Abstract
In 1949, Cade described “sedative effects” after injecting guinea pigs intraperitoneally with lithium (LTM) carbonate. Based on his experiments, he began treating psychiatric patients with LTM. This literature review aims to evaluate the clinical epidemiological profile, pathological mechanisms, and management of LTM-associated movement disorder (MD). Relevant reports in six databases (Excerpta Medica, Google Scholar, Latin American and Caribbean Health Sciences Literature, Medline, Scientific Electronic Library Online, and ScienceDirect) were identified and assessed by two reviewers without language restriction from 1949 to 2021. A total of 250 reports containing 1100 individuals who developed MD associated with LTM were identified. The MDs encountered 148 parkinsonism (PKN), 114 dyskinesia (DKN), 97 myoclonus, 22 dystonia (DTN), 20 Creutzfeldt–Jakob-like syndrome, 11 akathisia, 10 restless legs syndrome (RLS) symptoms, 6 tics, 5 cerebellar syndromes, and 3 stuttering. In the subgroup of cases not clearly defined, there were 320 individuals with extrapyramidal symptoms, 135 with DTN, 37 with DKN, 24 with PKN, and 7 with RLS. Other 141 individuals were only described as presenting an abnormal involuntary movement without further explanation. The mean age was 53.06 years (standard deviation [SD]: 15.64) and the predominant sex was female, i.e., 56.20% (154/274). The mean LTM dose was 963.03 mg/day (SD: 392.03). The mean serum LTM level was 1.53 mEq/L (SD: 1.08). The median onset time was 3 months (1 day to 40 years). The mean recovery time was 0.94 months (SD: 0.87). 45.94% had a full recovery. LTM-induced MD was extensively reported in the literature. Only general terms were used in the majority of the reports. LTM polytherapy probably affected the identification of the MD cause.
Keywords: Bipolar disorder, drug-induced, lithium, movement disorder, review
Introduction
Lithium (LTM) is an alkali metal and the lightest solid element (atomic number 3). There are many LTM salts, such as citrate, carbonate, and sulfate. Therapeutically, the most commonly used form is LTM carbonate. LTM does not occur freely in nature but can be found in pegmatite minerals. It is also present in springs and ocean waters because of its solubility as an ion and is frequently obtained from brines.[1]
The first major application of LTM was in the World War II, where it was used in greases for aircraft engines because this metal has the highest specific heat of any solid element.[1]
In medicine, LTM was used since 200 AD, when Greek physicians recommended alkaline water for psychiatric conditions. The use of LTM became a subject of study during the 19th century when James Parkinson created a pathogenic hypothesis based on “uric acid diathesis,” which stated that an excess of uric acid in the urine was responsible for many diseases, including depression and mania, also known as “brain gout.” Scientists performed experimental studies with LTM urate and demonstrated that it could dissolve uric acid deposits in the urinary tract and around the joints.[2]
Garrod published a study on gout in 1876 where he described “gouty mania,” which was characterized by psychiatric manifestations. He also recommended the use of LTM to treat this condition.[3]
In 1949, Cade described “sedative effects” after injecting guinea pigs intraperitoneally with LTM carbonate. Based on his experiments, he began treating 10 psychiatric patients with LTM. Years later, researchers thought that his initial experimental conclusions about LTM were accidental and that he probably noticed toxic instead of therapeutic effects in the guinea pigs. Even so, in his clinical observations with psychiatric patients, he documented significant improvement of mania.[4]
In 1954, the Danish psychiatrist Schou published the first randomized clinical trial with LTM treating 38 manic patients. Schou highlighted in his study the beneficial effects of LTM in manic-depressive psychosis. He also pointed out that the LTM mood-stabilizing properties differed from simple sedation. Patients experienced return to their previous level of functioning without the severe adverse effects they experienced when using barbiturates in high dosages, which was the standard treatment for psychosis at the time. Schou et al. also reported symptoms of LTM intoxication; “the most frequent toxic symptoms were nausea and vomiting, diarrhea, tremor of the hands, a feeling of general fatigue, and slight drowsiness. Occasionally, we observed blurred vision, vertigo, uncertain gait, slight confusion, and dryness of the mouth.”[2]
The study by Schou opened the venue for many subsequent clinical trials with LTM, and the drug started being studied extensively in psychiatry. Further studies showed that LTM has a narrow therapeutic index, with a therapeutic dose close to the toxic dose. Thus, patients require regular monitoring of LTM levels.[5]
Over the years, LTM demonstrated remarkable effectiveness in the maintenance treatment of bipolar disorders, preventing recurrent manic and depressive episodes, being considered by many psychiatrists as the mood stabilizer of choice.[6] Moreover, LTM has independent antisuicidal properties, which is essential considering the increased risk of suicide in psychiatric disorders.[7]
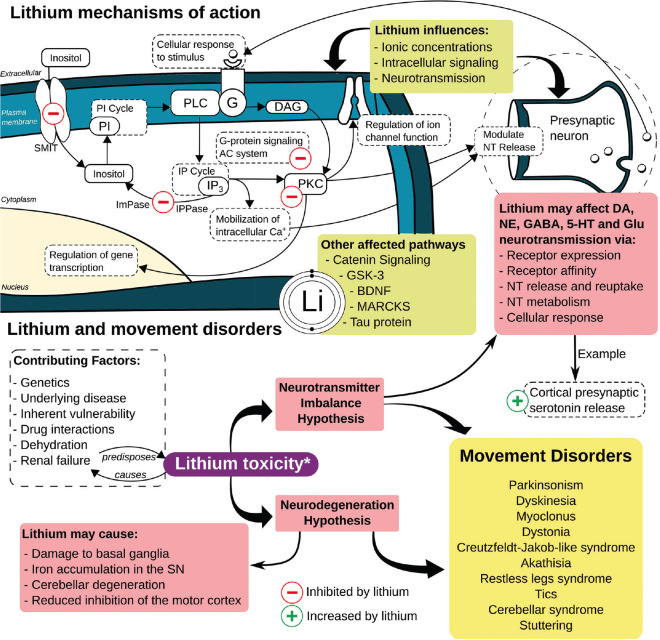
LTM's exact mechanisms of action in psychiatry are unknown. Unfortunately, LTM is significantly underpublicized, probably due to its low cost and the fact that it was never patented. According to international surveys, the percentage of LTM use in bipolar disorder in the USA is 17%, while it is 50% in other countries.[8] Nevertheless, preliminary studies suggested that inositol monophosphatase and magnesium-dependent phosphomonoesterases are direct LTM targets. It is also hypothesized that LTM inhibits glycogen synthase kinase-3 (GSK-3) directly and indirectly, while the latter occurs by inhibitory Ser21/9 phosphorylation. Valproic acid and ketamine also inhibit GSK-3 indirectly through Ser21/9 phosphorylation, suggesting a common mechanism involving GSK-3 [Figure 1].[8,9]
Figure 1.
Lithium mechanism of action and Lithium-induced movement disorders. 5-HT: 5-hydroxytryptamine (serotonin), BDNF: Brain-derived neurotrophic factor, DA: Dopamine, DAG: Diacylglycerol, G: G protein, GSK-3: Glycogen synthase kinase 3, ImPase: Inositol monophosphatase, IP: Inositol phosphate, IPPase: Inositol polyphosphate phosphatase, GABA: Gamma-aminobutyric acid, Glu: Glutamate, MARCKS: Myristoylated alanine-rich C kinase substrate, NE: Norepinephrine, NT: Neurotransmitter, PI: Phosphatidylinositol, PKC: Protein kinase C, PLC: Phospholipase C, SMIT: Sodium-myo-inositol co-transporter, SN: substantia nigra
In this context, movement disorders (MDs) secondary to LTM are not always easily diagnosed and treated. Psychiatric patients and their caregivers may have difficulties reporting symptoms. In this way, this literature review aims to evaluate the clinical epidemiological profile, pathological mechanisms, and management of LTM-associated MDs.
Methods
Search strategy
We performed a search on six databases to locate all the existing reports on MDs secondary to LTM published from 1949 to 2021 in electronic form. Excerpta Medica, Google Scholar, Latin American and Caribbean Health Sciences Literature, Medline, Scientific Electronic Library Online, and ScienceDirect were searched. Search terms were “parkinsonism, tics, dyskinesia, dystonia, stuttering, myoclonus, restless legs syndrome, akathisia, tremor, chorea, restlessness, ataxia, ballism, hyperkinetic, hypokinetic, bradykinesia, movement disorders.” These terms were combined with “lithium” [Supplemental Table 1].
Supplemental Table 1.
FreeText and MeSH search terms in the US National Library of Medicine
| Category | Search terms | Results |
|---|---|---|
| PKN | (“lithium”[MeSH Terms] OR “lithium”[All Fields] OR “lithium s”[All Fields] OR “lithiums”[All Fields]) AND (“parkinson disease”[MeSH Terms] OR (“parkinson”[All Fields] AND “disease”[All Fields]) OR “parkinson disease”[All Fields] OR “parkinsons”[All Fields] OR “parkinson”[All Fields] OR “parkinson s”[All Fields] OR “parkinsonian disorders”[MeSH Terms] OR (“parkinsonian”[All Fields] AND “disorders”[All Fields]) OR “parkinsonian disorders”[All Fields] OR “parkinsonism”[All Fields] OR “parkinsonisms”[All Fields] OR “parkinsons s”[All Fields]) | 285 |
| Tics | (“lithium”[MeSH Terms] OR “lithium”[All Fields] OR “lithium s”[All Fields] OR “lithiums”[All Fields]) AND (“tics”[MeSH Terms] OR “tics”[All Fields]) | 15 |
| DKN | (“lithium”[MeSH Terms] OR “lithium”[All Fields] OR “lithium s”[All Fields] OR “lithiums”[All Fields]) AND (“dyskinesiae”[All Fields] OR “dyskinesias”[MeSH Terms] OR “dyskinesias”[All Fields] OR “dyskinesia”[All Fields]) | 675 |
| DTN | (“lithium”[MeSH Terms] OR “lithium”[All Fields] OR “lithium s”[All Fields] OR “lithiums”[All Fields]) AND (“dystonia”[MeSH Terms] OR “dystonia”[All Fields] OR “dystonias”[All Fields] OR “dystonic disorders”[MeSH Terms] OR (“dystonic”[All Fields] AND “disorders”[All Fields]) OR “dystonic disorders”[All Fields]) | 57 |
| Stuttering | (“lithium”[MeSH Terms] OR “lithium”[All Fields] OR “lithium s”[All Fields] OR “lithiums”[All Fields]) AND (“stammerers”[All Fields] OR “stammers”[All Fields] OR “stutterer”[All Fields] OR “stutterer s”[All Fields] OR “stutterers”[All Fields] OR “stuttering”[MeSH Terms] OR “stuttering”[All Fields] OR “stammer”[All Fields] OR “stammering”[All Fields] OR “stutter”[All Fields] OR “stuttered”[All Fields] OR “stutters”[All Fields] OR “stutterings”[All Fields]) | 8 |
| MCL | (“lithium”[MeSH Terms] OR “lithium”[All Fields] OR “lithium s”[All Fields] OR “lithiums”[All Fields]) AND (“myoclonus”[MeSH Terms] OR “myoclonus”[All Fields]) | 43 |
| Restless legs syndrome | (“lithium”[MeSH Terms] OR “lithium”[All Fields] OR “lithium s”[All Fields] OR “lithiums”[All Fields]) AND (“restless legs syndrome”[MeSH Terms] OR (“restless”[All Fields] AND “legs”[All Fields] AND “syndrome”[All Fields]) OR “restless legs syndrome”[All Fields]) | 8 |
| AKT | (“lithium”[MeSH Terms] OR “lithium”[All Fields] OR “lithium s”[All Fields] OR “lithiums”[All Fields]) AND (“akathisias”[All Fields] OR “psychomotor agitation”[MeSH Terms] OR (“psychomotor”[All Fields] AND “agitation”[All Fields]) OR “psychomotor agitation”[All Fields] OR “akathisia”[All Fields]) | 153 |
| Tremor | (“lithium”[MeSH Terms] OR “lithium”[All Fields] OR “lithium s”[All Fields] OR “lithiums”[All Fields]) AND (“tremor”[MeSH Terms] OR “tremor”[All Fields] OR “tremors”[All Fields] OR “tremoring”[All Fields] OR “tremorous”[All Fields]) | 313 |
| Chorea | (“lithium”[MeSH Terms] OR “lithium”[All Fields] OR “lithium s”[All Fields] OR “lithiums”[All Fields]) AND (“chorea”[MeSH Terms] OR “chorea”[All Fields] OR “choreas”[All Fields]) | 93 |
| Restlessness | (“lithium”[MeSH Terms] OR “lithium”[All Fields] OR “lithium s”[All Fields] OR “lithiums”[All Fields]) AND (“psychomotor agitation”[MeSH Terms] OR (“psychomotor”[All Fields] AND “agitation”[All Fields]) OR “psychomotor agitation”[All Fields] OR “restlessness”[All Fields] OR “restless”[All Fields]) | 137 |
| ATX | (“lithium”[MeSH Terms] OR “lithium”[All Fields] OR “lithium s”[All Fields] OR “lithiums”[All Fields]) AND (“ataxia”[MeSH Terms] OR “ataxia”[All Fields] OR “ataxias”[All Fields]) | 118 |
| Ballism | (“lithium”[MeSH Terms] OR “lithium”[All Fields] OR “lithium s”[All Fields] OR “lithiums”[All Fields]) AND (“dyskinesias”[MeSH Terms] OR “dyskinesias”[All Fields] OR “ballism”[All Fields]) | 626 |
| Hyperkinetic | (“lithium”[MeSH Terms] OR “lithium”[All Fields] OR “lithium s”[All Fields] OR “lithiums”[All Fields]) AND (“hyperkinetic”[All Fields] OR “hyperkinetics”[All Fields]) | 12 |
| Hypokinetic | (“lithium”[MeSH Terms] OR “lithium”[All Fields] OR “lithium s”[All Fields] OR “lithiums”[All Fields]) AND (“hypokinesia”[MeSH Terms] OR “hypokinesia”[All Fields] OR “hypokinetic”[All Fields]) | 13 |
| Bradykinesia | (“lithium”[MeSH Terms] OR “lithium”[All Fields] OR “lithium s”[All Fields] OR “lithiums”[All Fields]) AND (”hypokinesia”[MeSH Terms] OR “hypokinesia”[All Fields] OR “bradykinesia”[All Fields]) | 13 |
| Movement disorder | ("lithium”[MeSH Terms] OR “lithium"[All Fields] OR “lithium s”[All Fields] OR “lithiums”[All Fields]) AND (”movement disorders”[MeSH Terms] OR (”movement”[All Fields] AND “disorders”[All Fields]) OR “movement disorders”[All Fields] OR ("movement”[All Fields] AND “disorder”[All Fields]) OR “movement disorder”[All Fields]) | 544 |
| Total | 3113 |
ATX: Ataxia, DKN: Dyskinesia, DTN: Dystonia, MCL: Myoclonus, PKN: Parkinsonism, AKT: Akathisia
Inclusion and exclusion criteria
Case reports, case series, original articles, letters to the editor, bulletins, and poster presentations published from 1949 to 2021, without language restriction to ensure a thorough review, were included. In the cases where the non-English literature was beyond the authors' proficiency (English, Portuguese, French, Spanish, and German) or when the English abstract did not provide enough data, such as articles in Polish, Dutch, Finnish, Japanese, Turkish, and Irish, Google Translate services were used.[10]
The authors independently screened the titles and abstracts of all articles found in the initial search. Disagreements between authors were solved through discussion. Cases where the cause of MD was already known and the motor symptoms were not worsened or were not related to LTM were excluded. Further, cases that were not accessible by electronic methods including after a formal request e-mailed to the authors were excluded. Cases that had more than one factor contributing to the MD were evaluated based on the probability of the event occurrence based on the Naranjo algorithm. Only adverse drug reactions classified as probable and definitive were included.
Data extraction
A total of 9,687 articles were found; 6,276 were inappropriate, and 3,161 were unrelated to the subject, duplicate, and inaccessible electronically, or provided insufficient data [Figure 2]. Data abstraction was performed. When provided, we extracted author, department, year of publication, country of occurrence, number of patients affected, LTM indication including off-label uses, time from first LTM dose till MD onset, time from LTM withdrawal to symptoms improvement, patient's status at follow-up, and important findings of clinical history and management. The data were extracted by two independent authors and double-checked to ensure matching.
Figure 2.
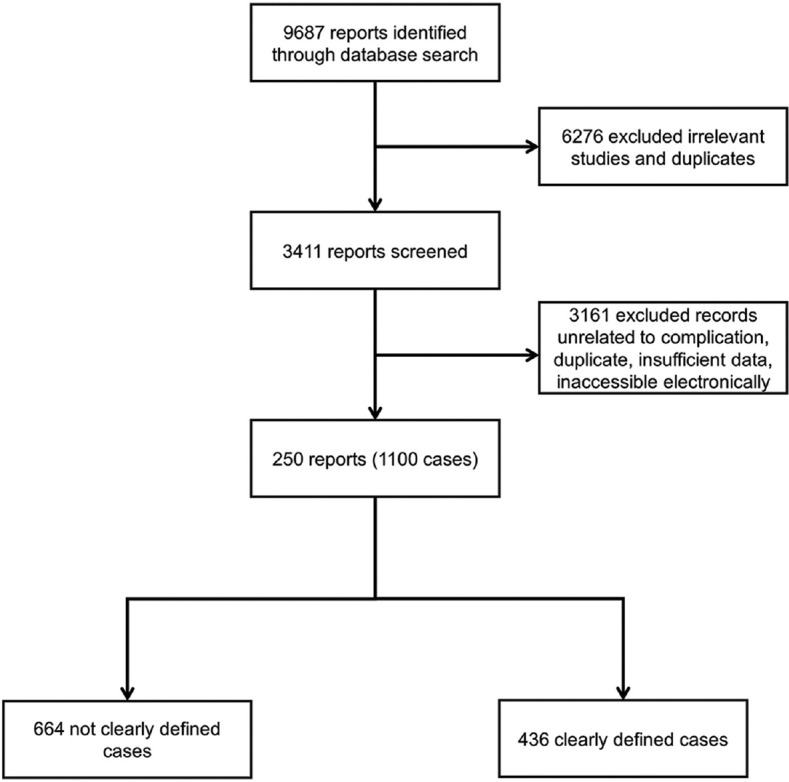
Flowchart of the screening process
Statistical analysis
Categorical variables were represented as proportions; continuous variables were represented as mean, standard deviation (SD), median, and range.
Definitions
The clinical characteristics and definitions of the MDs such as parkinsonism (PKN), tics, dyskinesia (DKN), dystonia, stuttering, myoclonus (MCL), restless legs syndrome (RLS), akathisia (AKT), tremor, chorea, ataxia, and ballism were obtained from Jankovic and Tolosa.[11] The clinical diagnosis for the psychiatric conditions was obtained from the Diagnostic and Statistical Manual of Mental Disorders (Fifth Edition).[12] The Naranjo algorithm was used for determining the likelihood of whether an adverse drug reaction was actually due to the drug rather than the result of other factors.[13]
Results
Between 1949 (December) and 2021 (June), a total of 250 reports of 1100 individuals who developed an MD associated with LTM were identified from 36 different countries [Supplemental Table 2 (12MB, pdf) ]. 830 subjects were from North American countries, 189 European, 67 Asian, 10 Australian, 3 South American, and 1 African. Figure 3 shows the number of articles published about MDs and LTM over time. The MDs cases associated with LTM encountered were 148 PKN, 114 DKN, 97 MCL, 22 dystonia (DTN), 20 Creutzfeldt–Jakob-like syndrome (CJLS), 11 AKT, 10 RLS symptoms, 6 tics, 5 cerebellar syndrome (CS), and 3 stuttering. In the subgroup of cases not clearly defined, there were 320 individuals with extrapyramidal symptoms, 135 DTN, 37 DKN, 24 PKN, and 7 RLS. Other 141 individuals were only described as presenting an abnormal involuntary movement without further explanation.
Figure 3.
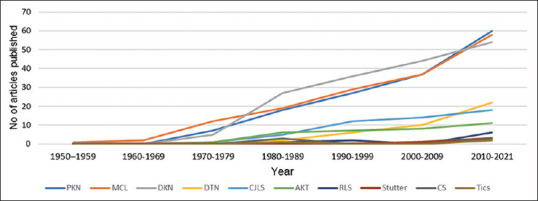
Line graph showing the cumulative number of publications regarding movement disorders and lithium throughout the decades. AKT: Akathisia, CJLS: Creutzfeldt–Jakob-like syndrome, CS: Cerebellar syndrome, DKN: Dyskinesia, DTN: Dystonia; MCL: Myoclonus; No: Cumulative number, PKN: Parkinsonism, RLS, Restless legs syndrome
The summary data about LTM-associated MDs are provided in Table 1. Herein, we describe the general data of all clearly defined cases.
Table 1.
Resume of lithium-associated movement disorder
| MD | PKN | DKN | MCL | DTN | CJLS | AKT | RLS | Tics | CS | Stutter | Others | General data |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases (%) | 148 (13.45) | 114 (10.36) | 97 (8.81) | 22 (2) | 20 (1.81) | 11 (1) | 10 (0.9) | 6 (0.05) | 5 (0.04) | 3 (0.02) | 664 (60.36) | 1100 (100) |
| Continent (%) | ||||||||||||
| Africa | 0 | 1 (0.87) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (0.09) |
| Australia | 1 (0.67) | 1 (0.87) | 6 (6.18) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 (0.30) | 10 (0.90) |
| Asia | 6 (4.05) | 17 (14.91) | 10 (10.30) | 11 (50) | 5 (25) | 4 (36.36) | 3 (30) | 0 | 4 (80) | 0 | 7 (1.05) | 67 (6.09) |
| Europe | 40 (27.02) | 26 (22.80) | 44 (45.36) | 4 (18.18) | 13 (65) | 2 (18.18) | 5 (50) | 0 | 1 (20) | 0 | 54 (8.13) | 189 (17.18) |
| N. America | 99 (66.89) | 68 (59.64) | 37 (38.14) | 7 (31.81) | 2 (10) | 5 (45.45) | 2 (20) | 6 (100) | 0 | 3 (100) | 601 (90.51) | 830 (75.45) |
| S. America | 2 (1.35) | 1 (0.87) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 (0.27) |
| Sex (%) | ||||||||||||
| Female | 47 (31.75) | 29 (25.43) | 52 (53.60) | 7 (31.81) | 11 (55) | 3 (27.27) | 3 (30) | 0 | 1 (20) | 1 (33.33) | 154 | |
| Male | 24 (16.21) | 34 (29.82) | 25 (25.77) | 13 (59.09) | 3 (15) | 7 (63.63) | 2 (20) | 6 (100) | 4 (80) | 2 (66.66) | 120 | |
| Unknown | 77 (52.02) | 51 (44.73) | 20 (20.61) | 2 (9.09) | 6 (30) | 1 (9.09) | 5 (50) | 0 | 0 | 0 | 162 | |
| Age (years) | ||||||||||||
| Rg | 34-89 | 16-81 | 20-86 | 3.5-69 | 52-78 | 37-73 | 18-51 | 8-9.6 | 32-56 | 10-86 | 3.5-89 (Md: 54.5) | |
| Mn | 54.58 | 56.50 | 53.06 | 37.37 | 67.71 | 55.7 | 40.75 | 9.33 | 42.6 | 48 | 53.06 (SD: 15.64) | |
| LTM-dose (Mn mg) | 1069.69 | 896.73 | 942.67 | 726.92 | 650 | 900 | 1000 | 300 | 1425 | 1500 | 963.03 (SD: 392.03; Rg: 250-2100; Md: 900) | |
| LTM-level (mEq/L) | 1.27 | 1.23 | 2.21 | 0.95 | 2.32 | 1.00 | 0.9 | 0.85 | 0.66 | 1.31 | 1.53 (SD: 1.08; Rg: 0.1-7.4; Md: 1.1) | |
| MD onset | ||||||||||||
| Range | 1 day-8 years | 2 days-24 years | 1 day-40 years | 1 day-25 years | 6 days-25 years | 3 days-4 years | 9 days-6 weeks | 2 months | 1 week-8 months | 1 month | 1 day-40 years | |
| Mean | 35.43 months | 21.05 months | 3.82 weeks | 1.53 weeks | 6.84 years | 10.83 days | 3.64 weeks | 2 months | 3.56 months | 1 month | 26.34 months (SD: 22.46; Md: 3 months) | |
| MD recovery | ||||||||||||
| Range | 5 days-9 months | 1 day-6 months | 1 day-3 months | 1 day-1 month | 5 days-4 weeks | 1 day-4 weeks | 1 day-2 weeks | 0 | 3 months-8 months | 2 days-2 weeks | 1 days-9 months | |
| Mean | 1.84 months | 1.42 months | 14.85 days | 8 days | 2.68 weeks | 1.43 months | 6 days | 0 | 5.5 months | 8 days | 0.94 months (SD: 0.87; Md: 0.33 months) | |
| Follow-up - % CR (number of reports) | 30 (8/26) | 42 (12/28) | 50 (18/36) | 60 (6/10) | 66 (2/3) | 50 (2/4) | 100 (3/3) | 0 | 0 (0/1) | 0 | 45.94 (51/111) |
In the “Others” subgroup, cases not specified about the movement disorder such as extrapyramidal symptoms. AKT: Akathisia, CJLS: Creutzfeldt–Jakob-like syndrome, CS: Cerebellar syndrome, DKN: Dyskinesia, DTN: Dystonia, MCL: Myoclonus, MD: Movement disorder, Md: Median, Mn: Mean, PKN: Parkinsonism, Rg: Range (minimum–maximum), RLS: Restless legs syndrome, SD: Standard deviation, CR: Complete recovery, LTM: Lithium, NA: Not available/not applicable
The mean age and median age were 53.06 (SD: 15.64) and 54.5 years (age range: 3.5–89), respectively. Subjects were predominant female, corresponding to 56.20% (154/274) of the cases. The most common indication of LTM was bipolar disorder. Some of the other LTM uses were unipolar disorder (major depression), agitated senile confusion, schizoaffective disorder, irritability, amyotrophic lateral sclerosis, to reduce hyperkinetic and hypokinetic symptoms. The mean and median doses of LTM, when the MD occurred, were 963.03 (SD: 392.03) and 900 mg/daily (LTM dose range: 250–2100), respectively. No significant relationship was found between LTM dose and age (r = −0.36) [Figure 4].
Figure 4.
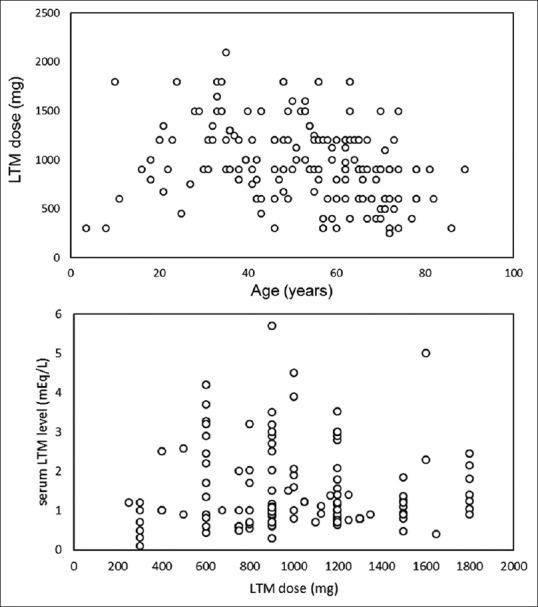
Scatterplot figures of LTM dose (mg) versus age (years) and serum LTM level (mEq/L) and LTM dose (mg). LTM: Lithium
Serum lithium concentration was reported in 279 cases, ranging from 0.1 to 7.4 mEq/L (median: 1.1 mEq/L; mean: 1.53 mEq/L). The majority of the patients were treated with LTM chronically (from months to years). No significant relationship was found between LTM serum concentration and dose (r = −0.07) Figure 4.
The mean and median time of LTM onset and LTM-associated MD were 26.34 (SD: 22.46) and 3 months (MD onset range: 1 day to 40 years). The mean and median recovery time after MD treatment were 0.94 (SD: 0.87) and 0.33 months (MD recovery range: 1 day to 9 months), in cases who achieved complete remission of the symptoms.
The MD management involved LTM discontinuation and intravenous fluid solutions according to serum electrolytes values in an attempt to decrease the toxicity of the drug. Some authors described LTM rechallenges, and they demonstrated the reoccurrence of MD.[14] Hemodialysis and peritoneal dialysis were reported. In MCL, some authors describe the use of benzodiazepines.[15] For PKN in the acute phase, benztropine was attempted.[16]
After treatment, 45.94% of the individuals had a full recovery. Three patients died at the follow-up. Lecamwasam et al. reported an individual who developed PKN symptoms and probably died from complications related to abnormal movement.[17] Campbell et al. described a suicide attempt, in which the patient developed orofacial DKN.[18] Isikay et al. showed a case of CJLS where the systemic symptoms led to poor outcomes.[19]
Discussion
General
The literature provides a large number of publications of LTM-associated MDs. In 2019, LTM was the 205th most commonly prescribed medication in the United States, with almost three million prescriptions. Furthermore, LTM is considered an essential medicine by the World Health Organization due to its efficacy, safety, and comparative cost-effectiveness.[20] Even though LTM effectiveness is well demonstrated, abnormal movements related to this drug were not thoroughly described. In the pioneer clinical trials that evaluated the side effects of LTM, only general terms were used. Table 2 summarizes the percentage of some abnormal movements secondary to LTM found in clinical trials and population-based studies.[16,21,22,23,24,25,26,27,28,29,30,31,32]
Table 2.
Incidence of some abnormal movements associated with lithium in the literature
| Reference | MD | Incidence (%) | NR | n | LTM indication |
|---|---|---|---|---|---|
| Shopsin et al. (1975) | Cogwheel rigidity alone | 59.25 | 16 | 27 | BD, unipolar, schizoaffective |
| Branchey et al. (1976) | Cogwheel rigidity + tremor | 8.33 | 3 | 36 | BD, schizoaffective |
| Action tremor alone | 41.66 | 15 | 36 | BD, schizoaffective | |
| Asnis et al. (1979) | Cogwheel rigidity alone | 39.17 | 38 | 97 | BD |
| Tremor alone | 35.05 | 34 | 97 | BD | |
| Himmelhoch et al. (1980) | DKN/PKN + dementia/confusion | 18.51 | 15 | 81 | BD |
| Lieberman et al. (1982) | Worse on–off phenomena of PKN | 8.33 | 1 | 12 | On–off phenomena |
| Perényi et al. (1983) | Tremor + rigidity | 20.83 | 5 | 24 | BD, unipolar, schizoaffective |
| Rao et al. (1983) | Tremor | 26.66 | 32 | 120 | BD, unipolar |
| Tardive DKN | 3.33 | 4 | 120 | BD, unipolar | |
| Gait disturbance | 0.83 | 1 | 120 | BD, unipolar | |
| Dyson et al. (1987) | Tremor + rigidity + bradykinesia | 3.57 | 1 | 28 | Intoxication |
| Tremor | 64.28 | 18 | 28 | Intoxication | |
| ATX | 50.00 | 14 | 28 | Intoxication | |
| Dysarthria | 35.71 | 10 | 28 | Intoxication | |
| MCL | 25.00 | 7 | 28 | Intoxication | |
| Nagaraja et al. (1987) | DKN | 0.61 | 3 | 488 | BD |
| Kerbeshian et al. (1988) | Worsening tics | 50.00 | 5 | 10 | Tourette disorder |
| Nasrallah et al. (1988) | DTN reaction | 26.08 | 12 | 46 | BD |
| Bender et al. (2004) | Oral DKN | 6.81 | 3 | 44 | Affective/aggressive symptoms |
| High-frequency hand tremor | 4.54 | 2 | 44 | Affective/aggressive symptoms | |
| MCL | 4.54 | 2 | 44 | Affective/aggressive symptoms | |
| Öhlund et al. (2018) | Tremor | 6.98 | 61 | 873 | BD |
| Restless legs | 0.57 | 5 | 873 | BD |
ATX: Ataxia, BD: Bipolar disorder, DKN: Dyskinesia, DTN: Dystonia, LTM: Lithium, MCL: Myoclonus, MD: Movement disorder, n: Number of individuals in the study using LTM, NR: Number of reports with the movement disorder, PKN: Parkinsonism
In our study, about 75% of the individuals affected by LTM-induced MDs are North American, which is interesting taking into account that newer mood stabilizers are much more publicized than LTM in the United States, and this country was one of the last to approve the drug to treat psychiatric disorders.[8] Moreover, LTM stands alone as a common therapy for the treatment of bipolar disorder in the rest of the world. Nardi et al. suggested that probably due to commercial interests rather than efficacy, LTM was left out of the treatment of many psychiatry patients, but it endured in the history of mood stabilizers due to the “test of time.”[33]
The present review demystified some of the initial beliefs around MDs secondary to LTM. First, older individuals may not be the most commonly affected by these side effects.[34,35] No correlation was found in our study between age and LTM-induced MDs. In fact, the reported cases were most frequently of middle-aged adults. Second, bipolar disorder probably is not directly associated with a higher percentage of adverse effects as reported in other studies.[36] This could be explained by the fact that bipolar disorder is the most common indication for LTM, which in the present study counted for more than 80% of the prescriptions. Nevertheless, the assumption of chronic use as a risk factor is probably true.[34,37] In our review, the majority of the individuals who developed an abnormal movement secondary to LTM used this drug for more than 2 years.
Based on the studies described in Table 1, we can present a hypothetical case report to illustrate our findings. A North American middle-aged female presented with tremor, bradykinesia, and rigidity. She was diagnosed with bipolar disorder and started on LTM (900 mg/day) 3 months ago. The psychiatrist recommended LTM withdrawal, and valproic acid was started. At the follow-up 1 month later, the patient presented amelioration of bradykinesia, but the tremor persisted.
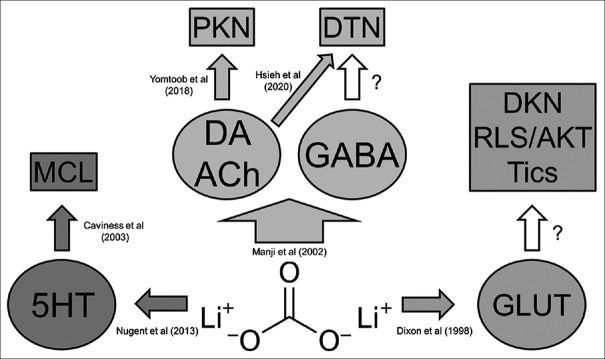
Figure 5 illustrates the pathways possibly associated with the pathophysiological mechanisms related to the LTM-induced MDs.[38,39,40,41,42,43] Herein, we discuss some of the MDs in subtopics to give a better overview of the data.
Figure 5.
Schematic diagram showing the neurotransmitter hypothesis of lithium-associated movement disorder. 5HT: 5-Hydroxytryptamine (serotonin), Ach: Acetylcholine, AKT: Akathisia, DA: Dopamine, DKN: Dyskinesia, DTN: Dystonia, GABA: Gamma-aminobutyric acid, GLUT: Glutamate, MCL: Myoclonus, PKN: Parkinsonism, RLS: Restless legs syndrome
Parkinsonism – The most common
Dalén et al. studied the efficacy of LTM in the management of hyperkinetic symptoms related to Parkinson's disease (PD). They observed that some of the individuals treated with LTM showed worsening cogwheel rigidity.[44] In the literature, PKN was the most commonly reported LTM-associated MD.
The mean age of the affected individuals was almost 55 years, which is important since it is more likely that the PKN was truly LTM-induced rather than PD. Another remarkable finding is the fact that the majority of the PKN subjects used high LTM doses. This is interesting because a similar association between high medication doses and PKN symptoms was already reported with other medications such as valproic acid.[45]
The pathophysiological mechanism of LTM-induced PKN is probably related to four main pathways. First, this salt showed to reduce the release of dopamine in the synaptic cleft of the striatum and limbic system in rat models.[41] Second, LTM has anticholinesterase activity, increasing the bioavailability of acetylcholine and causing a disbalance between dopamine–acetylcholine (DA-ACh) ratio in the basal ganglia region.[41,46] Some autopsy studies showed damage in the brain probably related to LTM, so another hypothesis could be the direct neuronal damage in dopaminergic neurons.[47] At last, the fact that the majority of the reports came from a similar population should highlight the association with a genetic predisposition.
Yomtoob et al. studied the use of dopamine transporter single-photon emission computed tomography (DAT-SPECT) imaging for distinguishing PD from drug-induced PKN. The targeted treatment according to neuroimaging features leads to beneficial outcomes. Furthermore, only one of every nine patients with LTM-induced PKN will have an abnormal DAT-SPECT, which would be a specificity of almost 90%.[43] Therefore, the neuroimage, when available, should be used to assist in establishing the most appropriate treatment.
LTM was discontinued in the majority of the patients; some subjects showed a dose-dependent side effect. In others, the PKN symptoms were related to the relapsing manic episode, which was described by the authors as not being a functional movement disorder.[48] Acute episodes of PKN were treated with benztropine showing good outcomes.[16] Nevertheless, PKN was the MD with the worst prognosis, in which only one of every three individuals achieved full recovery after clinical management. The majority of the patients remained with a fine tremor of low frequency. It is worth mentioning that individuals can present LTM-induced PKN even in the presence of normal serum LTM levels.[49] Thus, in the suspicion of this association, LTM should be promptly withdrawn without the awaiting biochemical results of its concentration.
Dyskinesia – The first
In 1949, Peters probably reported the first case of an MD secondary to LTM in the Wisconsin Medical Journal. They described a patient presenting with choreoathetosis during LTM therapy that showed a full recovery after the drug discontinuation.[50] In the literature, the clinical manifestations of DKN were chorea, choreoathetosis, orofacial DKN, Meige-like syndrome, rabbit syndrome, worsening PD dyskinetic symptoms, and tardive DKN.
DKN was reported to affect about 20% of individuals with bipolar disorder, in which aging and LTM therapy were related risk factors.[36] However, this finding occurred in patients who already used antipsychotics. Others believe that the dose of the neuroleptic drugs coadministered with LTM is the main predictor of MD rather than serum LTM level or LTM dose.[51] However, the fact that high-dose LTM showed excitotoxicity, leading to neuronal damage in the striatum and cortex, could explain the occurrence of MD even with mean serum LTM levels of 1.23 mEq/L encountered in this subgroup of patients.[39]
Myoclonus – The giant
MCL was the third most common MD reported. PKN, DKN, and MCL together represent 82.3% (359/436) of the LTM-associated MDs. The MCL was focal, multifocal, generalized, asterixis, and action. The source of MCL was cortical and subcortical. Electrodiagnostic studies revealed normal brain electrical activity and cortical activity without epileptic activity. Only some studies described electromyographic findings, which can affect our evaluation of the main MCL source.
Based on the electrodiagnostic studies, we can hypothesize that MCL could occur due to dysfunctional cerebellar output, leading to cortical hyperexcitability. Autopsy reports showed lesions in the cerebellum of individuals who presented MCL.[47] Moreover, the giant somatosensory evoked potentials are commonly reported as a remarkable MCL feature occurring with LTM monotherapy or in combination with antidepressants.[37] It is worth mentioning that this finding can occur even in the absence of any stimulus-sensitive MCL.
Another possible hypothesis besides the cerebellar output is serotonin augmentation. LTM, when added to antidepressants, showed to significantly raise the bioavailability of serotonin throughout the brain cortex.[42] Therefore, these increased serotonin levels could indirectly contribute to cortical hyperexcitability in some predisposed individuals due to chronic LTM exposure and other comedications.[38]
The most common management reported included discontinuation of the offending medication, intravenous fluid, and benzodiazepines.
Dystonia – The youngest
The individuals who developed DTN secondary to LTM were significantly younger than the subjects presenting other abnormal movements. Further, the recovery time after the management was the shortest, taking place within 1 week. These clinical findings were already reported with other medications such as bupropion,[52] cinnarizine, and flunarizine.[53]
DTN individuals presented with the subtypes: blepharospasm, worsening Pisa syndrome, bruxism, oromandibular, distal segmental, cervical, axial, lingual, and even status dystonics. Interestingly, the genetic factor appears to strongly contribute to the development of DTN. One of every two individuals was from Asia.
A possible mechanism explaining DTN could be related to the DA-ACh balance. Pharmacological studies showed that LTM increases acetylcholine concentrations by the inhibition of acetylcholinesterase, similar to LTM-induced PKN, and decreases the dopamine release in the presynaptic terminal.[41] Furthermore, other researchers revealed that chronic LTM use interferes with postsynaptic dopamine via protein kinase B and glycogen kinase-3.[54] Moreover, the fact that individuals using comedications with stronger cholinergic action had worse symptoms can support this finding.[40]
We would like to highlight the possible association of the γ-aminobutyric acid (GABA) in the pathogenesis of this abnormal movement, even though the gabaergic effect has not been reported by any of the authors.[55] In rat models, LTM significantly increases the GABA levels, mainly facilitating the release of GABA and upregulating the GABA-beta receptors.[56] In this way, we postulate that the inhibitory effects of this neurotransmitter interrupted both the direct and indirect pathways. However, the indirect pathway subactivity predominates leading to a disruption in the thalamocortical drive causing sustained muscle contractions. This mechanism was already reported in association with anticonvulsants such as topiramate.[57]
Creutzfeldt–Jakob-like syndrome – The diverse
CJLS and LTM association was not uncommonly reported in the literature. This could be explained by the fact that LTM can be neurotoxic, causing dementia and MDs.[58] The abnormal movements reported were diverse: tremor, PKN, MCL (most common), CS, and choreoathetosis. The majority of the patients had more than 60 years, which could have misled the diagnosis of LTM-induced dementia. Statistically, CJLS had the best prognosis, but this could not be assumed because we only have the report of recovery in approximately 10% of the cases. Moreover, it was already noted that patients who develop neuronal damage related to LTM have the worst prognosis.
Akathisia, restless legs syndrome, tics, cerebellar syndrome, stuttering – The rarest
AKT was not commonly reported in the literature. The fact that the majority of patients used polytherapy probably affected the identification of the AKT cause. Demir et al. and Scorr et al. revealed that their diagnoses were only assumed due to the use of LTM monotherapy in their study.[59,60] It is noteworthy that AKT secondary to LTM usually occurs days after the onset of LTM therapy. Yet, the recovery period after the MD management generally takes months. The pathophysiology of AKT may be related to the several interactions that LTM has different receptors, which explain the challenge to diagnose and differentiate AKT from RLS.[56] This overlapping is also known as AKT/RLS complex, and it was already proposed in patients who developed mirtazapine-associated MDs.[61]
Öhlund et al. studied the reasons for LTM therapy discontinuation in a retrospective cohort study.[32] They observed that about 1% of patients answered RLS symptoms as the cause. This is intriguing considering that, in the literature, there is only one reported case of LTM monotherapy causing RLS, two cases of RLS symptoms worsened by LTM, and two cases of LTM polytherapy causing RLS symptoms. The majority of the patients appear to have a good prognosis with an important percentage achieving full recovery.
The development of tics secondary to LTM was only described in the pediatric population. Kerbeshian and Burd documented a slight increase in tics frequency when LTM was introduced.[29] Potter et al. did not clearly describe the movement, but it occurred in a child on complex polytherapy.[62] Another interesting fact is that all the individuals had a diagnosis of attention-deficit hyperactivity disorder. A possible mechanism for tics can be postulated based on glutamate.[39] LTM is demonstrated to increase glutamate levels, which is related to the development of tics. The same pathophysiologic mechanism was already proposed for anticonvulsant drugs such as carbamazepine and lamotrigine.[63]
Five cases of CS were found. These individuals only had cerebellar symptoms at presentation including cerebellar ataxia, dysarthria, and nystagmus.[47] All of them were reported in the same year, which could be related to a biased observation. Further, Tesio et al. reported an Italian individual presenting with CS who further developed coma. They ingested a large amount of LTM in a suicide attempt.[64]
Stuttering secondary to LTM was observed in three cases. The description of stuttering and DTN is important for the development of hypothetical mechanisms. This is because they have almost contradictory pathophysiological mechanisms.[45] Therefore, we can postulate that the LTM-induced abnormal movements can occur differently depending on specific factors related to the individual per se.[56]
Conclusion
In sum, the MDs associated with LTM are, in order of frequency, PKN, DKN, MCL, DTN, CJLS, AKT, RLS, tics, CS, and stuttering. The mechanisms underlying these MDs are probably related to the neurotransmitters involved with the LTM complex mechanism of action. PKN may be associated with DA-ACh disbalance; DTN with DA-ACh disbalance and/or gabaergic effect; MCL with serotonin; and DKN, AKT-RLS, and tics with glutamate. In the literature, frequently, only general terms were used to describe abnormal movements. LTM polytherapy probably affected the identification of the MD cause, especially in AKT. Future studies need to further describe the clinical picture and the outcomes of each movement disorder to improve the management of patients affected by these conditions.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Clinical reports of lithium-associated movement disorder
Acknowledgments
We would like to acknowledge Dr. Alessandra Tomazeli (Faculdade de Medicina - Universidade Federal da Fronteira Sul) and Dr. Lucas Thomazi Ferron (Lucas Thomazi Ferron - Universidade de Passo Fundo).
References
- 1.PubChem. Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information; 2004. [Last accessed on 2021 Nov 23]. PubChem Element Summary for Atomic Number 3, Lithium. Available from: https://pubchem.ncbi.nlm.nih.gov/element/Lithium . [Google Scholar]
- 2.Schou M, Juel-Nielsen N, Stromgren E, Voldby H. The treatment of manic psychoses by the administration of lithium salts. J Neurol Neurosurg Psychiatry. 1954;17:250–60. doi: 10.1136/jnnp.17.4.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garrod AB. The Nature and Treatment of Gout, and Rheumatic Gout. Philadelphia: Longmans & Company; 1876. [Google Scholar]
- 4.Cade JF. Lithium salts in the treatment of psychotic excitement. Med J Aust. 1949;2:349–52. doi: 10.1080/j.1440-1614.1999.06241.x. [DOI] [PubMed] [Google Scholar]
- 5.Johnson FN, Amdisen A. The first era of lithium in medicine. An historical note. Pharmacopsychiatria. 1983;16:61–3. doi: 10.1055/s-2007-1017450. [DOI] [PubMed] [Google Scholar]
- 6.Jauhar S, Young AH. Controversies in bipolar disorder; role of second-generation antipsychotic for maintenance therapy. Int J Bipolar Disord. 2019;7:10. doi: 10.1186/s40345-019-0145-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barjasteh-Askari F, Davoudi M, Amini H, Ghorbani M, Yaseri M, Yunesian M, et al. Relationship between suicide mortality and lithium in drinking water: A systematic review and meta-analysis. J Affect Disord. 2020;264:234–41. doi: 10.1016/j.jad.2019.12.027. [DOI] [PubMed] [Google Scholar]
- 8.Pérez de Mendiola X, Hidalgo-Mazzei D, Vieta E, González-Pinto A. Overview of lithium's use: A nationwide survey. Int J Bipolar Disord. 2021;9:10. doi: 10.1186/s40345-020-00215-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Snitow ME, Bhansali RS, Klein PS. Lithium and therapeutic targeting of GSK-3. Cells. 2021;10:255. doi: 10.3390/cells10020255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Vries E, Schoonvelde M, Schumacher G. No longer lost in translation: Evidence that google translate works for comparative bag-of-words text applications. Polit Anal. 2018;26:417–30. [Google Scholar]
- 11.Jankovic J, Tolosa E. Parkinson's Disease and Movement Disorders. Philadelphia: Lippincott Williams & Wilkins; 2007. [Google Scholar]
- 12.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- 13.Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239–45. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- 14.Pikard JL, Oliver D, Saraceno J, Groll D. Lithium: Contributor to movement disorder sensitivity after anoxic brain injury? SAGE Open Med Case Rep. 2019;7 doi: 10.1177/2050313X18823101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosen PB, Stevens R. Action myoclonus in lithium toxicity. Ann Neurol. 1983;13:221–2. doi: 10.1002/ana.410130232. [DOI] [PubMed] [Google Scholar]
- 16.Shopsin B, Gershon S. Cogwheel rigidity related to lithium maintenance. Am J Psychiatry. 1975;132:536–8. doi: 10.1176/ajp.132.5.536. [DOI] [PubMed] [Google Scholar]
- 17.Lecamwasam D, Synek B, Moyles K, Ghose K. Chronic lithium neurotoxicity presenting as Parkinson's disease. Int Clin Psychopharmacol. 1994;9:127–9. doi: 10.1097/00004850-199400920-00010. [DOI] [PubMed] [Google Scholar]
- 18.Campbell WG, Raskind MA, Gordon T, Shaw CM. Iron pigment in the brain of a man with tardive dyskinesia. Am J Psychiatry. 1985;142:364–5. doi: 10.1176/ajp.142.3.364. [DOI] [PubMed] [Google Scholar]
- 19.Işikay CT, Yığıt A, Çelık T. Creutzfeldt-Jakob-like EEG changes in a case of fatal lithium toxicity. J Neurol Sci. 2011;28:604–8. [Google Scholar]
- 20.ClinCalc. The Top 300 of 2019: ClinCalc DrugStats Database. 2019. [Last accessed on 2021 Nov 23]. Available from: https://clincalc.com/DrugStats/Top300Drugs.aspx .
- 21.Branchey MH, Charles J, Simpson GM. Extrapyramidal side effects in lithium maintenance therapy. Am J Psychiatry. 1976;133:444–5. doi: 10.1176/ajp.133.4.444. [DOI] [PubMed] [Google Scholar]
- 22.Asnis GM, Asnis D, Dunner DL, Fieve RR. Cogwheel rigidity during chronic lithium therapy. Am J Psychiatry. 1979;136:1225–6. doi: 10.1176/ajp.136.9.1225. [DOI] [PubMed] [Google Scholar]
- 23.Himmelhoch JM, Neil JF, May SJ, Fuchs CZ, Licata SM. Age, dementia, dyskinesias, and lithium response. Am J Psychiatry. 1980;137:941–5. doi: 10.1176/ajp.137.8.941. [DOI] [PubMed] [Google Scholar]
- 24.Lieberman A, Gopinathan G. Treatment of “on-off” phenomena with lithium. Ann Neurol. 1982;12:402. doi: 10.1002/ana.410120416. [DOI] [PubMed] [Google Scholar]
- 25.Perényi A, Rihmer Z, Bánki CM. Parkinsonian symptoms with lithium, lithium-neuroleptic, and lithium-antidepressant treatment. J Affect Disord. 1983;5:171–7. doi: 10.1016/0165-0327(83)90010-1. [DOI] [PubMed] [Google Scholar]
- 26.Rao AV, Hariharasubramanian N, Sugumar A. A study of side effects of lithium. Indian J Psychiatry. 1983;25:87–93. [PMC free article] [PubMed] [Google Scholar]
- 27.Dyson EH, Simpson D, Prescott LF, Proudfoot AT. Self-poisoning and therapeutic intoxication with lithium. Hum Toxicol. 1987;6:325–9. doi: 10.1177/096032718700600410. [DOI] [PubMed] [Google Scholar]
- 28.Nagaraja D, Taly AB, Sahu RN, Channabasavanna SM, Narayanan HS. Permanent neurological sequelae due to lithium toxicity. Clin Neurol Neurosurg. 1987;89:31–4. doi: 10.1016/s0303-8467(87)80072-0. [DOI] [PubMed] [Google Scholar]
- 29.Kerbeshian J, Burd L. Differential responsiveness to lithium in patients with Tourette disorder. Neurosci Biobehav Rev. 1988;12:247–50. doi: 10.1016/s0149-7634(88)80052-6. [DOI] [PubMed] [Google Scholar]
- 30.Nasrallah HA, Churchill CM, Hamdan-Allan GA. Higher frequency of neuroleptic-induced dystonia in mania than in schizophrenia. Am J Psychiatry. 1988;145:1455–6. doi: 10.1176/ajp.145.11.1455. [DOI] [PubMed] [Google Scholar]
- 31.Bender S, Linka T, Wolstein J, Gehendges S, Paulus HJ, Schall U, et al. Safety and efficacy of combined clozapine-lithium pharmacotherapy. Int J Neuropsychopharmacol. 2004;7:59–63. doi: 10.1017/S1461145703003870. [DOI] [PubMed] [Google Scholar]
- 32.Öhlund L, Ott M, Oja S, Bergqvist M, Lundqvist R, Sandlund M, et al. Reasons for lithium discontinuation in men and women with bipolar disorder: A retrospective cohort study. BMC Psychiatry. 2018;18:37. doi: 10.1186/s12888-018-1622-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nardi AE, da Silva AG, Gentil Filho V. Where are lithium carbonate, typical antipsychotics, imipramine, and some other efficacious medications? Braz J Psychiatry. 2020;43:2–3. doi: 10.1590/1516-4446-2020-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fountoulakis KN, Tegos T, Kimiskidis V. Lithium monotherapy-induced tardive dyskinesia. J Affect Disord. 2019;244:78–9. doi: 10.1016/j.jad.2018.10.094. [DOI] [PubMed] [Google Scholar]
- 35.Axelsson R, Nilsson A. On the pathogenesis of abnormal involuntary movements in lithium-treated patients with major affective disorder. Eur Arch Psychiatry Clin Neurosci. 1991;241:1–7. doi: 10.1007/BF02193747. [DOI] [PubMed] [Google Scholar]
- 36.Dinan TG, Kohen D. Tardive dyskinesia in bipolar affective disorder: Relationship to lithium therapy. Br J Psychiatry. 1989;155:55–7. doi: 10.1192/bjp.155.1.55. [DOI] [PubMed] [Google Scholar]
- 37.Sarrigiannis PG, Zis P, Unwin ZC, Blackburn DJ, Hoggard N, Zhao Y, et al. Tremor after long term lithium treatment; is it cortical myoclonus? Cerebellum Ataxias. 2019;6:5. doi: 10.1186/s40673-019-0100-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Caviness JN, Evidente VG. Cortical myoclonus during lithium exposure. Arch Neurol. 2003;60:401–4. doi: 10.1001/archneur.60.3.401. [DOI] [PubMed] [Google Scholar]
- 39.Dixon JF, Hokin LE. Lithium acutely inhibits and chronically up-regulates and stabilizes glutamate uptake by presynaptic nerve endings in mouse cerebral cortex. Proc Natl Acad Sci U S A. 1998;95:8363–8. doi: 10.1073/pnas.95.14.8363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hsieh HT, Yeh YW. Dose-dependent effects of lithium treatment on the aggravation of antipsychotic-induced Pisa syndrome. Clin Neuropharmacol. 2020;43:90–1. doi: 10.1097/WNF.0000000000000388. [DOI] [PubMed] [Google Scholar]
- 41.Manji HK, Chen G. PKC, MAP kinases and the bcl-2 family of proteins as long-term targets for mood stabilizers. Mol Psychiatry. 2002;7(Suppl 1):S46–56. doi: 10.1038/sj.mp.4001018. [DOI] [PubMed] [Google Scholar]
- 42.Nugent AC, Carlson PJ, Bain EE, Eckelman W, Herscovitch P, Manji H, et al. Mood stabilizer treatment increases serotonin type 1A receptor binding in bipolar depression. J Psychopharmacol. 2013;27:894–902. doi: 10.1177/0269881113499204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yomtoob J, Koloms K, Bega D. DAT-SPECT imaging in cases of drug-induced Parkinsonism in a specialty movement disorders practice. Parkinsonism Relat Disord. 2018;53:37–41. doi: 10.1016/j.parkreldis.2018.04.037. [DOI] [PubMed] [Google Scholar]
- 44.Dalén P, Steg G. Lithium and levodopa in Parkinsonism. Lancet. 1973;1:936–7. doi: 10.1016/s0140-6736(73)91390-1. [DOI] [PubMed] [Google Scholar]
- 45.Rissardo JP, Caprara AL, Durante Í. Valproate-associated movement disorder: A literature review. Prague Med Rep. 2021;122:140–80. doi: 10.14712/23362936.2021.14. [DOI] [PubMed] [Google Scholar]
- 46.Jope RS. Anti-bipolar therapy: Mechanism of action of lithium. Mol Psychiatry. 1999;4:117–28. doi: 10.1038/sj.mp.4000494. [DOI] [PubMed] [Google Scholar]
- 47.Naramoto A, Koizumi N, Itoh N, Shigematsu H. An autopsy case of cerebellar degeneration following lithium intoxication with neuroleptic malignant syndrome. Acta Pathol Jpn. 1993;43:55–8. doi: 10.1111/j.1440-1827.1993.tb02914.x. [DOI] [PubMed] [Google Scholar]
- 48.Holroyd S, Smith D. Disabling Parkinsonism due to lithium: A case report. J Geriatr Psychiatry Neurol. 1995;8:118–9. doi: 10.1177/089198879500800208. [DOI] [PubMed] [Google Scholar]
- 49.Glass OM, Janjua AU, Hermida AP. Lithium-induced Parkinsonism in a bipolar patient. Am J Geriatr Psychiatry. 2016;24:S90. doi: 10.1017/S1041610216001101. [DOI] [PubMed] [Google Scholar]
- 50.Peters HA. Lithium intoxication producing chorea athetosis with recovery. Wis Med J. 1949;48:1075. [PubMed] [Google Scholar]
- 51.Huang SS. Prolonged dyskinesia following lithium intoxication in an elderly patient with bipolar I disorder. Kaohsiung J Med Sci. 2016;32:278–9. doi: 10.1016/j.kjms.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 52.Pitton Rissardo J, Fornari Caprara AL. Bupropion-associated movement disorders: A systematic review. Ann Mov Disord. 2020;3:86–98. [Google Scholar]
- 53.Rissardo JP, Caprara AL. Cinnarizine-and flunarizine-associated movement disorder: A literature review. Egypt J Neurol Psychiatry Neurosurg. 2020;56:1–23. [Google Scholar]
- 54.Beaulieu JM, Sotnikova TD, Yao WD, Kockeritz L, Woodgett JR, Gainetdinov RR, et al. Lithium antagonizes dopamine-dependent behaviors mediated by an AKT/glycogen synthase kinase 3 signaling cascade. Proc Natl Acad Sci U S A. 2004;101:5099–104. doi: 10.1073/pnas.0307921101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ng WX, Lau IY, Graham S, Sim K. Neurobiological evidence for thalamic, hippocampal and related glutamatergic abnormalities in bipolar disorder: A review and synthesis. Neurosci Biobehav Rev. 2009;33:336–54. doi: 10.1016/j.neubiorev.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 56.Malhi GS, Tanious M, Das P, Coulston CM, Berk M. Potential mechanisms of action of lithium in bipolar disorder. Current understanding. CNS Drugs. 2013;27:135–53. doi: 10.1007/s40263-013-0039-0. [DOI] [PubMed] [Google Scholar]
- 57.Rissardo JP, Caprara AL. Topiramate-associated movement disorder: Case series and literature review. Clin Neuropharmacol. 2020;43:116–20. doi: 10.1097/WNF.0000000000000395. [DOI] [PubMed] [Google Scholar]
- 58.Helmchen H, Kanowski S. EEG-changes during lithium therapy. Nervenarzt. 1971;42:144–8. [PubMed] [Google Scholar]
- 59.Demir B, Sancaktar M, Altindag A. Lithium-induced treatment-resistant akathisia: A case report and literature overview. Clin Neuropharmacol. 2021;44:112–3. doi: 10.1097/WNF.0000000000000453. [DOI] [PubMed] [Google Scholar]
- 60.Scorr L, Factor S. Non-tardive orofacial akathisia with dental sensory phenomenon: 719. Mov Disord. 2015;30:285. [Google Scholar]
- 61.Rissardo JP, Caprara AL. Mirtazapine-associated movement disorders: A literature review. Tzu Chi Med J. 2020;32:318–30. doi: 10.4103/tcmj.tcmj_13_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Potter PO, John N, Coffey DB. Onset of abnormal movements and cardiovascular symptoms after acute change in complex polypharmacy in a child with attention-deficit/hyperactivity disorder and mood symptoms. J Child Adolesc Psychopharmacol. 2012;22:388–92. doi: 10.1089/cap.2012.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rissardo JP, Caprara AL. Carbamazepine-, oxcarbazepine-, eslicarbazepine-associated movement disorder: A literature review. Clin Neuropharmacol. 2020;43:66–80. doi: 10.1097/WNF.0000000000000387. [DOI] [PubMed] [Google Scholar]
- 64.Tesio L, Porta GL, Messa E. Cerebellar syndrome in lithium poisoning: A case of partial recovery. J Neurol Neurosurg Psychiatry. 1987;50:235. doi: 10.1136/jnnp.50.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Clinical reports of lithium-associated movement disorder