Connectome-scale structural mapping is fundamental for understanding the underlying mechanisms of brain cognition and brain disease pathogenesis. By combining rapidly developing three-dimensional (3D) imaging techniques and big data analysis methods, researchers are working on mesoscale mapping of mammalian brains at an accelerated pace. Here, we briefly describe existing brain-wide imaging strategies, especially our recently established primate-optimized pipeline capable of pan-brain neuronal connectivity mapping at subcellular resolution, and further discuss their vast application prospects in the big data era of zoology.
As interdisciplinary technologies and “omics” analyses revolutionize many biological domains, connectomics research, i.e., the study of connectivity or “wiring” at different scales in the brain, has become a rapidly moving frontier. In particular, at the mesoscopic level, generating 3D cellular connectivity maps of the mammalian brain has been an overarching goal of major brain research initiatives in many countries (Poo et al., 2016). Because axons of individual neurons can target cells in multiple brain areas far from their soma, brain-wide maps of neuronal projections at subcellular or axonal resolution are essential. Such mapping efforts will establish a foundation for understanding the principles of brain wiring and information flow, and thus the mechanisms underlying specific brain functions and diseases.
Mesoscopic mapping of neuronal projections in the rodent brain has progressed considerably in the past decade (Gao et al., 2022; Peng et al., 2021; Winnubst et al., 2019), largely thanks to advances in imaging techniques such as fluorescence micro-optical sectioning tomography and serial two-photon tomography. With these techniques, the entire mouse brain can be imaged within several days. Another approach based on whole-brain tissue clearing and light-sheet microscopy (Ueda et al., 2020) can achieve high-speed imaging of the mouse brain at cellular resolution within hours. However, due to the large size and intrinsic inhomogeneity of brain tissue, this approach cannot achieve the subcellular resolution uniformity necessary for brain-wide axonal tracking.
For larger mammals, such as non-human primates, pan-brain connectome mapping is a major challenge. Compared to mouse brains, macaque brains are over 200-fold larger and require much higher speeds for 3D imaging at sufficient resolution. Furthermore, once monkey brain imaging is complete, the resulting petabytes of data require efficient algorithms to reconstruct accurate 3D structures and analyze neuronal fibers and connectivity.
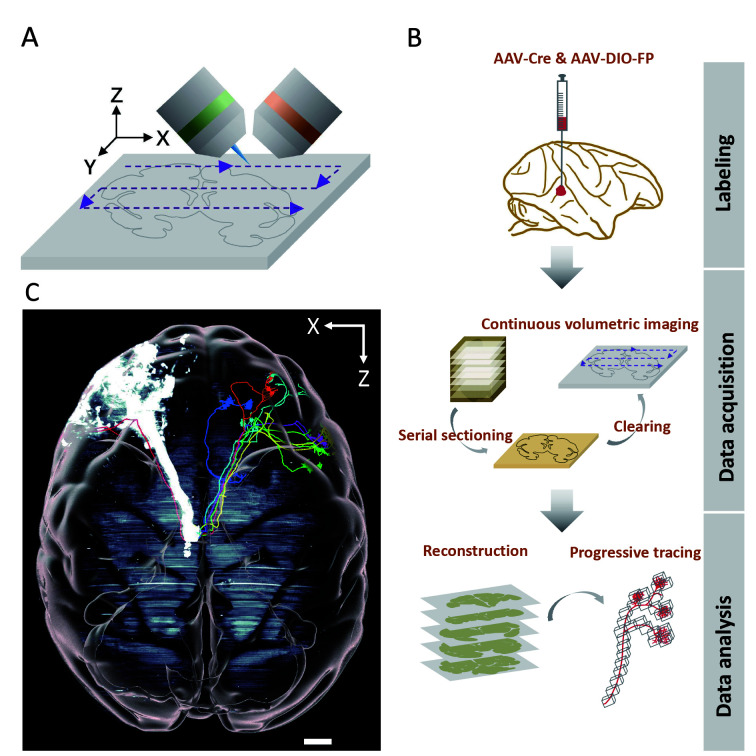
In a recent paper in Nature Biotechnology (Xu et al., 2021), we reported a systematic strategy to overcome the above bottlenecks (Figure 1) based on the Volumetric Imaging with Synchronized on-the-fly-scan and Readout (VISoR) approach. This scalable method enables ultra-high-speed 3D imaging of cleared brain slices with the continuous capture of blur-free, submicron-resolution images of moving samples (Figure 1A) (Wang et al., 2019). We improved the VISoR system and established a primate-optimized pipeline, i.e., serial Sectioning and clearing, 3D Microscopy with semi-Automated Reconstruction and Tracing (SMART) (Figure 1B) (Xu et al., 2021), enabling high-throughput connectome-scale mapping of macaque brains at micron resolution. With this approach, we first sectioned a whole rhesus macaque brain into consecutive coronal slices (300 μm thick), then cleared each slice independently with primate-optimized membrane permeabilization and high-refractive index matching solutions, allowing uniform transparency through both gray and white matter. With the improved VISoR system, we completed image acquisition of all cleared slices of the entire macaque brain within 100 hours. Notably, a new approach combining line-scan microscopy with integrated vibratome was recently developed for 3D imaging of large embedded samples at subcellular resolution (Zhou et al., 2022), which was capable of imaging an entire macaque brain within 70–80 days.
Figure 1.
Mapping axonal projections in a rhesus macaque brain with VISoR imaging and SMART pipeline
A: Schematic of VISoR microscope performing automated 3D imaging. B: SMART pipeline of serial sectioning and clearing, 3D microscopy, and semi-automated reconstruction and tracing. C: Axonal projections in macaque brain. Scale bar: 5 mm.
For the VISoR/SMART approach, we developed automated reconstruction software to stitch image sets from non-overlapping adjacent slices. We also developed semi-automated software capable of efficient progressive fiber tracking through the huge dataset of monkey brain images (Figure 1B). Using VISoR imaging and the SMART pipeline, we performed mesoscopic mapping of thalamocortical projections in a rhesus macaque brain (Figure 1C) (Xu et al., 2021), showcasing its potential capacity to construct detailed connectivity maps, especially when combined with sparse labeling strategies. In addition, we performed brain-wide single-fiber tracking based on the micron-resolution imaging accuracy and semi-automated annotation tools. Our preliminary observations indicated that thalamic neuronal axons have diverse projection patterns with unexpectedly complex routes, especially within cortical folding areas. Along these routes, we observed that some fibers turned sharply in white matter, which may be related to cortical folding (Xu et al., 2021). These findings demonstrate the important capability of whole-brain structural analysis at subcellular resolution, especially for understanding brain morphogenesis and neural circuit establishment (Van Essen, 2020).
Given the unique role of non-human primates as animal models for studies on human cognitive functions and diseases, it has become increasingly important to analyze their brain structures at different scales (Chen et al., 2020; Xu et al., 2021; Xu et al., 2022; Zhou et al., 2022). The high-throughput VISoR/SMART system enables structural studies of the primate brain at unprecedented scale and detail, e.g., large-scale pan-brain characterization of neuronal morphology, an essential step for understanding the principles of brain wiring diagrams. VISoR imaging can also be combined with other omics techniques to generate comprehensive brain maps, such as spatially defined gene expression atlases. Together with mesoscopic connectome mapping, these atlases could help reveal the neuroanatomic mechanisms underlying complex brain functions from a more holistic perspective, especially when complemented by functional approaches such as electrical microstimulation and functional magnetic resonance imaging (fMRI) (Xu et al., 2022). Furthermore, with recent advances in gene-editing in non-human primate brain disease models (Zhou et al., 2019), high-throughput connectome imaging could help unravel the complex circuit mechanisms of brain disorders in humans.
Beyond the brain, VISoR imaging can also be adapted to study diverse organs, even whole bodies of small and large animals, and thus promises to be useful tool for other zoological research such as animal development and systems physiology. As such, VISoR and other high-throughput microscopy methods are expected to generate an enormous amount of data across entire organs and systems with unprecedented cellular detail, which will undoubtedly lead to novel and groundbreaking discoveries. Furthermore, high-resolution imaging of large samples will also generate huge datasets. Extracting valuable information from these massive datasets will require efficient algorithms, including AI-based image processing, other systematic strategies such as crowdsourcing and manual computation (Gao et al., 2022), as well as dedicated high-performance computing resources. Going forward, this interdisciplinary synergy in the era of big data not only heralds potential new discoveries, but also a possible paradigm shift in zoological and biomedical research.
COMPETING INTERESTS
The authors declare that they have no competing interests.
AUTHORS’ CONTRIBUTIONS
Y.S. and G.Q.B. wrote the draft. All authors revised, read, and approved the final version of the manuscript.
Funding Statement
This work was supported by the Ministry of Science and Technology of China (2022ZD0205203)
References
- 1.Chen HZ, Yang HY, Zhong K, Li JL Preliminary study on fine structures of subcortical nuclei in rhesus monkeys by ex vivo 9.4 T MRI . Zoological Research. 2020;41(2):199–202. doi: 10.24272/j.issn.2095-8137.2020.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gao L, Liu S, Gou LF, Hu YC, Liu YH, Deng L, et al Single-neuron projectome of mouse prefrontal cortex. Nature Neuroscience. 2022;25(4):515–529. doi: 10.1038/s41593-022-01041-5. [DOI] [PubMed] [Google Scholar]
- 3.Peng HC, Xie P, Liu LJ, Kuang XL, Wang YM, Qu L, et al Morphological diversity of single neurons in molecularly defined cell types. Nature. 2021;598(7879):174–181. doi: 10.1038/s41586-021-03941-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poo MM, Du JL, Ip NY, Xiong ZQ, Xu B, Tan T China brain project: basic neuroscience, brain diseases, and brain-inspired computing. Neuron. 2016;92(3):591–596. doi: 10.1016/j.neuron.2016.10.050. [DOI] [PubMed] [Google Scholar]
- 5.Ueda HR, Dodt HU, Osten P, Economo MN, Chandrashekar J, Keller PJ Whole-brain profiling of cells and circuits in mammals by tissue clearing and light-sheet microscopy. Neuron. 2020;106(3):369–387. doi: 10.1016/j.neuron.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Essen DC A 2020 view of tension-based cortical morphogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2020;117(52):32868–32879. doi: 10.1073/pnas.2016830117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang H, Zhu QY, Ding LF, Shen Y, Yang CY, Xu F, et al Scalable volumetric imaging for ultrahigh-speed brain mapping at synaptic resolution. National Science Review. 2019;6(5):982–992. doi: 10.1093/nsr/nwz053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Winnubst J, Bas E, Ferreira TA, Wu ZH, Economo MN, Edson P, et al Reconstruction of 1, 000 projection neurons reveals new cell types and organization of long-range connectivity in the mouse brain. Cell. 2019;179(1):268–281.e13. doi: 10.1016/j.cell.2019.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu F, Shen Y, Ding LF, Yang CY, Tan H, Wang H, et al High-throughput mapping of a whole rhesus monkey brain at micrometer resolution. Nature Biotechnology. 2021;39(12):1521–1528. doi: 10.1038/s41587-021-00986-5. [DOI] [PubMed] [Google Scholar]
- 10.Xu R, Bichot NP, Takahashi A, Desimone R The cortical connectome of primate lateral prefrontal cortex. Neuron. 2022;110(2):312–327.e7. doi: 10.1016/j.neuron.2021.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou C, Yang XQ, Wu SH, Zhong QY, Luo T, Li AA, et al Continuous subcellular resolution three-dimensional imaging on intact macaque brain. Science Bulletin. 2022;67(1):85–96. doi: 10.1016/j.scib.2021.08.003. [DOI] [PubMed] [Google Scholar]
- 12.Zhou Y, Sharma J, Ke Q, Landman R, Yuan JL, Chen H, et al Atypical behaviour and connectivity in SHANK3-mutant macaques . Nature. 2019;570(7761):326–331. doi: 10.1038/s41586-019-1278-0. [DOI] [PubMed] [Google Scholar]