Abstract
Standardized data definitions are essential for assessing the quality of care and patient outcomes in observational studies and randomized controlled trials. The European Unified Registries for Heart Care Evaluation and Randomized Trials (EuroHeart) project of the European Society of Cardiology (ESC) aims to create contemporary pan-European data standards for cardiovascular diseases, including heart failure (HF). We followed the EuroHeart methodology for cardiovascular data standard development. A Working Group including experts in HF registries, representatives from the Heart Failure Association of the ESC, and the EuroHeart was formed. Using Embase and Medline (2016–21), we conducted a systematic review of the literature on data standards, registries, and trials to identify variables pertinent to HF. A modified Delphi method was used to reach a consensus on the final set of variables. For each variable, the Working Group developed data definitions and agreed on whether it was mandatory (Level 1) or additional (Level 2). In total, 84 Level 1 and 79 Level 2 variables were selected for nine domains of HF care. These variables were reviewed by an international Reference Group with the Level 1 variables providing the dataset for registration of patients with HF on the EuroHeart IT platform. By means of a structured process and interaction with international stakeholders, harmonized data standards for HF have been developed. In the context of the EuroHeart, this will facilitate quality improvement, international observational research, registry-based randomized trials, and post-marketing surveillance of devices and pharmacotherapies across Europe.
Keywords: Data standards, Variables, Data definitions, Heart failure, Quality of care, EuroHeart
Graphical Abstract
Graphical Abstract.
EuroHeart: European Unified Registries for Heart Care Evaluation and Randomized Trials.
 Listen to the audio abstract of this contribution.
Listen to the audio abstract of this contribution.
Introduction
Standardized data definitions are essential for the reliable monitoring and comparison of quality of care and outcomes in observational studies and form the basis for data management in randomized controlled trials. There is a lack of an international consensus about the use and description of heart failure (HF) variables including those pertinent to patient characteristics, care delivery, and outcomes.1 As such, heterogeneity exists in the selection and definitions of data which impedes benchmarking and leads to inconsistencies that impair the interpretation of clinical studies and the acceptance of their findings.1,2
The 2021 American College of Cardiology/American Heart Association Key Data Elements and Definitions for HF provides a comprehensive list of data variables relevant to the HF care process.3 It comprises around 295 data variables, but with no hierarchical specification as to which are of a greater importance—potentially limiting their uptake in clinical practice.3 Also, the dataset was developed in accordance with the North American Clinical Practice Guidelines and healthcare system characteristics and, unlike the European Society of Cardiology (ESC) recommendations, uses a locally proposed staging system for HF that has not been adopted widely outside North America. For Europe, the Cardiology Audit and Registration Data Standards project in 2004 defined a set of variables for acute coronary syndrome, percutaneous coronary intervention, and clinical electrophysiology, but not HF.4 The European Unified Registries for Heart Care Evaluation and Randomized Trials (EuroHeart) project is a new initiative to develop contemporary data standards for a range of cardiovascular diseases and interventions, and has to date developed international data standards for acute coronary syndrome, percutaneous coronary intervention, and atrial fibrillation, with plans for the same for transcatheter aortic valve implantation and cardiovascular outcomes among other cardiovascular areas.5,6 This document specifically presents the EuroHeart data standards for HF, which have been developed in collaboration with the Heart Failure Association (HFA) of the ESC.
Methods
A Data Science Group under the auspice of the EuroHeart project was established in August 2019. This comprised a project chair (C.P.G.), two medical experts (S.A. and G.B.), and a project manager.
Working and reference groups
A Working Group for the development of the 2021 EuroHeart data standards for HF was invited from members of the EuroHeart Data Science Group, HFA representatives, and selected HF experts who have experience in national or international HF registries. Names and affiliations of the Working Group members are provided in Table A1.
In addition, a Reference Group comprising 44 international HF experts from 34 countries was convened to review and provide feedback on the final set of variables, permissible values, and definitions.
EuroHeart methodology
We followed the EuroHeart methodology for cardiovascular data standard development.7 In brief, this methodology involves: (i) identification of a cardiovascular domain for development of data standards; (ii) conduction of a systematic review of the literature to synthesize a list of ‘candidate’ variables; (iii) selection and prioritization of variables by domain experts using a modified Delphi method; (iv) Reference Group feedback; and (v) programming the final data variables into the EuroHeart IT platform.7
Scope
From the outset, the Data Science Group consulted with the Working Group to decide upon the extent of the EuroHeart-HF registry. It was agreed that the registry should capture information relating to both in-patient and out-patient care because, unlike acute coronary syndrome for instance, HF is a chronic disease spanning multiple clinical settings, where treatment is often optimized and adjusted in response to disease progression, the development of comorbid disease, and the side-effects of therapy.
Systematic literature review
The EuroHeart Data Science Group conducted a systematic review of the literature on data definitions in HF (Appendix Table A2). The search included studies that defined variables relevant to HF published between 1 January 2016 and 10 January 2021. These dates were chosen to capture contemporary HF management and data variables. We included peer-reviewed randomized trials or prospective observational studies that provided definitions for at least one variable relevant to HF diagnosis, management, or outcomes. We also reviewed the data dictionaries of existing HF registries, as well as HF quality indicators and guidelines.8–10 Following the literature search, a ‘long-list’ of candidate HF variables was identified for potential inclusion in the EuroHeart-HF dataset.
Variable level
In the EuroHeart, variables are classified as Level 1 variables if they are used as quality indicators of HF care or are important for risk stratification, case-mix adjustment, or outcome evaluation. The EuroHeart provides clinical definitions for the Level 1 variables and implements them on the EuroHeart IT platform to facilitate their collection. Level 2 variables are further measures which may prove useful in selected areas or circumstances, but which are not universally available or useful. They complement quality assessment and may have a role in observational or randomized research. Level 2 variables are defined in the EuroHeart data standard documents, but are not implemented on the EuroHeart IT platform. Given that the end users of the EuroHeart will be healthcare providers, the EuroHeart platform allows for the addition of a third set of variables (Level 3) that can be centre- or country-specific, and may be needed for a national or local study or a quality improvement project.7 Level 3 variables are not defined or programmed by the EuroHeart.
Selection of the final set of variables
Using a modified Delphi method, the Working Group reviewed the list of candidate variables from the systematic review to select the final set, to decide whether they were Level 1 or Level 2, and to create permissible values and definitions. The EuroHeart criteria for data standard development (importance, evidence base, validity, reliability, feasibility, and applicability) were used to guide the selection process.7 In total, six virtual meetings were conducted between January 2021 and April 2021, with a large volume of e-mail correspondence between the meetings.
Implementation
After arriving at the final set of variables, the Data Science Group worked with the Registry Technology group of the EuroHeart project to programme the Level 1 variables into the EuroHeart IT platform. For each variable, details were provided to the IT team regarding the clinical setting(s) in which the variable is applicable, the permissible ranges for the numerical response options, and the inter-relationships between the chosen variables to facilitate the design of a logical prototype for data entry. In addition, the Registry Technology group integrated the specifications that are needed for the calculation of the ESC quality indicators for HF on the EuroHeart IT platform.9
Results
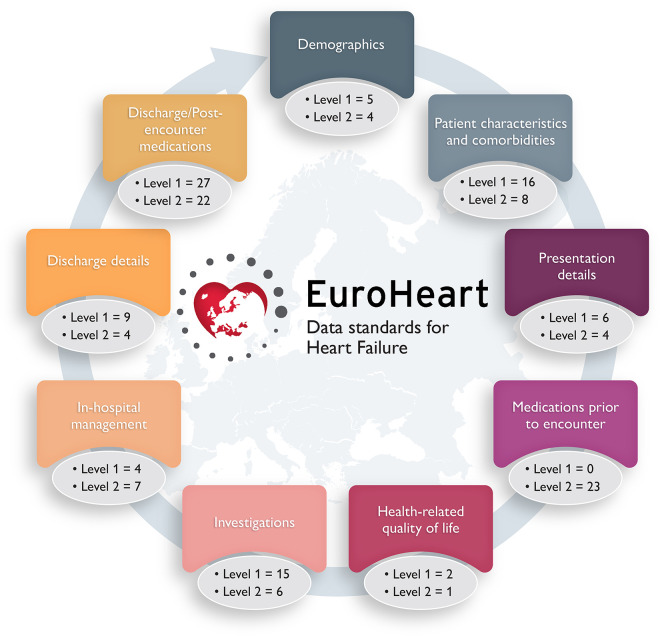
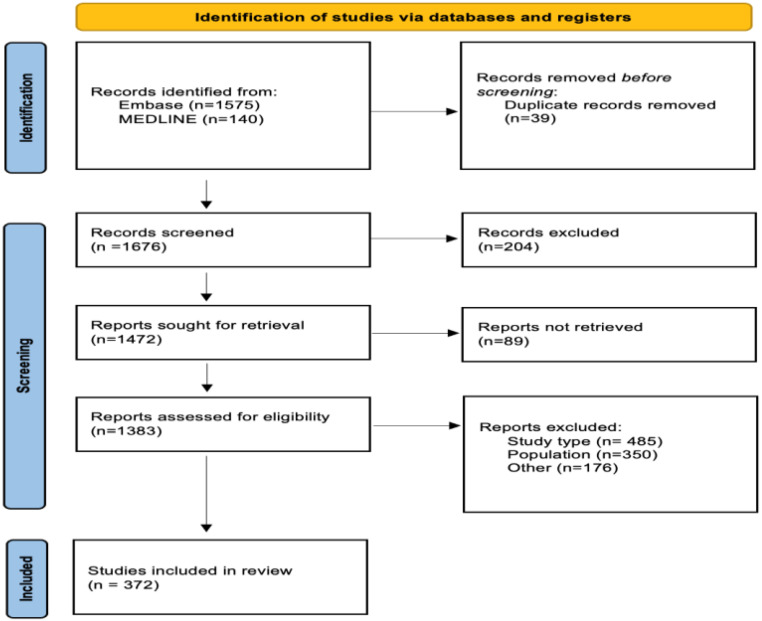
The systematic review retrieved 1715 articles. Of these, 372 met the inclusion criteria and were used to extract candidate variables (Appendix Figure A1). Of the 189 candidate variables considered for inclusion, 107 (57%) were obtained from the systematic review and 82 (43%) from the Clinical Practice Guidelines and quality indicators. Following the modified Delphi method, 84 Level 1 variables (see Supplementary material online, Table S1) and 79 Level 2 variables (see Supplementary material online, Table S2) were selected across nine domains of HF care (Graphical Abstract).
These key domains of HF care in the EuroHeart-HF registry are the following: (i) demographics, (ii) patient characteristics and comorbidities, (iii) presentation details, (iv) medications prior to encounter, (v) health-related quality of life, (vi) investigations, (vii) in-hospital management, (viii) discharge details, and (ix) discharge/post-encounter medications. With the exception of the ‘In-hospital management’ domain, all other domains comprise variables for both the in-patient and out-patient settings as shown in Supplementary material online, Tables S1 and S2.
Domain 1: demographics
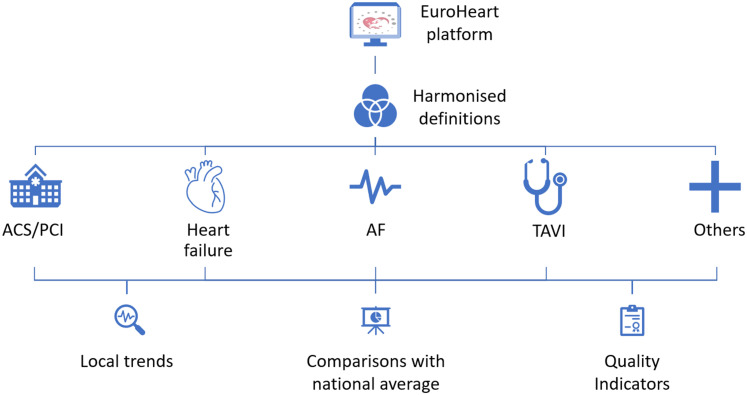
This domain was aligned with the EuroHeart acute coronary syndrome and percutaneous coronary intervention registries5 to minimize the burden of data collection when patients are enrolled in more than one EuroHeart registry (Figure 1). Some of the Level 1 variables within this domain capture patient-identifiable information to allow multi-source data linkage (see Supplementary material online, Table S1).11 Patient-identifiable data are stored and managed locally as required by each country’s data-sharing regulations. Anonymized data that are aggregated at the centre or the country level may be shared centrally with the EuroHeart Data Centre following an agreement from both parties.
Figure 1.
EuroHeart registry structure. ACS, acute coronary syndrome; AF, atrial fibrillation; PCI, percutaneous coronary intervention; TAVI, transcatheter aortic valve implantation.
Domain 2: patient characteristics and comorbidities
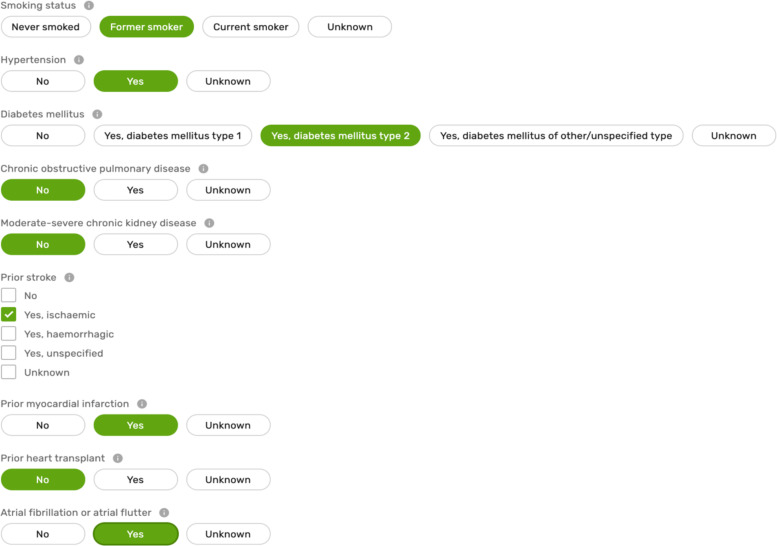
This domain contains data about the patient’s characteristics at the time of registration (e.g. weight), lifestyle habits (e.g. smoking), and medical history at the time of encounter with a healthcare professional (see Supplementary material online, Table S1). In addition, this domain captures information about comorbidities that may influence the decisions for patient care, improve the prediction of outcomes,12 or allow risk adjustments when variations in performance are evaluated (Figure 2).13 A wider list of characteristics (e.g. frailty) and comorbidities have also been selected as Level 2 variables and are presented in Supplementary material online, Table S2.
Figure 2.
Sample of the variables in patient characteristics and comorbidities domain.
Domain 3: presentation details
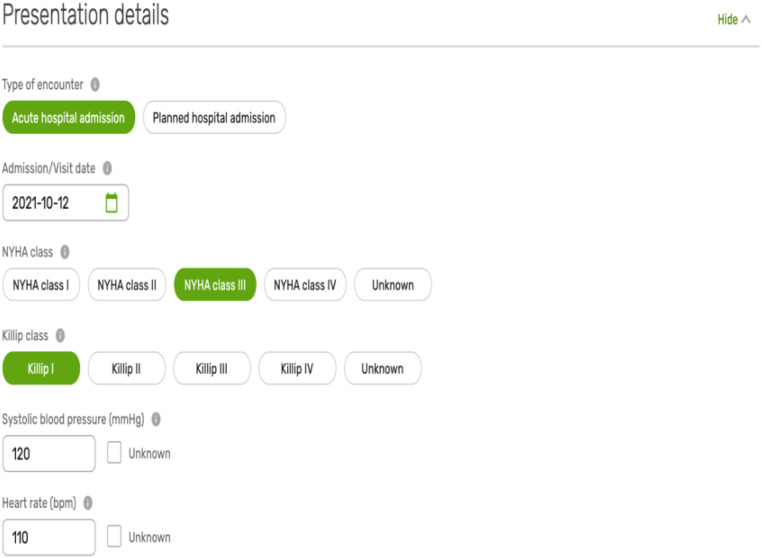
Many patients are likely to have received a diagnosis of HF many months or years prior to initial registration. Clinically stable patients may be enrolled in clinics. Patients with worsening chronic HF or new-onset HF may be enrolled in a variety of settings. This domain may be completed serially for each patient encounter if resources to do so exist.
The presentation details domain includes the type of the clinical encounter (in-patient/out-patient), as well as the patient’s clinical status at the time of assessment (initial or recurrent). Such information may be easily captured and is of a prognostic value for risk stratification and for determining the best treatment strategy (Appendix Figure A2).14 Examples of the variables in this domain include the New York Heart Association (NYHA) class prior to encounter, as well as heart rate, systolic blood pressure, and Killip class at the time of initial assessment (see Supplementary material online, Table S1).12,15
Domain 4: medications prior to encounter
While a patient’s pharmacotherapy prior to registration may provide an insight about the changes in treatment which have taken place during the episode of care, the Working Group raised concerns about the feasibility of collecting such information. Therefore, pre-registration medications were included as Level 2 variables (see Supplementary material online, Table S2), unlike medications at discharge (Domain 9).
Domain 5: health-related quality of life
Patient-reported outcome measures, particularly the health-related quality of life (HRQoL) are of importance to patients with HF.16 A number of validated HF-specific measurement tools exist for the measurement of HRQoL.17 The Kansas City Cardiomyopathy Questionnaire and the Minnesota Living with Heart Failure Questionnaire are commonly used HF-specific tools to measure the HRQoL.18 Other generic tool, such as the EuroQol-5 dimensions and the short-form survey have also been used to evaluate the HRQoL in patients with HF.19 For the EuroHeart-HF registry, information about whether the HRQoL was assessed at each encounter and which tool was used is captured as Level 1 category variables (see Supplementary material online, Table S1), with the results of the measurement as Level 2 variables (see Supplementary material online, Table S2). Notably, the EuroHeart IT platform allows the implementation of HRQoL questionnaires as Level 3 variables for those who have the desire, resources, and appropriate licensing permissions.
Domain 6: investigations
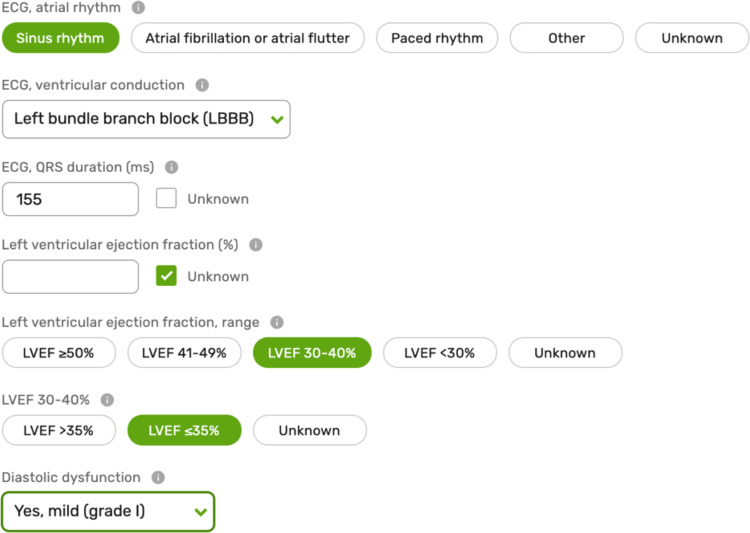
Tests such as the measurement of the left ventricular ejection fraction and plasma concentration of natriuretic peptides are important for the diagnosis of HF, assessing the effect of interventions, and evaluating prognosis.20 Other results including renal function, serum electrolytes,21,22 and electrocardiogram characteristics influence decisions about treatment8 and have a role in risk stratification (Figure 3).23,24 As such, these variables are included as Level 1 variables (see Supplementary material online, Table S1), with other investigations (e.g. C-reactive protein) placed as Level 2 variables (see Supplementary material online, Table S2).
Figure 3.
Sample of the variables in the investigations domain. LVEF, left ventricular ejection fraction.
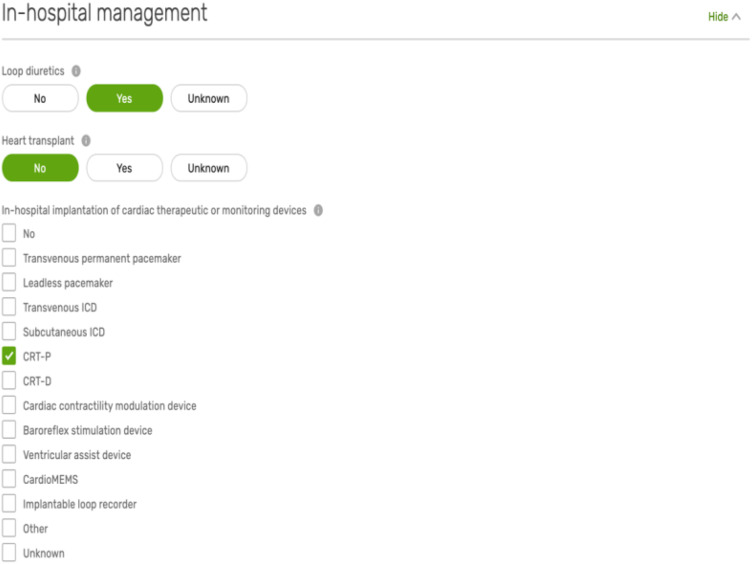
Domain 7: in-hospital management
This relates to the processes of care that are delivered during episodes of hospitalization with HF (Appendix Figure A3). That is, the prescription of loop diuretics, the implantation of cardiac therapeutic or monitoring devices, heart transplantation, and the performance of interventions such as percutaneous coronary intervention and transcatheter aortic valve implantation (see Supplementary material online, Table S1). Here, there are also a number of Level 2 variables capturing information about the use of circulatory support (mechanical and pharmacological), respiratory support, and renal replacement therapy during the hospital stay (see Supplementary material online, Table S2).25
Domain 8: discharge details
The ‘Discharge details' domain includes information about the length of hospital stay, in-hospital deaths, and discharge information, such as weight and plasma concentration of natriuretic peptides at the time of discharge (see Supplementary material online, Table S1). Such data are important for the evaluation of outcomes of care, but also may have a role in risk stratification.26 Moreover, an accumulating body of evidence supports the involvement of a multidisciplinary team (e.g. cardiac rehabilitation, HF clinics) in the management of HF and, after hospital discharge, early follow-up with a healthcare professional.8,27 Recently, these aspects of care have been proposed as ESC quality indicators for HF9 and are thus included as Level 1 variables (see Supplementary material online, Table S1). Less well-established assessments (e.g. NYHA class at discharge) or highly specialized interventions (e.g. referral to heart transplantation) are classified as Level 2 variables (see Supplementary material online, Table S2).
Domain 9: discharge/post-encounter medications
This domain forms the basis for the evaluation of performance and the assessment of adherence to the 2021 ESC Clinical Practice Guidelines and 2021 ESC quality indicators for HF.8,9 As such, for medications known to improve outcomes in patients with HF of any clinical type, data are collected not only about the class of the drug prescribed, but also about the generic name and the dose, recognizing that titration of medication may be incomplete at the time of discharge (from clinic or hospital) (see Supplementary material online, Table S1).8,9 These variables may also be of an importance for evaluating the changes in care and outcomes over time.29
While capturing information about existing contraindications to the guideline-recommended therapy for HF may provide a more meaningful assessment of care quality,29 it does increase the burden of data collection and can be difficult to obtain from routine medical records. Hence, variables that address the reason for not using guideline-recommended treatments for HF when apparently indicated are Level 2 (see Supplementary material online, Table S2).
Implementation
The Level 1 variables (see Supplementary material online, Table S1) were implemented on the EuroHeart IT platform with an interactive demonstration of the EuroHeart platform, which includes the HF registry, may be accessed at https://www.escardio.org/euroheart.
Discussion
Adoption of harmonized data collection is central to improving cardiovascular care.24 The lack of internationally recognized standardized data definitions has led to variability and inefficiencies in the monitoring of HF epidemiology and standards of care within and between countries.2,30 The EuroHeart project of the ESC, by means of a structured methodology and in collaboration with the HFA, has defined 84 Level 1 and 79 Level 2 variables for HF, which will be implemented on a bespoke IT platform to facilitate harmonized country-level quality improvement, international observational and registry-based randomized trials, and post-marketing surveillance of devices and pharmacotherapies.
The prevalence of HF and the healthcare resources required to manage it is increasing worldwide,31 including Europe.32–34 The emergence of novel therapies for HF in recent years8 and the emphasis on integrating these therapies with established care35 has shaped the need to develop systems that ensure a continuous supply of data to monitor and improve quality of care. Clinical registries for HF highlight gaps in care delivery and geographical variation in practice.13,28,36,37 However, the lack of harmonized data standards for HF limits the scalability of such registries, makes international comparisons less reliable, and hinders the development of registry-based randomized trials that could improve the efficiency and cost-effectiveness of both research and healthcare.38–40
The EuroHeart registry provides a unique opportunity to develop and maintain an infrastructure for the utilization of structured data through which generalizable evidence can be derived to inform the management and reduce the burden of cardiovascular disease.6 As opposed to a cross-sectional assessment, the EuroHeart model allows the collection and analysis of longitudinal observations of the characteristics of HF and the patterns of HF care and outcomes over time.
The EuroHeart data standards for HF have been developed in collaboration with the HFA and with involvement of experts in European HF registries and a panel of HF experts from 34 countries who have provided their feedback, taking the resources available in their own countries into consideration. Furthermore, these standards have been formally endorsed by the National Cardiac Societies from 13 ESC member countries, the ESC Patient Forum, and the ESC Committee for Young Cardiovascular Professional, highlighting the level of acceptance (and need) for the EuroHeart data standards for HF among the cardiovascular community.
The developed data standards for HF extends the existing literature by providing the European perspective to other HF quality registries, such as the Get With the Guidelines (GWTG) in the United States.41 Although the EuroHeart-HF and GWTG have similar mandatory variables, some differences exist. The EuroHeart-HF records information about whether patient-reported outcome measures (e.g. health-related quality of life) were collected, and about the tool(s) used to capture these measures as Level 1 variables. However, the GWTG does not collect mandatory data about health-related quality of life. On the contrary, the GWTG mandates the collection of variables capturing the rationale for not prescribing guideline-recommended therapies for HF (e.g. beta-blockers), while these are Levels 2 variables in the EuroHeart-HF.
The EuroHeart variables have been implemented on the EuroHeart IT platform to facilitate their integration with routine care. However, providing the computational phenotyping and coding details for these variables is beyond the scope of this project.42 The Working Group acknowledges that standardized data ontologies are needed to achieve semantic interoperability between registries, clinical trials and routinely collected electronic healthcare records (EHRs).43 While individual centres (or countries) may integrate the EuroHeart structured variables within their EHRs to allow the seamless exchange of data between both systems, this integration needs to be performed on the local level because of the substantial variation in the EHR software amongst centres. Furthermore, we recognize the limitations of the EuroHeart methodology for data standard development. Despite the conduct of a systematic review of the literature, we relied on expert opinion for the selection of the final set of variables and this selection may be biased. Nonetheless, we believe that a working collaboration with experts who have experience in national and international registries and quality improvement projects, and the wide representation of the Reference Group has enabled a degree of robustness to the selection process. Future Working Groups may benefit from the inclusion of patients and wider members of the multidisciplinary team for HF such as nurses, pharmacists, and primary care physicians.
Conclusions
This document presents the first EuroHeart international data standards for HF which have been developed in collaboration with the HFA using a standardized methodology. The 84 Level 1 variables have been implemented on the EuroHeart IT platform and can be adopted by HF registries around the world to harmonize the method by which HF data are captured.
Supplementary Material
Acknowledgements
We thank the ESC Patient Forum, the ESC Committee for Young Cardiovascular Professionals, and the following National Cardiac Societies for their endorsement to the 2021 ESC EuroHeart Data Standards for Heart Failure: Austrian Society of Cardiology, British Cardiovascular Society, Belgian Working Group of Heart Failure, Czech Heart Failure Association, Estonian Society of Cardiology Heart Failure, Heart Failure Working Group of Association of Cardiology of Bosnia & Herzegovina, Heart Failure Working Group of the Moldavian Society of Cardiology, Maltese Cardiac Society, Portuguese Society of Cardiology, Spanish Society of Cardiology, Syrian National Heart Failure Working Group, Ukrainian Heart Failure Association, Working Group of the Croatian Cardiology Society, and Working Group on Heart Failure and Heart Muscle Diseases of the Hungarian Society of Cardiology. We also thank the following heart failure experts for being part of the Reference Group for the 2021 ESC EuroHeart Data Standards for Heart Failure: Amr Abdin (Syria), Magdy Abdelhamid (Egypt), Saamay Abilova (Kyrgyzstan), Gani Bajraktari (Kosovo), Juan Luis Bonilla (Spain), Jasper Brugts (the Netherlands), Irina Cabac-Pogorevici (Moldova), Jelena Čelutkienė (Lithuania), Ovidiu Chioncel (Romania), Jose Gonzalez Costello (Spain), Catarina Dahlbom (Sweden), Mörtl Deddo (Austria), Märt Elmet (Estonia), Candida Fonseca (Portugal), Duska Glavas (Croatia), Greg Giamouzis (Greece), Morten Grundtvig (Norway), Plamen Gatzov (Bulgaria), Larisa Dizdarevic Hudic (Bosnia and Herzegovina), Inga Jóna Ingimarsdóttir (Iceland), Ginta Kamzola (Latvia), Mukhtar Kulimbet (Kazakhstan), Carolyn Lam (Singapore), Brian Bridal Løgstrup (Denmark), Przemyslaw Leszek (Poland), Tomas Lumbers (UK), Alejandro Recio Mayoral (Spain), Filip Malek (Czechia), Alice May Moore (Malta), Erkin Mirrakhimov (Kyrgyzstan), Jan Nilsson (Sweden), Olav Wendelboe Nielsen (Denmark), Markku Pentikäinen (Finland), Anne-Catherine Pouleur (Belgium), Pawel Rubis (Poland), Inge Schjødt (Denmark), Róbert Sepp (Hungary), Hamayak Sisakian (Armenia), Hadi Skouri (Lebanon), Pille Teppand (Estonia), and Leonid Voronkov (Ukraine).
Appendix
Figure A1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow chart for the systematic review.
Figure A2.
Sample of the variables in the presentation details domain.
Figure A3.
Sample of the variables in the in-hospital management domain.
Table A1.
Names and affiliations of the EuroHeart-Heart Failure Working Group members
| Name | Affiliation |
|---|---|
| Suleman Aktaa |
|
| Gorav Batra |
|
| John G.F. Cleland |
|
| Andrew Coats |
|
| Chris P. Gale |
|
| Lars H. Lund |
|
| Aldo P. Maggioni | National Association of Hospital Cardiologists Research Center (ANMCO), Florence, Italy. |
| Theresa McDonagh |
|
| Giuseppe Rosano |
|
| Petar Seferovic | Serbian Academy of Sciences and Arts, Heart Failure Center, Faculty of Medicine, Belgrade University Medical Center, Belgrade, Serbia. |
| Peter Vasko | Department of Medicine, Växjö Hospital, Växjö, Sweden. |
Table A2.
Terms used for the systematic review of the literature for the development of the EuroHeart-Heart Failure Data Standards
| Database: Embase <1996 to 2021 Week 2> 10 January 2021 | |
|---|---|
| 1 | *heart failure/or acute heart failure/or *forward heart failure/or *systolic dysfunction/(114036) |
| 2 | *Congestive Cardiomyopathy/or exp *Congestive Heart Failure/(39070) |
| 3 | exp Heart Failure/or exp Ventricular Dysfunction/or Cardiac Output, Low/(504308) |
| 4 | ((heart or cardiac or myocardial) adj2 (failure or decompensation)).ti. (111005) |
| 5 | ((congestive or acute or decompensat$) adj2 ‘heart failure’).ti, ab. (63281) |
| 6 | ((ventricular or ventricle$) adj2 (failure or insufficient$ or dysfunction$)).ti, ab. (45479) |
| 7 | ((heart* or cardia* or myocard*) adj3 (fail* or decompensat* or edema* or incompetence or insufficiency)).ti, ab. (299001) |
| 8 | or/1–7 (565730) |
| 9 | register/(112003) |
| 10 | disease registry/(16572) |
| 11 | registry.ti, ab. (202448) |
| 12 | registries.ti, ab. (40590) |
| 13 | register*.ti, ab. (275434) |
| 14 | database*.ti, ab. (742783) |
| 15 | exp cohort analysis/(718390) |
| 16 | exp longitudinal study/(150911) |
| 17 | exp prospective study/(675541) |
| 18 | exp follow-up/(1636601) |
| 19 | cohort$.tw. (1120767) |
| 20 | (random$ or placebo$ or single blind$ or double blind$ or triple blind$).ti, ab. (1644687) |
| 21 | or/9–20 (5059786) |
| 22 | exp common data elements/(387) |
| 23 | core data elements/(0) |
| 24 | exp nomenclature/(45465) |
| 25 | (data adj3 (harmoni? * or standard* or defin*)).ti, ab. (41970) |
| 26 | (data adj3 (element* or field* or variable*)).ti, ab. (33473) |
| 27 | data aggregation/(240) |
| 28 | big data/(3463) |
| 29 | data base/(235598) |
| 30 | clinical data repository/(1224) |
| 31 | data extraction/(17304) |
| 32 | data consistency/(234) |
| 33 | data collection method/(5237) |
| 34 | or/22–33 (372747) |
| 35 | (random sample$ or random digit$ or random effect$ or random survey or random regression).ti, ab. not exp randomized controlled trial/(122020) |
| 36 | news/or exp historical article/or Anecdotes as Topic/(35146) |
| 37 | (book or conference paper or review).pt. not exp randomized controlled trial/(2833116) |
| 38 | meta-analysis/(215159) |
| 39 | ‘systematic review’/or ‘review’/or ‘systematic review (topic)’/(2373930) |
| 40 | Congresses as Topic/(81476) |
| 41 | case report/or case study/(1932078) |
| 42 | (editorial or letter or comment*).ti. (219574) |
| 43 | (animal$ not human$).sh, hw. (2966520) |
| 44 | (animals/not humans/) or exp Animals, Laboratory/or exp Animal Experimentation/or exp Models, Animal/or exp Rodentia/or (rat or rats or mouse or mice).ti. (3598187) |
| 45 | or/35–44 (9129520) |
| 46 | 8 and 21 and 34 (9462) |
| 47 | 46 not 45 (7835) |
| 48 | limit 47 to English language (7773) |
| 49 | limit 48 to dd = 20160101-current (1575) |
|
Database: Ovid MEDLINE(R) ALL <1946 to January 09, 2021>
10 January 2021 | |
| 1 | heart failure/or acute heart failure/or *forward heart failure/or *systolic dysfunction/(98350) |
| 2 | *Congestive Cardiomyopathy/or exp *Congestive Heart Failure/(112088) |
| 3 | exp Heart Failure/or exp Ventricular Dysfunction/or Cardiac Output, Low/(164429) |
| 4 | ((heart or cardiac or myocardial) adj2 (failure or decompensation)).ti. (78454) |
| 5 | ((congestive or acute or decompensat$) adj2 ‘heart failure’).ti, ab. (49761) |
| 6 | ((ventricular or ventricle$) adj2 (failure or insufficient$ or dysfunction$)).ti, ab. (31951) |
| 7 | ((heart* or cardia* or myocard*) adj3 (fail* or decompensat* or edema* or incompetence or insufficiency)).ti, ab. (205332) |
| 8 | or/1–7 (284496) |
| 9 | register/(6642) |
| 10 | registry.ti, ab. (122780) |
| 11 | registries.ti, ab. (27497) |
| 12 | register*.ti, ab. (219437) |
| 13 | database*.ti, ab. (511239) |
| 14 | exp cohort analysis/(2172662) |
| 15 | exp longitudinal study/(147456) |
| 16 | exp prospective study/(584745) |
| 17 | cohort$.tw. (675517) |
| 18 | (random$ or placebo$ or single blind$ or double blind$ or triple blind$).ti, ab. (1334546) |
| 19 | or/9–18 (4064979) |
| 20 | exp common data elements/(118) |
| 21 | (data adj3 (harmoni? * or standard* or defin*)).ti, ab. (30832) |
| 22 | (data adj3 (element* or field* or variable*)).ti, ab. (27135) |
| 23 | data aggregation/(47) |
| 24 | big data/(1548) |
| 25 | data collection method/(90509) |
| 26 | Electronic Data Processing/(13290) |
| 27 | Routinely Collected Health Data/(29) |
| 28 | Data Collection/(90509) |
| 29 | Data Warehousing/(171) |
| 30 | or/20–29 (160215) |
| 31 | (random sample$ or random digit$ or random effect$ or random survey or random regression).ti, ab. not exp randomized controlled trial/(101589) |
| 32 | news/or exp historical article/or Anecdotes as Topic/(610698) |
| 33 | (book or conference paper or review).pt. not exp randomized controlled trial/(2825568) |
| 34 | meta-analysis/(137155) |
| 35 | ‘systematic review’/or ‘review’/or ‘systematic review (topic)’/(2888784) |
| 36 | Congresses as Topic/(28033) |
| 37 | case report/or case study/(2192999) |
| 38 | (editorial or letter or comment*).ti. (202576) |
| 39 | (animal$ not human$).sh, hw. (4815343) |
| 40 | (animals/not humans/) or exp Animals, Laboratory/or exp Animal Experimentation/or exp Models, Animal/or exp Rodentia/or (rat or rats or mouse or mice).ti. (5811211) |
| 41 | or/31-40 (11326251) |
| 42 | 8 and 19 and 30 (551) |
| 43 | 42 not 41 (447) |
| 44 | limit 43 to English language (432) |
| 45 | limit 44 to dt = 20160101–20210110 (140) |
Contributor Information
Suleman Aktaa, Leeds Institute of Cardiovascular and Metabolic Medicine, University of Leeds, Leeds, UK; Leeds Institute for Data Analytics, University of Leeds, Leeds, UK; Department of Cardiology, Leeds Teaching Hospitals NHS Trust, Leeds, UK.
Gorav Batra, Department of Medical Sciences, Cardiology and Uppsala Clinical Research Center, Uppsala University, Uppsala, Sweden.
John G F Cleland, Robertson Centre for Biostatistics and Glasgow Clinical Trials Unit, Institute of Health and Wellbeing, University of Glasgow, Glasgow Royal Infirmary, Glasgow & National Heart & Lung Institute, Imperial College London, London, UK.
Andrew Coats, University of Warwick, Coventry, UK; Heart Failure Association of the European Society of Cardiology.
Lars H Lund, Unit of Cardiology, Department of Medicine, Karolinska Institute, Stockholm, Sweden; Heart and Vascular Theme, Karolinska University Hospital, Stockholm, Sweden.
Theresa McDonagh, Department of Cardiology, King’s College Hospital London, Denmark Hill, Brixton, London, UK; School of Cardiovascular Medicine and Sciences, King’s College London British Heart Foundation Centre of Excellence, London, UK.
Giuseppe Rosano, IRCCS San Raffaele Roma, Rome, Italy.
Petar Seferovic, Serbian Academy of Sciences and Arts, Heart Failure Center, Faculty of Medicine, Belgrade University Medical Center, Belgrade, Serbia.
Peter Vasko, Department of Cardiology, University Hospital, Linköping, Sweden; SWEDEHEART – Swedish Web-system for Enhancement and Development of Evidence-Based Care in Heart disease Evaluated According to Recommended Therapies, Växjö, Sweden; SwedeHF – Swedish Heart Failure Registry, Växjö, Sweden.
Lars Wallentin, Department of Medical Sciences, Cardiology and Uppsala Clinical Research Center, Uppsala University, Uppsala, Sweden.
Aldo P Maggioni, National Association of Hospital Cardiologists Research Center (ANMCO), Florence, Italy.
Barbara Casadei, Division of Cardiovascular Medicine, NIHR Oxford Biomedical Research Centre, University of Oxford, Oxford, UK.
Chris P Gale, Leeds Institute of Cardiovascular and Metabolic Medicine, University of Leeds, Leeds, UK; Leeds Institute for Data Analytics, University of Leeds, Leeds, UK; Department of Cardiology, Leeds Teaching Hospitals NHS Trust, Leeds, UK.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
This work was supported by the European Society of Cardiology. G.R. was supported by the Italian Ministry of Health (Ricerca Corrente) 20/1819.
Conflict of interest: G.B. reports, outside the submitted work, personal fees (Boehringer Ingelheim, Pfizer, AsterZenica, and Novo Nordisk). B.C. is the ESC Board Member and Immediate Past President and reports, outside the submitted work, in kind support (Roche Diagnostics and iRhythm) for research projects and lecture fee (Menarini Foundation). A.C. reports, outside the submitted work, honoraria and/or lecture fees (Astra Zeneca, Bayer, Boehringer Ingelheim, Menarini, Novartis, Nutricia, Servier, Vifor, Abbott, Actimed, Arena, Cardiac Dimensions, Corvia, CVRx, Enopace, ESN Cleer, Faraday, Gore, Impulse Dynamics, Respicardia, Viatris). C.P.G. reports, outside the submitted work, consultancy (AstraZeneca, Bayer, Bristol Myers Squibb, Boehrinher-Ingleheim, Chiesi, Daiichi Sankyo, Menarini), speaker fees (AstraZeneca, Menarin, Raisio Group, Wondr Medical, Zydus), editorship (Deputy Editor: European Heart Journal Quality of Care and Clinical Outcomes, Oxford University Press), grants (British Heart Foundation, National Institute for Health Research, Horizon 2020, Abbott Diabetes, Bristol Myers Squibb), Advisory Board (Amgen, Bayer, Bristol Myers Squibb, Boehrinher-Ingleheim, Chiesi, Daiichi Sankyo, Menarini), leadership (NICE Indicator Advisory Committee, Chair ESC Quality Indicator Committee). L.H.L. reports, outside the submitted work, research grants (AstraZeneca, Vifor, Boston Scientific, Boehringer Ingelheim, Novartis), consulting fees (Merck, Vifor Pharma, AstraZeneca, Bayer, Pharmacosmos, MedScape, Sanofi, Lexicon, Myokardia, Boehringer Ingelheim, Servier), lecture fees (Abbott, MedScape, Radcliffe, AstraZeneca, Novartis, Bayer, TMA Academy, Boehringer Ingelheim, Orion Pharma), and stock (AnaCardio). G.R. reports, outside the submitted work, personal fees (Menarini, Vifor, Astra Zeneca, Servier, Cytokinetics).
Data availability
The data underlying this article are available in the article and in its online supplementary material.
References
- 1.Bozkurt B, Coats AJS, Tsutsui H, Abdelhamid CM, Adamopoulos S, Albert N, et al. Universal definition and classification of heart failure: a report of the heart failure society of America, heart failure association of the European society of cardiology, Japanese heart failure society and writing committee of the universal definition of heart failure. Eur J Heart Fail 2021;23:352–380. [DOI] [PubMed] [Google Scholar]
- 2.Seferović PM, Vardas P, Jankowska EA, Maggioni AP, Timmis A, Milinković I, et al. The heart failure association atlas: heart failure epidemiology and management statistics 2019. Eur J Heart Fail 2021;23:906–914. [DOI] [PubMed] [Google Scholar]
- 3.Bozkurt B, Hershberger RE, Butler J, Grady KL, Heidenreich PA, Isler ML, et al. 2021 ACC/AHA key data elements and definitions for heart failure: a report of the American College of Cardiology/American Heart Association task force on clinical data standards (Writing Committee to Develop Clinical Data Standards for Heart Failure). Circ Cardiovasc Qual Outcomes 2021;14:e000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flynn MR, Barrett C, Cosio FG, Gitt AK, Wallentin L, Kearney P, et al. The Cardiology Audit and Registration Data Standards (CARDS), European data standards for clinical cardiology practice. Eur Heart J 2005;26:308–313. [DOI] [PubMed] [Google Scholar]
- 5.Batra G, Aktaa S, Wallentin L, Maggioni AP, Ludman P, Erlinge D, et al. Data standards for acute coronary syndrome and percutaneous coronary intervention: The European Unified Registries for Heart Care Evaluation and Randomised Trials (EuroHeart). Eur Heart J 2022; ehac133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wallentin L, Gale CP, Maggioni A, Bardinet I, Casadei B. EuroHeart: European unified registries on heart care evaluation and randomized trials. Eur Heart J 2019;40:2745–2749. [DOI] [PubMed] [Google Scholar]
- 7.Batra G, Aktaa S, Wallentin L, Maggioni AP, Wilkinson C, Casadei B, et al. Methodology for the development of international clinical data standards for common cardiovascular conditions: European unified registries for heart care evaluation and randomised trials (EuroHeart). Eur Heart J Qual Care Clin Outcomes 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021; 42(36): 3599–3726. [DOI] [PubMed] [Google Scholar]
- 9.Aktaa S, Polovina M, Rosano G, Abdin A, Anguita M, Lainscak M, et al. European society of cardiology quality indicators for the care and outcomes of adults with heart failure: developed by the working group for heart failure quality indicators in collaboration with the heart failure association of the European society of cardiology. Eur J Heart Fail 2022;24:132–142. [DOI] [PubMed] [Google Scholar]
- 10.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129–2200. [DOI] [PubMed] [Google Scholar]
- 11.Mills S, Lee JK, Rassekh BM, Zorko Kodelja M, Bae G, Kang M, et al. Unique health identifiers for universal health coverage. J Health Popul Nutr 2019;38:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pocock SJ, Ariti CA, McMurray JJV, Maggioni A, Køber L, Squire IB, et al. Predicting survival in heart failure: a risk score based on 39 372 patients from 30 studies. Eur Heart J 2012;34:1404–1413. [DOI] [PubMed] [Google Scholar]
- 13.Emdin CA, Conrad N, Kiran A, Salimi-Khorshidi G, Woodward M, Anderson SG, et al. Variation in hospital performance for heart failure management in the National Heart Failure Audit for England and Wales. Heart 2017;103:55–62. [DOI] [PubMed] [Google Scholar]
- 14.Chioncel O, Mebazaa A, Maggioni AP, Harjola V-P, Rosano G, Laroche C, et al. Acute heart failure congestion and perfusion status – impact of the clinical classification on in-hospital and long-term outcomes; insights from the ESC-EORP-HFA Heart Failure Long-Term Registry. Eur J Heart Fail 2019;21:1338–1352. [DOI] [PubMed] [Google Scholar]
- 15.Meta-analysis Global Group in Chronic Heart Failure (MAGGIC) . The survival of patients with heart failure with preserved or reduced left ventricular ejection fraction: an individual patient data meta-analysis. Eur Heart J 2012;33:1750–1757. [DOI] [PubMed] [Google Scholar]
- 16.Johansson I, Joseph P, Balasubramanian K, McMurray JJV, Lund LH, Ezekowitz JA, et al. Health-related quality of life and mortality in heart failure: the global congestive heart failure study of 23 000 patients from 40 countries. Circulation 2021;143:2129–2142. [DOI] [PubMed] [Google Scholar]
- 17.Mackintosh A, Gibbons E, Fitzpatrick R, eds. A Structured Review of Patient-Reported Outcome Measures for People With Heart Failure: An Update 2009. Oxford: University of Oxford; 2009. [Google Scholar]
- 18.Burns DJP, Arora J, Okunade O, Beltrame JF, Bernardez-Pereira S, Crespo-Leiro MG, et al. International Consortium for Health Outcomes Measurement (ICHOM): standardized patient-centered outcomes measurement set for heart failure patients. JACC Heart Fail 2020;8:212–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moradi M, Daneshi F, Behzadmehr R, Rafiemanesh H, Bouya S, Raeisi M. Quality of life of chronic heart failure patients: a systematic review and meta-analysis. Heart Fail Rev 2020;25:993–1006. [DOI] [PubMed] [Google Scholar]
- 20.Roberts E, Ludman AJ, Dworzynski K, Al-Mohammad A, Cowie MR, McMurray JJV, et al. The diagnostic accuracy of the natriuretic peptides in heart failure: systematic review and diagnostic meta-analysis in the acute care setting. BMJ 2015;350:h910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rossignol P, Lainscak M, Crespo-Leiro MG, Laroche C, Piepoli MF, Filippatos G, et al. Unravelling the interplay between hyperkalaemia, renin-angiotensin-aldosterone inhibitor use and clinical outcomes. Data from 9222 chronic heart failure patients of the ESC-HFA-EORP Heart Failure Long-Term Registry. Eur J Heart Fail 2020;22:1378–1389. [DOI] [PubMed] [Google Scholar]
- 22.Ponikowski P, Kirwan BA, Anker SD, McDonagh T, Dorobantu M, Drozdz J, et al. Ferric carboxymaltose for iron deficiency at discharge after acute heart failure: a multicentre, double-blind, randomised, controlled trial. Lancet 2020;396:1895–1904. [DOI] [PubMed] [Google Scholar]
- 23.Collins SP, Pang PS. ACUTE heart failure risk stratification. Circulation 2019;139:1157–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mosterd A, Hoes AW. Clinical epidemiology of heart failure. Heart 2007;93:1137–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Collins SP, Jenkins CA, Harrell FE, Liu D, Miller KF, Lindsell CJ, et al. Identification of emergency department patients with acute heart failure at low risk for 30-day adverse events: the STRATIFY decision tool. JACC Heart Fail 2015;3:737–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sawano M, Shiraishi Y, Kohsaka S, Nagai T, Goda A, Mizuno A, et al. Performance of the MAGGIC heart failure risk score and its modification with the addition of discharge natriuretic peptides. ESC Heart Fail 2018;5:610–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Spall HGC, Rahman T, Mytton O, Ramasundarahettige C, Ibrahim Q, Kabali C, et al. Comparative effectiveness of transitional care services in patients discharged from the hospital with heart failure: a systematic review and network meta-analysis. Eur J Heart Fail 2017;19:1427–1443. [DOI] [PubMed] [Google Scholar]
- 28.Kapelios CJ, Laroche C, Crespo-Leiro MG, Anker SD, Coats AJS, Díaz-Molina B, et al. Association between loop diuretic dose changes and outcomes in chronic heart failure: observations from the ESC-EORP Heart Failure Long-Term Registry. Eur J Heart Fail 2020;22:1424–1437. [DOI] [PubMed] [Google Scholar]
- 29.Aktaa S, Batra G, Wallentin L, Baigent C, Erlinge D, James S, et al. European Society of Cardiology methodology for the development of quality indicators for the quantification of cardiovascular care and outcomes. Eur Heart J Qual Care Clin Outcomes 2022;8:4–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Timmis A, Townsend N, Gale CP, Torbica A, Lettino M, Petersen SE, et al. European Society of Cardiology: Cardiovascular Disease Statistics 2019. Eur Heart J 2020;41:12–85. [DOI] [PubMed] [Google Scholar]
- 31.Tromp J, Bamadhaj S, Cleland JGF, Angermann CE, Dahlstrom U, Ouwerkerk W, et al. Post-discharge prognosis of patients admitted to hospital for heart failure by world region, and national level of income and income disparity (REPORT-HF): a cohort study. Lancet Glob Health 2020;8:e411–e22. [DOI] [PubMed] [Google Scholar]
- 32.Conrad N, Judge A, Tran J, Mohseni H, Hedgecott D, Crespillo AP, et al. Temporal trends and patterns in heart failure incidence: a population-based study of 4 million individuals. Lancet 2018;391:572–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lawson CA, Zaccardi F, Squire I, Ling S, Davies MJ, Lam CSP, et al. 20-year trends in cause-specific heart failure outcomes by sex, socioeconomic status, and place of diagnosis: a population-based study. Lancet Public Health 2019;4:e406–e420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Savarese G, Lund LH. Global public health burden of heart failure. Card Fail Rev 2017;3:7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vaduganathan M, Claggett BL, Jhund PS, Cunningham JW, Pedro Ferreira J, Zannad F, et al. Estimating lifetime benefits of comprehensive disease-modifying pharmacological therapies in patients with heart failure with reduced ejection fraction: a comparative analysis of three randomised controlled trials. Lancet 2020;396:121–128. [DOI] [PubMed] [Google Scholar]
- 36.Chioncel O, Mebazaa A, Harjola V-P, Coats AJ, Piepoli MF, Crespo-Leiro MG, et al. Clinical phenotypes and outcome of patients hospitalized for acute heart failure: the ESC Heart Failure Long-Term Registry. Eur J Heart Fail 2017;19:1242–1254. [DOI] [PubMed] [Google Scholar]
- 37.Fu M, Vedin O, Svennblad B, Lampa E, Johansson D, Dahlström U, et al. Implementation of sacubitril/valsartan in Sweden: clinical characteristics, titration patterns, and determinants. ESC Heart Fail 2020;7:3633–3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parissis J, Farmakis D, Triposkiadis F. Heart failure registries: how far can we go? Eur J Heart Fail 2016;18:626–628. [DOI] [PubMed] [Google Scholar]
- 39.Bowman L, Baras A, Bombien R, Califf RM, Chen Z, Gale CP, et al. Understanding the use of observational and randomized data in cardiovascular medicine. Eur Heart J 2020;41:2571–2578. [DOI] [PubMed] [Google Scholar]
- 40.Lund LH, Oldgren J, James S. Registry-based pragmatic trials in heart failure: current experience and future directions. Curr Heart Fail Rep 2017;14:59–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Get With The Guidelines Heart Failure . https://www.heart.org/-/media/files/professional/quality-improvement/get-with-the-guidelines/get-with-the-guidelines-hf/clinical-tools/gwtghffullcrfjuly2018ucm_457483.pdf?la=en.
- 42.Liyanage H, Krause P, de Lusignan S. Using ontologies to improve semantic interoperability in health data. BMJ Health Care Inform 2015;22:309–315. [DOI] [PubMed] [Google Scholar]
- 43.Fitzpatrick T, Perrier L, Shakik S, Cairncross Z, Tricco AC, Lix L, et al. Assessment of long-term follow-up of randomized trial participants by linkage to routinely collected data: a scoping review and analysis. JAMA Netw Open 2018;1:e186019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material.