Abstract
Introduction
Olfactory dysfunction (OD) is a well know symptom of coronavirus disease 2019 (COVID-19), accounting for 48 to 85% of patients. In 1 to 10% of cases, patients develop a chronic olfactory dysfunction (COD), lasting more than 6 months. Recently, platelet-rich plasma (PRP) was used in patients with non-COVID-19 COD and authors reported encouraging results.
Methods
In the present study, we investigated the usefulness and safety of PRP injection in 56 patients with COVID-19 COD by the Sniffing Stick test (TDI score) and a linker-scale from 0 (none) to 3 (strong) and we compare the result to a control group.
Results
At 1 month post-PRP injection, the mean TDI scores significantly improved by 6.7 points in the PRP group (p < 0,001), the mean self-assessment of improvement in smell function was 1.8 (mild-to-moderate) in the PRP group, which was significantly higher than the score (0.3) in the control group (p < 0,001).
Conclusion
Our results showed that PRP in the olfactory cleft can increase the olfactory threshold 1 month after the injection. Moreover, our results suggest that timing of treatment may be an important factor and that PRP is a safe treatment, because no adverse effects were reported throughout the study.
Trial registration number: NCT05226546.
Keywords: COVID-19, Anosmia, Platelet rich plasma, Chronical olfactory dysfunction
Introduction
Olfactory dysfunction (OD) is a prevalent symptom of coronavirus disease 2019 (COVID-19), accounting for 48 to 85% of patients [1]. In 1 to 10% of cases, patients develop a chronic olfactory dysfunction (COD), lasting more than 6 months [2].
To date, there is no effective therapeutic approach for COD patients. Recently, platelet-rich plasma (PRP) was used in patients with non-COVID-19 COD and the authors reported encouraging results [3].
In the present study, we investigated the usefulness and safety of PRP injection in 56 patients with COVID-19 COD.
Methods
This study was approved by our Institutional Review Board (CHUSP2102028). Participants consented to participate.
From January to August 2021, adult patients with COVID-19 COD were prospectively recruited from the University Hospital of Brussels (CHU Saint-Pierre, Belgium). COVID-19 diagnosis was based on positive RT-PCR findings. Patients with abnormal findings on nasal endoscopy (e.g., polyposis, rhinosinusitis); blood disorders, or blood thinner use were excluded. Participants benefited from olfactory testing (Sniffin’ Sticks Test (Medisense, Groningen, Netherlands) resulting in the Threshold Discrimination Identification TDI score) at baseline and 1 month post-injection. The improvement of olfactory function was evaluated with a Likert-scale ranging from 0 (none) to 3 (strong).
1 mL of PRP was injected in each olfactory cleft via nasal endoscopy and under local anesthesia by the same physician (YS), following the protocol of Yan et al. (GS30-PURE II Protocol A: Emcyte, Ft Myers, Florida) [3]. According to the data distribution, the following tests were used: Wilcoxon signed-rank test, Mann–Whitney test, and Spearman correlation test. A level of significance of p < 0.05 was used.
We compare the result of the PRP group to a control group how underwent simple olfactory training for 1 month.
Results
Thirty-six patients received a PRP injection. Among those, six were lost to follow-up and, therefore, excluded. The control group matched for age, gender and TDI score at baseline included 26 patients with COVID-19 COD. Both groups were comparable regarding demographics, duration of OD, and TDI (Table 1).
Table 1.
Comparison of demographics, olfactory and subjective scores between PRP and control groups
| PRP group (n = 30) | Control group (n = 26) | p value | |
|---|---|---|---|
| Age (mean ± SD), years | 39 ± 12 | 44 ± 11 | 0.135 |
| Sex (F/M) | 16/14 | 20/6 | 0.66 |
| Duration of olfactory disorder (mean ± SD; min–max), months |
10.8 ± 2.5 7–16 |
9.7 ± 3.4 6–17 |
0.805 |
| TDI at baseline (mean ± SD) | 21.3 ± 7.4 | 24.5 ± 7.4 | 0.102 |
| TDI at 1 month (mean ± SD) | 28.0 ± 5.0 | 25.0 ± 7.7 | 0.226 |
| Subjective improvement (mean ± SD) | 1.8 ± 1.0 | 0.3 ± 0.6 | < 0.001 |
SD standard deviation, TDI Threshold–Discrimination–Identification
At 1 month post-PRP injection, the mean TDI scores significantly improved by 6.7 points in the PRP group (p < 0.001; Fig. 1), while there was no significant change in controls. There was a moderate negative correlation between TDI score difference and duration of OD in the PRP group (r = 0.387, p = 0.035) but not in controls. The mean self-assessment of improvement in smell function was 1.8 (mild-to-moderate) in the PRP group, which was significantly higher than the score (0.3) in the control group (p < 0.001). No adverse effects were reported throughout the study.
Fig. 1.
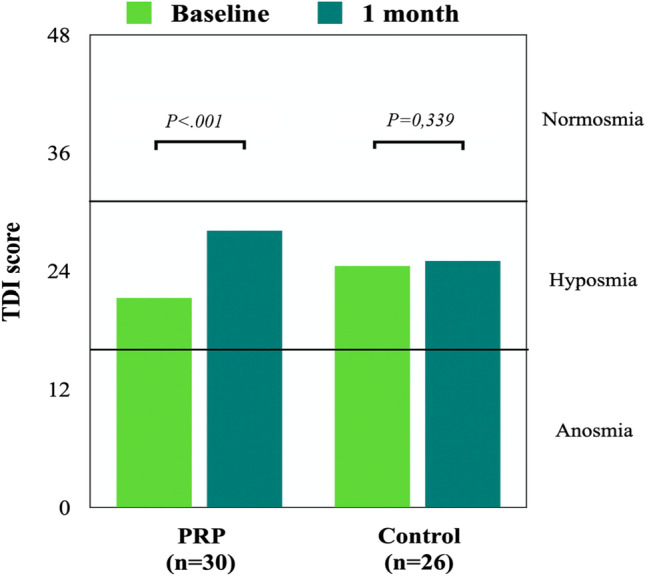
Evolution of TDI score at baseline and after 1 month in both PRP and control groups. PRP platelet-rich plasma, TDI Threshold–Discrimination–Identification
When we separate the patient considering the duration of olfactory loss, we did not find significant difference (p = 0.093) between the group with the loss of smell for less than 12 months and those since more than 12 months.
The average TDI gain at 1 month was 9 points in the 6–12 month group compared to 4 in the 12–18 month group.
Discussion
The main finding of the present preliminary study is the safety and potential usefulness of PRP as an in-office approach improving smell function in olfactory tests and subjective assessment. One month after PRP injection, mean olfactory score increased by 6.7 points, above the minimal clinically important difference of 5.5–6 for TDI [4].
In a recent study, De Melo et al. suggest that protracted viral infection and inflammation in the olfactory neuroepithelium may account for COVID-19 COD [5]. This might result from direct damage to the olfactory neuro-epithelium.
PRP is an autologous biologic product derived from fresh whole blood containing a high concentration of platelets. PRP is reported to have anti-inflammatory and pro-regenerative properties, including upregulation of growth factors and neuro-regeneration [6, 7]. Our results corroborate the findings of Yan et al. who reported a significant improvement in TDI score 1 month after PRP injection in the olfactory cleft of seven patients with non-COVID-19 COD [3]. The high concentration of growth factors in alpha granules in PRP, such as EGF and PDGF, has been found to enhance epithelial and neuro-regeneration [6, 7].
In comparison with other disease, such as atrophic rhinitis (AR), a recent study has shown that 1 injection of PRP is effective until 3 months [8]. Dr Yan et al. reported good result with a TDI score still increasing until 3 months of follow-up post-PRP injection. What could differentiate the AR from COVID-19 COD may be the mucosal surface affected by the disease. In COVID-19 COD the altered surface is much less than in atrophic rhinitis, which may explain our favorable results after only one injection.
However, it is possible that several injections of PRP allow for better and longer results on the olfactory function of the patients even though we had encouraging results after 1 month.
We reported several limitations in this study, first the lack of randomization. Second, we only have a follow-up of 1 month in this study, and finally our study concerns a small number of patients. So bigger sized studies, with long-term follow-up, are needed to confirm this encouraging result and to know if more than 1 injection is needed as other disease, such as AR.
Conclusions
Our results support that PRP may be of therapeutic interest for patients with COVID-19 COD, as the injection is made directly next to the olfactory cleft, where both olfactory neuro-epithelium and sustentacular cells have been shown to be injured by the virus. Moreover, our results suggest that timing of treatment may be an important factor in olfactory recovery.
Future randomized controlled studies are needed to confirm these results and explore long-term effect of this new approach.
Author contributions
YS: have made all the manipulation and the injection, also involved in the conception of the study, and drafting the manuscript. S-DL: have been involved in drafting the manuscript and revising it critically for important intellectual content. LP: have made substantial contributions to acquisition of the data (Sniffing stick test). AR: have been involved in drafting the manuscript and revising it critically for important intellectual content. JL: have been involved in drafting the manuscript and revising it critically for important intellectual content. SS: have given final approval of the version to be published. MH: have given final approval of the version to be published.
Funding
None.
Declarations
Conflict of interest
The author declares that they have no conflict of interest.
Ethic committee
This study was approved by the Institutional Review Board of the CHU Saint Pierre (CHUSP2102028).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Younès Steffens and Serge-Daniel Le Bon have contributed equally to this work.
References
- 1.Aziz M, Goyal H, Haghbin H, Lee-Smith WM, Gajendran M, Perisetti A. The association of “loss of smell” to COVID-19: a systematic review and meta-analysis. Am J Med Sci. 2021;361(2):216–225. doi: 10.1016/j.amjms.2020.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boscolo-Rizzo P, Guida F, Polesel J, et al. Self-reported smell and taste recovery in coronavirus disease 2019 patients: a one-year prospective study. Eur Arch Oto-Rhino-Laryngol. 2021 doi: 10.1007/s00405-021-06839-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yan CH, Mundy DC, Patel ZM. The use of platelet-rich plasma in treatment of olfactory dysfunction: a pilot study. Laryngoscope Investig Otolaryngol. 2020;5(2):187–193. doi: 10.1002/lio2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kattar N, Do TM, Unis GD, Migneron MR, Thomas AJ, McCoul ED. Olfactory training for postviral olfactory dysfunction: systematic review and meta-analysis. Otolaryngol Neck Surg. 2021;164(2):244–254. doi: 10.1177/0194599820943550. [DOI] [PubMed] [Google Scholar]
- 5.De Melo GD, Lazarini F, Levallois S, et al. COVID-19-related anosmia is associated with viral persistence and inflammation in human olfactory epithelium and brain infection in hamsters. Sci Transl Med. 2021;13(596):eabf8396. doi: 10.1126/scitranslmed.abf8396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Everts P, Onishi K, Jayaram P, Lana JF, Mautner K. Platelet-rich plasma: new performance understandings and therapeutic considerations in 2020. Int J Mol Sci. 2020;21(20):7794. doi: 10.3390/ijms21207794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farrag TY, Lehar M, Verhaegen P, Carson KA, Byrne PJ. Effect of platelet rich plasma and fibrin sealant on facial nerve regeneration in a rat model. Laryngoscope. 2007;117(1):157–165. doi: 10.1097/01.mlg.0000249726.98801.77. [DOI] [PubMed] [Google Scholar]
- 8.Kim DH, Lee MH, Lee J, Song EA, Kim SW, Kim SW. Platelet-rich plasma injection in patients with atrophic rhinitis. ORL J Otorhinolaryngol Relat Spec. 2021;83(2):104–111. doi: 10.1159/000513099. [DOI] [PubMed] [Google Scholar]


