Abstract
Most patients survive acute myocardial infarction (MI). Yet this encouraging development has certain drawbacks: heart failure (HF) prevalence is increasing and patients affected tend to have more comorbidities worsening economic strain on healthcare systems and impeding effective medical management. The heart’s pathological changes in structure and/or function, termed myocardial remodelling, significantly impact on patient outcomes. Risk factors like diabetes, chronic obstructive pulmonary disease, female sex, and others distinctly shape disease progression on the ‘road to HF’. Despite the availability of HF drugs that interact with general pathways involved in myocardial remodelling, targeted drugs remain absent, and patient risk stratification is poor. Hence, in this review, we highlight the pathophysiological basis, current diagnostic methods and available treatments for cardiac remodelling following MI. We further aim to provide a roadmap for developing improved risk stratification and novel medical and interventional therapies.
Keywords: Remodelling, Fibrosis, Heart failure, Myocardial Infarction
Graphical Abstract
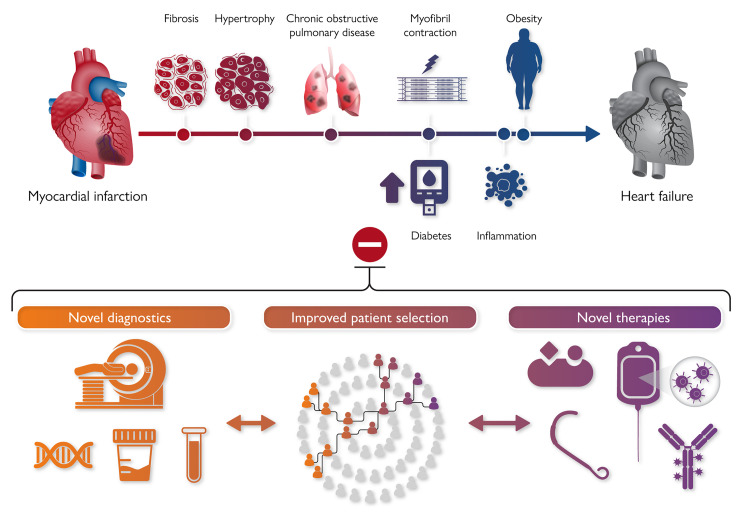
Graphical Abstract.
After a cardiac injury, comorbidites and various pathophysiologic mechanisms contribute to left ventricular remodelling and heart failure. Strategies to improve patient care beyond currently available treatment algorithms should include novel diagnostics, improved and treatment specific patient selection as well as novel therapies.
Introduction
Heart muscle comprises three important components that enable efficient contraction: cardiomyocytes, capillaries, and extracellular matrix (ECM), which consists of different collagen fibre types and provides structural integrity. Left ventricular (LV) remodelling describes the heart’s (mal)adaptation to mechanical, neurohormonal, and inherited changes by regulating ventricular size, shape, and function. While cardiomyocyte growth orchestrated by increased microcirculatory blood supply (e.g. during pregnancy, growth, or athletic training) is considered physiological and completely reversible, ‘adverse’ or ‘pathological’ remodelling following myocardial infarction (MI) confers disproportionate risk for heart failure (HF) and significantly decreases survival.1 This review focuses on pathophysiology, imaging, and management of LV remodelling preceding HF development. To date, therapies have sought to improve ‘mechanics’ (preload, afterload) and adjust molecular mechanisms of remodelling; novel treatment targets can encompass both strategies (Figure 1A).
Figure 1.
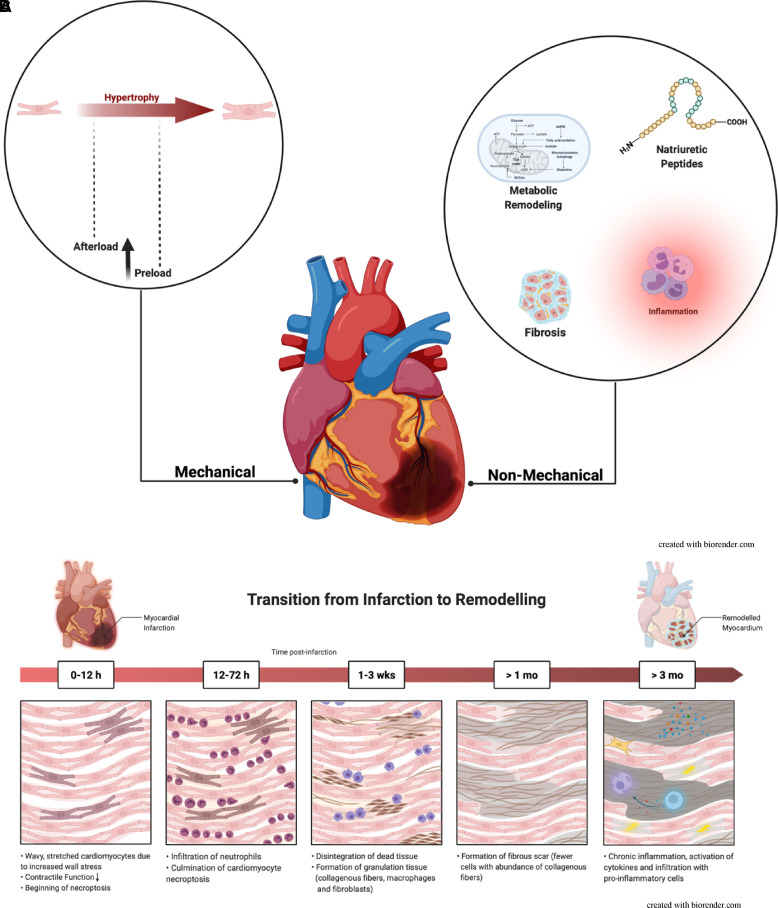
(A) Pathophysiology of left ventricular remodelling post-myocardial infarction. Schematic display of mechanical (left) and non-mechanical (right) pathophysiology leading to adverse left ventricular remodelling. Left: Persistent increase of afterload, and subsequently preload, promotes mechanical stretching of the tissue and activation of pro-hypertrophic pathways leading to pathological myocyte growth and the development of remodelling. Right: Derangements in energy metabolism leading to suboptimal energy production (metabolic remodelling) as well as activation of the renin–angiotensin–aldosterone system and sympathetic nervous system via natriuretic peptides, activation of pro-inflammatory pathways and changes in the extracellular matrix leading to myocardial fibrosis. These expand left ventricular remodelling post-myocardial infarction. (B) Histological changes in the heart following myocardial infarction. Following acute myocardial infarction, pressure and volume overload lead to increased wall stress and declining left ventricular function. An orchestrated process involving myocardial infiltration of different immune cells leads to scar tissue formation and progressive cardiomyocyte death. Chronic inflammation and other stimuli fuel the expansion of the extracellular matrix and promote chronic remodelling.
Epidemiology
Over the last decade, HF prevalence increased.2,3 Despite timely urgent revascularization and subsequent treatment strategies that significantly lower the mortality of acute MI, ischaemic heart disease remains the perennial cause of HF.2,3 Comorbidities predict HF development and severity. Indeed, >50% of HF patients have more than seven comorbidities,4,5 advanced age and female sex being the strongest individual HF predictors. Likewise, mortality post-MI correlates with advanced age, independent of infarct size,6 possibly because of a higher prevalence of cardiac hypertrophy but the reduced immune response, scar formation, and autophagy in senile hearts.7 Women are disproportionately affected by comorbid conditions.8 Concomitant diabetes makes women three times more likely to develop HF compared with men.9 Additionally, women are more likely to be obese and excess fat distribution is more disadvantageous following menopause, driving systemic inflammation.10 Other comorbidities conferring a significantly higher risk of developing HF post-MI are hypertension, chronic kidney disease (CKD), and chronic obstructive pulmonary disease (COPD). Predictors of improved left ventricular ejection fraction (LVEF) are HF duration <1 year, higher pre-discharge blood pressure and higher baseline LVEF.11
Pathophysiology
Understanding key pathophysiological mechanisms is essential for two reasons. Firstly, these mechanisms could source novel therapeutic targets. Secondly, some mechanisms can be used for diagnostic and prognostic purposes (biomarkers, imaging) to better tailor and adjust future medical treatment.
Changes in left ventricular geometry
Geometrical changes are a major stimulus for LV remodelling and the ‘Law of Laplace’ explains their progressive nature. Put simply, ventricular wall stress is directly related to LV pressure and radius and inversely proportional to twice the LV wall thickness. In the acute, early phase of MI, previously viable tissue decays, leading to loss of contractile function and secondarily increased LV volumes which in turn elevate wall stress and oxygen consumption. Weeks to months after MI, cardiac workload rises as the heart tries to compensate for higher pre- and afterloads.12
Myocardial hypertrophy
Myocardial hypertrophy is a distinct response to elevated workload and typically describes the volume expansion of terminally differentiated cardiomyocytes. This physiological, transient mechanism reduces wall stress and oxygen consumption to maintain cardiac output, as seen in pregnant and athletic individuals. Importantly, this process can occur and disappear without any permanent damage. On the contrary, pro-hypertrophic molecular pathways chronically triggered by either pressure/volume overload or MI frequently lead to overt HF. Physiologic hypertrophy proportionately expands chamber dimensions and wall thickness and since it includes adequate vascularization and no fibrosis, does not induce a specific pathological gene pattern.13 Conversely, pressure overload typically produces significant fibrosis paired with disproportionate increases in wall thickness compared with ventricular volumes, leading to activation of foetal genes, and HF associated with systolic and diastolic dysfunction.14
Myocardial fibrosis
Myocardial fibrosis inarguably influences LV remodelling. Fibrosis can be separated into two main groups, with considerable overlap.15 Interstitial fibrosis, which tends to occur earlier in response to various stimuli, describes collagen deposition by differentiated myofibroblasts and is, in principle, reversible. Replacement fibrosis, however, denotes collagen deposition following myocyte necroptosis and is considered irreversible. Following MI, the development and stimulation of collagen-producing myofibroblasts lead to progressive replacement fibrosis. Myocardial fibre stretching and the presence of inflammation are essential triggers for myofibroblast activation. Inflammatory reaction regulation is a finely tuned process as fibrosis is needed for sufficient scar formation but in excess will stiffen the heart, impairing oxygen diffusion, and thus hindering adequate oxygen supply. Fibrosis composition is tightly regulated by inflammatory and other cell types, paracrine mechanisms (e.g. transforming growth factors), and collagen-degrading enzymes like matrix metalloproteinases (MMPs). Detailed descriptions of molecular and cellular changes in the ECM exceed the scope of this review.16
Myocardial regeneration and proliferation
Cardiomyocyte necrosis precedes HF development. Research over the last two decades has, therefore, tried to compensate for myocyte loss by enhancing the regeneration of healthy myocardium. Zebrafish and, shortly after birth, also mammalian hearts can completely regenerate after cardiac injury,17 a capacity that, under certain circumstances, can be prolonged up to 4 weeks after birth.18 Yet the pathophysiologic principles underlying this complete regeneration remain largely elusive.19
Inflammation
A plethora of evidence confirms that MI triggers an inflammatory response which is primarily an orchestrated physiological process.20 Necrosis and apoptosis are essential to this process and occur simultaneously but nevertheless confer distinct changes. Apoptosis, programmed cell death without intracellular constituent release, happens either intrinsically when the cell detects damage or extrinsically after inflammatory cell interacts with so-called ‘death receptors’. Necrosis, the immediate predominant phenotype during MI, is an uncontrolled form of cell death with cell rupture. The released intracellular components activate the immune system via innate immune receptors. Inflammatory cells subsequently infiltrate to help clear the necroptotic cells and initiate a remedial response, thereby allowing adequate scar tissue formation.21 This complex response involves a variety of inflammatory cell types at different timepoints (Figure 1B). Some cell subtypes are pro-inflammatory and others mediate healing, and their differentiation and interaction are tightly regulated. Myocardial injury induces cardiac infiltration by neutrophils and macrophages that eliminate cell debris, drive inflammation by producing pro-inflammatory cytokines and further attract pro-inflammatory cells. After several days, neutrophils vanish and remedying macrophages appear, while T-cells regulate monocyte activation, which is pivotal for cardiac healing.22,23 T-cell activation takes place in heart-draining lymph nodes by autoantigens released from necroptotic myocytes, among other sources.24 In the final ‘remodelling phase’, after the formation of solid scar tissue, the non-infarcted myocardium recruits progressively more inflammatory cells.20 This chronic cytokine activation and myocardial infiltration by inflammatory cells can also be detected in HF patients. There are very limited experimental data on the underlying pathological changes and clinical implications of this chronic phase.25 However, the immune system might play a dual role: a chronic pro-inflammatory state drives maladaptive remodelling, whereas pro-angiogenic factors might contribute to healing.
Ischaemia/reperfusion injury and reactive oxygen species
Urgent coronary intervention in patients with ST-elevation MI (STEMI) is unequivocally beneficial, salvaging myocardium, reducing infarct size, and attenuating adverse LV remodelling. Paradoxically, inducing reperfusion to restore myocardial blood flow can expand infarct size, the so-called reperfusion injury, depending on ischaemia duration, severity, and residual blood flow level (for review see Heusch26). On a molecular level, succinate accumulation during ischaemia is suddenly oxidized following reperfusion, a process which ultimately mediates reactive oxygen species production.27 Chronically elevated generation of oxygen radicals may induce a vicious cycle of cardiac hypertrophy, myocyte death, and further remodelling via MMP activation.28 These changes lead to chronic mitochondrial remodelling, reduced energy production, and ultimately promote HF development.
Energy metabolism and mitochondria
Progressive metabolic remodelling is a pivotal driver of the transition to HF following MI.29 While it remains to be determined whether post-MI (mal)adaptive metabolic changes are causally responsible, it is clear that metabolic alterations aggravate LV remodelling development and progression. During periods of reduced oxygen supply (i.e. ischaemia), the heart enhances its glycogen storage and increasingly relies on glycolysis for more oxygen-efficient energy production.30 The peri-myocyte milieu (e.g. pressure overload, inflammation, hypoxia, etc.) and substrate availability regulate upstream genes resulting in a preference for carbohydrates over fatty acids for energy provision. This is a defining feature of the hypoxic ante-natal cardiac energy metabolism.31 Interestingly, repressing certain genes can revert the adult heart to this metabolic state, termed foetal gene pattern.32 Unfortunately, insulin resistance significantly reduces the availability of intracellular glucose, thereby activating mTOR and further promoting the development of fibrosis and apoptosis.33,34 Elevating fat oxidation and reducing over-reliance on glucose prevents remodelling and increases myocardial efficiency, possibly by restoring metabolic flexibility/homeostasis.35,36 In addition to substrate-level changes, acutely accumulated lactate leads to cytosolic and subsequently mitochondrial calcium overload which facilitates mitochondrial membrane leakage, decreasing both energy production and expression of pro-necroptotic and pro-inflammatory molecules (e.g. cytochrome c).37
Neurohormonal activation
The sympathetic nervous system (SNS) and renin–angiotensin–aldosterone system (RAAS) evolved to maintain cardiovascular equilibrium. When continuously activated by elevated circulating angiotensin II, the SNS and RAAS promote HF development, adverse remodelling and cell death in murine models.38,39 Moreover, SNS and RAAS activation levels in patients correlate with severity and outcomes in HF and predict poor prognosis.40–42 Impeding the deleterious effects of SNS and RAAS activation are the mainstay of present-day pharmacological HF treatment.43
Cardio-renal interplay
The close interplay between the heart and kidneys, often referred to as the cardio-renal axis, is key to HF development and progression.44 Multiple pathomechanisms, like oxidative stress and inflammation that are individually relevant for CKD and HF may also contribute to cardio-renal interplay; this is reviewed in more detail elsewhere.45 Mounting evidence, including from experiments using radiofrequency renal denervation (RDN), suggests direct cardio-renal interactions.
Natriuretic peptides
Increasing wall stress and stretching peri-MI leads to natriuretic peptide (NP) secretion from atrial and ventricular cardiomyocytes. The three identified isoforms, A-type NP (ANP), B-type NP (BNP), and C-type NP (CNP),46,47 primarily act as endocrine hormones and modulate diuresis, natriuresis, vasodilation, and inhibition of the SNS and RAAS.48,49 Additionally, certain NPs, such as BNP and NT-proBNP, are excellent prognosis predictors in patients after MI.50 Natriuretic peptide concentrations can reflect pro-fibrotic environments and could be used to stratify individuals at risk for remodelling, a patient group that currently cannot be adequately assessed by conventional imaging methods.51,52 Beyond their endocrine effects, NPs can also counter the negative impact of angiotensin II and endothelin-1 pro-hypertrophic signalling and seem to have an autocrine regulatory effect on myocyte size.53 A-type NP inhibits collagen synthesis, the main driver of cardiac fibrosis.54 Interestingly, pre-emptively injecting recombinant human BNP prior to coronary stent implantation appears to confer some degree of protection from myocardial injury, highlighting the NPs’ therapeutic potential.55
Imaging
In recent years, non-invasive cardiac imaging has significantly advanced, providing a range of increasingly accurate and sophisticated tools to characterize ventricular remodelling in vivo. As methodology has evolved, clinical applications have expanded. The primary purpose of clinical imaging remains to identify the presence and severity of remodelling in a given patient by assessing ventricular function and geometry using echocardiography or cardiac magnetic resonance (CMR). In clinical routine, echocardiographic biplane summation-of-disks methods are the standard two-dimensional (2D) volume measurement technique. If available and applied by experienced operators, the use of three-dimensional (3D) LV volume and function assessment is preferred over the summation-of disk method due to the better reproducibility.56 However, 3D techniques are heavily reliant on excellent imaging quality.57 Endocardial delineation can be further improved by the application of contrast media which enables levels of reliability when measuring ventricular function comparable to those of CMR.58 While CMR remains the gold standard for LV volume measurements, it is not as widely available, not easily applicable in patients with cardiac implanted devices and needs longer examination time when compared with routine echocardiography. Serially examining cardiac function and geometry may also help monitor the success of any given treatment strategy. Especially in Phase II clinical trials, more advanced and reproducible repetitive measurements of LV geometry (contrast echo, 3D echo, CMR) can help to improve detection of small treatment differences, understanding of mechanisms of action, and thereby improve patient selection for costly randomized Phase III trials.59 Yet, modern imaging has more to offer. Further steps after gross identification of remodelling may include (i) assessing cardiac and non-cardiac comorbidities as potential treatment targets, (ii) analysing aetiologic mechanisms of remodelling, (iii) evaluating the individual risk of disease progression and adverse outcomes, and (iv) specifically detecting targets for tailored therapies.60 To achieve these goals, an ever-increasing spectrum of advanced techniques such as strain imaging, CMR tissue characterization, and radionuclide-based molecular imaging is emerging, thereby establishing a foundation for implementing advanced personalized treatment strategies.61 Table 1 provides an overview of concurrent imaging applications in ventricular remodelling. Specific features of and recent developments in available methodologies are summarized below.
Table 1.
Non-invasive imaging modalities for assessment of left ventricular remodelling
| Imaging modality | Benefits | Limitations | Treatment/development implications |
|---|---|---|---|
| Echocardiography | |||
| 2D |
|
|
|
| 3D |
|
|
|
| Contrast echo |
|
|
|
| CMR | |||
| Imaging |
|
|
|
| LGE |
|
|
|
| Parametric mapping |
|
|
|
| MR spectroscopy |
|
|
|
| Nuclear imaging | |||
| SPECT/PET |
|
|
|
2D, two dimensional; 3D, three dimensional; 31P-MR, 31phosphorus magnetic resonance; CMR, cardiac magnetic resonance; Echo, echocardiography; ECV, extracellular volume, IMH, intramyocardial haemorrhage; LGE, late gadolinium enhancement; MRS, magnetic resonance spectroscopy; MR, magnetic resonance; MVO, microvascular obstruction; PET, positron emission tomography; RV, right ventricle; SPECT, single photon emission computed tomography.
Echocardiography
Standard 2D echocardiography is the first-line method to detect remodelling by determining contractile dysfunction and bi-ventricular geometry in clinical routine. Historically, HF is classified primarily by LVEF quantification,43 but additional functional parameters help define the severity of remodelling and may guide device therapies such as implantable cardioverter defibrillators (ICD) and cardiac resynchronization (CRT). Strain imaging allows more detailed analysis of myocardial contractility and provides excellent prognostic value in HF (Figure 2A). Trans-oesophageal and 3D echocardiography help detecting acquired valvular heart diseases such as ischaemic valvular regurgitation. Certainly, secondary/functional mitral regurgitation is associated with adverse HF outcomes and thus, may be a potential therapeutic target.62 Right ventricular (RV) assessments to further characterize remodelling and prognosis include functional parameters like tricuspid annular plane systolic excursion and fractional area change as well as Doppler methods. Effects can be two-fold as HF is regularly accompanied by pulmonary hypertension and pulmonary hypertension can equally worsen HF. This may further influence individualized therapeutic strategy.
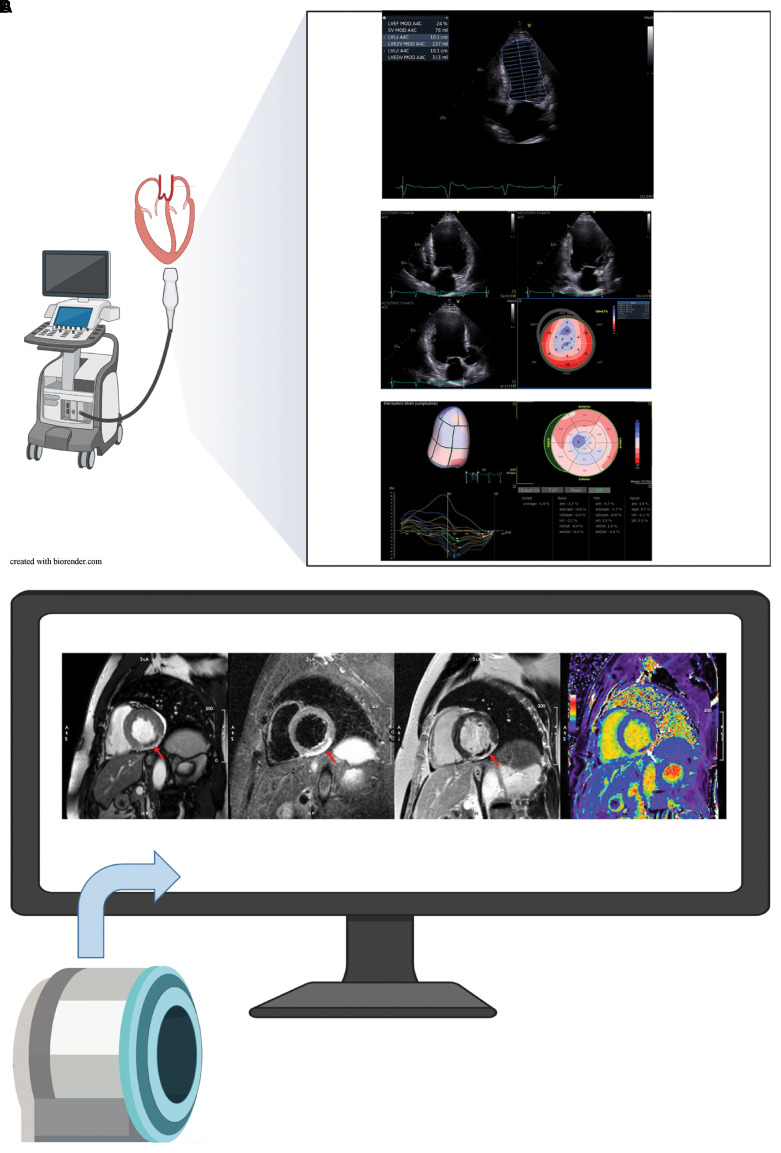
Figure 2.
(A) Detection and severity assessment of left ventricular remodelling by transthoracic echocardiography. (a) Quantification of left ventricular function showing severely reduced left ventricular ejection fraction, enlarged volume, and an apical aneurysm. (b) Assessment of myocardial deformation using two-dimensional strain imaging (same patient). The bulls-eye plot of segmental longitudinal strain is a composition of individual two-dimensional acquisitions and displays dyskinetic segments in blue. (c) Three-dimensional strain imaging provides simultaneous assessment of strain throughout the cardiac cycle in all myocardial regions, summarized in a volumetric display and a bulls-eye plot, with dyskinesia displayed in blue. (B) Post-infarct myocardial tissue characterization by cardiac magnetic resonance imaging. Short-axis views of inferior subacute myocardial infarction acquired using a 1.5 T system. (a) Mid-systolic frame of a cine sequence after administration of contrast agent; dark area indicates microvascular obstruction (see red arrow). (b) T2-weighted image sequence where bright area represents peri-infarction oedema (red arrow); grey area sub-endocardially indicates the presence of microvascular obstruction. (c) Late gadolinium enhancement acquisition following administration of gadolinium-based contrast agent; bright areas (red arrow) represent tissue necrosis/scar formation with an encapsulated dark area indicating microvascular obstruction. (d) Modified Look–Locker inversion recovery T1-map following administration of contrast media with bright green/red areas corresponding to scar and dark blue areas indicating microvascular obstruction.
Cardiovascular magnetic resonance
With the development of faster non-breathold sequences and highly reproducible post-processing, CMR remains the gold standard for assessing cardiac anatomy and function, particularly in patients with difficult anatomy (e.g. obesity, inherited disorders, etc.).63 Cardiac magnetic resonance not only detects the presence and severity of remodelling but also offers an array of novel approaches for differentiation of infarcted, viably injured, and non-infarcted myocardium (Figure 2B). Sophisticated techniques such as strain encoded-imaging or post-processed feature tracking can quantify myocardial deformation to gain information comparable to that provided by echocardiographic strain imaging.64,65 Cardiac magnetic resonance-measured LV volumes and certain infarct characteristics provide incremental post-MI prognostic information beyond LVEF.66 Cardiac magnetic resonance’s unique feature is its capacity to characterize myocardial tissue composition in order to determine reversible vs. irreversible injury: T2-imaging can show inflammation indicating reversible injury, while diffuse fibrosis identified by e.g. T1-mapping and/or focal scar revealed by late gadolinium enhancement (LGE) imaging demonstrate irreversible damage. Scar detection by LGE enables differentiation between ischaemic and non-ischaemic aetiology of HF. Furthermore, LGE is the reference standard for assessing myocardial viability and is used to guide coronary revascularization. Additionally, LGE can provide useful information for patient selection for CRT, as myocardial scar presence may be correlated to lower CRT response in patients with ischaemic heart failure with reduced ejection fraction (HFrEF).43 Equally, LGE facilitates early identification of papillary muscle infarction which may predict functional mitral regurgitation and adverse outcome post-STEMI. 67,68
Effective treatment of MI patients with non-obstructive coronary arteries (MINOCA) remains a major challenge. Even though MINOCA occurs in up to 13% of all MIs, its mechanisms are poorly understood and little is known about either the development of remodelling or prognosis in MINOCA patients.69 Cardiac magnetic resonance is an ideal technique for investigating the underlying aetiology and monitoring therapy to avoid progressive adverse remodelling e.g. by an undiagnosed scar.70,71
Importantly, LGE can also be used to identify post-MI microvascular obstruction (MVO) and/or intramyocardial haemorrhage (IMH) by visualizing the no-reflow phenomenon, which reveals an area of the infarct region with hypoenhancement indicating no blood supply despite successful revascularization. As this indicates a region of particularly severe damage, multiple underlying mechanisms such as increased inflammation-induced tissue pressure, arteriole occlusion, and haemorrhage due to capillary leakage may contribute to infarct expansion and thereby progression of adverse remodelling.72
Beyond LGE, MVO, and IMH, CMR tissue mapping may provide even more depth for phenotyping MI patients at risk for remodelling. The area at risk, characterized by the relation between T2-mapping-derived oedema and LGE-defined scar size after MI, predicts the outcome. Assessing alterations in non-infarcted myocardium by quantitative parametric mapping using a combination of T1-mapping and LGE also adds novel information on outcomes.73
Native T1-mapping may indicate different aetiologies of cardiac injury, whereas the calculation of extracellular volume (ECV) based on contrast-enhanced T1-mapping is often considered to be more specific for diffuse fibrosis.74 T2*-mapping is a novel technique for characterizing tissue iron content to identify a distinct subgroup of STEMI patients with IMH, which compromises myocardial salvage and drives infarct expansion after reperfusion.75 An approach combining MVO and IMH assessment with strain imaging was able to predict long-term recovery in STEMI patients after early beta-blocker therapy.76
Very recently, oxygenation-sensitive CMR identified inflammatory reactions, an important pathophysiologic mechanism driving adverse remodelling, in STEMI patients.77
Radionuclide-based molecular imaging
Nuclear cardiology employs radiolabelled biomolecules for non-invasive in vivo visualization. Unlike echocardiography or CMR, this method is not routinely used to detect remodelling or assess its severity. While standard clinical imaging of tissue perfusion and viability may help evaluate aetiology and guide revascularization, the development and implementation of novel molecular-targeted strategies have significantly advanced radionuclide imaging methodologies.78 The spectrum of molecular-targeted radiopharmaceuticals has been continuously growing, expanding the number of biological pathways that can be non-invasively interrogated by conventional scintigraphy or positron emission tomography (PET). Using the glucose analogue 18F-deoxyglucose, fatty acid analogues such as 11C-palmitate or the trycarboxylic acid cycle substrate 11C-acetate, PET elucidates detailed insights into metabolic substrate utilization and myocardial efficiency, and enables sensitive quantification of various drugs’ modifying effects.79–81 Furthermore, radiolabelled catecholamines can identify impaired myocardial sympathetic innervation, both in general and specifically in the viable infarct border zone, where it is a marker of arrhythmogenic risk and HF progression.82–84 As myocardial inflammation and fibrosis play key roles in repairing injured myocardium, their molecular hallmarks have emerged as targets for novel tracer-based approaches. These include targeted imaging of the chemokine receptors CXCR485 and CCR2,86 somatostatin receptors,87 the mitochondrial 18kD translocator protein TSPO,88 or other pro-inflammatory targets.89 Fibroblasts and the ECM may be interrogated through ligands binding to integrins, MMP90 or fibroblast activation protein.91 The tracer principle of nuclear cardiology theoretically facilitates labelling and visualizing almost any therapeutic molecule,92 nanoparticle or cellular system93 for non-invasive tracking of in vivo biodistribution and target area accumulation. Such molecular imaging approaches have so far been primarily applied to mechanistic studies, drug development, or trials of therapeutic effectiveness. However, as novel molecular therapeutics (RNA products, cells, nanoparticles) are costly and may not be beneficial in every disease stage and every individual, personalized and more complex diagnostic testing algorithms may be required (Figure 3). More specific molecular imaging-guided drug therapies may prevent post-infarct LV remodelling based on individual disease biology. Recently, PET imaging using a CXCR4-ligand, 68Ga-pentixafor, identified post-infarct inflammation as a marker of adverse outcome.94–96 This information was subsequently used to administer the CXCR4-blocker plerixafor to improve function only when PET indicated high expression of the CXCR4 target in myocardial tissue.94 This concept may be translated to other innovative pairs of imaging tests and drug interventions targeting distinct molecular or cellular mechanisms of cardiac repair and remodelling.97
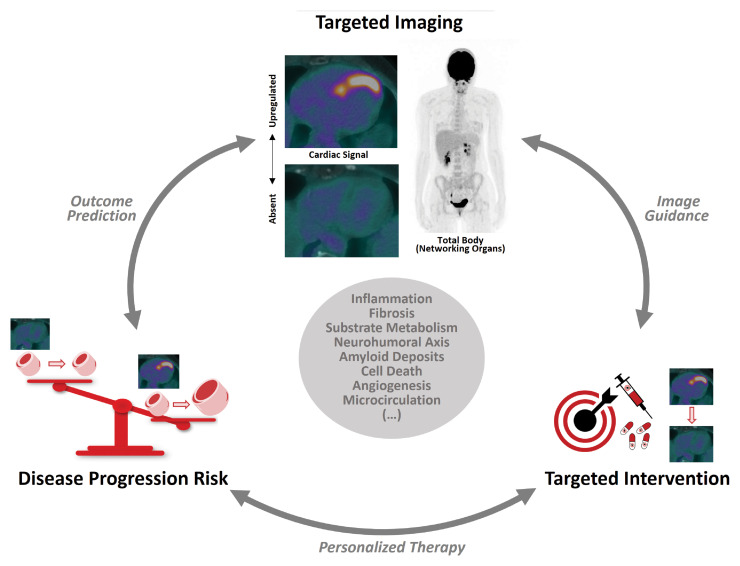
Figure 3.
Novel molecular imaging techniques. Schematic display of molecular imaging-guided, targeted therapy in left ventricular remodelling. Potential imaging targets are listed in the grey circle in the centre. Imaging visualizes the presence or absence of up-regulated pathways in the myocardium (global, regional) and interactions with other organs (top). Imaging signal strength predicts individual adverse outcomes and progressive myocardial remodelling (left). High personal risk triggers dedicated therapeutic intervention to attenuate target mechanisms and reduce risk (right). Repeat imaging may be used to monitor success.
Lastly, another strength of molecular imaging is that because it relies on the systemic biodistribution of labelled molecules, radionuclide imaging readily provides information on the entire body. This enables systemic analysis of the interrelationships between the heart and other organs, which may both expand mechanistic insights and help determine individual risk. Examples include interactions between myocardial inflammation, the haematopoietic system, and atherosclerosis98; interconnections among myocardial inflammation, remodelling, and neuroinflammation88,99; or the role of inflammation in cardio-renal crosstalk.100
Management
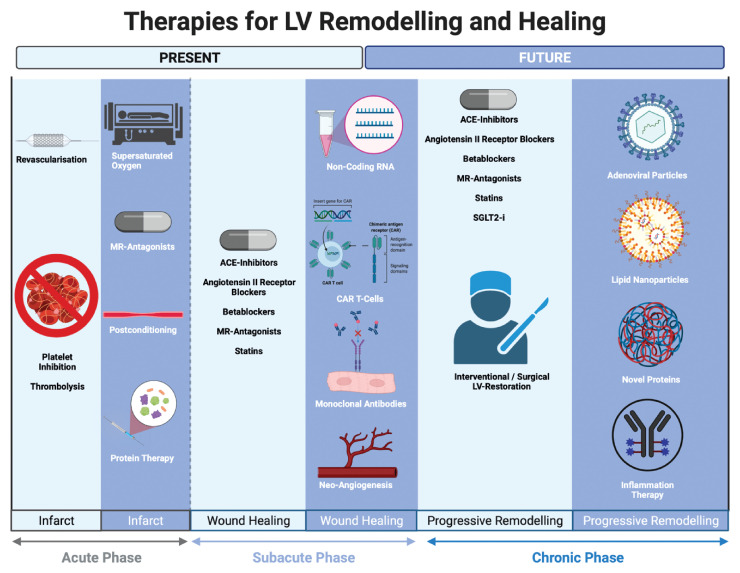
The first-line strategy for avoiding maladaptive LV remodelling is to treat reversible causes: revascularization should be performed during both acute and chronic conditions, valvular heart disease corrected, and hypertension adequately treated. As outlined above, changes in filling state, preload, and afterload are important contributors to LV remodelling. Several HF medications, including RAAS blockade, address this mechanism. Further, multiple mediators of LV remodelling contribute to current and future treatment strategies, independently of their haemodynamic effects, as discussed below (Figure 4).
Figure 4.
Therapies for left ventricular remodelling and healing. Overview of currently available and possible future treatments for left ventricular healing and remodelling following myocardial infarction.
Neurohormonal inhibition
Neurohormonal inhibition for LV recovery, remodelling and HF after MI is a classic example of successful bi-directional ‘bench-to-bedside’ translational science. Rodent models of post-MI ventricular remodelling provided the impetus for investigating captopril in patients, ultimately leading to angiotensin-converting enzyme (ACE) inhibitors becoming the standard indication after MI.101,102 Subsequently, in the VALIANT trial, valsartan commenced 0.5–10 days after acute MI in patients with HFrEF was as effective as captopril regarding total mortality, whereas combining both drugs increased the adverse event rate without improving survival.103 The mineralocorticoid receptor agonist eplerenone improved LV remodelling in rats with LV dysfunction after large MI. Combination therapy with an ACE inhibitor substantially increased this effect by preventing LV fibrosis, cardiac hypertrophy, and molecular alterations.104 These results provided mechanistic explanations for the reduced morbidity and mortality among patients with acute MI by eplerenone observed in EPHESUS.105
In addition to their anti-arrhythmic effects, betablockers also significantly improve LV remodelling, thereby lowering mortality and morbidity in patients with chronic HFrEF. However, despite recommendations to administer betablockers early to patients with acute MI, there is considerable controversy regarding if and how betablockers enhance LV healing and promote reverse remodelling post-MI. Recent work demonstrated that metoprolol, but not atenolol or propranolol, changed neutrophil dynamics and thus reduced infarct size and myocardial inflammatory infiltration in a mouse model of ischaemia/reperfusion.106
Angiotensin receptor-neprilysin inhibitors
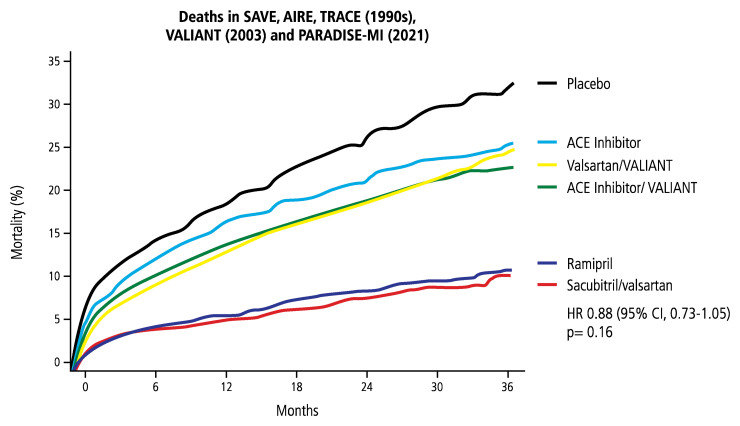
Because RAAS blockade markedly improved cardiac remodelling post-MI and angiotensin receptor-neprilysin inhibitor treatment reduced mortality/morbidity more effectively than ACE inhibition in chronic HFrEF in PARADIGM-HF107, subsequent trials sought to investigate the potential of sacubitril/valsartan in patients with HFrEF post-MI.108,109 In PROVE-HF, sacubitril/valsartan in HFrEF patients led to notably better echocardiographic indices of cardiac volume and function, results that correlated with lower NT-proBNP levels.110 In patients with asymptomatic LV dysfunction (EF ≤ 40%) ≥3 months after MI treated with ACE inhibition and betablockade, sacubitril/valsartan, compared with valsartan, did not significantly reduce the primary endpoint of LV end-systolic volume index (LVESVi) measured by CMR after 52 weeks.108 The PARADISE-MI109 trial randomized patients who survived acute MI and were at risk of developing symptomatic HF within 0.5–7 days to sacubitril/valsartan or ramipril. Compared to previous trials investigating ACE inhibitors or ARB post-MI, the event rates were substantially lower in PARADISE-MI, reflecting the totality of improved clinical care for these vulnerable patients (Figure 5). Sacubitril/valsartan did not significantly decrease the primary endpoints of adjudicated CV death or first worsening HF event but did limit both the total (including recurrent) adjudicated events as well as investigator-reported primary events.111,112
Figure 5.
Trials to evaluate left ventricular remodelling attenuation post-myocardial infarction. Markedly improved total mortality in patients at risk for heart failure after myocardial infarction in the last 30 years as reflected by declining mortality rates in trials of renin–angiotensin–aldosterone system inhibitors (modified from Pfeffer M, presented at ACC 2021). SAVE, AIRE, and TRACE showed significantly reduced mortality resulting from angiotensin-converting enzyme inhibitors vs. placebo; VALIANT demonstrated equivalence of angiotensin-converting enzyme inhibition and the angiotensin II receptor blocker valsartan; in PARADISE-MI sacubitril/valsartan was not superior to the angiotensin-converting enzyme inhibitor ramipril.
Sodium–glucose cotransporter 2 inhibitors/metabolism
Following the overwhelming success of sodium–glucose cotransporter 2 inhibitors (SGLT2-i) in patients with HF independent of underlying diabetes113–115, several studies have investigated these drugs’ potential to improve LV remodelling. In patients with HFrEF and Type 2 diabetes or prediabetes (SUGAR-DM-HF), empagliflozin vs. placebo was associated with reverse remodelling (reduced LVESVi and LVEDVi measured by CMR) and reduced NT-proBNP levels.116 In non-diabetic pigs with MI induced by transient balloon occlusion of the LAD, empagliflozin ameliorated adverse remodelling, measured by CMR at 2 months.117 Empagliflozin also switched post-MI myocardial fuel utilization away from glucose towards ketone bodies, free fatty acids, and branched-chain amino acids, thereby improving myocardial energetics. Two large trials are currently assessing SGLT2 inhibition in patients at risk for HF post-MI: EMPACT-MI (NCT04509674) includes 5000 patients with newly developed LVEF < 45% or signs/symptoms of congestion and, within 14 days after MI, randomizes them to receive 10 mg empagliflozin or placebo. The primary efficacy endpoint is the composite of time to first HF hospitalization or all-cause mortality. DAPA-MI (NCT04564742) includes 6400 patients with reduced LVEF (<50%, randomized within 10 days after MI) and investigates whether 10 mg dapagliflozin vs. placebo reduces the primary composite endpoint of time to first HF hospitalization or CV death.
Treatment with the anti-ischaemic agent trimetazidine may normalize energy supply118, reduce mitochondrial damage, and limit ischaemia/reperfusion injury in the acute MI phase.119 Whether or not this treatment confers protection against chronic post-MI remodelling is currently under investigation.120
Statins
Interestingly, statins, a standard treatment post-MI, also improve LV remodelling. In rats with large MI, statin treatment attenuated LV dilatation and LV end-diastolic pressure and was associated with reduced myocardial expression of foetal genes and collagens.121 These effects may be related to enhanced NO formation in statin-treated animals, as NOS inhibition abolished the positive results.122
Non-coding RNAs
Non-coding RNAs are important regulators of cardiac remodelling in chronic HF, post-MI and during pressure overload. Consequentially, silencing microRNAs in vivo using specific antisense inhibitors improves adverse myocardial remodelling.123,124 In large porcine studies using CMR and detailed molecular analyses, treatment with CDR132L, an antisense oligonucleotide drug directed against miR-132 (applied on days 3 and 28 post-MI) effectively prevented maladaptive growth, inhibited remodelling and restored LV function.125 Interestingly, delayed treatment after HF development (1 month post-MI) also significantly improved cardiac systolic and diastolic function and reversed cardiac remodelling at 3–5 months.126 In a small clinical trial, CDR132L was well tolerated in patients with stable HF on standard medications and seemed to improve cardiac function.127 CDR132L is now under investigation in a Phase II randomized, double-blind study in patients with reduced LVEF (≤45%) after MI.
Inflammation modulators
Although inflammation’s pathophysiologic contributions to LV remodelling have been appreciated since the early 1990s, anti-inflammatory drugs are not yet standard of care for HF patients. Early on, anti-tumour necrosis factor (TNF) treatment was studied in a HF population;128 however, all anti-TNF trials were futile, possibly due to poor patient selection and likely due to focusing on the wrong target, indicating the need for deeper pathophysiological understanding clinical trials. In recent years, a plethora of research has shown inflammation is pivotal during the remodelling process. Canakinumab, a monoclonal antibody targeting interleukin-1 beta, lowered CV events in a post-MI patient cohort,129 the first trial in cardiovascular medicine that improved outcomes only by inhibiting inflammation. Canakinumab also lowered the HF hospitalization rate.130
Novel pathways could be addressed via T-cell modulation, for example by using specific antibodies or by chimeric antigen receptor (CAR) T-cell technologies recently described in animals.131 Nanoparticles can facilitate targeted delivery of drugs and can be specifically designed to target inflammatory cells. In the cardiovascular field, though, nanoparticles have not yet been studied in patients.132,133 Selecting individual patients in need of anti-inflammatory treatment, possibly identified by enhanced molecular imaging strategies, is likely key to successful therapy.
Protein therapies
While angiogenic growth factors have huge therapeutic potential after MI, all clinical trials have unfortunately yielded neutral results, and paracrine-acting proteins may not be suitable for treating (sub)acute MI (for review see Wu et al.134). A more promising approach may be continuously delivering growth factor by subcutaneous infusion. Indeed, in a murine model of reperfused acute MI, prolonged infusions of myeloid-derived growth factor (MYDGF C19orf10) or endoplasmic reticulum membrane protein complex subunit 10 (EMC10) have beneficially affected cardiac remodelling and function as well as survival,135,136 though currently no clinical studies have been completed.
Gene editing and adenoviral and lipid nanoparticles
Over the past two decades, many attempts to improve outcomes post-MI have involved adenoviral-based gene therapy to induce angiogenesis in the ischaemic heart.137 However, beneficial effects in clinical studies appeared to be limited to enhanced perfusion reserve and angina relief in patients with chronic myocardial ischaemia.138,139 Genome-editing technologies have greatly progressed140 and may enable regenerative therapies that prevent/treat adverse cardiac remodelling after MI. While initially limited by the need to use adenoviral vectors to achieve in vivo genome editing, lipid nanoparticle (LNP)-based transfer of ribonucleic acid (RNA) may be much better suited to clinical applications. Nevertheless, any gene-editing approach crucially depends on identifying a single gene that determines pathophysiology, which seems unlikely for the complex process of post-infarction cardiac remodelling. However, the recently described attenuation of myocardial fibrosis and hypertrophy in a mouse model of cardiac hypertrophy by LNP-based RNA transfer to generate transient anti-fibrotic CAR T-cells in vivo131 may pave the way for addressing adverse remodelling post-MI.
Bone marrow-derived cell therapy
After encouraging early results from animal studies, bone marrow-derived cell (BMC) therapy was thoroughly investigated over the last 20 years and promoted as an innovative treatment for patients with STEMI. Regrettably, it now seems clear that this approach is not effective for acute MI, although there is still hope for potential application in refractory angina or chronic HFrEF.141,142 Following the neutral results of the BOOST-2 trial,143 neither the ALLSTAR nor BAMI trials detected significant benefits for BMC therapy in patients with MI.144,145
Transcatheter and surgical interventions
Addressing reperfusion injury hypothetically provides an attractive target to reduce infarct size and prevent adverse remodelling. Unfortunately, all strategies, such as ischaemic postconditioning, showing benefits in preclinical studies were futile in larger randomized controlled studies.26 In smaller studies, intracoronary infusion of hyperoxaemic blood (supersaturated oxygen therapy) after PCI in patients with anterior STEMI was associated with reduced infarct size and may be related to improved outcomes,146 but this method has not been subjected to large randomized studies to determine whether it can prevent or attenuate LV remodelling post-MI. Another potential way to reverse LV dilatation is surgical or interventional ventricular restoration. Surgically correcting LV geometry may be considered for large akinetic or aneurysmatic areas that increase wall stress. Despite the theoretical benefits of reconstructing normal LV architecture during surgical revascularization, this approach was not superior to revascularization alone.147 Therefore, this treatment is reserved for narrowly selected HF patients who are either refractory to standard of care or present with severe, malignant arrhythmias. In patients with chronic anteroseptal infarctions, a transjugular and left thoracotomy technique introduces anchor pairs to plicate the anterior and free wall LV scar against the RV septal scar in order to decrease cardiac volume (Revivent™).148 While results in carefully selected patients seem to be encouraging, randomized studies’ findings are still pending (REVIVE-HF, NCT03845127). Another interventionally placed device (AccuCinch® system) is attached to the inner LV wall and then cinched to reduce LV dilatation. The randomized CORCINCH-HF study (NCT04331769) investigating the device is ongoing. In HFrEF patients, RDN data consistently suggest beneficial effects on symptoms, HF biomarkers, and exercise capacity in different species.149–151 Renal denervation also improves cardiac stiffness and vascular resistance in HFpEF.152 Whether RDN augments post-MI remodelling and protects from HF development remains to be evaluated in clinical trials (COMBI-RDN, NCT02272920).
Conclusions
Left ventricular remodelling is an important determinant of morbidity and mortality. The original treatment strategy sought to reverse mechanical changes: e.g. reducing pre- and after- as well as volume load. Angiotensin-converting enzyme inhibitors are now a universal therapy for all forms of HFrEF. Independent of loading conditions, other pathophysiologic mechanisms like inflammation, metabolism, and fibrosis also contribute to adverse LV remodelling. Therefore, developing innovative drugs will depend on meticulously identifying novel targets beyond, taking into account that there may be large inter-individual differences regarding pathophysiologic mechanisms. In the above-mentioned CANTOS trial, pre-selecting patients based on CRP was key to success. Consequently, we recommend elucidating surrogate ‘biomarkers’ that indicate the pathomechanisms of LV remodelling. As circulating biomarkers reflect systemic changes but are not cardio-specific, novel imaging modalities might help reveal myocardial mechanisms and facilitate pathophysiologically guided individualized treatment (Graphical abstract).
Acknowledgements
The authors would like to thank Dr Caroline Morbach for providing the echo images.
Contributor Information
Stefan Frantz, Department of Internal Medicine I, Universitätsklinikum Würzburg, University Hospital Würzburg, University of Würzburg, Oberdürrbacher Str. 6, 97080 Würzburg, Germany.
Moritz Jens Hundertmark, Oxford Centre for Clinical Magnetic Resonance Research (OCMR), Division of Cardiovascular Medicine, Radcliffe Department of Medicine, University of Oxford, John Radcliffe Hospital, Oxford, UK.
Jeanette Schulz-Menger, Department of Cardiology and Nephrology, Experimental and Clinical Research Center, a Joint Cooperation between the Charité Medical Faculty and the Max-Delbrueck Center for Molecular Medicine and HELIOS Hospital Berlin Buch, Berlin, Germany.
Frank Michael Bengel, Department of Nuclear Medicine, Hannover Medical School, Hannover, Germany.
Johann Bauersachs, Department of Cardiology and Angiology, Hannover Medical School, Hannover, Germany.
Funding
S.F. was supported by the CRC1525 (project number 453989101). J.B. and F.M.B. were supported by the DFG, KFO311.
Conflict of interest: M.J.H. is supported by an industrial grant provided by IMBRIA pharmaceuticals, unrelated to this manuscript. F.M.B. receives research grants from Siemens Healthineers and GE Healthcare, which are not related to the topic of this manuscript. J.B. received honoraria for lectures/consulting from Novartis, Vifor, Bayer, Pfizer, Boehringer Ingelheim, AstraZeneca, Cardior, CVRx, BMS, Amgen, Corvia, not related to this article; and research support for the department from Zoll, CVRx, Abiomed, not related to this article. J.B. is a Scientific Advisory Board Member of Cardior and has patents PCT/EP2007/008772 and PCT/EP2009/051986 with royalties paid both on microRNA and downstream targets for diagnostic and therapeutic purposes. S.F. received honoraria for lectures/consulting from Amgen, AstraZeneca, Bayer Vital, Boehringer Ingelheim, Bristol-Meyers Squibb GmbH, Daiichi Sankyo, MSD, Novartis, Pfizer, Sanofi, Servier, and Vifor, not related to this article.
References
- 1.Bolognese L, Neskovic AN, Parodi G, Cerisano G, Buonamici P, Santoro GM, et al. Left ventricular remodeling after primary coronary angioplasty: patterns of left ventricular dilation and long-term prognostic implications. Circulation 2002;106:2351–2357. [DOI] [PubMed] [Google Scholar]
- 2.Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation 2020;141:e139–e596. [DOI] [PubMed] [Google Scholar]
- 3.Conrad N, Judge A, Tran J, Mohseni H, Hedgecott D, Crespillo AP, et al. Temporal trends and patterns in heart failure incidence: a population-based study of 4 million individuals. Lancet 2018;391:572–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stork S, Hense HW, Zentgraf C, Uebelacker I, Jahns R, Ertl G, et al. Pharmacotherapy according to treatment guidelines is associated with lower mortality in a community-based sample of patients with chronic heart failure: a prospective cohort study. Eur J Heart Fail 2008;10:1236–1245. [DOI] [PubMed] [Google Scholar]
- 5.Desta L, Jernberg T, Spaak J, Hofman-Bang C, Persson H. Risk and predictors of readmission for heart failure following a myocardial infarction between 2004 and 2013: a Swedish nationwide observational study. Int J Cardiol 2017;248:221–226. [DOI] [PubMed] [Google Scholar]
- 6.Maggioni AP, Maseri A, Fresco C, Franzosi MG, Mauri F, Santoro E, et al. Age-related increase in mortality among patients with first myocardial infarctions treated with thrombolysis. The Investigators of the Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico (GISSI-2). N Engl J Med 1993;329:1442–1448. [DOI] [PubMed] [Google Scholar]
- 7.Bujak M, Kweon HJ, Chatila K, Li N, Taffet G, Frangogiannis NG. Aging-related defects are associated with adverse cardiac remodeling in a mouse model of reperfused myocardial infarction. J Am Coll Cardiol 2008;51:1384–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lam CSP, Arnott C, Beale AL, Chandramouli C, Hilfiker-Kleiner D, Kaye DM, et al. Sex differences in heart failure. Eur Heart J 2019;40:3859–3868c. [DOI] [PubMed] [Google Scholar]
- 9.Kannel WB, Hjortland M, Castelli WP. Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol 1974;34:29–34. [DOI] [PubMed] [Google Scholar]
- 10.Ambikairajah A, Walsh E, Tabatabaei-Jafari H, Cherbuin N. Fat mass changes during menopause: a metaanalysis. Am J Obstet Gynecol 2019;221:393–409.e50. [DOI] [PubMed] [Google Scholar]
- 11.Albert J, Lezius S, Stork S, Morbach C, Guder G, Frantz S, et al. Trajectories of left ventricular ejection fraction after acute decompensation for systolic heart failure: concomitant echocardiographic and systemic changes, predictors, and impact on clinical outcomes. J Am Heart Assoc 2021;10:e017822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heusch G, Libby P, Gersh B, Yellon D, Bohm M, Lopaschuk G, et al. Cardiovascular remodelling in coronary artery disease and heart failure. Lancet 2014;383:1933–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beisvag V, Kemi OJ, Arbo I, Loennechen JP, Wisloff U, Langaas M, et al. Pathological and physiological hypertrophies are regulated by distinct gene programs. Eur J Cardiovasc Prev Rehabil 2009;16:690–697. [DOI] [PubMed] [Google Scholar]
- 14.Kehat I, Molkentin JD. Molecular pathways underlying cardiac remodeling during pathophysiological stimulation. Circulation 2010;122:2727–2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Treibel TA, Lopez B, Gonzalez A, Menacho K, Schofield RS, Ravassa S, et al. Reappraising myocardial fibrosis in severe aortic stenosis: an invasive and non-invasive study in 133 patients. Eur Heart J 2018;39:699–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frangogiannis NG, Kovacic JC. Extracellular matrix in ischemic heart disease, part 4/4: JACC Focus Seminar. J Am Coll Cardiol 2020;75:2219–2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN, et al. Transient regenerative potential of the neonatal mouse heart. Science 2011; 331:1078–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao M, Zhang E, Wei Y, Zhou Y, Walcott GP, Zhang J. Apical resection prolongs the cell cycle activity and promotes myocardial regeneration after left ventricular injury in neonatal pig. Circulation 2020;142:913–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang J, Bolli R, Garry DJ, Marban E, Menasche P, Zimmermann WH, et al. Basic and translational research in cardiac repair and regeneration: JACC State-of-the-Art Review. J Am Coll Cardiol 2021;78:2092–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Swirski FK, Nahrendorf M. Cardioimmunology: the immune system in cardiac homeostasis and disease. Nat Rev Immunol 2018;18:733–744. [DOI] [PubMed] [Google Scholar]
- 21.Frantz S, Falcao-Pires I, Balligand JL, Bauersachs J, Brutsaert D, Ciccarelli M, et al. The innate immune system in chronic cardiomyopathy: a European Society of Cardiology (ESC) scientific statement from the Working Group on Myocardial Function of the ESC. Eur J Heart Fail 2018;20:445–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fraccarollo D, Neuser J, Moller J, Riehle C, Galuppo P, Bauersachs J. Expansion of CD10(neg) neutrophils and CD14(+)HLA-DR(neg/low) monocytes driving proinflammatory responses in patients with acute myocardial infarction. Elife 2021;10:e66808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fraccarollo D, Thomas S, Scholz CJ, Hilfiker-Kleiner D, Galuppo P, Bauersachs J. Macrophage mineralocorticoid receptor is a pleiotropic modulator of myocardial infarct healing. Hypertension 2019;73:102–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rieckmann M, Delgobo M, Gaal C, Buchner L, Steinau P, Reshef D, et al. Myocardial infarction triggers cardioprotective antigen-specific T helper cell responses. J Clin Invest 2019;129:4922–4936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adamo L, Rocha-Resende C, Prabhu SD, Mann DL. Reappraising the role of inflammation in heart failure. Nat Rev Cardiol 2020;17:269–285. [DOI] [PubMed] [Google Scholar]
- 26.Heusch G. Myocardial ischaemia-reperfusion injury and cardioprotection in perspective. Nat Rev Cardiol 2020;17:773–789. [DOI] [PubMed] [Google Scholar]
- 27.Chouchani ET, Pell VR, Gaude E, Aksentijevic D, Sundier SY, Robb EL, et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature 2014;515:431–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsutsui H, Kinugawa S, Matsushima S. Mitochondrial oxidative stress and dysfunction in myocardial remodelling. Cardiovasc Res 2009;81:449–456. [DOI] [PubMed] [Google Scholar]
- 29.Peterzan MA, Lygate CA, Neubauer S, Rider OJ. Metabolic remodeling in hypertrophied and failing myocardium: a review. Am J Physiol Heart Circ Physiol 2017;313:H597–H616. [DOI] [PubMed] [Google Scholar]
- 30.Nascimben L, Ingwall JS, Lorell BH, Pinz I, Schultz V, Tornheim K, et al. Mechanisms for increased glycolysis in the hypertrophied rat heart. Hypertension 2004;44:662–667. [DOI] [PubMed] [Google Scholar]
- 31.Lopaschuk GD, Collins-Nakai RL, Itoi T. Developmental changes in energy substrate use by the heart. Cardiovasc Res 1992;26:1172–1180. [DOI] [PubMed] [Google Scholar]
- 32.Razeghi P, Young ME, Alcorn JL, Moravec CS, Frazier OH, Taegtmeyer H. Metabolic gene expression in fetal and failing human heart. Circulation 2001;104:2923–2931. [DOI] [PubMed] [Google Scholar]
- 33.Rosenblatt-Velin N, Montessuit C, Papageorgiou I, Terrand J, Lerch R. Postinfarction heart failure in rats is associated with upregulation of GLUT-1 and downregulation of genes of fatty acid metabolism. Cardiovasc Res 2001;52:407–416. [DOI] [PubMed] [Google Scholar]
- 34.Gibb AA, Hill BG. Metabolic coordination of physiological and pathological cardiac remodeling. Circ Res 2018;123:107–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choi YS, de Mattos AB, Shao D, Li T, Nabben M, Kim M, et al. Preservation of myocardial fatty acid oxidation prevents diastolic dysfunction in mice subjected to angiotensin II infusion. J Mol Cell Cardiol 2016;100:64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kolwicz SC J, Olson DP, Marney LC, Garcia-Menendez L, Synovec RE, Tian R. Cardiac-specific deletion of acetyl CoA carboxylase 2 prevents metabolic remodeling during pressure-overload hypertrophy. Circ Res 2012;111:728–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol Cell Physiol 2004;287:C817– C833. [DOI] [PubMed] [Google Scholar]
- 38.Bohm M, Ettelbruck S, Flesch M, van Gilst WH, Knorr A, Maack C, et al. Beta-adrenergic signal transduction following carvedilol treatment in hypertensive cardiac hypertrophy. Cardiovasc Res 1998;40:146–55. [DOI] [PubMed] [Google Scholar]
- 39.Olivetti G, Capasso JM, Meggs LG, Sonnenblick EH, Anversa P. Cellular basis of chronic ventricular remodeling after myocardial infarction in rats. Circ Res 1991;68:856–869. [DOI] [PubMed] [Google Scholar]
- 40.Giannoni A, Emdin M, Bramanti F, Iudice G, Francis DP, Barsotti A, et al. Combined increased chemosensitivity to hypoxia and hypercapnia as a prognosticator in heart failure. J Am Coll Cardiol 2009;53:1975–1980. [DOI] [PubMed] [Google Scholar]
- 41.Cohn JN, Levine TB, Olivari MT, Garberg V, Lura D, Francis GS, et al. Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N Engl J Med 1984;311:819–823. [DOI] [PubMed] [Google Scholar]
- 42.Francis GS, Benedict C, Johnstone DE, Kirlin PC, Nicklas J, Liang CS, et al. Comparison of neuroendocrine activation in patients with left ventricular dysfunction with and without congestive heart failure. A substudy of the Studies of Left Ventricular Dysfunction (SOLVD). Circulation 1990;82:1724–1729. [DOI] [PubMed] [Google Scholar]
- 43.McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021; 42:3599–3726. [DOI] [PubMed] [Google Scholar]
- 44.Sharp T, Lefer DJ. Renal denervation to treat heart failure. Annu Rev Physiol 2021;83:39–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zannad F, Rossignol P. Cardiorenal syndrome revisited. Circulation 2018;138:929–944. [DOI] [PubMed] [Google Scholar]
- 46.Kangawa K, Fukuda A, Kubota I, Hayashi Y, Minamitake Y, Matsuo H. Human atrial natriuretic polypeptides (hANP): purification, structure synthesis and biological activity. J Hypertens Suppl 1984;2:S321–S323. [PubMed] [Google Scholar]
- 47.Kuhn M. Molecular physiology of membrane guanylyl cyclase receptors. Physiol Rev 2016;96:751–804. [DOI] [PubMed] [Google Scholar]
- 48.Kasama S, Furuya M, Toyama T, Ichikawa S, Kurabayashi M. Effect of atrial natriuretic peptide on left ventricular remodelling in patients with acute myocardial infarction. Eur Heart J 2008;29:1485–1494. [DOI] [PubMed] [Google Scholar]
- 49.Chen HH, Redfield MM, Nordstrom LJ, Horton DP, Burnett JC Jr. Subcutaneous administration of the cardiac hormone BNP in symptomatic human heart failure. J Card Fail 2004;10:115–119. [DOI] [PubMed] [Google Scholar]
- 50.Wright GA, Struthers AD. Natriuretic peptides as a prognostic marker and therapeutic target in heart failure. Heart 2006;92:149–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goetze JP, Bruneau BG, Ramos HR, Ogawa T, de Bold MK, de Bold AJ. Cardiac natriuretic peptides. Nat Rev Cardiol 2020;17:698–717. [DOI] [PubMed] [Google Scholar]
- 52.Chen Y, Burnett JC Jr. Biochemistry, therapeutics, and biomarker implications of neprilysin in cardiorenal disease. Clin Chem 2017;63:108–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Horio T, Nishikimi T, Yoshihara F, Matsuo H, Takishita S, Kangawa K. Inhibitory regulation of hypertrophy by endogenous atrial natriuretic peptide in cultured cardiac myocytes. Hypertension 2000;35:19–24. [DOI] [PubMed] [Google Scholar]
- 54.Maki T, Horio T, Yoshihara F, Suga S, Takeo S, Matsuo H, et al. Effect of neutral endopeptidase inhibitor on endogenous atrial natriuretic peptide as a paracrine factor in cultured cardiac fibroblasts. Br J Pharmacol 2000;131:1204–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liang L, Tang R, Xie Q, Han J, Li W. The clinical effect of recombinant human brain natriuretic peptide on asymptomatic peri-procedural myocardial injury after percutaneous transluminal coronary angioplasty. Sci Rep 2020;10:15902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Galderisi M, Cosyns B, Edvardsen T, Cardim N, Delgado V, Di Salvo G, et al. Standardization of adult transthoracic echocardiography reporting in agreement with recent chamber quantification, diastolic function, and heart valve disease recommendations: an expert consensus document of the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2017;18:1301–1310. [DOI] [PubMed] [Google Scholar]
- 57.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28:1–39.e14. [DOI] [PubMed] [Google Scholar]
- 58.Hoffmann R, von Bardeleben S, ten Cate F, Borges AC, Kasprzak J, Firschke C, et al. Assessment of systolic left ventricular function: a multi-centre comparison of cineventriculography, cardiac magnetic resonance imaging, unenhanced and contrast-enhanced echocardiography. Eur Heart J 2005;26:607–616. [DOI] [PubMed] [Google Scholar]
- 59.Shah SJ, Fonarow GC, Gheorghiade M, Lang RM. Phase II trials in heart failure: the role of cardiovascular imaging. Am Heart J 2011;162:3–15.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bengel FM, George RT, Schuleri KH, Lardo AC, Wollert KC. Image-guided therapies for myocardial repair: concepts and practical implementation. Eur Heart J Cardiovasc Imaging 2013;14:741–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fertin M, Dubois E, Belliard A, Amouyel P, Pinet F, Bauters C. Usefulness of circulating biomarkers for the prediction of left ventricular remodeling after myocardial infarction. Am J Cardiol 2012;110:277–283. [DOI] [PubMed] [Google Scholar]
- 62.Goliasch G, Bartko PE, Pavo N, Neuhold S, Wurm R, Mascherbauer J, et al. Refining the prognostic impact of functional mitral regurgitation in chronic heart failure. Eur Heart J 2018;39:39–46. [DOI] [PubMed] [Google Scholar]
- 63.Friedrich MG. The future of cardiovascular magnetic resonance imaging. Eur Heart J 2017;38:1698–1701. [DOI] [PubMed] [Google Scholar]
- 64.Bucius P, Erley J, Tanacli R, Zieschang V, Giusca S, Korosoglou G, et al. Comparison of feature tracking, fast-SENC, and myocardial tagging for global and segmental left ventricular strain. ESC Heart Fail 2020;7:523–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lim C, Blaszczyk E, Riazy L, Wiesemann S, Schuler J, von Knobelsdorff-Brenkenhoff F, et al. Quantification of myocardial strain assessed by cardiovascular magnetic resonance feature tracking in healthy subjects-influence of segmentation and analysis software. Eur Radiol 2021;31:3962–3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Weir RAP, Clements S, Steedman T, Dargie HJ, McMurray JJV. Prognostic value of cardiac magnetic resonance parameters and biomarkers following myocardial infarction; 10-year follow-up of the Eplerenone Remodelling in Myocardial Infarction without Heart Failure trial. Eur J Heart Fail 2022;24:393–395. [DOI] [PubMed] [Google Scholar]
- 67.Klug G, Feistritzer HJ, Reinstadler SJ, Reindl M, Tiller C, Holzknecht M, et al. Impact of posteromedial papillary muscle infarction on mitral regurgitation during ST-segment elevation myocardial infarction. Int J Cardiovasc Imaging 2020;36:503–511. [DOI] [PubMed] [Google Scholar]
- 68.Eitel I, Gehmlich D, Amer O, Wohrle J, Kerber S, Lauer B, et al. Prognostic relevance of papillary muscle infarction in reperfused infarction as visualized by cardiovascular magnetic resonance. Circ Cardiovasc Imaging 2013;6:890–898. [DOI] [PubMed] [Google Scholar]
- 69.Agewall S, Beltrame JF, Reynolds HR, Niessner A, Rosano G, Caforio AL, et al. ESC working group position paper on myocardial infarction with non-obstructive coronary arteries. Eur Heart J 2017;38:143–153. [DOI] [PubMed] [Google Scholar]
- 70.Dastidar AG, Rodrigues JCL, Johnson TW, De Garate E, Singhal P, Baritussio A, et al. Myocardial infarction with nonobstructed coronary arteries: impact of CMR early after presentation. JACC Cardiovasc Imaging 2017;10:1204–1206. [DOI] [PubMed] [Google Scholar]
- 71.Pathik B, Raman B, Mohd Amin NH, Mahadavan D, Rajendran S, McGavigan AD, et al. Troponin-positive chest pain with unobstructed coronary arteries: incremental diagnostic value of cardiovascular magnetic resonance imaging. Eur Heart J Cardiovasc Imaging 2016;17:1146–1152. [DOI] [PubMed] [Google Scholar]
- 72.Galea N, Dacquino GM, Ammendola RM, Coco S, Agati L, De Luca L, et al. Microvascular obstruction extent predicts major adverse cardiovascular events in patients with acute myocardial infarction and preserved ejection fraction. Eur Radiol 2019;29:2369–2377. [DOI] [PubMed] [Google Scholar]
- 73.Puntmann VO, Carr-White G, Jabbour A, Yu CY, Gebker R, Kelle S, et al. Native T1 and ECV of noninfarcted myocardium and outcome in patients with coronary artery disease. J Am Coll Cardiol 2018;71:766–778. [DOI] [PubMed] [Google Scholar]
- 74.Messroghli DR, Moon JC, Ferreira VM, Grosse-Wortmann L, He T, Kellman P, et al. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: a consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI). J Cardiovasc Magn Reson 2017;19:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu T, Howarth AG, Chen Y, Nair AR, Yang HJ, Ren D, et al. Intramyocardial hemorrhage and the “Wave Front” of reperfusion injury compromising myocardial salvage. J Am Coll Cardiol 2022;79:35–48. [DOI] [PubMed] [Google Scholar]
- 76.Hamirani YS, Wong A, Kramer CM, Salerno M. Effect of microvascular obstruction and intramyocardial hemorrhage by CMR on LV remodeling and outcomes after myocardial infarction: a systematic review and meta-analysis. JACC Cardiovasc Imaging 2014;7:940–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shi K, Ma M, Yang MX, Xia CC, Peng WL, He Y, et al. Increased oxygenation is associated with myocardial inflammation and adverse regional remodeling after acute ST-segment elevation myocardial infarction. Eur Radiol 2021;31:8956–8966. [DOI] [PubMed] [Google Scholar]
- 78.Werner RA, Thackeray JT, Diekmann J, Weiberg D, Bauersachs J, Bengel FM. The changing face of nuclear cardiology: guiding cardiovascular care toward molecular medicine. J Nucl Med 2020;61:951–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nielsen R, Moller N, Gormsen LC, Tolbod LP, Hansson NH, Sorensen J, et al. Cardiovascular effects of treatment with the ketone body 3-hydroxybutyrate in chronic heart failure patients. Circulation 2019;139:2129–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Peterson LR, Herrero P, Schechtman KB, Racette SB, Waggoner AD, Kisrieva-Ware Z, et al. Effect of obesity and insulin resistance on myocardial substrate metabolism and efficiency in young women. Circulation 2004;109:2191–2196. [DOI] [PubMed] [Google Scholar]
- 81.Tuunanen H, Engblom E, Naum A, Nagren K, Scheinin M, Hesse B, et al. Trimetazidine, a metabolic modulator, has cardiac and extracardiac benefits in idiopathic dilated cardiomyopathy. Circulation 2008;118:1250–1258. [DOI] [PubMed] [Google Scholar]
- 82.Fallavollita JA, Heavey BM, Luisi AJJR, Michalek SM, Baldwa S, Mashtare TL Jr, et al. Regional myocardial sympathetic denervation predicts the risk of sudden cardiac arrest in ischemic cardiomyopathy. J Am Coll Cardiol 2014;63:141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jacobson AF, Senior R, Cerqueira MD, Wong ND, Thomas GS, Lopez VA, et al. Myocardial iodine-123 meta-iodobenzylguanidine imaging and cardiac events in heart failure. Results of the prospective ADMIRE-HF (AdreView Myocardial Imaging for Risk Evaluation in Heart Failure) study. J Am Coll Cardiol 2010;55:2212–2221. [DOI] [PubMed] [Google Scholar]
- 84.Simoes MV, Barthel P, Matsunari I, Nekolla SG, Schomig A, Schwaiger M, et al. Presence of sympathetically denervated but viable myocardium and its electrophysiologic correlates after early revascularised, acute myocardial infarction. Eur Heart J 2004;25:551–557. [DOI] [PubMed] [Google Scholar]
- 85.Thackeray JT, Derlin T, Haghikia A, Napp LC, Wang Y, Ross TL, et al. Molecular imaging of the chemokine receptor CXCR4 after acute myocardial infarction. JACC Cardiovasc Imaging 2015;8:1417–1426. [DOI] [PubMed] [Google Scholar]
- 86.Heo GS, Kopecky B, Sultan D, Ou M, Feng G, Bajpai G, et al. Molecular imaging visualizes recruitment of inflammatory monocytes and macrophages to the injured heart. Circ Res 2019;124:881–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tarkin JM, Calcagno C, Dweck MR, Evans NR, Chowdhury MM, Gopalan D, et al. 68Ga-DOTATATE PET identifies residual myocardial inflammation and bone marrow activation after myocardial infarction. J Am Coll Cardiol 2019;73:2489–2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Thackeray JT, Hupe HC, Wang Y, Bankstahl JP, Berding G, Ross TL, et al. Myocardial inflammation predicts remodeling and neuroinflammation after myocardial infarction. J Am Coll Cardiol 2018;71:263–275. [DOI] [PubMed] [Google Scholar]
- 89.Thackeray JT, Bengel FM. Molecular imaging of myocardial inflammation with positron emission tomography post-ischemia: a determinant of subsequent remodeling or recovery. JACC Cardiovasc Imaging 2018;11:1340–1355. [DOI] [PubMed] [Google Scholar]
- 90.Thorn SL, Barlow SC, Feher A, Stacy MR, Doviak H, Jacobs J, et al. Application of hybrid matrix metalloproteinase-targeted and dynamic (201)Tl single-photon emission computed tomography/computed tomography imaging for evaluation of early post-myocardial infarction remodeling. Circ Cardiovasc Imaging 2019;12:e009055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Diekmann J, Koenig T, Zwadlo C, Derlin T, Neuser J, Thackeray JT, et al. Molecular imaging identifies fibroblast activation beyond the infarct region after acute myocardial infarction. J Am Coll Cardiol 2021;77:1835–1837. [DOI] [PubMed] [Google Scholar]
- 92.Teyssier VR, Tournoux F, Simard JM, Gaudette F, Boudjemeline M, Petrenyov DR, et al. Novel O-[(11)C]-methylated derivatives of the neprilysin inhibitor sacubitril: Radiosynthesis, autoradiography and plasma stability evaluation. Nucl Med Biol 2021;102–103:34–44. [DOI] [PubMed] [Google Scholar]
- 93.Polyak A, Bankstahl JP, Besecke KFW, Hozsa C, Triebert W, Pannem RR, et al. Simplified (89)Zr-labeling protocol of oxine (8-Hydroxyquinoline) Enabling prolonged tracking of liposome-based nanomedicines and cells. Pharmaceutics 2021;13:1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hess A, Derlin T, Koenig T, Diekmann J, Wittneben A, Wang Y, et al. Molecular imaging-guided repair after acute myocardial infarction by targeting the chemokine receptor CXCR4. Eur Heart J 2020;41:3564–3575. [DOI] [PubMed] [Google Scholar]
- 95.Werner RA, Koenig T, Diekmann J, Haghikia A, Derlin T, Thackeray JT, et al. CXCR4-targeted imaging of post-infarct myocardial tissue inflammation: prognostic value after reperfused myocardial infarction. JACC Cardiovasc Imaging 2022;15:372–374. [DOI] [PubMed] [Google Scholar]
- 96.Reiter T, Kircher M, Schirbel A, Werner RA, Kropf S, Ertl G, et al. Imaging of C-X-C motif chemokine receptor CXCR4 expression after myocardial infarction with [(68)Ga]Pentixafor-PET/CT in correlation with cardiac MRI. JACC Cardiovasc Imaging 2018;11:1541–1543. [DOI] [PubMed] [Google Scholar]
- 97.Hess A, Thackeray JT, Wollert KC, Bengel FM. Radionuclide image-guided repair of the heart. JACC Cardiovasc Imaging 2020;13:2415–2429. [DOI] [PubMed] [Google Scholar]
- 98.Nahrendorf M, Frantz S, Swirski FK, Mulder WJ, Randolph G, Ertl G, et al. Imaging systemic inflammatory networks in ischemic heart disease. J Am Coll Cardiol 2015;65:1583–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bascunana P, Hess A, Borchert T, Wang Y, Wollert KC, Bengel FM, et al. 11C-Methionine PET identifies astroglia involvement in heart-brain Inflammation networking after acute myocardial infarction. J Nucl Med 2020;61:977–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Werner RA, Hess A, Koenig T, Diekmann J, Derlin T, Melk A, et al. Molecular imaging of inflammation crosstalk along the cardio-renal axis following acute myocardial infarction. Theranostics 2021;11:7984–7994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pfeffer JM, Pfeffer MA, Braunwald E. Influence of chronic captopril therapy on the infarcted left ventricle of the rat. Circ Res 1985;57:84–95. [DOI] [PubMed] [Google Scholar]
- 102.Pfeffer MA, Braunwald E, Moye LA, Basta L, Brown EJJR, Cuddy TE, et al. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. The SAVE Investigators. N Engl J Med 1992;327:669–677. [DOI] [PubMed] [Google Scholar]
- 103.Pfeffer MA, McMurray JJ, Velazquez EJ, Rouleau JL, Kober L, Maggioni AP, et al. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med 2003;349:1893–1906. [DOI] [PubMed] [Google Scholar]
- 104.Fraccarollo D, Galuppo P, Hildemann S, Christ M, Ertl G, Bauersachs J. Additive improvement of left ventricular remodeling and neurohormonal activation by aldosterone receptor blockade with eplerenone and ACE inhibition in rats with myocardial infarction. J Am Coll Cardiol 2003;42:1666–1673. [DOI] [PubMed] [Google Scholar]
- 105.Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 2003;348:1309–1321. [DOI] [PubMed] [Google Scholar]
- 106.Clemente-Moragon A, Gomez M, Villena-Gutierrez R, Lalama DV, Garcia-Prieto J, Martinez F, et al. Metoprolol exerts a non-class effect against ischaemia-reperfusion injury by abrogating exacerbated inflammation. Eur Heart J 2020;41:4425–4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.McMurray JJ, Packer M, Solomon SD. Neprilysin inhibition for heart failure. N Engl J Med 2014;371:2336–2337. [DOI] [PubMed] [Google Scholar]
- 108.Docherty KF, Campbell RT, Brooksbank KJM, Dreisbach JG, Forsyth P, Godeseth RL, et al. Effect of neprilysin inhibition on left ventricular remodeling in patients with asymptomatic left ventricular systolic dysfunction late after myocardial infarction. Circulation 2021;144:199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jering KS, Claggett B, Pfeffer MA, Granger C, Kober L, Lewis EF, et al. Prospective ARNI vs. ACE inhibitor trial to DetermIne Superiority in reducing heart failure Events after Myocardial Infarction (PARADISE-MI): design and baseline characteristics. Eur J Heart Fail 2021;23:1040–1048. [DOI] [PubMed] [Google Scholar]
- 110.Januzzi JL J, Prescott MF, Butler J, Felker GM, Maisel AS, McCague K, et al. Association of change in N-terminal Pro-B-type natriuretic peptide following initiation of sacubitril-valsartan treatment with cardiac structure and function in patients with heart failure with reduced ejection fraction. JAMA 2019;322:1085–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pfeffer MA, Claggett B, Lewis EF, Granger CB, Kober L, Maggioni AP, et al. Angiotensin receptor-neprilysin inhibition in acute myocardial infarction. N Engl J Med 2021;385:1845–1855. [DOI] [PubMed] [Google Scholar]
- 112.Pfeffer MA, Claggett B, Lewis EF, Granger CB, Kober L, Maggioni AP, et al. Impact of sacubitril/valsartan versus ramipril on total heart failure events in the PARADISE-MI Trial. Circulation 2022;145:87–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Bohm M, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med 2021;385:1451–1461. [DOI] [PubMed] [Google Scholar]
- 114.McMurray JJV, Solomon SD, Inzucchi SE, Kober L, Kosiborod MN, Martinez FA, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019;381:1995–2008. [DOI] [PubMed] [Google Scholar]
- 115.Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 2020;383:1413–1424. [DOI] [PubMed] [Google Scholar]
- 116.Lee MMY, Brooksbank KJM, Wetherall K, Mangion K, Roditi G, Campbell RT, et al. Effect of empagliflozin on left ventricular volumes in patients with type 2 diabetes, or prediabetes, and heart failure with reduced ejection fraction (SUGAR-DM-HF). Circulation 2021;143:516–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Santos-Gallego CG, Ibanez JAR, Antonio RS, Ishikawa K, Watanabe S, Botija MBP, et al. Empagliflozin induces a myocardial metabolic shift from glucose consumption to ketone metabolism that mitigates adverse cardiac remodeling and improves myocardial contractility. J Am Coll Cardiol 2018;71:A674. [Google Scholar]
- 118.Kantor PF, Lucien A, Kozak R, Lopaschuk GD. The antianginal drug trimetazidine shifts cardiac energy metabolism from fatty acid oxidation to glucose oxidation by inhibiting mitochondrial long-chain 3-ketoacyl coenzyme A thiolase. Cir Res 2000;86:580–588. [DOI] [PubMed] [Google Scholar]
- 119.Lee L, Horowitz J, Frenneaux M. Metabolic manipulation in ischaemic heart disease, a novel approach to treatment. Eur Heart J 2004;25:634–641. [DOI] [PubMed] [Google Scholar]
- 120.Wu L, Luan Y, Li Y, Wang M, He J, Jin C, et al. Effects of trimetazidine on ventricular remodeling in coronary artery disease patients with left ventricular hypertrophy: the rationale and design of a randomized controlled trial. BMC Cardiovasc Disord 2020;20:273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bauersachs J, Galuppo P, Fraccarollo D, Christ M, Ertl G. Improvement of left ventricular remodeling and function by hydroxymethylglutaryl coenzyme a reductase inhibition with cerivastatin in rats with heart failure after myocardial infarction. Circulation 2001;104:982–985. [DOI] [PubMed] [Google Scholar]
- 122.Nahrendorf M, Hu K, Hiller KH, Galuppo P, Fraccarollo D, Schweizer G, et al. Impact of hydroxymethylglutaryl coenzyme a reductase inhibition on left ventricular remodeling after myocardial infarction: an experimental serial cardiac magnetic resonance imaging study. J Am Coll Cardiol 2002;40:1695–1700. [DOI] [PubMed] [Google Scholar]
- 123.Thum T, Gross C, Fiedler J, Fischer T, Kissler S, Bussen M, et al. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature 2008;456:980–984. [DOI] [PubMed] [Google Scholar]
- 124.Kumarswamy R, Thum T. Non-coding RNAs in cardiac remodeling and heart failure. Circ Res 2013;113:676–689. [DOI] [PubMed] [Google Scholar]
- 125.Foinquinos A, Batkai S, Genschel C, Viereck J, Rump S, Gyongyosi M, et al. Preclinical development of a miR-132 inhibitor for heart failure treatment. Nat Commun 2020;11:633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Batkai S, Genschel C, Viereck J, Rump S, Bar C, Borchert T, et al. CDR132L improves systolic and diastolic function in a large animal model of chronic heart failure. Eur Heart J 2021;42:192–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Taubel J, Hauke W, Rump S, Viereck J, Batkai S, Poetzsch J, et al. Novel antisense therapy targeting microRNA-132 in patients with heart failure: results of a first-in-human Phase 1b randomized, double-blind, placebo-controlled study. Eur Heart J 2021;42:178–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mann DL, McMurray JJ, Packer M, Swedberg K, Borer JS, Colucci WS, et al. Targeted anticytokine therapy in patients with chronic heart failure: results of the Randomized Etanercept Worldwide Evaluation (RENEWAL). Circulation 2004;109:1594–602. [DOI] [PubMed] [Google Scholar]
- 129.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017;377:1119–1131. [DOI] [PubMed] [Google Scholar]
- 130.Everett BM, Cornel JH, Lainscak M, Anker SD, Abbate A, Thuren T, et al. Anti-Inflammatory therapy with canakinumab for the prevention of hospitalization for heart failure. Circulation 2019;139:1289–1299. [DOI] [PubMed] [Google Scholar]
- 131.Rurik JG, Tombacz I, Yadegari A, Mendez Fernandez PO, Shewale SV, Li L, et al. CAR T cells produced in vivo to treat cardiac injury. Science 2022;375:91–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Duivenvoorden R, Senders ML, van Leent MMT, Perez-Medina C, Nahrendorf M, Fayad ZA, et al. Nanoimmunotherapy to treat ischaemic heart disease. Nat Rev Cardiol 2019;16:21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Passaro F, Tocchetti CG, Spinetti G, Paudice F, Ambrosone L, Costagliola C, et al. Targeting fibrosis in the failing heart with nanoparticles. Adv Drug Deliv Rev 2021;174:461–481. [DOI] [PubMed] [Google Scholar]
- 134.Wu X, Reboll MR, Korf-Klingebiel M, Wollert KC. Angiogenesis after acute myocardial infarction. Cardiovasc Res 2021;117:1257–1273. [DOI] [PubMed] [Google Scholar]
- 135.Korf-Klingebiel M, Reboll MR, Klede S, Brod T, Pich A, Polten F, et al. Myeloid-derived growth factor (C19orf10) mediates cardiac repair following myocardial infarction. Nat Med 2015;21:140–149. [DOI] [PubMed] [Google Scholar]
- 136.Reboll MR, Korf-Klingebiel M, Klede S, Polten F, Brinkmann E, Reimann I, et al. EMC10 (Endoplasmic Reticulum Membrane Protein Complex Subunit 10) is a bone marrow-derived angiogenic growth factor promoting tissue repair after myocardial infarction. Circulation 2017;136:1809–1823. [DOI] [PubMed] [Google Scholar]
- 137.Korpela H, Hatinen OP, Nieminen T, Mallick R, Toivanen P, Airaksinen J, et al. Adenoviral VEGF-B186R127S gene transfer induces angiogenesis and improves perfusion in ischemic heart. iScience 2021;24:103533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hartikainen J, Hassinen I, Hedman A, Kivela A, Saraste A, Knuuti J, et al. Adenoviral intramyocardial VEGF-DDeltaNDeltaC gene transfer increases myocardial perfusion reserve in refractory angina patients: a phase I/IIa study with 1-year follow-up. Eur Heart J 2017;38:2547–2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Leikas AJ, Hassinen I, Hedman A, Kivela A, Yla-Herttuala S, Hartikainen JEK. Long-term safety and efficacy of intramyocardial adenovirus-mediated VEGF-D(DeltaNDeltaC) gene therapy eight-year follow-up of phase I KAT301 study. Gene Ther 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Musunuru K. Moving toward genome-editing therapies for cardiovascular diseases. J Clin Invest 2022;132:e148555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bolli R. Cell therapy for acute myocardial infarction: requiescat in pace. Eur Heart J 2020;41:3711–3714. [DOI] [PubMed] [Google Scholar]
- 142.Sanz-Ruiz R, Fernandez-Aviles F. Cardiovascular regenerative and reparative medicine: is myocardial infarction the model? Eur Heart J 2020;41:3459–3461. [DOI] [PubMed] [Google Scholar]
- 143.Wollert KC, Meyer GP, Muller-Ehmsen J, Tschope C, Bonarjee V, Larsen AI, et al. Intracoronary autologous bone marrow cell transfer after myocardial infarction: the BOOST-2 randomised placebo-controlled clinical trial. Eur Heart J 2017;38:2936–2943. [DOI] [PubMed] [Google Scholar]
- 144.Makkar RR, Kereiakes DJ, Aguirre F, Kowalchuk G, Chakravarty T, Malliaras K, et al. Intracoronary ALLogeneic heart STem cells to Achieve myocardial Regeneration (ALLSTAR): a randomized, placebo-controlled, double-blinded trial. Eur Heart J 2020;41:3451–3458. [DOI] [PubMed] [Google Scholar]
- 145.Mathur A, Fernandez-Aviles F, Bartunek J, Belmans A, Crea F, Dowlut S, et al. The effect of intracoronary infusion of bone marrow-derived mononuclear cells on all-cause mortality in acute myocardial infarction: the BAMI trial. Eur Heart J 2020;41:3702–3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chen S, David SW, Khan ZA, Metzger DC, Wasserman HS, Lotfi AS, et al. One-year outcomes of supersaturated oxygen therapy in acute anterior myocardial infarction: The IC-HOT study. Catheter Cardiovasc Interv 2021;97:1120–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Jones RH, Velazquez EJ, Michler RE, Sopko G, Oh JK, O’Connor CM, et al. Coronary bypass surgery with or without surgical ventricular reconstruction. N Engl J Med 2009;360:1705–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Biffi M, Loforte A, Folesani G, Ziacchi M, Attina D, Niro F, et al. Hybrid transcatheter left ventricular reconstruction for the treatment of ischemic cardiomyopathy. Cardiovasc Diagn Ther 2021;11:183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Davies JE, Manisty CH, Petraco R, Barron AJ, Unsworth B, Mayet J, et al. First-in-man safety evaluation of renal denervation for chronic systolic heart failure: primary outcome from REACH-Pilot study. Int J Cardiol 2013;162:189–192. [DOI] [PubMed] [Google Scholar]
- 150.Sharp TE III, Polhemus DJ, Li Z, Spaletra P, Jenkins JS, Reilly JP, et al. Renal denervation prevents heart failure progression via inhibition of the renin-angiotensin system. J Am Coll Cardiol 2018;72:2609–2621. [DOI] [PubMed] [Google Scholar]
- 151.Booth LC, de Silva RAU, Pontes RB, Yao ST, Hood SG, Lankadeva YR, et al. Renal, cardiac, and autonomic effects of catheter-based renal denervation in ovine heart failure. Hypertension 2021;78:706–715. [DOI] [PubMed] [Google Scholar]
- 152.Kresoja KP, Rommel KP, Fengler K, von Roeder M, Besler C, Lucke C, et al. Renal sympathetic denervation in patients with heart failure with preserved ejection fraction. Circ Heart Fail 2021;14:e007421. [DOI] [PubMed] [Google Scholar]