on female cognitive behavior (Hamson et al, 2016), only male tree shrews were included in this study. Nineteen domesticated adult male tree shrews (aged 8–12 months, obtained from the breeding colony at the Animal House Center of the Kunming Institute of Zoology, Yunnan, China) were trained to perform “go/no go” visual-discrimination tasks with “worm picture” and “apple picture” stimuli (Figure 1A). During training, the two visual cues were randomly displayed in two fixed touchscreen windows. When the correct picture was touched, the animal received a food reward, otherwise, the animal was punished by an audible sound and no reward.
Figure 1.
Variations in collaborative network patterns during visual discrimination learning and reversal learning (RL) in brains of tree shrews
A: Schematics of visual discrimination tasks and 18F-FDG PET/CT imaging. B: Voxel-wise comparisons between RN and LE stage. Significant hyper-metabolism region was found in left NAc in RN stage (P<0.05, FWE-corrected, cluster size>50).T value, value of two-sample t-test. C: Sub-region location of RL network in tree shrew brain. D: Venn diagram showing overlap of metabolic networks involved in LE, RN, and RE stages. E: Schematic of functional systems engaged in dynamic visual discrimination tasks. LN, learning naive; LE, learning expert; RN, reversal naive; RE, reversal expert. MFC, medial frontal cortex; DFC, dorsal frontal cortex; Cg, cingulate cortex; OFC, orbital frontal cortex; TC, temporal cortex; RSg, retrosplenial granular cortex; Pir, piriform cortex; PPC, posterior parietal cortex; SMC, sensorimotor cortex; VC, visual cortex; AuC, auditory cortex; Ent, entorhinal cortex; Ins, insular cortex; PRh, perirhinal cortex; PrS, presubiculum; Hip, hippocampus; Amy, amygdala; IC, inferior colliculus; SC, superior colliculus; NAc, accumbens nucleus; Cl, claustrum; Str, striatum; Cb, cerebellum. L, left hemisphere; R, right hemisphere.
Daily training was performed on each tree shrew and terminated after 30 min or 40 touches. In the initial training, the “worm picture” was set as the correct option. Animals were considered to have moved from the learning naive (LN) to LE stage after reaching >85% correct touch rate. After ca. six days of training, all 19 tree shrews reached the LE stage. An additional 5 days of training was performed to ensure stability of the LE stage. On the last day of LE stage training, behavioral performance accuracy reached 95.64%±1.41% and trial number reached 38.00±1.05. Stimulus-reward mapping was then switched (“rule-switch”) by setting the “apple picture” as the correct option. The tree shrews initially showed decreased performance after reversal, but eventually relearned the new stimulus-reward mapping from the RN to RE stages. After seven days of training, 15 of the 19 tree shrews reached the RN to RE performance criteria. On the last day of RE stage training, behavioral performance accuracy reached 91.71%±2.23% and trial number reached 39.13±0.84. The RN stage is a critical period for animal adaptation to rule switching, thus FDG-PET brain images obtained on the second day of rule switch training were selected as representative of the RN stage, with behavioral performance accuracy of 32.68%±4.87% and trial number of 20.87±3.14 (Supplementary Figure S1A, B). These behavioral data were further analyzed. When tree shrews reached either LE or RE, no significant differences were found in total training days ( P=0.053, Student’s t-test; Supplementary Figure S1C) or number of correct trials (P=0.812, Student’s t-test; Supplementary Figure S1E). However, total number of trials (*: P=0.042, Student’s t-test; Supplementary Figure S1D) and number of error trials (***: P<0.001, Student’st-test; Supplementary Figure S1F) during RL were significantly higher than during initial learning, which was to be expected as animals tend to persevere with original mapping before switching. The tree shrews showed good executive capacity in the RL paradigm, indicating their suitability as a model animal in visual-based decision-making studies (Mustafar et al, 2018).
Four FDG-PET/CT scans were performed during the baseline, LE, RN, and RE stages, respectively (see Supplementary Materials for details, Figure 1A). Compared to LE, tree shrews encountered an unexpected “rule-switch” in the RN stage, and significant hyper-metabolism was found in the left nucleus accumbens (NAc) (P<0.05, family-wise error (FWE) corrected; Figure 1B, Supplementary Figure S2A and Table S1). A significant positive correlation was found between the standard uptake value ratio (SUVR) of the left NAc and the error rate (P=0.001, Pearson correlation analysis; Supplementary Figure S3A). These results suggest that the left NAc may be involved in the reversed stimulus-reward process. As a major part of the ventral striatum, the NAc is regarded as an essential site for flexibly dealing with contingencies in both positive and negative situations (Floresco, 2015). Our results are consistent with previous brain lateralization studies showing that the left ventral striatum is critical for processing emotions elicited by food rewards (Güntürkün et al, 2020).
Therefore, the left NAc was selected as the seed region to generate a group-wide whole-brain metabolic connectivity map based on PET images at the RN stage. In detail, Pearson correlation coefficients were calculated between the SUVR of the seed region and every other voxel (Yakushev et al, 2017), with significance defined at P<0.05 (false discovery rate (FDR) corrected). Hence, a significant potential RL network was constructed (Figure 1C; Supplementary Table S2 and Figure S4). We then computed three different properties of the RL network during the baseline, LE, RN, and RE stages to examine network specificity. Average network degree, global efficiency, and synchronization of the RL network in the RN stage were all significantly higher than those in the other three stages (see Supplementary Materials for details; Supplementary Figure S5), suggesting that the RL network was functionally highly integrated in the RN stage. Furthermore, these results indicate that the RL network in the tree shrew brain specifically responds to “rule-switch”. This RL network contained several prefrontal regions, consistent with the function of the PFC in error detection and value information storage (Izquierdo et al, 2017). Furthermore, corresponding key nodes of three PFC networks (i.e., fronto-parietal network (FPN), cingulo-opercular network (CON), and dorsal attention network (DAN)) were also contained in the RL network, including the dorsal frontal and posterior parietal cortices, cingulate and medial frontal cortices, and visual and dorsal frontal cortices, respectively (Menon & D’Esposito, 2022). This finding supports the view that the implementation of cognitive control in a constantly changing environment depends on the dynamic and flexible organization of the PFC networks (Menon & D’Esposito, 2022). More importantly, the recruitment of these PFC networks is closely related to NAc activation, reflecting the role of the NAc in triggering prefrontal functional networks to process contingencies.
To explore the potential dynamic interconnections of functional components that underpin cognitive status switching, we also constructed metabolic networks for the LE and RE stages (see Supplementary Materials for details, Supplementary Figures S6–S8). The learning networks in the LE and RE stages were nearly identical, and included cortical areas associated with executive control, such as the PFC, and classically recognized structures of the memory system, such as the hippocampus and amygdala (Figure 1D). This is not unexpected, as the animals were proficient at performing learned stimulus-reward mapping in both the LE and RE stages, which primarily involved stored-memory retrieval and behavioral execution. We also summarized the variations in brain networks in the LE, RN, and RE stages. The neural correlates involved in the three cognitive states represented the neural networks responsible for learned-memory storage and retrieval, internal and external environmental monitoring, and behavioral execution processing, respectively (Figure 1E). Interestingly, we found that the overlapping regions of the three metabolic networks contained key structures of the three PFC networks, as described above. Hence, the PFC functional networks were involved in both the performance of new learning and acquired cognitive task execution and may act as functional interfaces in different systems to deal with both contingencies and normal situations flexibly and effectively.
In conclusion, this study revealed that the NAc and core PFC executive control systems are involved in achieving RL. Multiple functional networks cooperate to accomplish a series of cognitive processes associated with RL, memory retrieval, performance monitoring, behavioral control, and updated memory storage. These findings highlight the complex brain network patterns of cognition and provide valuable hints regarding the potential pathophysiological sites associated with impaired cognitive flexibility in neuropsychiatric disorders.
SUPPLEMENTARY DATA
Supplementary data to this article can be found online.
COMPETING INTERESTS
The authors declare that they have no competing interests.
AUTHORS’ CONTRIBUTIONS
T.T.P. and H.L. designed the experiment; T.T.P., C.L., and Q.X.Z. conducted the experiments; T.T.P., T.H.Z., W.Z., S.L.Z., and B.B.N. analyzed the data; D.M.L., G.H.Z., B.C.S., and L.X. conceived and supervised the project; T.T.P. and H.L. wrote the manuscript. All authors read and approved the final version of the manuscript.
ACKNOWLEDGEMENTS
We thank Mr. Jun-Bo Sun and Ms. Gui-Fen Xie for their excellent technical assistance in animal experiments.
Funding Statement
This work was supported by the National Natural Science Foundation of China (11975249, 81771923, 12175268, 32071029, 31861143037), China Postdoctoral Science Foundation (2021T140668), and Strategic Priority Research Program of the Chinese Academy of Sciences (XDB32020000)
Contributor Information
Hua Liu, Email: liuhua@ihep.ac.cn.
Gao-Hong Zhu, Email: 1026909611@qq.com.
Lin Xu, Email: lxu@vip.163.com.
Bao-Ci Shan, Email: shanbc@ihep.ac.cn.
References
- 1.Floresco SB The nucleus accumbens: An interface between cognition, emotion, and action. Annual Review of Psychology. 2015;66:25–52. doi: 10.1146/annurev-psych-010213-115159. [DOI] [PubMed] [Google Scholar]
- 2.Güntürkün O, Ströckens F, Ocklenburg S Brain lateralization: a comparative perspective. Physiological Reviews. 2020;100(3):1019–1063. doi: 10.1152/physrev.00006.2019. [DOI] [PubMed] [Google Scholar]
- 3.Hamson DK, Roes MM, Galea LAM Sex hormones and cognition: Neuroendocrine influences on memory and learning. Comprehensive Physiology. 2016;6(3):1295–1337. doi: 10.1002/cphy.c150031. [DOI] [PubMed] [Google Scholar]
- 4.Izquierdo A, Brigman JL, Radke AK, Rudebeck PH, Holmes A The neural basis of reversal learning: an updated perspective. Neuroscience. 2017;345:12–26. doi: 10.1016/j.neuroscience.2016.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mar AC, Horner AE, Nilsson SRO, Alsiö J, Kent BA, Kim CH, et al The touchscreen operant platform for assessing executive function in rats and mice. Nature Protocols. 2013;8(10):1985–2005. doi: 10.1038/nprot.2013.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Menon V, D'Esposito M The role of PFC networks in cognitive control and executive function. Neuropsychopharmacology. 2022;47(1):90–103. doi: 10.1038/s41386-021-01152-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mustafar F, Harvey MA, Khani A, Arató J, Rainer G Divergent solutions to visual problem solving across mammalian species. eNeuro. 2018;5(4):ENEURO.0167–18.2018. doi: 10.1523/ENEURO.0167-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schumacher JW, McCann M, Maximov KJ, Fitzpatrick D. 2021. Selective enhancement of neural coding in V1 underlies fine discrimination learning in tree shrew. bioRxiv,doi: 10.1101/2021.01.10.426145.
- 9.Uddin LQ Cognitive and behavioural flexibility: neural mechanisms and clinical considerations. Nature Reviews Neuroscience. 2021;22(3):167–179. doi: 10.1038/s41583-021-00428-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yakushev I, Drzezga A, Habeck C Metabolic connectivity: methods and applications. Current Opinion in Neurology. 2017;30(6):677–685. doi: 10.1097/WCO.0000000000000494. [DOI] [PubMed] [Google Scholar]
- 11.Yao YG Creating animal models, why not use the Chinese tree shrew (Tupaia belangeri chinensis)? . Zoological Research. 2017;38(3):118–126. doi: 10.24272/j.issn.2095-8137.2017.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data to this article can be found online.