A new outbreak of monkeypox virus (MPV) is currently occurring in several regions of the world. Since the beginning of May 2022, 3 040 cases have been reported to the World Health Organization (WHO) globally. Outside of its usual “endemic” base in Africa, the virus has so far been detected in 47 countries, including in Europe, America, Oceania, and Asia, where monkeypox is uncommon or previously unreported. On 25 June 2022, the WHO Emergency Committee declared that the outbreak does not currently constitute a Public Health Emergency of International Concern, but unanimously acknowledged the emergency nature of the event and that controlling further spread will require a vigorous response. Recently, new cases of MPV infection have been confirmed in Korea, Singapore, and Taiwan, China. Thus, a better understanding of MPV and enhanced surveillance are essential for preventing disease recurrence.
MPV, which belongs to the genus Orthopoxvirus in the family Poxviridae, is an enveloped double-stranded DNA virus with a large genome of approximately 200 000 nucleotide bases. Viral sequence comparison confirmed that MPV is a distinct species of Orthopoxvirus rather than a direct ancestor or descendent of the variola virus (Shchelkunov et al., 2002). MPV was discovered in 1958 and initially found to cause rash disease in non-human primates, with human MPV infection first confirmed in 1970 by the WHO Commission to Certify Smallpox Eradication. As a zoonotic virus, human MPV outbreaks have mainly occurred in the tropical rainforests of West and Central Africa, including the Congo, Sierra Leone, Ghana, Central African Republic, Nigeria, and Sudan. The first outbreak outside Africa was reported in the USA in 2003, which included 72 confirmed or suspected cases following the importation of MPV-infected animals from Ghana (Ligon, 2004). In 2018 and 2021, human-to-human transmission outside Africa was also reported among healthcare workers and family members of travelers from Nigeria (Hobson et al., 2021; Vaughan et al., 2020).
Among contacts who have not been vaccinated against smallpox, MPV carries a secondary attack rate of about 10% and a case fatality rate of 1%–11% (Beer & Rao, 2019). MPV infection induces a series of smallpox-like symptoms in the skin, muscles, and digestive, respiratory, circulatory, and nervous systems. Initially, MPV infection presents with skin eruptions and fever with diaphoresis and rigors. The skin lesions progress from papules to vesiculopustules to eschars. Lymphadenopathy in the early stage of the disease is the most important sign differentiating human MPV from other pox-like illnesses (Ježek et al., 1987). Clinical symptoms such as dental ulcers, genital ulcers, muscle ache, sepsis, vomiting, and diarrhea are also frequently observed in infected humans. Of particular concern, MPV infection can induce severe encephalitis, miscarriage, and fetal death, with profound implications for human society.
To clarify the pathological characteristics of MPV infection and evaluate the protective effects of vaccines and antiviral medications, several MPV-infected animal models have been established, including non-human primates (e.g., Macaca fascicularis and M. mulatta), wild rodents (e.g., Graphiurus kelleni and Cynomys ludovicianus), and transgenic mice (e.g., STAT1 knockout mice). Results from animal models indicate that MPV infection can induce reduced activity, weight loss, viral pneumonia, fibrinonecrotic bronchopneumonia, immunoactivation in lymph nodes, and cell apoptosis in the spleen, with viral replication in the lungs, spleen, lymph nodes, nasal mucosa, and abdominal organs. Current therapeutic strategies, including cidofovir, tecovirimat, resveratrol, ribavirin, neutralizing antibodies, and short interfering RNA (siRNA), have shown effective anti-MPV activity in vivo and in vitro. Certain vaccines (e.g., inactivated, DNA, and subunit recombinant vaccines) have demonstrated protection in animal models during poxviridae challenge. Notably, previous smallpox vaccination appears to confer 85% protection against MPV infection in individuals with or without vaccination scars (Fine et al., 1988).
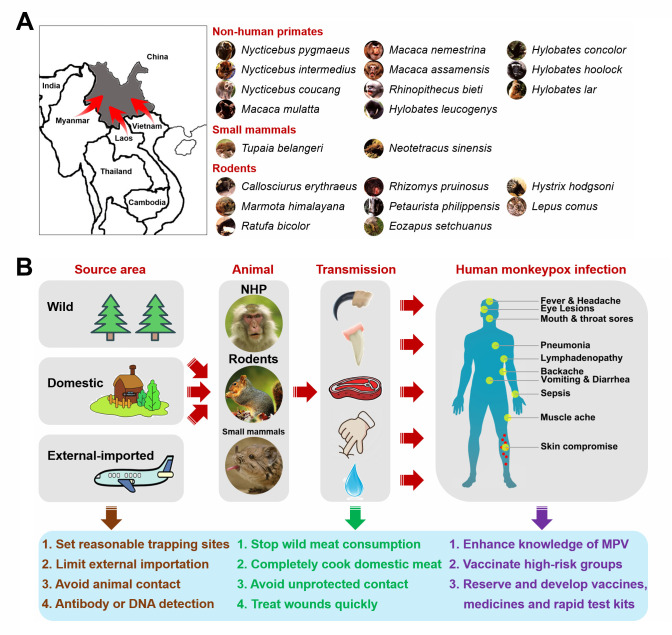
Human MPV infection can spread through animal-to-human and human-to-human transmission (Doty et al., 2017; Learned et al., 2005). Compared with human-to-human transmission, wild and domestic animals may transmit MPV to humans more easily and directly, resulting in a higher proportion of animal-induced cases (Ježek et al., 1988). Various wild animals, including non-human primates (e.g., Pan troglodytes, Cercocebus torquatus, and Papio ursinus), rodents (e.g., Graphiurus lorraineus, Cynomys ludovicianus, and Funisciurus sp.), and small mammals (e.g., Petrodromus tetradactylus), have been shown to support natural MPV infection. The mountainous region of Yunnan in southwestern China is known as the “Kingdom of Animals and Plants”. The forest area ranks second in China, with approximately 250 species of mammals, 360 species of fish, 140 species of reptiles, 90 species of amphibians, and 780 species of birds. Importantly, several MPV-sensitive species, including non-human primates (e.g., M. mulatta, M. nemestrina, and M. assamensis), small mammals (e.g., Tupaia belangeri), and rodents (e.g., Callosciurus erythraeus, Ratufa bicolor, and Marmota himalayana), are found in the tropical forests of Yunnan, implying a high risk of animal-to-human MPV transmission (Figure 1A). In addition, south and southeast Yunnan adjoins Laos and Vietnam, while southwest and west Yunnan shares a long border with Myanmar. Animals from bordering countries could potentially induce MPV transmission through external importation. Wild and imported animals may further induce animal-to-animal transmission to domestic livestock, which may induce secondary transmission to humans. Therefore, we propose several recommendations for the prevention and control of animal-to-human MPV transmission in Yunnan (Figure 1B). ANIMALS: (1) Establish trapping sites in forested areas near villages and the southwestern frontier for wild animal collection; (2) Limit importation of external animals, especially rodents and primates, which may damage the balance between supply and demand of domestic experimental primates; (3) Avoid domestic animal contact with wild or imported animals; (4) Perform specific antibody and viral DNA detection. TRANSMISSION: (1) Strictly prohibit wild meat consumption; (2) Ensure meat from domestic animals is completely cooked; (3) Avoid direct and unprotected contact with wild and domestic animals; (4) Treat wounds rapidly using efficient and easy-to-use treatments. PREPARATION: (1) Enhance knowledge and awareness of MPV; (2) Vaccinate high-risk groups (e.g., forest dwellers, animal breeders, veterinarians, geological investigators, animal researchers, and pet traders); (3) Strategically stockpile vaccines and medicines; (4) Develop specific vaccines, medicines, and rapid test kits against MPV.
Figure 1.
Possibility and prevention of animal-to-human MPV transmission in Yunnan
A: Potential animals supporting natural MPV infection in Yunnan. B: Proposals for prevention and control of animal-to-human MPV transmission in Yunnan.
COMPETING INTERESTS
The authors declare that they have no competing interests.
AUTHORS’ CONTRIBUTIONS
Y.T.Z. and T.Z.S. initiated and conceived the current work. T.Z.S. and Y.T.Z. prepared and wrote the manuscript. All authors read and approved the final version of the manuscript.
Funding Statement
This work was supported by the Yunnan Major Science and Technique Programs (202103AC100005) and Yunnan Key Research and Development Program (202103AQ100001)
References
- 1.Beer EM, Rao VB A systematic review of the epidemiology of human monkeypox outbreaks and implications for outbreak strategy. PLoS Neglected Tropical Diseases. 2019;13(10):e0007791. doi: 10.1371/journal.pntd.0007791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doty JB, Malekani JM, Kalemba LN, Stanley WT, Monroe BP, Nakazawa YU, et al Assessing monkeypox virus prevalence in small mammals at the human-animal interface in the Democratic Republic of the Congo. Viruses. 2017;9(10):283. doi: 10.3390/v9100283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fine PEM, Jezek Z, Grab B, Dixon H The transmission potential of monkeypox virus in human populations. International Journal of Epidemiology. 1988;17(3):643–650. doi: 10.1093/ije/17.3.643. [DOI] [PubMed] [Google Scholar]
- 4.Hobson G, Adamson J, Adler H, Firth R, Gould S, Houlihan C, et al Family cluster of three cases of monkeypox imported from Nigeria to the United Kingdom, May 2021. Euro Surveillance. 2021;26(32):2100745. doi: 10.2807/1560-7917.ES.2021.26.32.2100745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ježek Z, Grab B, Szczeniowski M, Paluku KM, Mutombo M Clinico-epidemiological features of monkeypox patients with an animal or human source of infection. Bulletin of the World Health Organization. 1988;66(4):459–464. [PMC free article] [PubMed] [Google Scholar]
- 6.Ježek Z, Szczeniowski M, Paluku KM, Mutombo M Human monkeypox: clinical features of 282 patients. The Journal of Infectious Diseases. 1987;156(2):293–298. doi: 10.1093/infdis/156.2.293. [DOI] [PubMed] [Google Scholar]
- 7.Learned LA, Reynolds MG, Wassa DW, Li Y, Olson VA, Karem K, et al Extended interhuman transmission of monkeypox in a hospital community in the Republic of the Congo, 2003. The American Journal of Tropical Medicine and Hygiene. 2005;73(2):428–434. doi: 10.4269/ajtmh.2005.73.428. [DOI] [PubMed] [Google Scholar]
- 8.Ligon BL Monkeypox: a review of the history and emergence in the western hemisphere. Seminars in Pediatric Infectious Diseases. 2004;15(4):280–287. doi: 10.1053/j.spid.2004.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shchelkunov SN, Totmenin AV, Safronov PF, Mikheev MV, Gutorov VV, Ryazankina OI, et al Analysis of the monkeypox virus genome. Virology. 2002;297(2):172–194. doi: 10.1006/viro.2002.1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vaughan A, Aarons E, Astbury J, Brooks T, Chand M, Flegg P, et al Human-to-human transmission of monkeypox virus, United Kingdom, October 2018. Emerging Infectious Diseases. 2020;26(4):782–785. doi: 10.3201/eid2604.191164. [DOI] [PMC free article] [PubMed] [Google Scholar]