Abstract
INTRODUCTION:
We studied the relationship of acute ischemic stroke (AIS) large-vessel occlusion clot composition with vessel recanalization and preprocedure imaging.
SUBJECTS AND METHODS:
Individual clots from AIS patients who underwent mechanical thrombectomy (MT) between September 2016 and September 2018 were examined. Clot composition was analyzed histologically through a trichrome staining and image segmentation, and the area occupied by red blood cells (RBCs), fibrin, or mixed composition was quantified.
RESULTS:
Forty-three patients (65.4 ± 12.7 years, 39% of females) who underwent 92 retrieval passes (mean 2.14, range 1–6) were included in this study. Fibrin (44%) occupied the greatest area, followed by mixed composition (34%) and RBCs (22%). A stent retriever was deployed in 81% of cases, 20 patients (47%) achieved first-pass efficacy (FPE) (thrombolysis in cerebral infarction [TICI] 2b-3 after first pass), 41 (95%) achieved successful revascularization (TICI 2b-3), and 21 (49%) had good outcome (modified Rankin Scale [mRS] ≤2) at 90 days. Hyperdense artery sign (HAS) on initial computed tomography was correlated with mixed clot composition (P = 0.01) and lack of fibrin content (P = 0.03). In the univariate analysis, FPE was associated with RBC clot area, atrial fibrillation, and occlusion location but not with fibrin clot area, mixed clot area, stroke etiology, thrombectomy technique, distal emboli, or 90-day mRS. In the multivariate analysis, FPE was significantly correlated with low RBC clot area (odd ratio = 0.96, confidence interval [0.92.99], P = 0.034) but not with atrial fibrillation or location.
CONCLUSION:
Our results suggest that HAS is correlated with mixed clot composition and lower fibrin content and that lower RBC clot composition is associated with FPE in patients undergoing MT.
Keywords: Acute ischemic stroke, clot, clot composition, fibrin, large-vessel occlusion, mechanical thrombectomy, red blood cell
Introduction
Acute ischemic stroke (AIS) is a leading cause of death and disability worldwide. In 2015, five randomized controlled trials (RCT) established mechanical thrombectomy (MT) as the treatment of choice for AIS due to anterior circulation large-vessel occlusion (LVO),[1,2,3,4,5] and subsequently, DEFUSE 3 and DAWN trials have expanded the thrombectomy window in selected patients.[6,7] The HERMES meta-analysis and the five RCTs found that recanalization failed in 29% of LVO patients.[8] Understanding the relationship between clot histology and other clinical indicators such as initial CT findings, underlying stroke etiology, and the ease or difficulty of mechanical revascularization is pertinent. Prior clot analysis studies have suggested a relationship between retrieved LVO clot composition and reperfusion success as well as the association of clot subtype with underlying stroke etiology.[9,10] Some have suggested that increasing the percentage of red blood cell (RBC) composition within retrieved clots portends a higher likelihood of first-pass efficacy (FPE), which is defined as achieving a modified thrombolysis in cerebral infarction (TICI) score of 2b-3.[9,10] Overall, however, the data regarding clot histology and clinical factors lack consensus.[9]
The present study evaluates the composition of blood clots retrieved from the intracranial circulation during LVO mechanical thrombectomies and analyzes whether clot composition subtype differs between underlying stroke etiologies, whether clot composition impacts FPE, and whether any clot subtype is associated with dense vessel sign on the initial brain computed tomography (CT). Understanding the association of clot composition with preintervention patient characteristic, imaging findings, and reperfusion success may inform MT strategy and decision-making.
Subjects and Methods
A total of 43 ischemic stroke patients who underwent MT for acute stroke over 2 years between September 2016 and September 2018, with sufficient clot retrieved to facilitate histological evaluation, were included in the analysis. For patients with more than one clot retrieved, all retrieved clots were analyzed together, and the cumulative breakdown in composition was calculated. All patients were confirmed to have LVO on CT angiography (CTA). We retrospectively reviewed clinical, radiological, and procedural data from our prospectively maintained interventional stroke database. Five patients were excluded as insufficient clot material needed to perform a histological analysis was retrieved. The study was approved by the institutional review board, and no procedures were performed in this study involving humans or animals.
The patient's data gathered included demographic information, cerebrovascular risk factors, and initial stroke severity through NIHSS scores [Table 1]. The patient outcome was assessed by modified Rankin Scale (mRS) score at 90 days, and mRS score 0–2 considered a good outcome. Radiological data including the Alberta Stroke Program Early CT score (ASPECTS, hyperdense artery sign (HAS) on initial CT, and location of the LVO on CTA were reported by the reading neuroradiologist.
Table 1.
Patient and procedural characteristics
| n=43 | |
|---|---|
| Mean age (range) | 65.4±12.7 (40-88) |
| Female (%) | 39.5 |
| Risk factors (%) | |
| Atrial fibrillation | 46.5 |
| Hypertension | 69.8 |
| Hyperlipidemia | 37.2 |
| Diabetes | 39.5 |
| Smoking | 32.6 |
| Hypercoagulable | 2.3 |
| Stroke etiology (TOAST classification) (%) | |
| Cardioembolic | 58.1 |
| LAA | 14.0 |
| Other determined etiology* | 11.6 |
| Undetermined etiology | 16.3 |
| Location (%) | |
| ICA | 23.3 |
| M1 | 55.8 |
| M2 | 14.0 |
| Basilar | 7.0 |
| Thrombectomy technique (%) | |
| ADAPT | 18.6 |
| Solumbra/Trelumbra | 37.2 |
| SRBG | 23.3 |
| TRAP | 20.9 |
| Mean NIHSS on admission (range) | 16.1±5.3 (6-29) |
| Premorbid mRS 0-2 (%) | 93.0 |
| IV tPA (%) | 58.1 |
| IV Heparin | 2.3 |
| Distal emboli during the procedure (%) | 14 |
| Procedural time parameters (min) | |
| Mean LSW to puncture | 309.9±265.4 |
| Mean puncture to final reperfusion | 52.5±28.9 |
| Mean LSW to final reperfusion | 362.1±279.0 |
| Outcomes (%) | |
| FPE | 46.5 |
| Final successful reperfusion (TICI 2b-3) | 95.3 |
| Mean NIHSS at discharge (range) | 10.2±8.9 (0-30) |
| 90 days mRS ≤2 | 48.7 |
| Postprocedure sICH† | 9.3 |
*Five patients had stroke of “other” etiology (two dissections, one malignancy, one hypercoagulable state, one secondary to oral contraceptive pills), †One patient had HI1, two patients had HI2, and one patient had PH 1. n: Sample size, HI1: Hemorrhagic infarction type 1, HI2: Hemorrhagic infarction type 2, PH 1: Parenchymal hematoma type 1, TOAST: Trial of ORG 10172 in acute stroke treatment, ICA: Internal carotid artery, NIHSS: National Institute of Health Stroke Scale, mRS: Modified Rankin Scale, IV tPA: Intravenous tissue plasminogen activator, LSW: Last-seen-well, sICH: Symptomatic intracerebral hemorrhage, TICI: Thrombolysis in cerebral infarction, LAA: Large artery atherosclerosis, FPE: First-pass efficacy, ADAPT: A direct first-pass aspiration, SRBG: Stent retriever with balloon guide catheter, TRAP: Trevo aspiration proximal flow control technique
In accordance with our institutional protocol, noncontrast-enhanced CT of the brain and CTA were performed to guide decisions regarding thrombolysis and thrombectomy. Intravenous tissue plasminogen activator (IV tPA) was administered in all eligible patients prior to neurointervention. Inclusion criteria for MT included presentation within 6 h of time last-seen-well (LSW), NIHSS ≥4 or <4 with disabling neurological deficit, the presence of LVO on CTA, no hemorrhage on noncontrast CT, ASPECTS score ≥6, and premorbid mRS score of ≤2. Beyond 6 h and <24 h from time LSW, LVO-confirmed patients additionally underwent CT perfusion imaging and were deemed candidates for MT based on core versus penumbra ratio as described in the DAWN study and adjudicated by neuroradiology, stroke neurology, and the interventionalist.[6] Acute stroke subtypes were classified as cardioembolic (CE), large artery atherosclerosis (LAA), small-vessel occlusion, other rare causes, or undetermined cause as classified in the Trial of ORG 10172 in Acute Stroke Treatment (TOAST).[11]
At the interventionalist's discretion, Trevo (Concentric Medical Inc., Mountain View, CA), Solitaire (AB or FR, Covidien, Irvine, CA), Penumbra reperfusion catheter (Penumbra, Alameda, CA), and Catalyst catheters (Stryker) were used to perform MT. Reperfusion strategies utilized included aspiration alone (ADAPT), balloon guide catheter + stent retriever (SRBG), aspiration catheter + stent retriever (Solumbra or Trelumbra), BGC + aspiration catheter + stent retriever (TRAP), and supplemental tirofiban infusion. The strategies used were at the discretion of the interventionalist, and strategies were often switched after an initial unsuccessful pass. Angiographic outcome was rated on the TICI score, and MT was considered successful if the score was 2b or 3. Postprocedural symptomatic hemorrhage was defined as any hemorrhage associated with an increase in NIHSS score ≥4 within 24 h. Carotid tortuosity anatomic variation was scored post hoc.[12] Two fellowship-trained endovascular neurosurgeons independently evaluated all radiological outcomes and came to a consensus on final scores at adjudication sessions.
Thrombi retrieved immediately post procedure were placed in formalin solution, later photographed while in formalin, and sectioned and prepared for histology. Clots were sectioned along their longest axis. A trichrome staining method (Martius Scarlet Blue) was used to identify each clot element. Following staining, mature fibrin appeared blue, fresh fibrin was red, and RBC were bright yellow [Figure 1]. The images were analyzed with the Orbit Image Analysis software.
Figure 1.
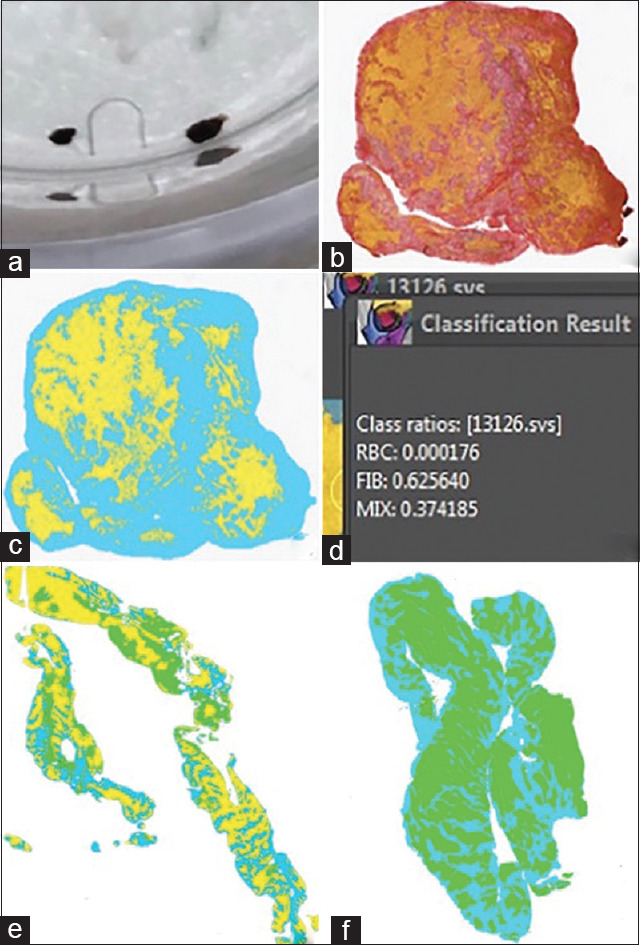
(a) A study subject clot sample prior to sectioning. (b) Clot sample with MSB stain. (c) Clot sample after segmentation. RBCs were highlighted green, mature and fresh fibrin were grouped and highlighted blue, and “mixed” area was highlighted yellow. (d) Quantification of clot sample in panel C showing it is fibrin rich. (e) Example of mixed-type clot. (f) Example of RBC-rich clot. RBC: Red blood cells, MSB Martius Scarlett Blue
The segmentation process began by loading the selected raw image into the field of view and identifying each clot component. In each case, there were 3–5 different components that were named and designated a specific color. An “Exclusion model” was first prepared by highlighting all of the components (background, RBC, fibrin, and mixed areas). The model was “trained and classified,” which solidified the total segmented area across the entire image. Once the exclusion model had been saved, then the “Inclusion model” was prepared, and the segmentation process was performed identically to the previous method. Once the inclusion model was complete, both models were layered to then compute the segmentation area. The total amount of segmented area was quantified per component, and the value of each segment was converted to a percentage.
We analyzed the radiological and clinical data for the 43 patients, and clot composition was analyzed histologically. The area of the clot occupied by RBC, fibrin, or mixed composition was quantified according to the established criteria.[13] RBC-rich clots were defined as clots with >15% RBC compared to fibrin, and fibrin-rich clot was defined as clots with >15% fibrin compared to RBC. Those not meeting either of these criteria were deemed as mixed. Differences in continuous variables were analyzed using one-way ANOVA with the post hoc Tukey's test. Categorical variables were compared using the Chi-squared test. Linear regression was performed to determine the correlation between the number of passes and procedure time to RBC/fibrin/mixed clot area. We performed a univariate analysis of RBC/fibrin/mixed clot area as well as other variables to find any association with FPE (association defined as P ≤ 0.25). Finally, a multivariate regression analysis was conducted on the associated variables. Statistical outliers, defined as points that fall more than 1.5 times the interquartile range above the third quartile or below the first quartile, were excluded during analysis. Statistical significance was set at P < 0.05.
Results
In a 43-patient cohort, the mean age was 65 (±12.7) years, 26 (61%) were male, 20 (47%) had atrial fibrillation, and 33% were smokers [Table 1]. The mean NIHSS at presentation was 16.1 ± 5.3 (range 6–29), and 58% received IV tPA before MT. TOAST criteria stroke subtypes classifications are as follows: 25 (58%) CE, 6 (14%) LAA, 5 (12%) other etiology, and 7 (16%) with undetermined etiology. 19% of patients received ADAPT, 37% received Solumbra/Trelumbra, 23% received SRBG, and 21% received TRAP (81% of patients were treated with a stent retriever). The meantime from LSW to puncture was 310 min (±265.4), puncture to reperfusion was 52.5 min (± 28.9), and LSW to reperfusion was 362.1 min (±279.0). A total of 92 thrombectomy passes were performed in 43 subjects (mean: 2.14, range: 1–6), with successful FPE in 20 (47%) patients. Successful revascularization (TICI 2b-3) was achieved in 41 (95%) of patients, and 21 (49%) patients had a good functional outcome at 3 months (mRS 0–2). Of the 21 patients with good functional outcomes, 11 (52%) had achieved FPE. A HAS was present in 20 (47%) patients on initial plain CT. On a 5-point internal carotid artery tortuosity scale, the mean score was 3.03 (±0.96), and tortuosity score was not associated with successful revascularization (P = 0.19).[14]
On average, fibrin occupied the greatest area of the retrieved clots, comprising 44%, followed by mixed comprising 34% and RBC comprising 22%. Based on the criteria described earlier, 14% of clots were classified as RBC rich, 49% were classified as fibrin rich, and 37% were classified as mixed. As shown in Table 2, there was no significant difference in clot classification between the categories of stroke etiology (P = 0.17) or between the different thrombectomy techniques (P = 0.81).
Table 2.
Clot classification per stroke etiology and thrombectomy technique
| Clot classification | |||
|---|---|---|---|
|
| |||
| RBC rich (n=6), n (%) | Fibrin rich (n=21), n (%) | Mixed (n=16), n (%) | |
| Stroke etiology | |||
| CE (n=25) | 1 (4) | 15 (60) | 9 (36) |
| LAA (n=6) | 1 (16.7) | 3 (50) | 2 (33.3) |
| Other (n=5) | 1 (20) | 1 (20) | 3 (60) |
| Undetermined (n=7) | 3 (42.9) | 2 (28.6) | 2 (28.6) |
| Thrombectomy technique | |||
| ADAPT | 1 (17) | 3 (14) | 4 (25) |
| Solumbra/Trelumbra | 2 (33) | 10 (48) | 4 (25) |
| SRBG | 1 (17) | 5 (24) | 4 (25) |
| TRAP | 2 (33) | 3 (14) | 4 (25) |
RBC: Red blood cell, CE: Cardioembolic, LAA: Large artery atherosclerosis, ADAPT: A direct first-pass aspiration, TRAP: Trevo aspiration proximal flow control technique, SRBG: Stent retriever with balloon guide catheter
There was no difference in number of passes versus RBC/fibrin/mixed clot area (P > 0.05), clot classification (P = 0.45), stroke etiology (P = 0.25), occlusion location (P = 0.31), or final reperfusion status (P = 0.18). Similarly, there was no difference in procedure time versus RBC/fibrin/mixed clot area (P > 0.05), clot classification (P = 0.25), stroke etiology (P = 0.58), occlusion location (P = 0.41), or final reperfusion status (P = 0.94) [Table 3]. There was a significantly higher percent of fibrin content in patients without a HAS (52%) than in patients with the sign (35%) (P = 0.03), and a significantly higher percentage of mixed content in patients with the HAS (46%) than in patients without the sign (24%) (P = 0.01). Clot classification did not correlate significantly with achieving successful reperfusion (P = 0.26).
Table 3.
Retrieval passes and procedure time analysis per subgroup
| Total number of retrieval passes (per patient) | Mean procedure time (mean±SD) | |
|---|---|---|
| All patients (n=43) | 92 (2.1) | 52.5±28.9 |
| Clot classification | P=0.45 | P=0.25 |
| RBC rich (n=6) | 17 (2.8) | 64.0±22.1 |
| Fibrin rich (n=21) | 44 (2.1) | 44.7±22.3 |
| Mixed (n=16) | 31 (1.9) | 53.4±28.9 |
| Stroke etiology | P=0.25 | P=0.58 |
| CE (n=25) | 56 (2.2) | 49.0±30.5 |
| LAA (n=6) | 9 (1.5) | 55.5±18.0 |
| Other etiology (n=5) | 7 (1.4) | 47.6±27.9 |
| Undetermined etiology (n=7) | 20 (2.9) | 65.9±32.2 |
| Occlusion locations | P=0.31 | P=0.41 |
| ICA terminus (n=10) | 23 (2.3) | 62.8±29.7 |
| M1 (n=24) | 57 (2.4) | 52.9±29.9 |
| M2 (n=6) | 9 (1.5) | 39.7±26.7 |
| Basilar (n=3) | 3 (1) | 40.7±14.0 |
| Final reperfusion | P=0.18 | P=0.94 |
| TICI <2b (n=2) | 7 (3.5) | 54.0±38.2 |
| TICI 2b-3 (n=41) | 85 (2.1) | 52.4±28.9 |
n: Sample size, SD: Standard deviation, RBC: Red blood cell, CE: Cardioembolic, LAA: Large artery atherosclerosis, ICA: Internal carotid artery, TICI: Thrombolysis in cerebral infarction
In the univariate analysis, FPE was associated (P < 0.25) with RBC clot area, atrial fibrillation, and occlusion location but not with fibrin clot area, mixed clot area, stroke etiology, thrombectomy technique, distal emboli, or 90-day mRS. In the multivariate analysis, FPE was significantly correlated with RBC clot area (odd ratio [OR] = 0.96, confidence interval [CI] [0.92.99], P = 0.034) but not with atrial fibrillation (P = 0.07) or location (P = 0.32). The odds ratio being <1 indicates that having less RBC clot area correlates to greater FPE success. Clots in cases that achieved FPE had a mean RBC clot area of 16.7%, while clots in cases that did not achieve FPE had a mean RBC clot area of 27.3%. On the other hand, fibrin and mixed composition clot area was similar in cases that achieved and did not achieve FPE (FPE achieved: fibrin – 46.1%, mixed – 37.1; FPE not achieved: fibrin – 41.4%, mixed – 31.3%).
Further statistical analysis showed no correlation between clot composition and distal emboli (P = 0.97), mean NIHSS at discharge (P = 0.59), 90-day mRS.(P = 0.15), LSW to puncture time (P = 0.47), or tPA administration (P = 0.74). There was also no correlation between tPA administration and FPE (P = 0.40). HAS did not correlate with 90-day mRS (P = 0.34) or mean NIHSS at discharge (P = 0.35).
Discussion
Understanding the underlying histology of AIS thrombi and the relationship to clinical factors may aid clinical decision-making. Our analysis of blood clots retrieved from 43 patients undergoing MT demonstrated RBC-rich clots were less likely to be retrieved successfully on the first pass. Mixed clot type was associated with HAS on CT, whereas high fibrin content was associated with lack of HAS. Further, histological clot composition was not different between stroke etiologies.
The predominant clot component in the current study was fibrin which occupied 44% of the overall clot area, followed by mixed (34%) and RBC (22%). Fibrin was found to occupy the greatest percentage of the clots retrieved, which is consistent with other clot analysis studies of a similar methodology. As previously described, clots were further classified as RBC rich – 14%, fibrin rich – 49%, and mixed – 37%. Our analysis found no significant difference in clot composition (RBC rich, fibrin rich, and mixed) between the categories of stroke etiology categorized by TOAST criteria Table 2. Prior clot analysis studies have reported inconsistent associations of clot subtype with underlying stroke etiology, with some describing an association of RBC rich clots with CE, others reporting LAA etiology associated with RBC-rich content clots, and one report found an association between WBC-rich clots with CE source; however, the majority of studies report no association between stroke underlying etiology and clot subtype.[15,16,17]
In prior studies, high RBC content clots have been positively correlated with response to IV tPA and postIV tPA thrombus migration prior to MT.[10,17] A recent histopathological study of 39 patients found increasing RBC-rich clot area to be a successful recanalization during intra-arterial thrombectomy.[9] Our multivariate analysis found that increased RBC content was negatively associated with FPE. Other studies have demonstrated an association of high RBC content with decreased MT procedure times, whereas high fibrin content is associated with longer procedure times and increased clot fragmentation.[9,13,18] Fibrin-rich clots created in the laboratory were shown to have a higher coefficient of friction than RBC-rich thrombus and have stronger interactions with the vessel wall, increased elasticity, and are harder to remove from the vessel wall.[13,19] This is contrary to our findings of increased RBC content being associated with a reduced likelihood of FPE. Studies have also shown that aspiration is more effective for RBC clots, whereas stent-retriever use is more effective for RBC-poor clots. In our study, only 19% of patients received ADAPT, while 81% of patients underwent stent retriever thrombectomy. The inverse relationship between RBC content and FPE could be a result of a high percentage of stent retriever thrombectomy in our study, again confirming prior studies that stent retriever is perhaps better for retrieval of RBC-poor clots (only 14% of retrieved clots in our study were classified as RBC rich). Similar to prior reports, our analysis found no association between clot subtype and overall revascularization success, nor functional outcome.[16]
HAS was noted in 20 (47%) patients on their initial plain CT. A mixed subtype of clot was significantly correlated with HAS on plain CT, and fibrin content correlated with lack of a HAS. Other studies have reported similar findings with RBC-rich and mixed clots more likely to be reported as hyperdense on pre-procedure plain CT.[13,16,18,19] Sporns et al.[14] found a strong correlation between low vessel attenuation on CT and low fibrin clot content and conversely reported a high RBC content with high vessel attenuation.[12] One study negatively correlated platelet content with HAS.[20] A recent systematic review found a positive correlation between HAS on CT and RBC content, and meta-analysis of four eligible studies demonstrated that patients with HAS artery on CT had a higher odd of having RBC rich thrombus than those without (OR: 9.0, 95% CI: 2.6–31.2, P < 0.01).[16] Our findings of low clot fibrin content being associated with a lack of dense vessel sign on CT are consistent with prior studies. Together, these indicate that we can predict clot composition based on CT imaging, which can potentially inform us about the reperfusion rate and guide our thrombectomy techniques.
In this study, we found no association of proximal vessel tortuosity score[12] with vessel recanalization rates or first-pass MT success.[15,16,20,21] The tortuosity score used in this study was only based on tortuosity of the carotid siphon, and other anatomical factors such as tortuosity beyond (e.g., M1) or proximal (e.g., cervical carotid) in the arterial tree that could impact FPE and final recanalization were not accounted for. Perhaps, a tortuosity score that captures both extra and intracranial tortuosity should be used in future studies to further assess the relationship between tortuosity and recanalization.
This study has several limitations which should be considered when interpreting the results. First, the design is a retrospective analysis of data prospectively collected at a single center with a moderate sample size for this type of study. Second, some clot subtype numbers such as RBC rich clots were low (n = 6), which may impact generalizability. Third, there was heterogeneity of thrombus retrieval techniques utilized, and the impact of thrombectomy technique on intracranial clots during MT is not well established, but there some some data to suggest that aspiration may affect clot composition compared to stent retriever. Finally, there is a degree of selection bias inherent in this type of study because patients who undergo MT where no clot is retrieved are not included in the histological analysis; therefore, the properties of those clots are unable to be assessed.
Conclusion
Our analysis suggests that mixed clot subtype is associated with HAS, while increased fibrin content negatively correlates with HAS. Further, increased clot RBC content reduced the chance of FPE. We found no association between clot histology and clot origin. This work furthers our knowledge regarding the relationship between thrombus histology, imaging, and reperfusion factors that may inform clinical decision-making. Future research is needed to determine whether certain reperfusion devices or combinations are more effective in treating particular thrombi subtypes and whether these subtypes can be predicted by preprocedural imaging.
Financial support and sponsorship
This study received unrestricted educational grant from Stryker Neurovascular ($50,000). Stryker participated neither in the data collection, data analysis, nor in the review of the results.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372:11–20. doi: 10.1056/NEJMoa1411587. [DOI] [PubMed] [Google Scholar]
- 2.Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372:1019–30. doi: 10.1056/NEJMoa1414905. [DOI] [PubMed] [Google Scholar]
- 3.Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, Rovira A, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372:2296–306. doi: 10.1056/NEJMoa1503780. [DOI] [PubMed] [Google Scholar]
- 4.Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, et al. Stent-retriever thrombectomy after intravenous t-PA vs.t-PA alone in stroke. N Engl J Med. 2015;372:2285–95. doi: 10.1056/NEJMoa1415061. [DOI] [PubMed] [Google Scholar]
- 5.Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372:1009–18. doi: 10.1056/NEJMoa1414792. [DOI] [PubMed] [Google Scholar]
- 6.Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378:708–18. doi: 10.1056/NEJMoa1713973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378:11–21. doi: 10.1056/NEJMoa1706442. [DOI] [PubMed] [Google Scholar]
- 8.Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: A meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387:1723–31. doi: 10.1016/S0140-6736(16)00163-X. [DOI] [PubMed] [Google Scholar]
- 9.Shin JW, Jeong HS, Kwon HJ, Song KS, Kim J. High red blood cell composition in clots is associated with successful recanalization during intra-arterial thrombectomy. PLoS One. 2018;13:e0197492. doi: 10.1371/journal.pone.0197492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi MH, Park GH, Lee JS, Lee SE, Lee SJ, Kim JH, et al. Erythrocyte fraction within retrieved thrombi contributes to thrombolytic response in acute ischemic stroke. Stroke. 2018;49:652–9. doi: 10.1161/STROKEAHA.117.019138. [DOI] [PubMed] [Google Scholar]
- 11.Adams HP, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. trial of org 10172 in acute stroke treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 12.Vijaywargiya M, Deopujari R, Athavale SA. Anatomical study of petrous and cavernous parts of internal carotid artery. Anat Cell Biol. 2017;50:163–70. doi: 10.5115/acb.2017.50.3.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Niesten JM, van der Schaaf IC, van Dam L, Vink A, Vos JA, Schonewille WJ, et al. Histopathologic composition of cerebral thrombi of acute stroke patients is correlated with stroke subtype and thrombus attenuation. PLoS One. 2014;9:e88882. doi: 10.1371/journal.pone.0088882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sporns PB, Jeibmann A, Minnerup J, Broocks G, Nawabi J, Schön G, et al. Histological clot composition is associated with preinterventional clot migration in acute stroke patients. Stroke. 2019;50:2065–71. doi: 10.1161/STROKEAHA.118.023314. [DOI] [PubMed] [Google Scholar]
- 15.Maekawa K, Shibata M, Nakajima H, Mizutani A, Kitano Y, Seguchi M, et al. Erythrocyte-rich thrombus is associated with reduced number of maneuvers and procedure time in patients with acute ischemic stroke undergoing mechanical thrombectomy. Cerebrovasc Dis Extra. 2018;8:39–49. doi: 10.1159/000486042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brinjikji W, Duffy S, Burrows A, Hacke W, Liebeskind D, Majoie CBLM, et al. Correlation of imaging and histopathology of thrombi in acute ischemic stroke with etiology and outcome: A systematic review. J Neurointerv Surg. 2017;9:529–34. doi: 10.1136/neurintsurg-2016-012391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gersh KC, Nagaswami C, Weisel JW. Fibrin network structure and clot mechanical properties are altered by incorporation of erythrocytes. Thromb Haemost. 2009;102:1169–75. doi: 10.1160/TH09-03-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh P, Doostkam S, Reinhard M, Ivanovas V, Taschner CA. Immunohistochemical analysis of thrombi retrieved during treatment of acute ischemic stroke: Does stent-retriever cause intimal damage? Stroke. 2013;44:1720–2. doi: 10.1161/STROKEAHA.113.000964. [DOI] [PubMed] [Google Scholar]
- 19.Liebeskind DS, Sanossian N, Yong WH, Starkman S, Tsang MP, Moya AL, et al. CT and MRI early vessel signs reflect clot composition in acute stroke. Stroke. 2011;42:1237–43. doi: 10.1161/STROKEAHA.110.605576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fitzgerald S, Dai D, Wang S, Douglas A, Kadirvel R, Layton KF, et al. Platelet-rich emboli in cerebral large vessel occlusion are associated with a large artery atherosclerosis source. Stroke. 2019;50:1907–10. doi: 10.1161/STROKEAHA.118.024543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sporns PB, Hanning U, Schwindt W, Velasco A, Buerke B, Cnyrim C, et al. Ischemic stroke: Histological thrombus composition and pre-interventional CT attenuation are associated with intervention time and rate of secondary embolism. Cerebrovasc Dis. 2017;44:344–50. doi: 10.1159/000481578. [DOI] [PubMed] [Google Scholar]


