Abstract
Background
Inverted left atrial appendage (ILAA) is a rare condition following cardiac surgery. Failure to recognize the condition or making misdiagnosis of a tumour, a thrombus or vegetation can lead to unnecessary and potentially adverse events. We present a case of ILAA after surgical repair of an atrial septal defect (ASD) in a young female.
Case summary
A 3-year-old caucasian female was admitted for surgical repair of an ASD. The intraoperative course was uneventful until the opening of the right atrium (RA) after the commencement of cardiopulmonary bypass (CPB) and vacuum application, where the inferior vena cava (IVC) cannula was seen displaced in the RA. Cannula was repositioned, and ASD was repaired. On post-CPB transesohageal echocardiography (TEE), a newly developed mass was revealed in the left atrium (LA). The heart was re-arrested, and LA was re-assessed with unexpected finding of ILAA. ILAA was everted. RA was closed and CPB weaned off. Repeated post-CPB TEE showed no mass in the LA. No recurrence of mass was demonstrated on follow-up transthoracic echocardiography (TTE).
Discussion
The incidence of ILAA is rare. Therefore, it is usually forgotten and not anticipated as a complication during heart surgery using CPB. In our case, dislodgement of the IVC cannula into the RA in combination with vacuum application in the setting of an ASD may have resulted in ILAA. This has not been reported in previous cases. ILAA should be suspected on intraoperative TEE if the mass is newly developed. Visual inspection of the left atrium appendage (LAA) in situ is recommended before chest closure.
Keywords: Inverted LAA, Cardiac surgery, Cardiopulmonary bypass, Case report
Learning points.
Vacuum application for better venous drainage in combination with dislodgement of the inferior vena cava cannula into the right atrium may result in inverted left atrial appendage (ILAA) in the setting of an atrial septum defect.
ILAA must be suspected on an intraoperative transesohageal echocardiography with a newly appearing left atrium (LA) mass.
Visual inspection of the LA appendage (LAA) in situ is recommended before chest closure
Introduction
Left atrial appendage (LAA) is a finger-like outward extension of the main body of the left atrium (LA). Rarely, it is seen inverted into the LA after cardiac surgery.1 Failure to recognize the condition can lead to unnecessary interventions and potentially adverse events.2 We present a case of an inverted LAA (ILAA) after surgical repair of an atrial septal defect (ASD) in a child.
Timeline
| Time | Event |
|---|---|
| Pre-operative assessment | No clinical symptoms. Transthoracic echocardiography (TTE) with significantly large atrial septum defect (ASD). No mass is identified in any of the chambers. |
| Day 0 Intraoperative course
|
Day of surgical repair of ASD. Dislodgement of inferior vena cava (IVC) cannula into RA is acknowledged. Cannula is repositioned into IVC. Routine transesophageal echocardiography (TEE) reveals a newly developed mass in the left atrium (LA). LA is re-assessed with unexpected finding of inverted left atrial appendage. The appendage is everted. No mass in the LA. The chest is closed. |
| Postoperative day 4 | Discharged with no LA mass in follow-up TTE. |
Case presentation
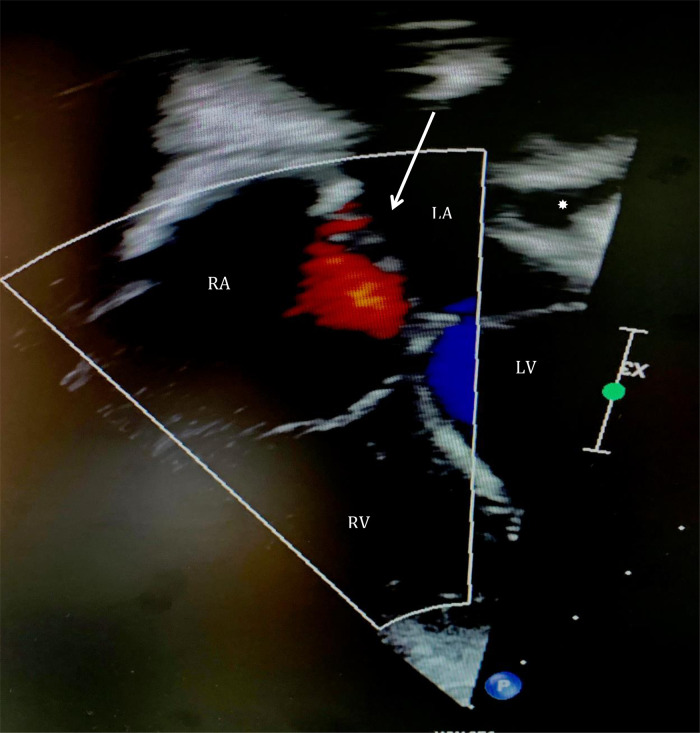
A 3-year-old caucasian female with no previous medical history was admitted for surgical repair of an accidental finding of secundum ASD after a year of routine examinations in paediatric outpatient clinic. The patient, weighing 15.2 kg (70 percentile), presented with no clinical symptoms. On cardiovascular examination, a soft systolic murmur was audible along the left lower sternal border. The oxygen saturation in ambient air was 99% at rest. A chest X-ray revealed cardiomegaly and pulmonary plethora. Preoperative transthoracic echocardiography (TTE) demonstrated a large defect in the atrial septum (14 × 12 mm) consisting of multiple fenestrations with a significant unidirectional shunt from left to right (Figure 1). The right ventricle (RV) was moderately volume overloaded, but with normal function. No sign of pulmonary artery pressure was noted, and no valve pathology was found. No masses were identified in any of the cardiac chambers. A decision was made to surgically operate the ASD by our Heart Team.
Figure 1.
Preoperative transthoracic echocardiography demonstrating a large defect in atrial septum (14 × 12 mm) consisting of multiple fenestrations with a significant unidirectional shunt from left to right (arrow). No mass is present in any of the chambers. Left atrium appendage is seen in its anatomical position (*). LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
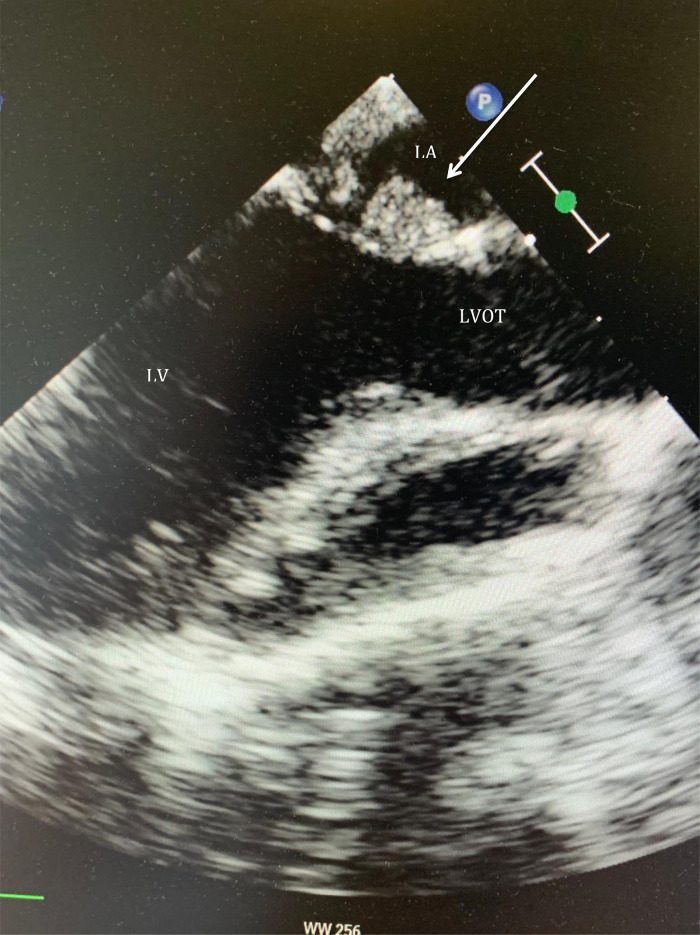
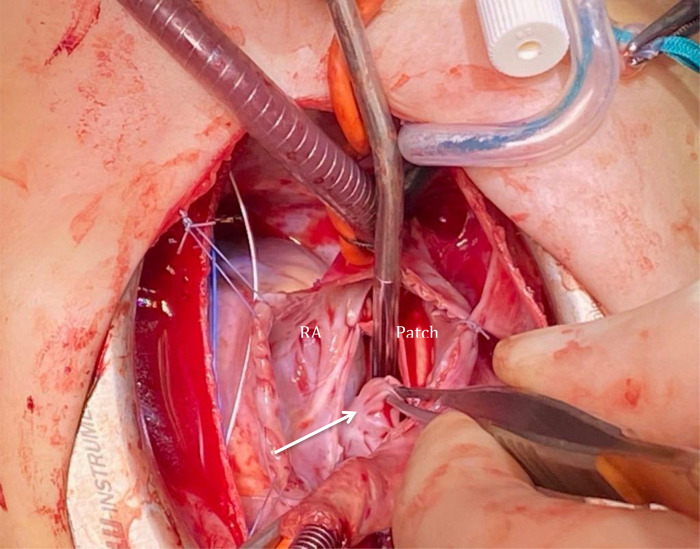
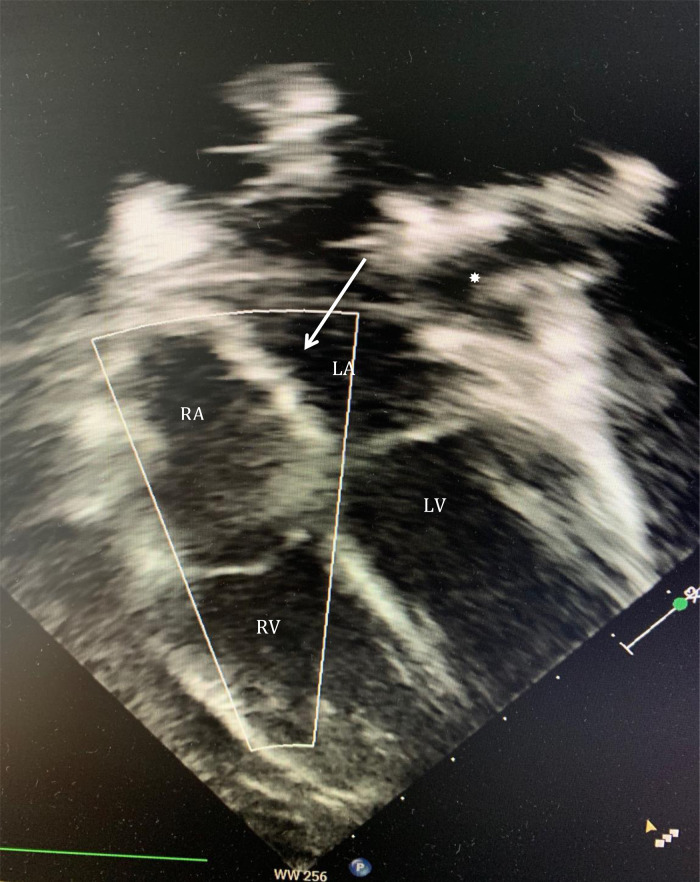
A median sternotomy was performed followed by systemic heparinization and standard aortic-bicaval cannulation. Hypothermic (34° Celsius) cardiopulmonary bypass (CPB) was commenced. Vacuum was applied to improve the venous drainage. The aorta was cross-clamped, and cold blood-cardioplegia was infused in the aortic root for immediate cardiac arrest. Both venae cavae were snared, and the right atrium (RA) was opened along the atrio-ventricular groove. At this point, air in the inferior vena cava (IVC) cannula was noted. The cannula was quickly clamped, and perfusion flow was simultaneously reduced briefly. The IVC cannula was seen displaced into the RA and was re-positioned into the IVC before full perfusion flow was resumed. No change in the cerebral near-infrared spectroscopy was noted. No venting catheter was inserted in the LA or left ventricle (LV). The remnants of the septum primum were removed to establish a single large atrial defect, which was subsequently closed with a tailored bovine pericardial patch. The RA atriotomy was closed, and the heart de-aired before cross-clamp release. A routine transesophageal echocardiography (TEE) before weaning from the CPB was performed; however, the assessment was inconclusive due to the low filling of the chambers. CPB was then weaned off uneventfully. A standard post-CPB TEE revealed no residual ASD defect. However, there was a well-defined rounded heterogenous mass (2 × 3 cm) arising from the left atrial wall in close proximity to the anterior mitral leaflet (AML) and below the level of pulmonary veins with no evidence of internal flow (Figure 2). It appeared freely mobile and pendulated into the anterolateral commissure (Video 1). No mitral valve stenosis or regurgitation could be demonstrated. The decision was made to re-arrest the heart and examine the LA. Unexpectedly, the LAA was found inverted into the LA (Figure 3). The appendage was everted to its anatomical position with no further interventions. Repeated post-CPB TEE showed no mass in the LA. The postoperative course was uneventful, and the patient was discharged on the fourth postoperative day (POD). The follow-up TTE after 4th and 21st revealed no LA mass, and LAA was seen in its anatomical position (Figure 4).
Figure 2.
Location of a 2 × 3 cm long mass arising from the left atrial wall near the anterior mitral leaflet and below the pulmonary veins with no evidence of flow on Doppler colour (arrow). LA, left atrium; LV, left ventricle; LVOT, left ventricle outflow tract.
Figure 3.
Explorative re-surgery revealing inverted left atrial appendage into the left atrium (arrow). RA, right atrium.
Figure 4.
Transthoracic echocardiography on fourth postoperative day with no detectable masses in any of the chambers. Left atrium appendage is in its anatomical position (*). Arrow is demonstrating repaired atrial septal defect with patch. LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
Discussion
Cases of ILAA have been described in both paediatric and adult patients undergoing cardiac surgery with the use of CPB.1–4 The most likely explanation postulated is that LV vent creates negative pressure in left side of the heart during weaning off the CPB, thus resulting in ILAA.3–5 Another hypothesis is that the inversion may occur during de-airing manoeuvers.3–5 In our case, we did not use a LV vent, but a de-airing manoeuver cannot be excluded as a cause of ILAA. Nevertheless, we believe that the reason for the inversion in our case was the dislodgement of the IVC cannula into the RA during commencement of the CPB and the vacuum applied to venous drainage. This created a substantial negative pressure in both atria due to the ASD resulting in suction of the LAA into the LA. We purposely did not empty the LA in order to avoid excess intracardiac air, which also makes direct pump sucker an unlikely cause of ILAA. A focused inspection of LAA’s anatomical position was not done before closing the ASD. To best of our knowledge, inversion of LAA as a result of IVC cannula dislodgement in combination with vacuum application has not been reported previously.
The differential diagnosis of a mass in the LA includes a tumour, a vegetation, a remnant of the pulmonary vein, a thrombus, or an ILAA.2 In our case, the mass was newly developed excluding the first three possibilities. The likelihood of thrombus formation was low as the patient was fully heparinized before initiating CPB, and the mass did not present with typical thrombi features upon TEE. ILAA was not anticipated, and therefore, the condition was not diagnosed on the post-CPB TEE. To determine the nature of the mass and because it was interacting closely with the mitral valve, we decided to re-arrest the heart and examine the LA. If the diagnosis of ILAA had been made on post-CPB TEE, we may have opted for a different approach such as digital manipulation or forceps eversion of the LAA without re-arresting the heart.7 Our case underlines the importance of performing a post-CPB TEE in addition to keeping an ILAA in mind as a complication to CPB and thereby avoiding unnecessary re-interventions. There is no consensus on whether to evert or ligate the LAA in order to avoid LAA’s re-inversion.1–6 As the LAA was positioned in its anatomical place in preoperative TTE and we had an explanation for its inversion, we decided to evert the LAA without finding it necessary to ligate it. The treatment of a confirmed ILAA after chest closure is still debated due to its rare occurrence. Some investigators argue towards a conservative treatment as spontaneous eversion has been documented, while others find surgical correction necessary.1,7 Meticulous assessment of impending complications, such as mural thrombus formation with the associated risk of thromboembolism or mitral valve inflow obstruction leading to functional MV stenosis, pulmonary hypertension, and RV failure, are important and will guide the treatment strategy.1,7 If ILAA recurs, it may be necessary to surgically ligate or percutaneously occlude the LAA to avoid these complications.
In conclusion, the combination of IVC cannula dislodgement and vacuum application for better venous drainage in the setting of an ASD may have resulted in ILAA. ILAA must be suspected on an intraoperative TEE with a newly appearing LA mass. Visual inspection of the LAA in situ is recommended before chest closure.
Acknowledgements
Conception and design: Hans Gustav H. Thyregod, Sana N. Buttar. Collection and assembly of data: Sana N. Buttar. Manuscript writing: Sana N. Buttar. Final approval of manuscript: Hans Gustav H. Thyregod. Accountable for all aspects of the work: All authors.
Contributor Information
Sana N Buttar, Department of Cardiothoracic Surgery, University Hospital of Copenhagen, Rigshospitalet, Denmark.
Henrik Ø Andersen, Department of Cardiothoracic Surgery, University Hospital of Copenhagen, Rigshospitalet, Denmark.
Jesper B Poulsen, Department of Cardiothoracic-Anesthesia, University Hospital of Copenhagen, Rigshospitalet, Denmark.
Hans Gustav H Thyregod, Department of Cardiothoracic Surgery, University Hospital of Copenhagen, Rigshospitalet, Denmark.
Lead author biography
 Sana N. Buttar graduated from University of Copenhagen in 2016. Currently, she is a senior resident in Cardiothoracic Surgery at the University Hospital of Copenhagen, Rigshospitalet.
Sana N. Buttar graduated from University of Copenhagen in 2016. Currently, she is a senior resident in Cardiothoracic Surgery at the University Hospital of Copenhagen, Rigshospitalet.
Consent: The authors confirm that written consent for submission and publication of this case report including imaging and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: None declared.
Funding: None declared.
References
- 1.Cohen AJ, Tamir A, Yanai O, Houri S, Schachner A. Inverted left atrial appendage presenting as a left atrial mass after cardiac surgery. Ann Thorac Surg 1999;67:1489–1491. [DOI] [PubMed] [Google Scholar]
- 2.Troise D, Errico G, Ranieri L, Civita A, Mele D, Lasaracina C, Arciprete P. Inversion of the left atrial appendage following cardiac surgery. Ital Heart J 2003;4:129–133. [PubMed] [Google Scholar]
- 3.Allen BS, Ilbawi M, Hartz RS, Kumar S, Thoele D. Inverted left atrial appendage: an unrecognized cause of left atrial mass. J Thorac Cardivasc Surg 1997;114:278–280. [DOI] [PubMed] [Google Scholar]
- 4.Bouzas-Mosquera A, Alvarez-García N, Cuenca-Castillo JJ. Inverted left atrial appendage. Heart 2008;94:1064. [DOI] [PubMed] [Google Scholar]
- 5.Leong MC, Latiff HA, Hew CC, Mazlan SL, Osman H. Inverted left atrial appendage masquerading as a cardiac mass. Echocardiography 2013;30:E33–E35. [DOI] [PubMed] [Google Scholar]
- 6.Meredith DS, Kimball TR. Spontaneous inversion of the left atrial appendage. J Am Soc Echocardiogr 2004;17:901–904. [DOI] [PubMed] [Google Scholar]
- 7.Ekanem E, Gattani R, Shea J, Zhao Q, Singh R, Liam RP. Left atrial appendage inversion presenting as acute right ventricular failure after left ventricular assist device implantation. CASE (Phila) 2020;4:221–225. [DOI] [PMC free article] [PubMed] [Google Scholar]